Abstract
OBJECTIVE— Obesity and diabetes are characterized by the incapacity to use fat as fuel. We hypothesized that this reduced fat oxidation is secondary to a sedentary lifestyle.
RESEARCH DESIGN AND METHODS— We investigated the effect of a 2-month bed rest on the dietary oleate and palmitate trafficking in lean women (control group, n = 8) and the effect of concomitant resistance/aerobic exercise training as a countermeasure (exercise group, n = 8). Trafficking of stable isotope–labeled dietary fats was combined with muscle gene expression and magnetic resonance imaging–derived muscle fat content analyses.
RESULTS— In the control group, bed rest increased the cumulative [1-13C]oleate and [d31]palmitate appearance in triglycerides (37%, P = 0.009, and 34%, P = 0.016, respectively) and nonesterified fatty acids (NEFAs) (37%, P = 0.038, and 38%, P = 0.002) and decreased muscle lipoprotein lipase (P = 0.043) and fatty acid translocase CD36 (P = 0.043) mRNA expressions. Plasma NEFA-to-triglyceride ratios for [1-13C]oleate and [d31]palmitate remained unchanged, suggesting that the same proportion of tracers enters the peripheral tissues after bed rest. Bed rest did not affect [1-13C]oleate oxidation but decreased [d31]palmitate oxidation by −8.2 ± 4.9% (P < 0.0001). Despite a decreased spontaneous energy intake and a reduction of 1.9 ± 0.3 kg (P = 0.001) in fat mass, exercise training did not mitigate these alterations but partially maintained fat-free mass, insulin sensitivity, and total lipid oxidation in fasting and fed states. In both groups, muscle fat content increased by 2.7% after bed rest and negatively correlated with the reduction in [d31]palmitate oxidation (r2 = 0.48, P = 0.003).
CONCLUSIONS— While saturated and monounsaturated fats have similar plasma trafficking and clearance, physical inactivity affects the partitioning of saturated fats toward storage, likely leading to an accumulation of palmitate in muscle fat.
In our search of the environmental factors that fuelled the pandemic of obesity, we face a paradox. Although sedentary lifestyle has been highlighted for decades as one of the main factors triggering weight gain, the physiology of physical inactivity has received little attention (1). Clearly, the causal relationships between sedentary behaviors and obesity are essentially based on epidemiological studies or on the indirect beneficial effects of exercise training (2). None of these studies provide evidence to support a cause-and-effect relationship.
Obesity is a fat storage disease characterized by insulin resistance and a decreased capacity to oxidize lipids (3) in fasting (4) and postprandial (5) conditions. Because weight reduction was not associated with improvement in fat utilization (6), it was suggested as a primary impairment in the etiology of obesity, rather than an adaptive response. Consequently, the delineation of the causes responsible for this reduced capacity to oxidize fat appears to be a fundamental prerequisite to develop efficient strategies against obesity.
We previously extended the early Mayer hypothesis (7) and hypothesized that the decreased fat oxidation observed in obese and postobese subjects is due to the generalized adoption of sedentary behaviors (8). Using strict bed rest as a model, we showed that physical inactivity, per se (i.e., independent of the known physical inactivity-induced energy balance changes), lowers fasting and postprandial fat oxidation (9). Unexpectedly, whereas monounsaturated dietary fat (oleate) oxidation remained unaffected by bed rest, saturated fat (palmitate) oxidation decreased by 11% (9). These results are interesting when considering the north/south gradient in obesity prevalence in France that was not associated with the overall energy intake but in the greater amount of saturated fat in the diet (10).
The main objective of our present study was to investigate the mechanisms involved in metabolism of dietary fat during 2 months of bed rest in women. The key for obesity prevention is to determine the minimal volume of exercise that will restore fat oxidation. Since aerobic exercise training protocols in sedentary lean (11) or obese individuals (12,13) result in increased fat oxidation during exercise, we tested, as a second objective, the efficacy of combined aerobic and resistance exercise training on fat oxidation.
RESEARCH DESIGN AND METHODS
Sixteen women volunteered for a 60-day bed rest study. For logistical reasons, the study was divided into two sessions separated by 2 months, each involving half of the volunteers. The subjects were selected if they engaged in at least 30 min of moderate activity per day, this being achieved either with structured exercise or with activities of daily living. Athletes and extremely fit individuals were excluded. The subjects were nonsmokers, were free of clinical or biomedical diseases, and were asked to stop birth control pills 3 months before the study. The study was approved by the local institutional review board (Midi-Pyrénées I, France).
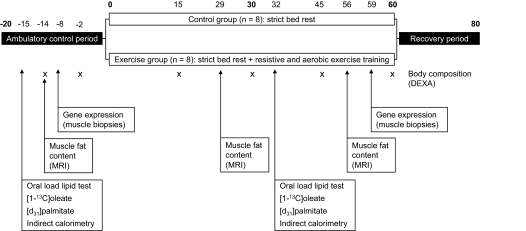
The bed rest in head-down tilt position (−6°) was preceded and followed by two 20-day periods of ambulatory control and recovery, respectively. This duration was necessary for the different teams involved in the study to complete baseline data collection. During the control period, subjects were asked, under professional supervision, to exercise in order to help offset detraining possibly occurring while living confined to the institute. After the 20-day control period, the subjects were randomly divided into a control group that remained in bed and an exercise group that was subjected to combined supine resistance and aerobic exercise training for 60 days (n = 8, each). Subjects were continuously in bed 24 h a day and standing or seated positions were forbidden. A general overview of the protocols, indicating the specific days of the tests, is illustrated in Fig. 1.
FIG. 1.
Overview of the protocols conducted during the study.
For the exercise group, resistance training was performed at maximal effort on a flywheel ergometer (14) to train the thigh muscle groups using supine squat exercises. Nineteen training sessions of 35 min were programmed every 3 days, as previously described (9,15). Aerobic training was performed three to four times per week (29 sessions of 50 ± 2 min total) on a vertical treadmill in a lower-body negative-pressure chamber, with intensities varying from 40 to 80% pre–bed rest maximal oxygen uptake, as previously described (15–17).
Throughout the experiment, we aimed to maintain energy balance. Energy requirements during control and recovery periods were calculated as resting metabolic rate (RMR) times a physical activity level of 1.4 selected for individuals with a low level of activity (18). During bed rest, a physical activity level of 1.2 was selected based on a previous bed rest experiment (19). RMR was measured twice in each period to adjust intake for changes in fat-free mass (FFM). Water intake was provided at 3 l/day. The macronutrient composition of the diet was set at 30% fat, 15% protein, and 55% carbohydrate. The subjects were asked to finish all food given. Snacks were provided to the subjects to compensate for leftovers, if any, and maintain energy balance after the exercise sessions in the exercise group and more generally when the subjects felt hungry. Fat mass (FM) and FFM were measured twice during the ambulatory period and every 15 days during the bed rest period by dual-energy X-ray absorptiometry on a QDR 4500 W scanner using the version software 11.2 (Hologic France).
Vastus lateralis biopsies were obtained before and after 59 days of bed rest, 4 h after lunch, and at least 24 h from the last exercise session, as previously described (20). Due to tissue sharing, we obtained biologic material for only five subjects in both groups. After total RNA extraction, the relative expression levels of ACADL, CD36, carnitine palmitoyl transferase 1 (CPT1) B, COX4I1, GPD1, and lipoprotein lipase (LPL) were analyzed on the ABI 7900HT Sequence Detection System, as previously described (21), and RT-PCR was performed by using a random primer from a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) to reverse the RNA. TaqMan Universal PCR Master Mix and Assays-on-Demand Gene Expression probes (Applied Biosystems) were used for the PCR step and by using the comparative amplification detection threshold of target gene expression (Ct) method for analysis. Helicase with zinc finger was taken as an internal standard. Thus, mRNA levels were measured by determining the cycle number at which Ct was reached. In each sample, Ct was normalized to helicase with zinc finger expression, performed in parallel (ΔCt). Normalized ΔCt values from each time point in the samples from “ambulatory control period” were then subtracted from each time point in the samples from “bed rest” (ΔΔCt) to determine the relative abundance values (2−ΔΔCT). Results were expressed in percentage changes from the ambulatory period.
The gastrocnemius/soleus muscle fat content was estimated 14 days before and after 29 and 56 days of bed rest from axial T1-spin echo-weighted images obtained from the superior aspect of the calcaneus to the proximal tibia of both legs (Philips Intera 1.5 T magnetic resonance imaging), as previously described (22). We used a relaxation time of 425 ms and an excitation time of 16 ms based on one excitation. The field of view was 400 mm × 400 mm, matrix size of 192 × 384, with a slice thickness of 7 mm and spacing of 1 mm.
Dietary fat oxidation and trafficking were measured 15 days before and after 32 days of bed rest. Upon waking (0600 h), an intravenous catheter was inserted into the forearm vein for arterialized blood sampling. Baseline breath and urine samples and fasting blood sample were collected. The subjects then ingested 0.4 g/kg of H218O (10% enriched; CIL, Andover, MA) to measure total body water (TBW), and, subsequently, RMR was measured for 1 h using indirect calorimetry (Deltatrac II; General Electric). Then, the participants ingested a fixed, moderately high-fat breakfast (41%), representing ∼50% of each subject's RMR in energy (i.e., 3.1 MJ in the ambulatory period for both groups and 2.7 and 3.1 MJ during bed rest for control and exercise groups, respectively). The breakfast included a liquid replacement meal in which 15 mg/kg of [d31]palmitic acid (>98% enriched; CIL) and 10 mg/kg of [1-13C]oleic acid (>99% enriched; CIL) were homogenized. Following ingestion of the meal, hourly breath and urine samples were collected for 12 h. Equilibration time for H218O was taken at 3 and 4 h postdose. During the 10 h of the test, total substrate use and nonprotein respiratory quotient (NPRQ) were measured hourly by continuous indirect calorimetry and nitrogen excretion. Blood samples were collected every hour for 10 h to assess the postprandial response of metabolites and hormones. At 1300 h, subjects were given a moderately high carbohydrate (58%) lunch (2.7 MJ in the ambulatory period for both groups and 2.7 and 2.4 MJ during bed rest for control and exercise groups, respectively).
Breath sample 13CO2-to-12CO2 ratios were measured on a continuous-flow inlet system connected to an isoprime isotope ratio mass spectrometer (GV Instruments). The oxidation rate of monounsaturated fat was inferred from the recovery of [1-13C]oleate calculated as the instantaneous recovery of 13C in expired CO2 expressed as a percentage of the dose and corrected for isotope sequestration by assuming an acetate correction factor of 51% (23). 2H/1H from urine samples was analyzed, as previously described (24). The oxidation rate of saturated fat was inferred from the cumulative recovery of 2H in TBW. The method was validated at rest and during exercise by comparison with classical 13C-labeling corrected for isotopic sequestration (25,26). 18O enrichments in TBW urine samples were decolorized by black carbon and reduced to CO by carbon reduction at 1,400°C in an elemental analyzer (Flash HT; ThermoFisher) coupled to a Delta V isotope ratio mass spectrometer. TBW was determined using the 18O isotope dilution method. The detailed calculations of the percentage recoveries are described in detail elsewhere (25).
To determine dietary [d31]palmitate and [1-13C]oleate trafficking, total lipids were extracted from plasma. NEFA and triglyceride fractions were separated by solid-phase extraction and derivatized to methyl esters (27). The absolute concentrations of the individual fatty acids were calculated by reference to internal standards. To assess both the isotopic enrichment and the individual fatty acid concentrations (both unlabeled and labeled) in the same gas chromatography/mass spectrometry (Agilent 5975 Inert XL), we designed a dual acquisition program in single-ion monitoring mode. The following m/z ratios were acquired: 296 and 297 for oleate and 270 and 301 for palmitate. The concentration of each labeled fatty acid was calculated by multiplying its molar percent enrichment (MPE) by the concentration of its corresponding unlabeled compound.
Insulin was measured by radioimmunoassay (DSL), and glucose (Biomérieux), NEFAs (Wako), and triglycerides (Biomérieux) were measured by enzymatic methods. Total fat, carbohydrate, and protein oxidation rates, as well as the NPRQ, were calculated from indirect calorimetry data and urinary nitrogen (28).
All variables were analyzed by a multiple ANOVA, with time as the repeated measure (ambulatory versus bed rest), group (control versus exercise) as main effect, and sessions as covariate. The changes in gene expressions were analyzed using a Wilcoxon rank-sign test to determine the bed rest effect (ambulatory versus bed rest) and a Mann-Whitney test to analyze the between-group differences because normality was not respected. Statistics were performed using Statistica version 7.1.515.0 (Statsoft), and reported values are means ± SD, unless otherwise stated.
RESULTS
The baseline characteristics of the volunteers, energy intake, and diet composition provided to the control and exercise groups during the study are summarized in Table 1. No between-group differences were observed at baseline.
TABLE 1.
Characteristics of the participants in the ambulatory period and body mass and composition and dietary intake evolution during bed rest
| Ambulatory period
|
30 days of bed rest
|
60 days of bed rest
|
MANOVA (P value)
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | Exercise | Control | Exercise | Control | Exercise | Bed rest effect | Group effect | Bed rest–by–group interaction | |
| Characteristics | |||||||||
| n | 8 | 8 | 8 | 8 | 8 | 8 | |||
| Age (years) | 34 ± 4 | 33 ± 4 | |||||||
| Height (m) | 1.63 ± 0.06 | 1.65 ± 0.07 | |||||||
| BMI (kg/m2) | 21.3 ± 1.4 | 21.7 ± 1.4 | |||||||
| Vo2peak (l/min) | 1.9 ± 0.4 | 2.1 ± 0.4 | |||||||
| Vo2peak (ml · kg−1 · min−1) | 34.5 ± 7.7 | 35.1 ± 4.4 | |||||||
| Body mass and composition | |||||||||
| Body mass (kg) | 55.6 ± 3.8 | 58.4 ± 6.5 | 52.9 ± 4.1 | 55.8 ± 6.0 | 52.3 ± 3.8 | 54.9 ± 6.1 | 0.0001 | NS | NS |
| FFM (kg) | 40.8 ± 3.1 | 43.8 ± 5.8 | 38.3 ± 3.2 | 42.7 ± 5.5 | 38.0 ± 3.0 | 42.5 ± 5.7 | 0.0001 | NS | 0.0006 |
| FM (kg) | 14.8 ± 3.7 | 14.5 ± 3.2 | 14.7 ± 3.8 | 13.1 ± 3.5 | 14.3 ± 3.5 | 12.4 ± 3.6 | 0.001 | NS | 0.005 |
| FM (%) | 26.4 ± 5.5 | 25.0 ± 5.0 | 27.5 ± 5.8 | 23.5 ± 5.8 | 27.1 ± 5.5 | 22.6 ± 6.2 | 0.06 | NS | 0.006 |
| Dietary intake | |||||||||
| Energy intake (MJ/day) | 7.5 ± 0.2 | 7.6 ± 0.5 | 6.5 ± 0.2 | 7.4 ± 0.8 | 6.5 ± 0.2 | 7.4 ± 0.8 | 0.0001 | 0.039 | 0.004 |
| Carbohydrates (g/day) | 259 ± 7 | 261 ± 16 | 219 ± 9 | 252 ± 27 | 219 ± 9 | 252 ± 27 | 0.0001 | 0.030 | 0.002 |
| % | 58 ± 1 | 58 ± 1 | 56 ± 1 | 57 ± 1 | 56 ± 1 | 57 ± 1 | 0.0001 | NS | 0.002 |
| Lipids (g/day) | 59 ± 2 | 59 ± 4 | 52 ± 2 | 59 ± 6 | 52 ± 2 | 59 ± 6 | 0.001 | 0.027 | 0.0018 |
| % | 29 ± 0 | 30 ± 0 | 30 ± 0 | 30 ± 0 | 30 ± 0 | 30 ± 0 | 0.0001 | NS | NS |
| Proteins (g/day) | 56 ± 4 | 59 ± 6 | 54 ± 4 | 56 ± 6 | 54 ± 4 | 56 ± 6 | 0.01 | NS | NS |
| % | 13 ± 1 | 12 ± 1 | 14 ± 1 | 13 ± 1 | 14 ± 1 | 13 ± 1 | 0.0002 | NS | 0.0001 |
Data are means ± SD. No differences were noted between groups (t test) in the ambulatory period. The effect of bed rest was determined by multiple MANOVA. The between-group differences in the energy intake corresponds to the estimated cost of the exercise training protocol. Vo2peak, peak O2 consumption.
Body composition changes.
After bed rest, body weight decreased by 4.6% in both groups (Table 1). The loss in body weight was essentially due to a 6.2% reduction of FFM in the control group but to a 9.9% decrease in FM in the exercise group. Exercise training partially counteracted the loss in FFM compared with the control group (−2.5% vs. −6.2%).
Insulin resistance and shift in substrate utilization.
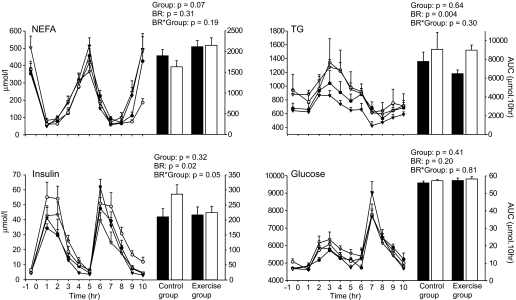
After 1 month of inactivity, we observed an increase in fasting triglycerides (37%; P = 0.025), insulin (22%; P = 0.014), and homeostasis model assessment (38%; P = 0.01) in both the control and exercise groups (Fig. 2), whereas fasting glucose and NEFAs remained unchanged. NPRQ increased from 0.791 ± 0.033 to 0.859 ± 0.030 during bed rest in the control group (P < 0.0001). This shift in fasting substrate use was mitigated by exercise training (4% increase in NPRQ vs. 9% in control group; bed rest–by–group interaction: P = 0.037) and was not explained by a shift in macronutrient composition of the diet, as the food quotient remained close to 0.88 in all subjects throughout the study.
FIG. 2.
Time course of NEFAs, triglycerides (TG), insulin, and glucose concentrations (μmol/l) in the control (n = 8) and exercise (n = 8) groups 15 days before bed rest (BR) and after 32 days of bed rest. Time 0 corresponds to the standard breakfast ingestion. The postprandial cumulative responses of these parameters were calculated by the area under the curve (AUC) over 10 h postdose. Data are means ± SE. ▪, ambulatory period; •, control group; ▾, exercise group; □, bed rest period; ○, control group; ▿, exercise group.
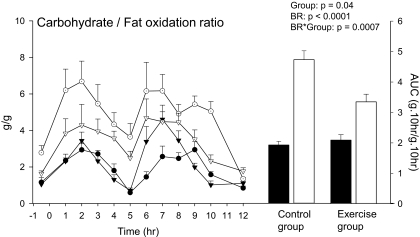
After meal ingestion, the cumulative glucose and NEFA responses did not vary and triglyceride concentration increased significantly by 27% in both groups during bed rest (Fig. 2). Bed rest induced a 36% increase in postprandial insulin concentration in the control group that was not seen following exercise training, as evidenced by a significant bed rest–by–group interaction (Fig. 2). After bed rest, the ratio of cumulative carbohydrate to fat oxidation increased significantly by 2.5-fold in the control group and by 1.6-fold in the exercise group. The significant bed rest–by–group interaction suggests, however, that exercise training partially attenuated the effect of sedentariness (Fig. 3).
FIG. 3.
Time course of the ratio between carbohydrate and fat oxidations (g/g) in the control (n = 8) and exercise (n = 8) groups 15 days before bed rest (BR) and after 32 days of bed rest. Time 0 corresponds to the standard breakfast ingestion. The ratio of the postprandial cumulative carbohydrate to cumulative fat oxidation were calculated by the area under the curve (AUC) over 10 h postdose. Data are means ± SE. ▪, ambulatory period; •, control group; ▾, exercise group; □, bed rest period; ○, control group; ▿, exercise group.
Proportion of dietary fatty acid uptake.
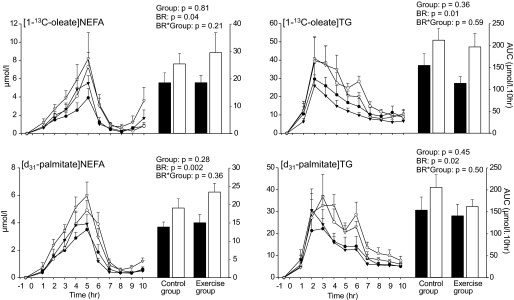
The concentrations of [1-13C]oleate and [d31]palmitate in plasma triglycerides and NEFAs were not different between the control and exercise groups (Fig. 4). Cumulative dietary labeled oleate and palmitate in triglycerides increased significantly by 51 and 25%, respectively, during bed rest in both groups. Similarly, a 1.5-fold increase in both cumulative oleate- and palmitate-labeled NEFA concentrations were noted during bed rest (Fig. 4). In the exercise and control groups, the NEFA-to-triglyceride ratio remained unchanged during bed rest for both [1-13C]oleate (14 vs. 13% during ambulatory conditions) and [d31]palmitate (10 vs. 11%).
FIG. 4.
Time course of labeled dietary [1-13C]oleate and [d31]palmitate in triglycerides (TG) and NEFAs in the control (n = 8) and exercise (n = 8) groups 15 days before bed rest (BR) and after 32 days of bed rest. Time 0 corresponds to the standard breakfast ingestion. The cumulative responses of these parameters were calculated by the area under the curve over 10 h postdose. Data are means ± SE. ▪, ambulatory period; •, control group; ▾, exercise group; □, bed rest period; ○, control group; ▿, exercise group.
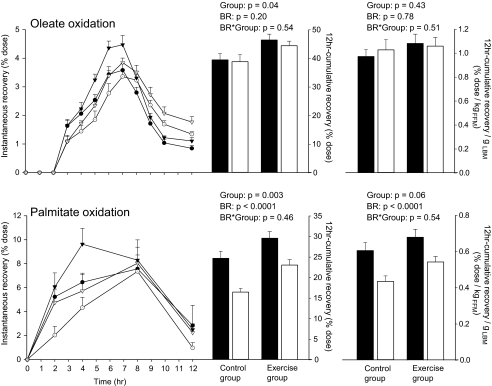
Palmitate and oleate oxidation.
An overall group effect was noted for both oleate and palmitate oxidation. The higher oxidation values observed in the exercise group were essentially accounted for by differences in initial FFM (Fig. 5). In both the exercise and control groups, bed rest decreased palmitate oxidation by −8.2 ± 4.9% and −6.4 ± 4.8% of the dose, respectively (Fig. 5). Conversely, bed rest did not affect the 12-h postdose cumulative recovery of [1-13C]oleate in either group. The differential oxidation rates between oleate and palmitate was not partly attributable to differences in the kinetics of oxidation, as suggested from the 12-h instantaneous percentage recovery curves (Fig. 5). Indeed, breath and urine samples collected 24 h postdose confirmed that in both groups oleate remained unaffected by physical inactivity (−0.70 ± 8.6%, bed rest effect: P = 0.8), whereas palmitate oxidation decreased by −6.0 ± 8.1% (bed rest effect: P = 0.01).
FIG. 5.
Hourly instantaneous percent recovery of [1-13C]oleate and [d31]palmitate in the control (n = 8) and exercise (n = 8) groups 15 days before bed rest (BR) and after 32 days of bed rest. Time 0 corresponds to the standard breakfast ingestion. Recoveries of [1-13C]oleic and [d31]palmitic acids were calculated as the instantaneous recovery of 13C in expired CO2 hourly sampled over the 12 h of the test and as the cumulative recovery of 2H in total body water hourly sampled through urine, respectively. Both of these recoveries are expressed as a percentage of the dose. The cumulative percent recoveries of [1-13C]oleate and [d31]palmitate were calculated by the area under the curve over the 12 h postdose and then normalized by kilograms of FFM. Data are means ± SE. ▪, ambulatory period; •, control group; ▾, exercise group; □, bed rest period; ○, control group; ▿, exercise group.
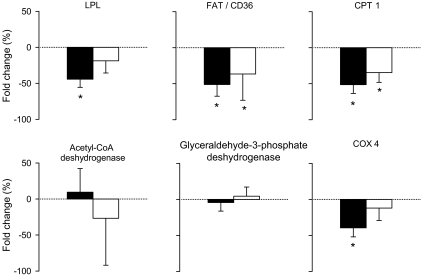
Muscle expression of key lipid metabolism proteins.
Muscle mRNA LPL expression decreased after bed rest in the control group (P = 0.043; Fig. 6) but not in the exercise group. Fatty acid translocase CD36 (FAT/CD36) (P = 0.043, for each group) and CPT1 (P = 0.043, for each group) mRNA expressions decreased in both groups, whereas mRNA expressions of ACADL and GPD1 remained unchanged. COX4 mRNA expression was decreased during bed rest in the control group (P = 0.043) but not in the exercise group. No significant group differences were noted using a Mann-Whitney test.
FIG. 6.
Bed rest–induced changes in expression of skeletal muscle LPL, fatty acid transporter CD36 (FAT/CD36), CPT1, acetyl-CoA dehydrogenase, GPD1, and COX4 mRNAs measured between 8 days before bed rest and after 59 days of bed rest and expressed in percentage of fold change in the control (n = 5) and exercise (n = 5) groups. Data are means ± SE. ▪, control group; □, exercise group. *P < 0.05 vs. ambulatory period. Group effect: NS.
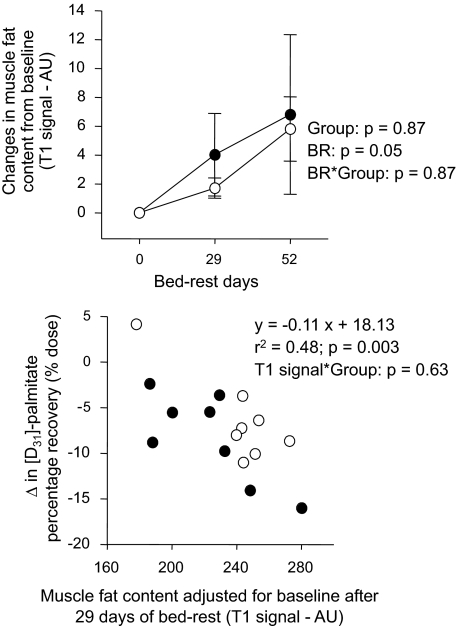
Muscle fat content.
Bed rest induced a significant 2.7% increase in muscle fat content in both groups (Fig. 7). The T1 signal values measured after 1 month of bed rest and adjusted for baseline values negatively correlated with the reduction in palmitate oxidation (Fig. 7). Both groups were combined for this analysis since no bed rest–by–group interaction was observed for both palmitate oxidation (P = 0.87) (Fig. 5) and T1 signal (P = 0.54) (Fig. 7). This demonstrates no between-group differences in the slopes of the relationships. No relation was noted between the T1 signal adjusted for baseline and the changes in oleate oxidation after bed rest (r2 = 0.02, P = 0.68). An outlier test failed to reveal extreme values in both these relationships.
FIG. 7.
Changes in the gastrosoleus muscular fat content during bed rest (BR) from ambulatory period (top). Regression analysis between bed rest–induced changes in cumulative [d31]palmitate (bottom) percentage recoveries (% dose) and the gastrosoleus muscle fat content after 1 month of bed rest. Muscle fat content was measured before bed rest and after 1 and 2 months of bed rest by magnetic resonance T1 signal intensity (arbitrary units) in the control (n = 8) and exercise (n = 8) groups. Bed rest induced a significant increase in muscle fat content in both groups (P = 0.051). After 1 month of bed rest, T1 signal adjusted for baseline values negatively correlated with the reduction in palmitate (y = −0.11 × +18.13) (r2 = 0.48, P = 0.003). Data are means ± SE. ○, control group; •, exercise group.
DISCUSSION
The present study extends our previous results obtained during a 90-day bed rest in men (9) to women. Extreme physical inactivity, independent of changes in energy balance and macronutrient intake, induces hypertriglyceridemia, a decrease in insulin sensitivity, and a decrease in total lipid oxidation in favor of an increase in total carbohydrate oxidation in both fasted and fed states. As in men, inactivity decreases saturated but not monounsaturated fatty acid oxidation. The present study suggests some mechanisms involved in these metabolic alterations.
Bed rest induces an increase in plasma triglyceride concentrations, which may be due to a greater lipid absorption in enterocytes and/or a lower clearance of triglycerides. The head-down position used to simulate microgravity may induce a larger blood volume in the visceral areas (29), leading to greater macronutrient absorption. Our data suggest that hydrolysis of triglyceride-rich lipoproteins by LPL may be reduced by bed rest, which is caused by both muscle atrophy and the reduction in muscle LPL gene expression. Using the hindlimb tail rat model, Bey and Hamilton (30) already demonstrated that acute or chronic periods of inactivity decrease muscle LPL activity. The chylomicron-released NEFAs can either enter the plasma pool or the peripheral cells (adipocyte or myocyte). During physical inactivity, we observed a greater spillover of the dietary NEFAs, indicating a lower uptake by the peripheral tissues. At the muscle level, FAT/CD36 gene expression dropped during bed rest, which reduces muscle uptake of NEFAs, as was previously reported in inactive muscle of rats (31). Despite the greater dietary NEFA spillover, we did not observe greater plasma NEFA concentrations. Both the higher insulin concentration in the postprandial period acting on the adipose tissue and the VLDL metabolism may be involved. The NEFA pool constitutes the major source for VLDL synthesis in the liver (32). Because our volunteers developed insulin resistance during bed rest, it is likely that the lower NEFA concentration and the higher triglyceride concentration are interrelated and involve VLDL synthesis. In support of that, Hodson et al. (33) showed that the higher triglyceride concentrations observed in the insulin-resistant male subjects is attributed to a higher postprandial VLDL concentration. Further investigations are required to delineate the chylomicrons and VLDL metabolism during physical inactivity.
Extreme physical inactivity induces a shift in substrate use with a decrease in lipid oxidation in favor of carbohydrate oxidation in both fasting and fed states. This shift was observed during all bed rest studies and seems unrelated to energy balance as it was observed in conditions of positive, stable, or negative energy balance (34). This phenomenon is partially due to the muscle atrophy associated with a shift in the muscle fiber types characterized by a decrease in the oxidative twitch fibers (myosin heavy chain [MHC]-I and MHCIIa) and an increase in the glycolytic twitch fibers (MHCIIx) (35,36). Such a fiber type pattern with a higher proportion of glycolytic fibers in skeletal muscle was observed in obese and diabetic subjects (37). Moreover, the decrease in CPTI mRNA expression could likely explain the blunted fat oxidation. Using the paradigm of suspension in rat and Affimetrix technology, Stein et al. (38) showed an increase in glycolytic capacity and a drop in oxidative capacity in atrophied slow-type soleus muscle. In our study, we failed to show such changes in GPD1 and ACADL gene expressions in the mixed-muscle type vastus lateralis. Further investigations are required to better understand the physical inactivity–induced impairments of the mechanisms regulating the metabolism of carbohydrates and lipids.
Physical inactivity does not affect dietary oleate oxidation but decreases palmitate oxidation by −8%, which is in accordance with the 11% decrease observed in the 3-month bed rest in men (9). In the present study, labeled oleate and palmitate incorporation into the NEFA and triglyceride fractions in the ambulatory and bed rest periods were similar, suggesting that oleate and palmitate present a similar absorption rate, plasma trafficking, and clearance as already reported by Evans et al. (39) under normal conditions. The labeled NEFA-to-triglyceride ratio can be considered an index of the proportion of NEFAs from LPL-mediated hydrolysis of triglyceride-rich lipoprotein entering the NEFA pool. In our study, the ratios indicated a similarity of the two dietary fatty acids with regard to uptake by the peripheral tissues in both ambulatory and bed rest conditions.
To our knowledge, no differences between oleate and palmitate binding affinities were reported in the human fatty acid binding proteins (FABPs) studied so far, including liver, muscle (40), and adipose tissue (41). Furthermore, studies that have measured fasting fractional uptake of fatty acids by heart (42), liver, or forearm (43) have failed to demonstrate any difference between palmitate and oleate. Taken together, these results suggest that the differential metabolism between the dietary saturated and monounsaturated fats is more likely due to differences in the handling between storage and oxidation within the myocytes than to a differential plasma trafficking.
Interestingly, we found a significant correlation between the decrease in palmitate oxidation and the gastrosoleus fat accumulation induced by physical inactivity, suggesting a preferential channelling of palmitate toward muscle fat. Gaster et al. (44) have previously reported similar results in cultured myotubes from diabetic subjects expressed as a reduction in palmitate oxidation and no change in oleate oxidation associated with a differential handling by myotubes: palmitate accumulates as diglycerides and triglycerides, whereas oleate accumulates as intracellular free fatty acids. The key is to understand the mechanisms of regulation, which are negatively impacted by the physical inactivity, or by type 2 diabetes, that may explain this differential oxidation and partitioning in myocytes. It was found that rat liver CPT1 has a lower affinity for oleyl-CoA than palmitoyl-CoA (45) and that enzyme activity toward oleyl-CoA was more sensitive to inhibition by malonyl-CoA than was the activity toward palmitoyl-CoA (46). Both of these findings would favor oxidation of palmitate. Recently, it has been shown that a mitochondrial isoform of glycerol-3-phosphate acyltransferase (mtGPAT) may direct the flux of fatty acids toward glycerolipid synthesis and away from β-oxidation (47). Interestingly, mtGPAT also presents a higher affinity for saturated than for monounsaturated fatty acids (47). Because both mtGPAT and CPT1 are located on the outer mitochondrial membrane, they can compete for acyl coenzyme A. In fact, AMP-activated kinase (AMPK) reciprocally regulates triglyceride synthesis and fat oxidation in liver and muscle via an inverse regulation of CPT1 and mtGPAT (48). During exercise, AMPK is activated (49) and decreases triglyceride synthesis and upregulates β-oxidation. Since muscle unloading downregulates AMPK (50), an opposite process under physical inactivity would represent a good hypothesis for explaining the fat accumulation that we measured in muscles and the negative correlation between muscle fat content and reduced saturated fat oxidation. Despite some changes in the kinetics of oleate oxidation, the daily oleate oxidation was unchanged by physical inactivity, likely due to a preferential accumulation of oleate as free fatty acids (44). Because a clear relationship is observed between intramuscular triglycerides (IMTGs) and their derivated products such as diglycerides or ceramides and the development of insulin resistance in diabetic subjects (51,52), further investigations are clearly required to better understand the relationship between IMTG and dietary fatty acids according to their nature.
In this present study, we tested the efficacy of combined resistance and aerobic exercise training, performed concomitantly with bed rest, to mitigate the deleterious effects of physical inactivity. Similar to what we observed during the 90-day bed rest performed in men (9), the bed rest–induced hypertriglyceridemia was not counteracted by exercise training. Such a lack of effect might be partially attributed to the absence of recent exercise (36 h) before the test (53). Nevertheless, the combined aerobic/resistive exercise training partially maintained the muscle mass, as did the resistance training alone, but also partially protected the muscle fiber–type pattern (15,35,36), the amount of mitochondria, and the oxidative capacity, as expressed by the maintenance of COX4 gene expression. Interestingly, contrary to the resistance exercise training performed during the previous bed rest (9), we observed that this exercise training protocol partially counteracted the shift in substrate utilization in the fasting and fed states and the higher postprandial insulin concentration observed in the control group. Consequently, the comparison of the results from these two bed rests may highlight the major role of the aerobic type exercise and its effect on energy balance in the protection of the muscle metabolic pattern. It also suggests that rather than the muscle mass, the anatomic and metabolic characteristics of the muscle may be the key factors determining macronutrient oxidation. However, only ∼50% of the capacity to use fat as fuel was maintained by exercise training, suggesting at least some metabolic alterations in the exercise group. Indeed, the exercise training only tended to mitigate the LPL gene expression changes and had no effect on the muscle FAT/CD36 or CPT1 expression alterations. Contrary to the total lipid oxidation, no beneficial effect of the exercise training was detected on the exogenous palmitate oxidation. However, the dietary palmitate oxidation normalized for FFM decreased by 20% in the exercise group compared with 29% in the control group, suggesting a slight protective effect of training. Yet, the question remains regarding how much physical activity is required to maintain both total and exogenous lipid oxidation. Lastly, the relationship between muscle fat accumulation and bed rest–induced reduced dietary palmitate oxidation was also observed in the exercise group. Because high IMTGs content was observed in both sedentary obese subjects and athletes (54), it is likely that this relationship does not have the same impact in health outcomes such as insulin resistance. However, further studies are needed to ascertain this relationship as well as to better understand the interactions between physical activity and total and exogenous lipid oxidation.
Several limitations of our study have to be considered. First, because multiple research teams participated in this study, there were limits on tissue availability and assays (i.e., no VLDL separation from chylomicrons possible). Second, the strict bed rest paradigm used in this study created a physical inactivity at the extreme level of what is typically seen in our society. Although this model is relevant to highlight mechanisms of regulation underlying the lipid metabolism, further studies are required on the general population.
Despite tight monitoring of the diet, the volunteers of the exercise group did not ingest the entire energy intake that they required to maintain their initial FM and, consequently, were in negative energy balance, which does not allow us to clearly dissociate the effect of negative energy balance on substrate use from those solely due to exercise. Indeed, based on the changes in body composition, we estimated an average energy deficit of 1.48 ± 0.54 MJ/day in the exercise group compared with 0.54 ± 0.38 MJ/day in the control group. These estimations match to 88.5 and 98.7% of the energy balance measured from intake and doubly labeled water derived total energy expenditure in the control and exercise groups, respectively. Interestingly, the negative energy balance of the exercise group was due to increased leftovers, not false energy intake prescription, suggesting a strong effect of the bed rest/countermeasure on satiety (data not shown). Nevertheless, it is important to note that the previous bed rest studies reported that the substrate shift was observed in subjects being in positive, negative, or neutral energy balance (34). This suggests that the effect of physical inactivity might be, to some extent, independent of major changes in energy balance.
The present study clearly shows that extreme physical inactivity, independent of its effects on energy balance, impacts the partitioning of saturated fatty acids toward storage versus oxidation likely via a preferential accumulation of palmitate in muscle fat. Additionally, saturated and monounsaturated fatty acids present a similar absorption rate, trafficking, and uptake by peripheral tissues independently of the physical activity level. Thus, our study provides interesting areas for future research in insulin resistance since it strongly correlated with IMTG. Further investigations are indeed necessary to better understand the mechanisms involved in the trafficking of the dietary fatty acids toward the different peripheral tissues (muscle or adipose tissue) according to their nature and in the lipid metabolism at the muscle level. Although this study also highlights the key role of the aerobic-type exercise training in the regulation of the lipid oxidation, investigations on the relationship between the type of exercise and the exogenous lipid oxidation are also required.
Acknowledgments
The global cost of the 60-day bed rest study was supported by the European, French, American, and Canadian Space Agencies. The present study was funded by grants from the Centre National d'Etudes Spatiales, the Centre National de la Recherche Française, l’Université Louis Pasteur, and the Canadian Institutes of Health Research. G.C.B. receives salary support from the British Heart Foundation.
No potential conflicts of interest relevant to this article were reported.
The authors are indebted to the administrative and medical staff of the Institute of Space Medicine for the outstanding organization of the bed rest supported by CNES, ESA, NASA, and CSA. We particularly thank Eliane Mioskowski for performing hormones and metabolites assays, Caroline Childs for her precious advice on the development of the solid phase extraction method, Martin Lecompte for Magnetic Resonance imaging, Hakim Louati and Liz Coletta for MR muscle fat measures, and Georges Favre for his help in data analysis. The most credit, however, must be given to the participants of the study.
Published ahead of print at http://diabetes.diabetesjournals.org on 18 November 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Hamilton MT, Hamilton DG, Zderic TW: Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 56: 2655–2667, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Booth FW, Gordon SE, Carlson CJ, Hamilton MT: Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol 88: 774–787, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Schutz Y, Flatt JP, Jequier E: Failure of dietary fat intake to promote fat oxidation: a factor favoring the development of obesity. Am J Clin Nutr 50: 307–314, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Colberg SR, Simoneau JA, Thaete FL, Kelley DE: Skeletal muscle utilization of free fatty acids in women with visceral obesity. J Clin Invest 95: 1846–1853, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Binnert C, Pachiaudi C, Beylot M, Hans D, Vandermander J, Chantre P, Riou JP, Laville M: Influence of human obesity on the metabolic fate of dietary long- and medium-chain triacylglycerols. Am J Clin Nutr 67: 595–601, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Faraj M, Jones P, Sniderman AD, Cianflone K: Enhanced dietary fat clearance in postobese women. J Lipid Res 42: 571–580, 2001 [PubMed] [Google Scholar]
- 7.Mayer J, Marshall NB, Vitale JJ, Christensen JH, Mashayekhi MB, Stare FJ: Exercise, food intake and body weight in normal rats and genetically obese adult mice. Am J Physiology 177: 544–548, 1954 [DOI] [PubMed] [Google Scholar]
- 8.Egger GJ, Vogels N, Westerterp KR: Estimating historical changes in physical activity levels. MJA 175: 635–636, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Bergouignan A, Schoeller DA, Normand S, Gauquelin-Koch G, Laville M, Shriver T, Desage M, Maho YL, Ohshima H, Gharib C, Blanc S: Effect of physical inactivity on the oxidation of saturated and monounsaturated dietary fatty acids: results of a randomized trial. PLoS Clin Trials 1: e27, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon C, Arveiler D, Ruidavets JB, Amouyel P, Bingham A, Schlienger JL: Evolution pondérale de 1986 à 1996 dans 3 régions françaises. Nutrition clinique et métabolisme 11(Suppl.): 325, 1997. [in French] [Google Scholar]
- 11.Achten J, Jeukendrup AE: Optimizing fat oxidation through exercise and diet. Nutrition 20: 716–727, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Wolfe RR, Kelley DE: Effects of obesity on substrate utilization during exercise. Obes Res 10: 575–584, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Van Aggel-Leijssen DP, Saris WH, Wagenmakers AJ, Senden JS, Van Baak A: Effect of exercise training at different intensities on fat metabolism of obese men. J Appl Physiol 92: 1300–1309, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Alkner BA, Tesch PA: Efficacy of a gravity-independent resistance exercise device as a countermeasure to muscle atrophy during 29-day bed rest. Acta Physiol Scand 181: 345–357, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Trappe SW, Creer A, Slivka D, Minchev K, Trappe TA: Single muscle fiber function with concurrent exercise or nutrition countermeasures during 60 days of bed rest in women. J Appl Physiol 103: 1242–1250, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Watenpaugh DE, Ballard RE, Schneider SM, Lee SM, Ertl AC, William JM, Boda WL, Hutchinson KJ, Hargens AR: Supine lower body negative pressure exercise during bed rest maintains upright exercise capacity. J Appl Physiol 89: 218–227, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Cao P, Kimura S, Macias BR, Ueno T, Watenpaugh DE, Hargens AR: Exercise within lower body negative pressure partially counteracts lumbar spine deconditioning associated with 28-day bed rest. J Appl Physiol 99: 39–44, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Food and Nutrition Board: Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids. Institute of Medicine of the National Academies, Washington, DC, National Academies Press, 2005
- 19.Gretebeck RJ, Schoeller DA, Gibson EK, Lane HW: Energy expenditure during antiorthostatic bed rest (simulated microgravity). J Appl Physiol 78: 2207–2211, 1995 [DOI] [PubMed] [Google Scholar]
- 20.Bergstrom J: Muscle electrolytes in man. Scand J Clin Lab Invest 68: 7–110, 1962 [Google Scholar]
- 21.Winer J, Jung CK, Shackel I, Williams PM: Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem 270: 41–49, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Elliott JM, Galloway GJ, Jull GA, Noteboom JT, Centeno CJ, Gibbon WW: Magnetic resonance imaging analysis of the upper cervical spine extensor musculature in an asymptomatic cohort: an index of fat within muscle. Clin Radiol 60: 355–363, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Bergouignan A, Schoeller DA, Votruba S, Simon C, Blanc S: The acetate recovery factor to correct tracer-derived dietary fat oxidation in humans. Am J Physiol Endocrinol Metab 294: E645–E653, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Schoeller DA, Colligan AS, Shriver T, Avak H, Bartok-Olson C: Use of an automated chromium reduction system for hydrogen isotope ratio analysis of physiological fluids applied to doubly labeled water analysis. J Mass Spectrom 35: 1128–1132, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Votruba SB, Zeddun SM, Schoeller DA: Validation of deuterium labeled fatty acids for the measurement of dietary fat oxidation: a method for measuring fat-oxidation in free-living subjects. Int J Obes Relat Metab Disord 25: 1240–1245, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Raman A, Blanc S, Adams A, Schoeller DA: Validation of deuterium-labeled fatty acids for the measurement of dietary fat oxidation during physical activity. J Lipid Res 45: 2339–2344, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Burdge GC, Wright P, Jones AE, Wooton SA: A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by sold-phase extraction. Br J Nutr 84: 781–787, 2000 [PubMed] [Google Scholar]
- 28.Frayn KN: Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55: 628–634, 1983 [DOI] [PubMed] [Google Scholar]
- 29.Convertino VA, Bloomfield SA, Greenleaf JE: An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc 29: 187–190, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Bey L, Hamilton MT: Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: a molecular reason to maintain daily low-intensity activity. J Physiol 551: 673–682, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koonen DP, Benton CR, Arumugam Y, Tandon NN, Calles-Escandon J, Glatz JF, Luiken JJ, Bonen A: Different mechanisms can alter fatty acid transport when muscle contractile activity is chronically altered. Am J Physiol Endocrinol Metab 286: E1042–E1049, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Barrows BR, Parks EJ: Contributions of different fatty acid sources to very low-density lipoprotein-triacylglycerol in the fasted and fed states. J Clin Endocrinol Metab 91: 1446–1452, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Hodson L, Bickerton AS, McQuaid SE, Roberts R, Karpe F, Frayn KN, Fielding BA: The contribution of splanchnic fat to VLDL triglyceride is greater in insulin-resistant than insulin-sensitive men and women: studies in the postprandial state. Diabetes 56: 2433–2441, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Stein TP, Wade CE: Metabolic consequences of muscle disuse atrophy. J Nutr 135: 1824S–1828S, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Trappe SW, Creer A, Minchev K, Slivka D, Louis E, Luden N, Trappe TA: Human soleus single muscle fiber function with exercise or nutrition countermeasures during 60 days of bed rest. Am J Physiol Regul Integr Comp Physiol R939–R947, 2008 [DOI] [PubMed]
- 36.Salanova M, Schiffl G, Puttmann B, Schoser BG, Blottner D: Molecular biomarkers monitoring human skeletal muscle fibres and microvasculature following long-term bed rest with and without countermeasures. J Anat 212: 306–318, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, Swanson MS, Houmard JA: Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 282: E1191–E1196, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Stein T, Schluter M, Galante A, Soteropoulos P, Tolias P, Grindeland R, Moran M, Wang T, Polansky M, Wade C: Energy metabolism pathways in rat muscle under conditions of simulated microgravity. J Nutr Biochem 13: 471, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Evans K, Burdge GC, Wootton SA, Clark ML, Frayn KN: Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes 51: 2684–2690, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Maatman RG, van Moerkerk HT, Nooren IM, van Zoelen EJ, Veerkamp JH: Expression of human liver fatty acid-binding protein in Escherichia coli and comparative analysis of its binding characteristics with muscle fatty acid-binding protein. Biochim Biophys Acta 1214: 1–10, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Richieri GV, Ogata RT, Kleinfeld AM: Equilibrium constants for the binding of fatty acids with fatty acid-binding proteins from adipocyte, intestine, heart, and liver measured with the fluorescent probe ADIFAB. J Biol Chem 269: 23918–23930, 1994 [PubMed] [Google Scholar]
- 42.Wisneski JA, Gertz EW, Neese RA, Mayr M: Myocardial metabolism of free fatty acids: studies with 14C-labeled substrates in humans. J Clin Invest 79: 359–366, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagenfeldt L, Wahren J, Pernow B, Raf L: Uptake of individual free fatty acids by skeletal muscle and liver in man. J Clin Invest 51: 2324–2330, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaster M, Rustan AC, Beck-Nielsen H: Differential utilization of saturated palmitate and unsaturated oleate: evidence from cultured myotubes. Diabetes 54: 648–656, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Gavino GR, Gavino VC: Rat liver outer mitochondrial carnitine palmitoyltransferase activity towards long-chain polyunsaturated fatty acids and their CoA esters. Lipids 26: 266–270, 1991 [DOI] [PubMed] [Google Scholar]
- 46.Ide T, Murata M, Sugano M: Octadecatrienoic acids as the substrates for the key enzymes in glycerolipid biosynthesis and fatty acid oxidation in rat liver. Lipids 30: 755–762, 1995 [DOI] [PubMed] [Google Scholar]
- 47.Coleman RA, Lee DP: Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res 43: 134–176, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Muoio DM, Seefeld K, Witters LA, Coleman RA: AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J 338: 783–791, 1999 [PMC free article] [PubMed] [Google Scholar]
- 49.Winder WW, Hardie DG: Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol 270: E299–E304, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Han B, Zhu MJ, Ma C, Du M: Rat hindlimb unloading down-regulates insulin like growth factor-1 signaling and AMP-activated protein kinase, and leads to severe atrophy of the soleus muscle. Appl Physiol Nutr Metab 32: 1115–1123, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Goodpaster BH, Kelley DE: Skeletal muscle triglyceride: marker or mediator of obesity-induced insulin resistance in type 2 diabetes mellitus? Curr Diab Rep 2: 216–222, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Kelley DE, Goodpaster BH, Storlien L: Muscle triglyceride and insulin resistance. Annu Rev Nutr 22: 325–346, 2002 [DOI] [PubMed] [Google Scholar]
- 53.Gill JM, Hardman AE: Exercise and postprandial lipid metabolism: an update on potential mechanisms and interactions with high-carbohydrate diets (Review). J Nutr Biochem 14: 122–132, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Goodpaster BH, He J, Watkins S, Kelley DE: Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86: 5755–5761, 2001 [DOI] [PubMed] [Google Scholar]