Abstract
OBJECTIVE— Insulin stimulates glucose uptake in skeletal muscle and adipose tissues primarily by stimulating the translocation of vesicles containing a facilitative glucose transporter, GLUT4, from intracellular compartments to the plasma membrane. The formation of stable soluble N-ethyl-maleimide–sensitive fusion protein [NSF] attachment protein receptor (SNARE) complexes between vesicle-associated membrane protein-2 (VAMP-2) and syntaxin-4 initiates GLUT4 vesicle docking and fusion processes. Additional factors such as munc18c and tomosyn were reported to be negative regulators of the SNARE complex assembly involved in GLUT4 vesicle fusion. However, despite numerous investigations, the positive regulators have not been adequately clarified.
RESEARCH DESIGN AND METHODS— We determined the intracellular localization of DOC2b by confocal immunoflorescent microscopy in 3T3-L1 adipocytes. Interaction between DOC2b and syntaxin-4 was assessed by the yeast two-hybrid screening system, immunoprecipitation, and in vitro glutathione S-transferase (GST) pull-down experiments. Cell surface externalization of GLUT4 and glucose uptake were measured in the cells expressing DOC2b constructs or silencing DOC2b.
RESULTS— Herein, we show that DOC2b, a SNARE-related protein containing double C2 domains but lacking a transmembrane region, is translocated to the plasma membrane upon insulin stimulation and directly associates with syntaxin-4 in an intracellular Ca2+-dependent manner. Furthermore, this process is essential for triggering GLUT4 vesicle fusion. Expression of DOC2b in cultured adipocytes enhanced, while expression of the Ca2+-interacting domain mutant DCO2b or knockdown of DOC2b inhibited, insulin-stimulated glucose uptake.
CONCLUSIONS— These findings indicate that DOC2b is a positive SNARE regulator for GLUT4 vesicle fusion and mediates insulin-stimulated glucose transport in adipocytes.
Insulin stimulates glucose uptake in skeletal muscles and adipose tissues primarily by stimulating the translocation of vesicles containing a facilitative glucose transporter, GLUT4, from intracellular compartments to the plasma membrane (1,2). In addition to this translocation step, membrane fusion processes are also controlled by insulin (3,4). Like other regulated exocytotic processes in many cell types, the formation of stable soluble N-ethyl-maleimide–sensitive fusion protein [NSF] attachment protein receptor (SNARE) complexes between vesicle-associated membrane protein-2 (VAMP-2) and syntaxin-4 initiates GLUT4 vesicle docking and fusion processes (5). However, the precise mechanism by which insulin regulates SNARE complex assembly remains poorly understood.
In neurons, Ca2+ triggers exocytotic membrane fusion of synaptic vesicles to the plasma membrane, and calcium sensor proteins such as synaptotagmins have critical roles in this process (6,7). Similar mechanisms result in GLUT4 vesicle fusion in adipocytes and muscle cells. Whitehead et al. (8) demonstrated, and we confirmed, that reduction of intracellular Ca2+ ([Ca2+]i) using the membrane-permeable Ca2+-chelating agent BAPTA-AM diminished insulin-stimulated glucose transport, whereas this reagent did not inhibit GLUT4 translocation to the plasma membrane (i.e., GLUT4 vesicle trafficking was not impaired) (8) (N.F., M.E., unpublished observation). These observations suggest that an appropriate intracellular Ca2+ level may be required for the final docking/fusion steps of GLUT4 vesicles in adipocytes.
The universal role of Ca2+ as a trigger for regulated exocytosis predicts the existence of conserved proteins capable of activating the fusion machinery upon binding Ca2+. Although many proteins have been suggested to play such a role, synaptotagmins have attracted the most attention as putative calcium sensor proteins functioning in regulated exocytosis (9). Synaptotagmin family proteins have tandem C2 domains at the C-terminus. These two domains, C2A and C2B, are conserved in all 13 synaptotagmins described to date and constitute Ca2+-binding modules (10). Many proteins have been identified as being involved in the GLUT4 vesicle fusion machinery in adipocytes. However, neither synaptotagmins nor other calcium sensor proteins have as yet been reported to regulate GLUT4 vesicle fusion.
We investigated, in detail, the mechanisms of Ca2+-dependent GLUT4 vesicle fusion in adipocytes. We searched for double C2 domain proteins as candidate Ca2+ sensor proteins suitable for the relatively slow (on the order of several minutes) SNARE complex formation, and we found that DOC2b bound syntaxin-4 upon insulin stimulation in an intracellular Ca2+-dependent manner and mediated GLUT4 vesicle fusion. DOC2b may be a downstream target of the insulin signal and a positive regulator of SNARE assembly involving regulated exocytosis in adipocytes.
RESEARCH DESIGN AND METHODS
Mouse DOC2a and DOC2b cDNA constructs were kindly provided by Dr. R.R. Duncan (University of Edinburgh, Edinburgh, U.K.). Mouse munc18c cDNA construct was kindly provided by Dr. T. Takuma (School of Dentistry, Health Sciences University of Hokkaido, Hokkaido, Japan).
Cell culture.
3T3-L1 fibroblasts were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) at 37°C. The cells (3–5 days after confluence) differentiated into adipocytes with incubation in the same DMEM, containing 0.5 mmol/l isobutylmethylxanthine, 0.25 μmol/l dexamethasone, and 4 μg/ml insulin, for 3 days and were then grown in DMEM with 10% FBS for an additional 5–8 days.
Plasmids and antibodies.
Wild-type DOC2b was subcloned into peGFP-C2, pDsRed2-C1 (Clontech, Palo Alto, CA), and pGEX-6P1 (GH Healthcare, Buckinghamshire, U.K.) vectors. Calcium interacting domain mutants (CIMs) of DOC2b (D157N, D163N, D297N, and D303N) were subcloned into peGFP-C2 and pGEX-6P1 vectors. We also constructed syntaxin-4 and a series of deletion mutants of DOC2b corresponding to Δmunc13 interaction domain (MID) (amino acids 36–413), ΔC2A (2–120 and 252–413), and ΔC2B (2–264) in a peGFP-C2 vector. Myc-tagged DOC2b (wild-type or CIM) was subcloned into a pcDNA3 vector. All chemically synthesized and PCR-derived DNA sequences were verified by DNA sequencing.
Rabbit polyclonal DOC2b antibody was generated against the peptide sequence CGARDDDEDVDQL specific for DOC2b isoform. This antibody was found to cross-react minimally (online appendix Fig. S1 [available at http://dx.doi.org/10.2337/db08-0303). The following antibodies were used: monoclonal anti-GLUT4 (clone 1F8) (R&D systems, Minneapolis, MN), polyclonal anti-GLUT4, anti-GST (Santa Cruz Biotechnology, Santa Cruz, CA), anti-myc (clone 9E10) (Covance, Princeton, NJ), polyclonal anti–syntaxin-4 (Synaptic Systems, Gottingen, Germany), and fluorescent-conjugated and horseradish peroxidase–conjugated secondary antibodies (Jackson Immuno Research, West Grove, PA).
DOC2b shRNA construct.
Short-hairpin RNA (shRNA) specific for mouse DOC2b was designed to have a 5′-GCCAGATGTAGACAAGAAATC-3′ sequence. Synthetic complementary single-stranded DNA of the target sequence was annealed, and the double-stranded DNA was inserted into a pcPUR+U6i cassette (11). This shRNA decreased DOC2b protein expression to 10–20% of the control level within 74 h. A same cassette encoding nonspecific scramble sequence was used as a negative control.
Preparation of recombinant adenovirus vectors.
Adenovirus producing enhanced green fluorescent protein (eGFP), myc-tagged DOC2b (wild type, CIM mutant), and shRNA (DOC2b, control) were prepared using an AdEasy adenovirus vector system according to the manufacturer's instructions (Stratagene, Cedar Creek, TX). All amplified viruses were purified by the cesium chloride centrifugation method and stored at −80°C.
Live cell imaging of DOC2b.
The peGFP-DOC2b was electroporated into 3T3-L1 adipocytes, which were then reseeded onto 0.1-mm glass-bottom dishes (Matsunami, Tokyo, Japan). At 24–48 h after electroporation, cells were serum starved for 3–4 h in DMEM and then incubated at 37°C for 2 h in Krebs-Ringer HEPES buffer (130 mmol/l NaCl, 5 mmol/l KCl, 1.3 mmol/l CaCl2, 1.3 mmol/l MgSO4, 25 mmol/l HEPES [pH 7.4]). The cells were treated with 100 nmol/l of insulin at 37°C for the time indicated and observed by laser confocal microscopy (LSM510 Pascal; Carl Zeiss, Oberkochen, Germany). For the translocation analysis, fluorescent intensities at three distinct areas in plasma membrane, cytosol, and nucleus (nine areas per cell each) of three independent cells were analyzed by Photoshop software CS2.
Yeast two-hybrid screening.
The Matchmaker Yeast Two-Hybrid System (Clontech) was used for determination of DOC2b-binding partners. Full-length DOC2b and munc18c cDNA were subcloned into a pLexA vector and full-length syntaxin-4 and munc18c cDNA into a pB42AD vector. A standard lithium acetate/single-stranded carrier DNA/polyethylene glycol method for transformation into yeast strain EGY48 (p8op-lacZ) was used, and these proteins were expressed in this strain. Transcriptional activation of LacZ was determined by an X-Gal assay. β-Galactosidase activity was detected within 16 h of reaction at 30°C.
In vitro GST pull-down assay.
Glutathione S-transferase (GST) fusion proteins of wild-type and CIM DOC2b were purified according to the manufacturer's instructions. GST syntaxin-4 was cleaved with PreScission Protease (2 units/μl) (GH Healthcare) in buffer containing 50 mmol/l Tris-HCl (pH 7.0), 150 mmol/l NaCl, 1 mmol/l EDTA, and 1 mmol/l dithiothreitol at 4°C for 16 h. At the end of incubation, cleaved syntaxin-4 protein was further purified using Amicon Ultra filter devices (Millipore, Danvers, MA).
Recombinant individual GST-DOC2bs or GST (1 μg each) were incubated with 1 μg of recombinant syntaxin-4 in 1 ml of Tris-buffered saline (20 mmol/l Tris, pH 7.4, 150 mmol/l NaCl) plus 0.5% Triton X-100 in the presence of 2 mmol/l EDTA or 1 mmol/l CaCl2 for 4–6 h. This mixture was immunoprecipitated by incubating with Glutathion Sepharose 4B (GE Healthcare) for 1 h. The precipitates were washed four times and analyzed by SDS-PAGE and immunoblotting. Approximately 5% of syntaxin-4 was pulled down by the GST-DOC2b.
Northern blotting.
Total RNAs were prepared from 3T3-L1 fibroblasts, 3T3-L1 adipocytes (days 3 and 9 of differentiation) or rat epididymal fat, and mouse brain using ISOGEN (Nippongene, Tokyo, Japan) and denatured in formaldehyde/formamide, resolved by electrophoresis, and transferred to hybond-N membranes (GH Healthcare). The membranes were hybridized with α-[32P]–labeled full-length DOC2a and DOC2b cDNAs as probes and then washed three times with 1× saline sodium citrate buffer (15 mmol/l NaCl, 15 mmol/l sodium citrate [pH 7.0], and 0.1% SDS) at 65°C. Radioisotopic measurements were conducted using a Phosphorimager FLA2000 (Fuji film, Tokyo, Japan).
Immunoprecipitation and immunoblotting.
A 10-cm plate of cells was lysed in 1 ml of lysis buffer (20 mmol/l HEPES [pH 7.2], 100 mmol/l NaCl, 25 mmol/l NaF, 1 mmol/l sodium vanadate, 1 mmol/l benzamidine, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 1 mmol/l phenylmethylsulphonyl fluoride, 1 mmol/l dithiothreitol, and 0.5% NP-40) in the presence of 2 mmol/l EDTA or 1 mmol/l CaCl2 and centrifuged for 15 min at 15,000g. The postnuclear lysates were used for the following experiments. The protein concentration was measured with a bicinchoninic acid protein assay reagent (Pierce, IL). For immunoprecipitation, the cell lysates were preincubated with protein-G/A–Sepharose at 4°C for 30 min to remove nonspecific bound proteins. Then, samples were incubated with primary antibodies at 4°C for 8–12 h followed by incubation with protein-G/A–Sepharose. Lysates and immunoprecipitates were resolved by SDS-PAGE and transferred to a polyvinylidene fluoride membrane (GH Healthcare). The membranes were incubated with primary antibodies for 8–12 h. Protein signals were visualized using horseradish peroxidase–conjugated secondary antibodies and an enhanced chemiluminescence substrate kit (GH Healthcare). The efficiencies of immunoprecipitation of the associated protein in Fig. 3C–E were ∼1, 0.5, and 0.6%, respectively. All the images in figures are representative, and we repeated the immunoblots at least three times and found similar results.
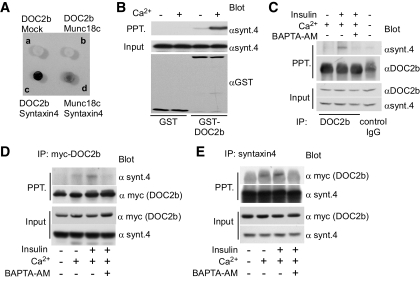
FIG. 3.
Insulin promotes Ca2+-dependent interaction between DOC2b and syntaxin-4. A: pLexA containing DOC2b or munc18c and pB42AD containing munc18c, syntaxin-4 (synt.4) or empty vectors were coexpressed in the yeast strain of EGY48 (p8op-lacZ), followed by incubation at 30°C for 16 h. Transcriptional activation of LacZ was determined by β-galactosidase assay. B: GST-tagged DOC2b and syntaxin-4 were bacterially expressed. Syntaxin-4 protein was cleaved by PreScession protease and further purified with Amicon Ultra filter devices. Both proteins (1 μg each) were mixed in Tris-buffered saline in the presence of 2 mmol/l EDTA or 1 mmol/l CaCl2 and pulled down by glutathione sepharose. The precipitates were analyzed by Western blotting with anti–syntaxin-4 and anti-GST antibodies. C: 3T3-L1 adipocytes were serum-starved for 3–4 h and pretreated with or without 50 μmol/l of BAPTA-AM before insulin treatment. The DOC2b–syntaxin-4 interaction was determined by immunoprecipitation using anti-DOC2b antibody. The immunoprecipitated proteins were immunoblotted with anti–syntaxin-4 and anti-DOC2b antibodies. D and E: Myc-tagged DOC2b was expressed by adenovirus vector in 3T3-L1 adipocytes. After serum starvation and pretreatment with or without 50 μmol/l of BAPTA-AM, before insulin treatment, the cells were stimulated with or without 100 nmol/l of insulin for 20 min and then immunoprecipitated with anti-myc or anti–syntaxin-4 antibodies. The immunoprecipitated proteins were immunoblotted with anti-myc and anti–syntaxin-4 antibodies.
Immunofluorescence microscopy and digital image analysis.
Differentiated 3T3-L1 adipocytes were left untreated or electroporated by eGFP-DOC2b (wild type, CIM, ΔMID, ΔC2A, and ΔC2B), eGFP alone, DsRed2-DOC2b (wild type, CIM), shRNAs (control, DOC2b), or myc-GLUT4-eGFP. The cells were then replated onto coverslips and allowed to recover for 48 h. Cells were preincubated in the presence or absence of 50 μmol/l of BAPTA-AM for 10 min, followed by incubation with or without insulin for 20 min at 37°C. Next, the cells were fixed with 3.7% formaldehyde in PBS and permeabilized with buffer A (0.5% Triton X-100, 1% FBS in PBS) for 15 min. For the detection of endogenous proteins, the coverslips were incubated for 2 h with primary antibodies at room temperature. The cells were washed and incubated with an appropriate secondary antibody for 30 min. The coverslips were washed thoroughly again and mounted on glass slides. Immunostained cells were observed at room temperature with an LSM 5 PASCAL laser-scanning confocal microscope and its two-channel–scanning module (Carl Zeiss) equipped with an inverted Zeiss Axiovert 200M using the 63× oil objective lens (numerical aperture 1.4) run by LSM 5 processing software and Adobe Photoshop CS2. At least five cells were observed in a condition. The experiments were repeated at least three times, unless stated otherwise. All the images in figures are representative, and the conclusions are based on qualitative visual impression. The cell surface myc-GLUT4-eGFP was measured by subtracting the internal myc signal from total myc signal of electroporated cells. The plasma membrane eGFP content was also measured. The cell surface GLUT4 was calculated as (total myc − internal myc)/(total eGFP − internal eGFP) (22).
2-Deoxy-glucose uptake.
Differentiated adipocytes were prepared in 24-well plates. Cells were infected with the recombinant adenoviruses. Two days thereafter, the cells were serum starved for 2 h at 37°C in Krebs-Ringer HEPES buffer. Then, the cells were stimulated with or without 100 nmol/l of insulin for 10 min, and 2-deoxy-glucose uptake was determined by 2-deoxy-d-[2,6 3H] glucose incorporation. Nonspecific glucose uptake was measured in the presence of 20 μmol/l cytochalasin B and subtracted from each determination to obtain specific uptake. The results were normalized by the protein amount.
Statistical analysis.
Multiple comparisons among groups were performed using one-way ANOVA (post hoc test: Tukey-Kramer). Results are presented as means ± SD. Values of P < 0.05 were considered statistically significant.
RESULTS
DOC2b translocates to the plasma membrane in response to insulin.
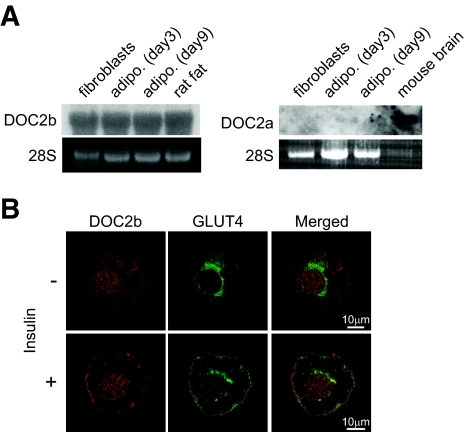
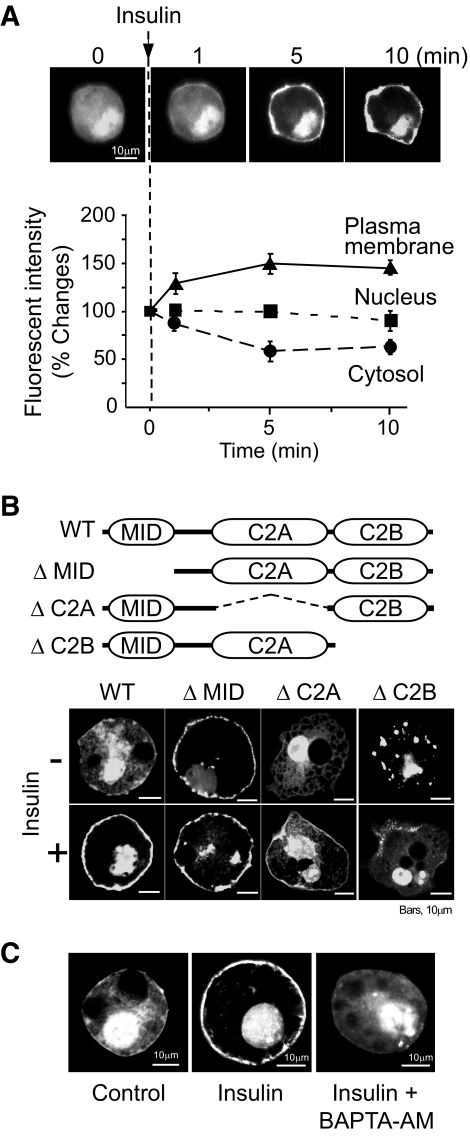
Type C tandem C2 domain proteins are classified into three groups (i.e., synaptotagmin and synaptotagmin-like protein and DOC2 family proteins). The first two groups of proteins regulate relatively fast membrane fusion (on the order of milliseconds to a few seconds) (12,13). This time scale is not suitable for GLUT4 vesicle fusion. Therefore, we focused on DOC2 family proteins as candidate regulators of GLUT4 vesicle fusion. First, we determined the expression profile of DOC2 mRNA in adipocytes. As shown in Fig. 1A, DOC2a was not expressed in 3T3-L1 adipocytes. According to a previous study, DOC2γ is localized to the nucleus and has no Ca2+-binding activity because of amino acid substitutions at the Ca2+-binding sites (14). Thus, we investigated the function of the DOC2b isoform involved in GLUT4 membrane fusion. Next, we examined the intracellular localization of DOC2b in differentiated 3T3-L1 adipocytes using anti-DOC2b antibody (Fig. 1B) or by expressing an eGFP fused to DOC2b (Fig. 2A). As shown in Fig. 1B, DOC2b results in a fine punctate or granular appearance throughout the cytoplasm under basal conditions. In contrast, the addition of insulin yields relatively slow (∼5 min) translocation of DOC2b to the cell periphery (Fig. 2A). This time scale of translocation was very similar to that of GLUT4 vesicles. It is noteworthy that deletion of the MID enhanced plasma membrane localization even in the absence of insulin and that the C2B domain is necessary for membrane targeting (Fig. 2B). These observations are interesting in considering the distinct roles of the C2A and C2B domains and the negative regulatory function of MID. We performed additional experiments to determine the role of calcium in the translocation of DOC2b. As shown in Fig. 2C, the cell membrane permeable Ca2+-chelating agent BAPTA-AM inhibited insulin-dependent translocation of DOC2b.
FIG. 1.
The expression profiles and intracellular localization of DOC2b in 3T3-L1 adipocytes. A: The expressions of DOC2a and DOC2b in 3T3-L1 fibroblasts, 3T3-L1 adipocytes (days 3 and 9 of differentiation), rat epididymal fat, and mouse brain were analyzed by mRNA blot. B: Differentiated 3T3-L1 adipocytes were serum-starved for 3–4 h and then stimulated with 100 nmol/l of insulin for 20 min, fixed, and immunostained. The endogenous DOC2b and GLUT4 were visualized using anti-DOC2b and anti-GLUT4 antibodies and observed by confocal microscopy. (Please see http://dx.doi.org/10.2337/db08-0303 for a high-quality digital representation of this figure.)
FIG. 2.
Insulin augments DOC2b translocation to the plasma membrane. A: 3T3-L1 adipocytes were electroporated with eGFP-DOC2b and then treated with 100 nmol/l of insulin for the times indicated. Live fluorescent images of the cells were captured by confocal microscopy. Relative fluorescences of the plasma membrane, nucleus, and cytosol were each measured in three distinct areas. At least three cells were analyzed for each condition. Results are means ± SD from three independent experiments. B: 3T3-L1 adipocytes expressing eGFP-DOC2b (WT [wild type], ΔMID, ΔC2A, or ΔC2B) were treated with or without 100 nmol/l of insulin and observed by confocal microscopy. C: 3T3-L1 adipocytes were electroporated with eGFP-DOC2b and then pretreated with 50 μmol/l of BAPTA-AM for 10 min, before treatment with insulin and observed under a confocal microscope.
DOC2b binds syntaxin-4 upon stimulation with insulin.
Since DOC2b is thought to be a soluble calcium-sensing protein, compartment-specific targeting must be achieved through interaction with membrane-bound proteins such as SNARE and SNARE-related proteins. Therefore, we attempted to identify DOC2b-binding partners among these proteins using a yeast two-hybrid system. We found a very strong interaction between DOC2b and syntaxin-4 compared with the already known binding between munc18c and syntaxin4 (Fig. 3A). Interestingly, this t-SNARE protein is reportedly a key molecule for GLUT4 vesicle fusion in response to insulin (5,15). Although this interaction was very strong, SNARE proteins are quite “sticky” and can on occasion bind with many proteins nonspecifically. Therefore, we performed the following three additional experiments. First, we determined the direct interaction in vitro using recombinant proteins, GST-tagged DOC2b, and synthesized syntaxin-4. As shown in Fig. 3B, the interaction was easily detected by immunoblotting in the presence of calcium. Second, we examined endogenous protein-protein interactions by immunoprecipitation experiments using polyclonal anti-DOC2b. As shown in Fig. 3C, insulin treatment increased DOC2b–syntaxin-4 binding, and BAPTA-AM abolished this interaction. Since the molecular weights of DOC2b and the IgG heavy chain are quite similar (computed molecular weight of DOC2b and syntaxin-4 are 46 and 34 kDa, respectively), it was difficult to perform a reverse immunoprecipitation experiment. Third, our results were confirmed by immunoprecipitation experiments using adipocytes expressing myc-tagged DOC2b (Fig. 3D and E). It is important to note that we could not detect the interaction between DOC2b and syntaxin-4 in the buffer containing EDTA (data not shown). Furthermore, we could not find interaction between DOC2b and syntaxin6 in 3T3-L1 adipocytes (online appendix Fig. S2).
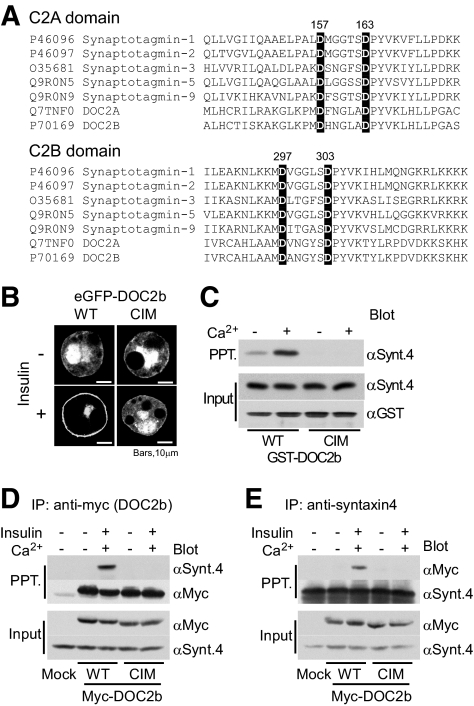
To better assess the calcium dependency of DOC2b translocation and interaction with syntaxin-4 in adipocytes, we conducted the following additional experiments using a Ca2+-unbound mutant. Based on the information from crystallographic analysis of synaptotagmin-1 (16–18), we created mutations in the putative Ca2+-binding sites of DOC2b (i.e., C2A [D157N, D163N] and C2B [D297N, D303N]) and designated the product obtained the CIM (Fig. 4A). This type of mutant reportedly loses its calcium-dependent phospholipids-targeting capacity (19,20). As shown in Fig. 4B, the CIM mutation markedly inhibited insulin-induced DOC2b translocation. Furthermore, CIM-DOC2b also failed to interact with syntaxin-4 in both the in vivo and the in vitro setting (Fig. 4C–E). These results raise the possibility that Ca2+ binding is essential for insulin-stimulated DOC2b translocation as well as the interaction with syntaxin-4.
FIG. 4.
Calcium binding to DOC2b is essential for its translocation and interaction with syntaxin-4. A: ClustalW sequence alignment of C2A and C2B domains from synaptotagmin-1, -2, -3, -5, and -9 and DOC2a and DOC2b. Two aspartic acid residues in each C2 domain (shown on a black background) are well-conserved putative Ca2+-binding sites. These four aspartic acid residues were mutated into asparagine for CIM-DOC2b (D157N, D163N, D297N, and D303N) construction. B: Wild-type (WT) or calcium-interacting domain mutants (CIM) of eGFP-DOC2b were expressed in 3T3-L1 adipocytes, which were then treated with insulin and observed under a confocal microscope. Figures show representative images of three independent experiments. C: Purified GST-DOC2b (WT or CIM) and syntaxin-4 were mixed in the presence of 2 mmol/l EDTA or 1 mmol/l of CaCl2 and pulled down with glutathione sepharose. The precipitates were analyzed by Western blotting with anti–syntaxin-4 and anti-GST antibodies. D and E: Myc-tagged DOC2b (WT or CIM) was expressed in 3T3-L1 adipocytes. After serum starvation, the cells were stimulated with or without 100 nmol/l of insulin for 20 min and then immunoprecipitated with anti-myc or anti–syntaxin-4 antibodies. The immunoprecipitated proteins were immunoblotted with anti-myc and anti–syntaxin-4 antibodies.
DOC2b regulates the step of GLUT4 vesicle fusion in response to insulin.
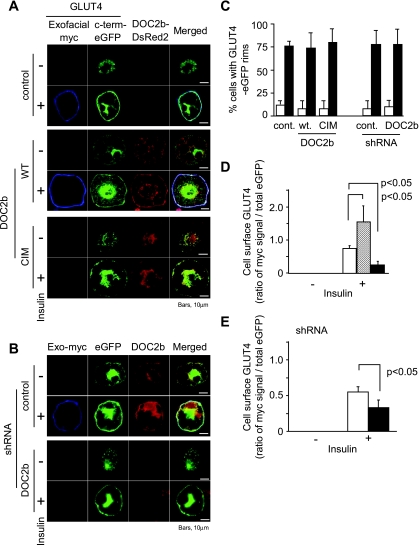
One interpretation of the above findings is that DOC2b may act as a Ca2+ sensor protein for SNARE complexes triggering GLUT4 vesicle fusion. To assess the role of DOC2b in GLUT4 vesicle fusion to the plasma membrane, we utilized a GLUT4 construct containing four myc epitopes in the first exofacial loop of GLUT4 and an eGFP fusion at its cytoplasmic COOH terminus. These exofacial tags are easily detected by anti-myc antibody but only when GLUT4 vesicles are fused to the plasma membrane (21). Adipocytes expressing this GLUT4 construct and DsRed2-DOC2b (wild type or CIM) or shRNAs were observed by confocal microscopy. As shown in Fig. 5A and B, the externalized GLUT4 was increased in the cells coexpressing wild-type DOC2b, while being decreased in those coexpressing CIM-DOC2b or shRNA (shRNADOC2b) compared with the control cells. Since the above conclusions were based on qualitative visual impression, we next estimated the fluorescent signals of the cellular rims in Fig. 5A and B in two ways. First, we counted the number of the cells with eGFP rims (50 cells in each condition) in the cells expressing myc-GLUT4-eGFP. As shown in Fig. 5C, just translocated or docked GLUT4 detected by eGFP fluorescence did not change under either condition. Second, we quantified the ratio of cell surface GLUT4 (myc sinal) to GLUT4 translocated to plasma membrane (eGFP signal) as previously described (22). As shown in Fig. 5D and E, cell surface GLUT4 was increased in the cells expressing wild-type DOC2b but decreased in those expressing CIM-DOC2b and silenced DOC2b (Fig. 5D and E). These results, taken together with the data shown in Fig. 5A, are consistent with the idea that DOC2b is a calcium-sensing protein and regulates the GLUT4 vesicle fusion step in response to insulin.
FIG. 5.
DOC2b enhances GLUT4 vesicle fusion in 3T3-L1 adipocytes. A and B: Exofacial myc-tagged GLUT4-eGFP and DsRed2-DOC2b or pcPUR-U6i-shRNADOC2b,control were coelectroporated into 3T3-L1 adipocytes. The cells were serum starved for 2–4 h and either untreated or treated with 100 nmol/l of insulin for 20 min. The cells were then fixed and stained with anti-myc antibody and Cy5-labeled secondary antibody without detergents. In the cells electroporated with shRNAs, endogenous DOC2b were visualized with anti-DOC2b antibody, followed by Cy3-labeled secondary antibody. Stained cells were observed by confocal microscopy. This shRNA system decreased DOC2b protein expression to 10–20% of control level. Images are representative of three independent experiments. C–E: Percents of cells with GLUT4-eGFP rims and the cell surface myc-GLUT4 contents (the ratio of myc signal/eGFP signal at plasma membrane rims) in Fig. 5A was calculated as described under research design and methods. C: □, −insulin; ▪, +insulin. D: □, control; ▒, wild type; ▪, CIM. E: □, shRNA control; ▪, shRNA DOC2b. The graphs represent values from at least 3–5 independent experiments, and error bars show SD. (Please see http://dx.doi.org/10.2337/db08-0303 for a high-quality digital representation of this figure.)
Role of DOC2b in glucose transport in 3T3-L1 adipocytes.
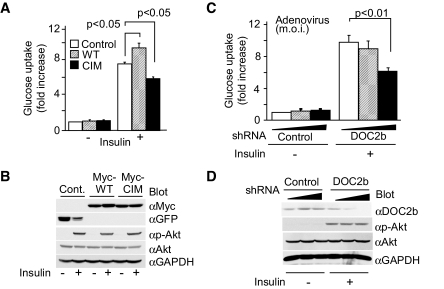
We next focused on the role of DOC2b in insulin-dependent glucose uptake in adipocytes expressing wild-type DOC2b and CIM-DOC2b. As shown in Fig. 6A, overexpression of wild-type DOC2b increased insulin-stimulated glucose uptake to 122% of the control level. In contrast, overexpression of CIM-DOC2b decreased to 78% of the control level. Furthermore, we introduced shRNAs (shRNADOC2b, control) by adenovirus vectors into cultured adipocytes to induce specific degradation of the DOC2b mRNA. DOC2b protein expression was decreased to 50 and 10% of the control level in the cells infected with viruses at multiplicity of infection (MOI) of 20 and 50, respectively (Fig. 6B). As expected, glucose uptake was decreased from 87 to 60% in adipocytes infected with the adenovirus vectors at MOI of 20–50 (Fig. 6B). To confirm the specificity of silencing, we conducted add-back style rescue experiment using adenovirus vector containing wild-type DOC2b. As shown in online appendix Fig. S3, overexpression of DOC2b rescued the inhibitory effect on glucose uptake in DOC2b-silenced cells. Under these conditions, DOC2b overexpression and silencing had no effects on serine phosphorylation of Akt (Fig. 6A and B and online appendix Fig. S3). These results, taken together with the data presented in Figs. 2–5, suggest that DOC2b regulates glucose transport through modulating vesicle fusion processes but not insulin signaling.
FIG. 6.
DOC2b regulates insulin-stimulated glucose uptake in 3T3-L1 adipocytes. 3T3-L1 adipocytes were infected with recombinant adenovirus vectors encoding eGFP, myc-tagged DOC2b (WT, CIM) at MOI of 50 (A and B) or adenovirus vectors encoding shRNA specific for DOC2b or nontargeting control at MOI of 0, 20, and 50 (C and D). After serum starvation, the cells were treated with or without 100 nmol/l of insulin for 10 min. A: □, control; ▒ wild type;; ▪, CIM. C: □, 0; ▒, 20; ▪, 50. 2-Deoxy-glucose uptake was measured under each condition. Results are presented as means ± SD of at least five independent experiments. B and D: The cell lysates were also immunoblotted with anti-myc, anti-GFP, anti-DOC2b, anti-Akt, anti–phosphoserine-Akt, and anti–glyceraldehyde-3-phosphate dehydrogenase antibodies. Immunoblots were representative of at least three independent experiments.
DISCUSSION
Regulation of glucose uptake in muscle and adipose tissues by insulin is of fundamental importance for proper maintenance of postprandial hyperglycemia. This hormone stimulates translocation of the GLUT4 glucose transporter from the intracellular membrane to the cell surface (1,2). In addition to this movement of intracellular vesicles containing GLUT4, it has been suggested that the docking and fusion step of GLUT4 vesicles is also critically regulated by insulin (3,4,23). However, the precise mechanism by which insulin regulates vesicle fusion is still largely unknown.
A key finding of this study is identification of the double C2 domain protein DOC2b, which mediates insulin-regulated GLUT4 vesicle fusion. Like other membrane fusion processes, GLUT4 vesicle fusion occurs essentially through the formation of a “core complex” consisting of syntaxin-4 and VAMP-2 (5). In general, however, a number of additional factors are required to bring about SNARE-mediated membrane fusion in vivo. Many of these factors, which can collectively be called SNARE regulators (e.g., munc18, synaptotagmin, munc13, GATE-16/Apg8, LMA1, synaptophysin, tomosyn, and Vsm1/Ddi1), bind directly to SNARE proteins and are involved in membrane trafficking and fusion events (24). Among these SNARE regulators, munc18c and tomosyn were reported to be negative regulators of the SNARE complex assembly involved in GLUT4 vesicle fusion (25–27). Despite numerous investigations, the positive SNARE regulators for GLUT4 vesicle fusion have not been adequately clarified. In this report, we have shown that DOC2b mediates insulin-stimulated GLUT4 membrane fusion in adipocytes, while having no effect on the GLUT4 vesicle translocation step. These data are consistent with the hypothesis that DOC2b regulates insulin-stimulated GLUT4 vesicle fusion. DOC2b may be a positive SNARE regulator for vesicle fusion processes in adipocytes.
A second significant finding reported herein is the identification of a DOC2b binding partner. DOC2b interacts with t-SNARE syntaxin-4 upon stimulation with insulin in the presence of calcium. Syntaxin-4 is thought to be a SNARE protein on the target membrane for GLUT4 vesicle fusion (28,29). As shown in Fig. 3A, this interaction appears to be very strong compared with that between munc18c and syntaxin-4 demonstrated by the yeast two-hybrid method. Although this interaction appeared to be very strong, SNARE proteins are quite sticky and can on occasion bind with many proteins nonspecifically. Therefore, we performed three additional experiments. As shown in Fig. 3B–E, we confirmed the interaction between DOC2b and syntaxin-4 in both the in vivo and the in vitro setting. Furthermore, changes in the intracellular localization of DOC2b also supported the functional interaction. As shown in Fig. 2A, DOC2b translocates to the plasma membrane in response to insulin stimulation. Importantly, the time scale of DOC2b translocation coincides with relatively slow externalization of GLUT4 vesicles. Taken together, our data are consistent with the aforementioned hypothesis that DOC2b regulates GLUT4 vesicle fusion by triggering SNARE complex assembly.
Another interesting observation made in this study is the essential role of [Ca2+]i in insulin-stimulated GLUT4 vesicle fusion. In 2001, Whitehead et al. (8) first reported that a calcium chelator, BAPTA-AM, inhibited GLUT4 externalization and glucose uptake. However, the precise mechanism underlying calcium-dependent GLUT4 vesicle fusion remains unknown because no studies have as yet focused on Ca2+-sensing proteins in adipocytes. DOC2b is structurally similar to the well-known calcium-sensing SNARE regulator synaptotagmins (Fig. 4A). Taking these observations together, we hypothesized that DOC2b is a Ca2+-sensing protein that regulates GLUT4 vesicle fusion in adipocytes. To better assess the role of Ca2+ in GLUT4 vesicle fusion, we confirmed the following. First, DOC2b translocation and its binding to syntaxin-4 were shown to be [Ca2+]i dependent. Second, mutations in calcium-binding sites on C2 domains of DOC2b resulted in loss of syntaxin-4 binding, GLUT4 externalization, and glucose uptake. In contrast, we also observed DOC2b–syntaxin-4 binding, without insulin action, in a GST pull-down assay, suggesting that [Ca2+]i is necessary for this interaction in vitro (Fig. 3B). In parallel, we also found that insulin initiates the DOC2b translocation to plasma membrane and promotes interaction between DOC2b and syntaxin-4 in the presence of Ca2+ ions. Based on the above observations, we propose a simple model whereby insulin regulates SNARE regulator DOC2b, and basal level of [Ca2+]i may act in a constitutive manner to promote DOC2b–syntaxin-4 interaction involved in insulin-stimulated GLUT4 vesicle fusion.
A recent report (30) suggests that DOC2 proteins remove munc18 from syntaxin, thereby regulating core SNARE complex formation. This interpretation is very attractive because munc18c is thought to be a negative regulator of GLUT4 vesicle fusion. Although we have no direct data pertaining to munc18c, it is possible that munc18c and DOC2b may modulate SNARE assembly in a counterregulatory manner. Very recently, Ke et al. (31) reported that DOC2b bound munc18c, but not syntaxin-4, in pancreatic β-cells. This apparent discrepancy on syntaxin-4 binding may be attributable to the different experimental conditions. They performed most of their experiments using a buffer without calcium (i.e., Nonidet P-40 lysis buffer). Since DOC2b has conserved Ca2+-binding sites and is thought to be a Ca2+ sensor protein in other cell types such as neuron (32) and chromaffin cells (33), Ca2+ might be important for the physiological properties of DOC2b in β-cells. Further work is required to uncover the underling mechanisms by which DOC2b regulates SNARE assembly in response to insulin.
In summary, we have identified DOC2b as a syntaxin-4 binding protein in adipocytes. This protein regulates GLUT4 vesicle fusion as well as glucose uptake in response to insulin stimulation. We have further revealed that DOC2b requires [Ca2+]i and positively regulates the step of GLUT4 vesicle fusion.
Supplementary Material
Acknowledgments
This work was partly supported by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to M.E. and Y.T.); from the Takeda Scientific Foundation (to M.E.); and by the Research Grant for Longevity Science (to Y.T.).
No potential conflicts of interest relevant to this article were reported.
We thank Dr. R.R. Duncan for the DOC2a,b constructs, Dr. T. Takuma for the munc18c construct, Dr. K. Taira for the pcPUR+U6i cassette, and Dr. M. Fukuda for the anti-DOC2b antibody. We are also very grateful to Dr. Teruo Nishida for generously supporting us in the experiments using the confocal microscope and to Y. Kora, M. Kaneko, K. Yamada, and S. Ota for their technical supports.
Published ahead of print at http://diabetes.diabetesjournals.org on 25 November 2008.
Additional information for this article can be found in an online appendix at: http://dx.doi.org/10.2337/db08-0303.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Yang J, Holman, GD: Comparison of GLUT4 and GLUT1 subcellular trafficking in basal and insulin-stimulated 3T3-L1 cells. J Biol Chem 268: 4600–4603, 1993 [PubMed] [Google Scholar]
- 2.Czech MP, Corvera S: Signaling mechanisms that regulate glucose transport. J Biol Chem 274: 1865–1868, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Koumanov F, Jin B, Yang J, Holman GD: Insulin signaling meets vesicle traffic of GLUT4 at a plasma-membrane-activated fusion step. Cell Metab 2: 179–189, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez E, McGraw TE: Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell 17: 4484–4493, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheatham B, Chuk AV, Kahn CR, Wang L, Rhodes CJ, Klip A: Insulin-stimulated translocation of GLUT4 glucose transporters requires SNARE-complex proteins. Proc Natl Acad Sci U S A 93: 15169–15173, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgoyne RD, Morgan A: Secretory granule exocytosis. Physiol Rev 83: 581–632, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Tucker WC, Weber T, Chapman ER: Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304: 435–438, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Whitehead JP, Molero JC, Clark S, Meneilly MS, James DE: The role of Ca2+ in insulin-stimulated glucose transport in 3T3-L1 cells. J Biol Chem 276: 27816–27824, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Perin MS, Fried VA, Mignery GA, Jahn R, Sudhof TC: Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature 345: 260–263, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Rickman C, Craxton M, Osborne S, Davletov B: Comparative analysis of tandem C2 domains from the mammalian synaptotagmin family. Biochem J 378: 681–686, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyagishi M, Taira K: U6 promoter-driven siRNAs with four uridine 3′ overhangs efficiently suppress targeted gene expression in mammalian cells. Nat Biotechnol 20: 497–500, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Kuroda TS, Fukuda M: Rab27A-binding protein Slp2-a is required for peripheral melanosome distribution and elongated cell shape in melanocytes. Nat Cell Biol 6: 1195–1203, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Hui E, Bai J, Wang P, Sugimori M, Llinas RR, Chapman ER: Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis. Proc Natl Acad Sci U S A 102: 5210–5214, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda M, Saegusa C, Kanno E, Mikoshiba K: The C2A domain of double C2 protein γ contains a functional nuclear localization signal. J Biol Chem 276: 24441–24444, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Volchuk A, Wang Q, Ewart HS, Liu Z, He L, Bennett MK, Klip A: Syntaxin 4 in 3T3-L1 adipocytes: regulation by insulin and participation in insulin-dependent glucose transport. Mol Biol Cell 7: 1075–1082, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutton RB, Davltov BA, Berghuis AM, Sudhof TC, Sprang S: Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell 80: 929–938, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Shao X, Davletov BA, Sutton RB, Sudhof TC, Rizo J: Bipartite Ca2+-binding motif in C2 domains of synaptotagmin and protein kinase C. Science 273: 248–251, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Fernandez I, Arac D, Ubach J, Gerber SH, Shin O, Gao Y, Anderson R, Sudhof TC, Rizo J: Three-dimensional structure of the synaptotagmin 1 C2B-domain: synaptotagmin 1 as a phospholipid binding machine. Neuron 32: 1057–1069, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Fukuda M, Kojima T, Mikoshiba K: Regulation by bivalent cations of phospholipid binding to the C2A domain of synaptotagmin III. Biochem J 323: 421–425, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackler JM, Drummond JA, Loewen CA, Robinson IM, Reist NE: The C2B Ca2+-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature 418: 340–344, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Bogan JS, McKee AE, Lodish HF: Insulin-responsive compartments containing GLUT4 in 3T3-L1 and CHO cells: regulation by amino acid concentrations. Mol Cell Biol 21: 4785–4806, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JG, Bose A, Leszyk J, Czech MP: PYK2 as a mediator of endothelin-1/G alpha 11 signaling to GLUT4 glucose transporters. J Biol Chem 276: 47751–47754, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Lizunov VA, Matsumoto H, Zimmerberg J, Cushman SW, Frolov VA: Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J Cell Biol 169: 481–489, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerst JE: SNARE regulators: matchmakers and matchbreakers. Biochim Biophys Acta 1641: 99–110, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Thurmond DC, Ceresa BP, Okada S, Elemendorf JS, Coker K, Pessin JE: Regulation of insulin-stimulated GLUT4 translocation by Munc18c in 3T3-L1 adipocytes. J Biol Chem 273: 33876–33883, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Kanda H, Tamori Y, Shinoda H, Yoshikawa M, Sakaue M, Udagawa J, Otani H, Tashiro F, Miyazaki J, Kasuga M: Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J Clin Invest 115: 291–301, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Widberg CH, Bryant NJ, Girotti M, Res S, James DE: Tomosyn interacts with the t-SNAREs syntaxin4 and SNAP23 and plays a role in insulin-stimulated GLUT4 translocation. J Biol Chem 278: 35093–35101, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Tellam JT, Macaulay SL, Mcintosh S, Hewish DR, Ward CW, James DE: Characterization of munc-18c and syntaxin-4 in 3T3-L1 adipocytes. J Biol Chem 272: 6179–6186, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Yang C, Coker KJ, Kim JK, Mora S, Thurmond DC, Davis AC, Yang B, Williamson RA, Shulman GI, Pessin JE: Syntaxin 4 heterozygous knockout mice develop muscle insulin resistance. J Clin Invest 107: 1311–1318, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhage M, de Vries KJ, Roshol H, Burbach JP, Gispen WH, Sudhof TC: DOC2 proteins in rat brain: complementary distribution and proposed function as vesicular adapter proteins in early stages of secretion. Neuron 18: 453–461, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Ke B, Oh E, Thurmond DC: Doc2beta is a novel munc18c-interacting partner and positive effector of syntaxin 4-mediated exocytosis. J Biol Chem 282: 21786–21797, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Groffen AJ, Friedrich R, Brian EC, Ashery U, Verhage M: DOC2A and DOC2B are sensors for neuronal activity with unique calcium-dependent and kinetic properties. J Neurochem 97: 818–833, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Friedrich R, Groffen AJ, Connell E, van Weering JR, Gutman O, Henis YI, Davletov B, Ashery U: DOC2B acts as a calcium switch and enhances vesicle fusion. J Neurosci 28: 6794–6806, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.