Abstract
OBJECTIVE— Somatostatin (SST) is secreted by islet δ-cells and by extraislet neuroendocrine cells. SST receptors have been identified on α- and β-cells, and exogenous SST inhibits insulin and glucagon secretion, consistent with a role for SST in regulating α- and β-cell function. However, the specific intraislet function of δ-cell SST remains uncertain. We have used Sst−/− mice to investigate the role of δ-cell SST in the regulation of insulin and glucagon secretion in vitro and in vivo.
RESEARCH DESIGN AND METHODS— Islet morphology was assessed by histological analysis. Hormone levels were measured by radioimmunoassay in control and Sst−/− mice in vivo and from isolated islets in vitro.
RESULTS— Islet size and organization did not differ between Sst−/− and control islets, nor did islet glucagon or insulin content. Sst−/− mice showed enhanced insulin and glucagon secretory responses in vivo. In vitro stimulus-induced insulin and glucagon secretion was enhanced from perifused Sst−/− islets compared with control islets and was inhibited by exogenous SST in Sst−/− but not control islets. No difference in the switch-off rate of glucose-stimulated insulin secretion was observed between genotypes, but the cholinergic agonist carbamylcholine enhanced glucose-induced insulin secretion to a lesser extent in Sst−/− islets compared with controls. Glucose suppressed glucagon secretion from control but not Sst−/− islets.
CONCLUSIONS— We suggest that δ-cell SST exerts a tonic inhibitory influence on insulin and glucagon secretion, which may facilitate the islet response to cholinergic activation. In addition, δ-cell SST is implicated in the nutrient-induced suppression of glucagon secretion.
Islets of Langerhans are heterogeneous cell aggregates containing β-, α-, δ-, and PP cells, which secrete insulin, glucagon, somatostatin (SST), and pancreatic polypeptide, respectively. The different cell types within the islet are affected by changes in the extracellular glucose concentration. Thus, elevations in circulating glucose stimulate insulin secretion from islet β-cells and inhibit glucagon secretion from α-cells as part of the reciprocal regulation of blood glucose by insulin and glucagon. SST secretion from islet δ-cells is also stimulated by increased extracellular glucose, although the threshold concentration for δ-cells to respond to glucose is lower than that for β-cells (1), and the ionic events in stimulus-response coupling differ between β- and δ-cells (2).
Rodent islets have a defined architecture with a β-cell core surrounded by a mantle of non–β-cells (3), whereas human islets do not show such pronounced anatomical subdivisions (4). The anatomical organization of islets is important for their correct functioning, and there is much evidence to suggest that cell-cell interactions within islets are crucial for normal function (5–9). Interactions between islet β-cells have been investigated extensively, and we have previously demonstrated the importance of homotypic β-cell interactions in the regulation of insulin secretion (10,11). Similarly, there have been numerous studies of possible interactions between α- and β-cells within islets (9,12–15). Less attention has been paid to the possible intraislet roles of δ-cell–derived SST. δ-Cells comprise 5–10% of the islet endocrine cells (3), but the peptide hormone SST is also synthesized and secreted by neuroendocrine cells in the central nervous system and the gastrointestinal system, and the latter is the major contributor to circulating SST (16,17). SST often acts as an inhibitory regulator of endocrine systems, for example, as a hypothalamic factor to suppress growth hormone secretion from the anterior pituitary (18), or as a local inhibitor of the release of gastrointestinal peptide hormones (19). SST receptors (SSTR1–5) have been identified on both α- and β-cells (20), and exogenously administered SST or SST analogs inhibit glucose-induced insulin secretion and arginine-induced glucagon secretion both in vitro and in vivo (21–24). In addition, studies using SSTR-deficient mice (SSTR1, -2, or -5) revealed changes in both basal and stimulated insulin secretion (21,25,26). However, studies using SSTR knockout models are difficult to interpret because mouse islets express all five SSTRs (20) and reduced expression of one receptor subtype may be compensated for by the overexpression of another. Therefore, although current data are consistent with a negative regulatory role for SST in islet secretory function, it is unclear whether this effect can be ascribed to circulating SST or to locally released δ-cell SST.
The study of δ-cell function within islets is complicated by the possibility of multiple heterotypic interactions between islet cell types in experiments using intact primary islets. Studies of islet SST have also been hampered by the lack of selective and potent SST receptor antagonists. To investigate the role of locally released δ-cell SST, we have therefore used a mouse model in which disruption of the SST gene produced a SST-deficient phenotype (27). Our results suggest that locally released δ-cell SST exerts a tonic inhibitory influence on insulin and glucagon secretion and that this inhibitory effect of δ-cell SST may be involved in facilitating the insulin secretory response to cholinergic activation and the nutrient-induced suppression of glucagon secretion.
RESEARCH DESIGN AND METHODS
Sst−/− mice on a C57BL/6J background were generated as described previously (27) and subsequently rederived onto a CBA/Ca × C57BL/10 F1 background by backcrossing to CBA/Ca × C57BL/10 F1 mice for 10 generations, followed by intercrossing to generate Sst−/− mice on a mixed CBA/Ca × C57BL/10 F1 background. Age-matched CBA/Ca × C57BL/10 F1 mice served as controls.
Islet isolation.
Islets from 8- to 12-week-old female Sst−/− or control mice were isolated by collagenase digestion (1 mg/ml; type XI; Sigma, St. Louis, MO) and separated from exocrine pancreatic tissue on a histopaque gradient (Sigma). Islets were incubated overnight at 37°C (5% CO2) in RPMI 1640 (10% FBS, 2% glutamine, 100 units/ml penicillin/0.1 mg/ml streptomycin, and 11 mmol/l glucose) before experiments.
PCR.
RNA was extracted from islets using an RNAeasy kit (Qiagen, Sussex, U.K.). Reverse transcription to cDNA and RT-PCR amplification were performed as described previously (28) using the primer sequences shown in Table 1. All reactions were carried out for 40 cycles. Products were separated on agarose gels and visualized by staining with 0.5 μg/ml ethidium bromide.
TABLE 1.
SST and SSTR1–5 mRNA expression in control and Sst−/− islets
| Primer sequence | Product (bp) | Tann (C°) | mRNA expression
|
||
|---|---|---|---|---|---|
| Sst+/+ | Sst−/− | ||||
| SST | F: ccagactccgtcagtttc | 124 | 56 | + | − |
| R: gctccagggcatcattct | |||||
| SSTR1 | F: ttttgtcatctgctggatgc | 167 | 59.9 | + | + |
| R: ggaaagagcgcttgaagttg | |||||
| SSTR2 | F: acgccaagatgaagaccatc | 197 | 55.7 | + | + |
| R: catgaccgtcaagcagaaga | |||||
| SSTR3 | F: tggtgatctacgtggtcctg | 163 | 59.9 | + | + |
| R: agacggcacatgagagatcc | |||||
| SSTR4 | F: tgctaactgctggcatgaag | 154 | 59.9 | + | + |
| R: ttcagcactgacaccaggag | |||||
| SSTR5 | F: cgacttcgtacagcaatcca | 213 | 58 | + | + |
| R: ctcacagaggttggctcaca | |||||
SST mRNA was detected by RT-PCR in control but not in Sst−/− mouse islet extracts, confirming the absence of endogenous δ-cell SST in Sst−/− mice. SSTR1–5 mRNAs were identified in both control and Sst−/− islet extracts. F, forward primer, R, reverse primer.
Immunohistochemistry and morphometric analysis.
An estimate of islet size and number per mm2 pancreas was obtained by morphometric analysis of fixed pancreatic sections labeled for insulin by immunohistochemistry. The entire paraffin-embedded pancreas from each animal was cut into 5-μm sections onto microscope slides and sections selected for staining and morphometry. Sections were systematically sampled throughout the entire pancreas to give 20 sections per animal for analysis. Sections were dewaxed, hydrated, and labeled with a mouse monoclonal antibody to insulin (Sigma), and positive cells were visualized with a peroxidase-conjugated secondary antibody to mouse IgG and diaminobenzidine as substrate. Sections were counterstained with hematoxylin, and digital images of sections were used for image analysis. Diameters of islets and area of insulin- and glucagon-labeled cells within islets on each section were measured with the aid of image analysis software (ImageJ). Numbers of islets, defined as clusters of five or more β-cells on the section, were determined relative to pancreatic area. For assessment of the organization of α- and β-cells within the islets, 10 sections per animal were labeled with mouse monoclonal antibodies to glucagon (Sigma) or insulin, respectively, and positive cells were visualized as described above.
Insulin and glucagon secretion
In vivo studies.
Eight- to 12-week-old female mice were fasted overnight before the experiments. The animals were anesthetized with sodium pentobarbital (667 μg · 100 μl−1 · 10 g−1 mouse i.p.), and a cannula was inserted into the jugular vein. Glucose (0.5 g/kg) or arginine (0.25 g/kg) was injected, and blood samples were withdrawn at time points indicated in the legend of Fig. 2. For insulin clearance tests, nonfasted mice were injected intravenously with 0.4 unit/kg insulin, and blood samples were withdrawn as indicated in the legend of Fig. 2. Samples were centrifuged, and blood glucose was measured in the plasma using a glucose analyzer (Analox).
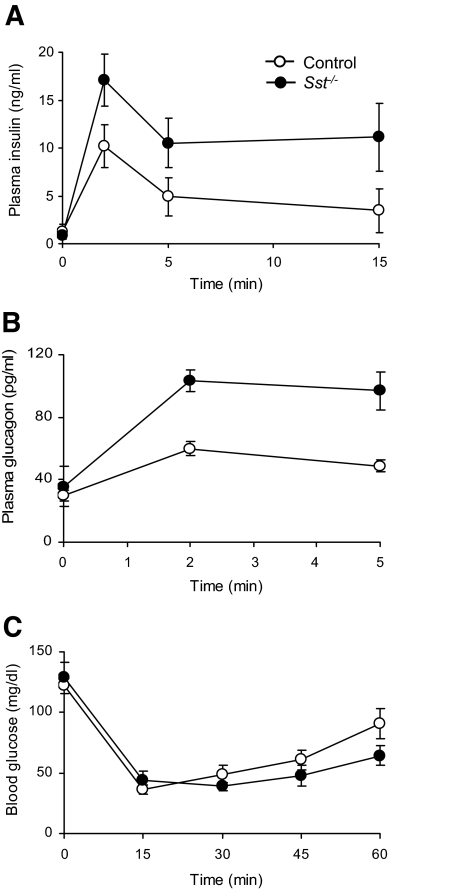
FIG. 2.
Insulin and glucagon secretion in vivo. A: Plasma insulin levels in Sst−/− mice and control mice 0, 2, 5, and 15 min after an intravenous glucose challenge (0.5 g/kg). Points show means ± SE for four to six separate animals. B: Plasma glucagon levels in Sst−/− mice and control mice 0, 2, and 5 min after injection of intravenous arginine (0.25 g/kg). Points show means ± SE for 5 and 14 separate animals (Sst−/− and control, respectively). C: Plasma glucose levels in Sst−/− mice and control mice 0, 15, 30, 45, and 60 min after injection of intravenous insulin (0.4 units/kg). Points show means ± SE for five separate animals.
In vitro studies.
For measuring the dynamics of insulin or glucagon release, islets were transferred into chambers of a temperature-controlled multichannel perifusion system as described previously (29) and perifused for 70 min at 37°C with a bicarbonate-buffered physiological salt solution (30) containing 2 mmol/l glucose. The tissue was subsequently perifused (0.5 ml/min) with salt solution containing agents of interest, and fractions were collected at 2-min intervals or as indicated on graphs. For static secretion experiments, islets were preincubated for 60 min in buffer containing 2 mmol/l glucose (or 1 mmol/l for SST secretion experiments), after which batches of 15 islets were incubated for 60 min in 0.5-ml salt solution containing agents of interest. The SST 14 isoform (SST-14; St. Helens, Bachem, U.K.) was used as an exogenous source of SST. For assessment of islet hormone content, islets were pelleted by centrifugation, washed with PBS, lysed in acidified ethanol, and sonicated.
Hormone immunoassays.
Insulin and glucagon content of incubation medium and islet extracts and insulin content of plasma samples were assessed by radioimmunoassay (RIA) using in-house assays, as described previously (31,32). SST and plasma glucagon levels were measured using commercially available RIA kits (SST: Euro-Diagnostica, Malmö, Sweden; Glucagon: Linco, St. Charles, MO).
Data analysis.
Data are expressed as means ± SE and analyzed statistically using Student's t test, one- or two-way ANOVA, and Bonferroni's multiple comparisons test, as appropriate. For statistical analysis of dynamic hormone release, data were expressed as the area under the curve after subtraction of basal secretion values. Differences between treatments were considered significant at P < 0.05.
RESULTS
Phenotypic characterization of control and Sst−/− mice, pancreata, and islets.
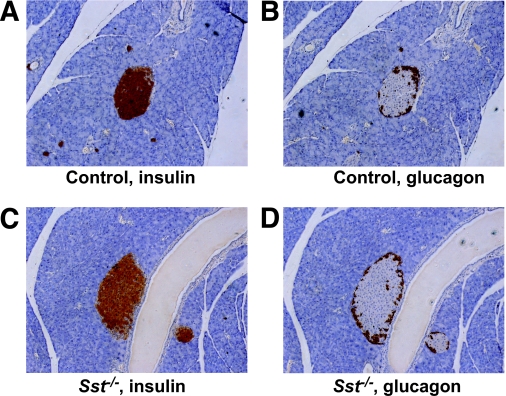
There were no significant differences in growth or body weight in the Sst−/− female mice versus controls at the ages studied (20 ± 0.5 vs. 20.9 ± 0.5 g, Sst−/− vs. control, n = 6, P > 0.2), nor did the Sst−/− animals show any overt behavioral dissimilarities or any differences in resting normoglycemia (8.1 ± 0.28 vs. 7.4 ± 0.14 mmol/l glucose, Sst−/− vs. control, n = 36–41, P > 0.2). Insulin tolerance tests showed no differences in sensitivity or maximal glucose suppression between female Sst−/− and controls (Fig. 2C; P > 0.2). Histological analysis of pancreatic sections found no significant differences in the size distribution of islets in control and Sst−/− pancreata (control, 97.2 ± 3.5 μm; Sst−/−, 95.6 ± 4.7, n = 88–217 islets from three animals per group, P > 0.2) nor in the number of islets per mm2 pancreas (control, 0.739 ± 0.054 islets/mm2; Sst−/−, 0.617 ± 0.064, n = 3, P > 0.2). Furthermore, the ratio of α- to β-cell area (control, 0.267 ± 0.031; Sst−/−, 0.282 ± 0.048, n = 32–34 sections from three animals per group, P > 0.2) and the cellular organization of α- and β-cells within the islet were similar in islets from control and Sst−/− animals. Thus, in both control and Sst−/− islets, glucagon-positive α-cells were located primarily at the periphery, whereas β-cells formed a central core (Fig. 1).
FIG. 1.
Intraislet organization of α- and β-cells from control and Sst−/− islets. Consecutive sections of control (A and B) or Sst−/− (C and D) mouse islets were stained for insulin (A and C) or glucagon (B and D), respectively, and are representative of sections from three different animals. (Please see http://dx.doi.org/10.2337/db08-0792 for a high-quality digital representation of this figure.)
In vivo islet hormone secretion from control and Sst−/− mice.
To assess the importance of SST in regulating islet hormone release, we initially examined the in vivo islet hormone secretory responses in female mice in the presence or absence of endogenous SST. No differences were detected in basal plasma levels of insulin and glucagon (Fig. 2; P > 0.2), but plasma levels of both hormones were significantly elevated in Sst−/− mice when compared with control mice after intravenous administration of glucose (0.5 g/kg; Fig. 2A; P < 0.01) or arginine (0.25 g/kg; Fig. 2B; P < 0.001), respectively. In separate experiments, plasma insulin levels were also enhanced in male Sst−/− mice compared with control animals in response to glucose (data not shown).
In vitro hormone secretion from control and Sst−/− islets.
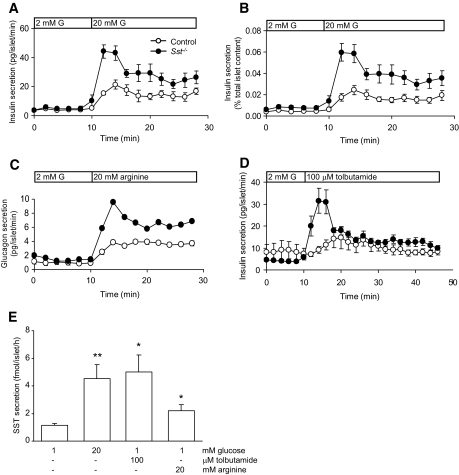
To determine whether the enhanced secretory responses in Sst−/− mice could be attributable to a specific lack of δ-cell SST rather than a global absence of circulating SST, in vitro secretion studies were carried out using islets isolated from female Sst−/− and control mice, as shown in Fig. 3. There was no significant difference between the basal rate of hormone secretion from control and Sst−/− islets (Fig. 3A–C; P > 0.2), whereas stimulus-induced insulin and glucagon release was significantly enhanced in the Sst−/− islets (Fig. 3A–D). Thus, Sst−/− islets showed approximately twofold greater insulin and glucagon secretory responses to elevations in glucose and arginine, respectively, compared with control islets (glucose, P < 0.05; arginine, P < 0.001). The augmented secretory responses did not reflect increased islet hormone content because the content of both insulin (control, 66.0 ± 8.6 ng/islet; Sst−/−, 50.0 ± 7.2, n = 6, P > 0.1) and glucagon (control, 3.5 ± 0.5 ng/islet; Sst−/−, 2.8 ± 0.5, n = 7, P > 0.1) did not differ significantly between genotypes. As a consequence, the differences in secretory responses between genotypes were observed whether secretion was expressed as a rate (Fig. 3A) or as a percentage of total insulin content (Fig. 3B). Enhanced insulin secretion from Sst−/− islets was not dependent on a nutrient stimulus, because similar effects were observed when secretion was initiated by the sulfonylurea tolbutamide, which bypasses glucose transport and metabolism and stimulates insulin secretion by direct effects on plasma membrane ATP-sensitive K+ channels (Fig. 3D; Sst−/− vs. control; P < 0.01). Thus, the first-phase of tolbutamide-induced insulin secretion was improved in Sst−/− islets compared with control islets. Parallel experiments using control islets demonstrated that δ-cells also responded to the secretagogues, which stimulate insulin and glucagon secretion, as shown in Fig. 3E. Accordingly, glucose, tolbutamide, and arginine all caused significant increases in SST secretion from islets in vitro by 4-, 4.5-, and 2-fold, respectively.
FIG. 3.
Hormone secretion from control and SST−/− islets. A: Insulin secretion from control and Sst−/− mouse islets at a substimulatory concentration of glucose (2 mmol/l G, 0–10 min) and at a stimulatory concentration of glucose (bar, 20 mmol/l G). Points show means ± SE, n = 7–8 separate perifusion channels in each experiment, typical of 10 separate experiments. In B, the same results are expressed as a percentage of total insulin content. C: Arginine-induced (20 mmol/l, bar) glucagon secretion from Sst−/− islets and control islets. Points show means ± SE, n = 4 perifusion channels. Where no error bars are shown, they are smaller than the size of the symbols. D: Insulin secretory responses to the sulfonylurea tolbutamide (100 μmol/l, bar) in the presence of 2 mmol/l glucose from Sst−/− islets and control islets. Points show means ± SE, n = 4 perifusion channels. E: SST secretion from control islets in response to glucose, tolbutamide, and arginine. Bars represent means ± SE, n = 8–9 in one experiment typical of three separate experiments. *P < 0.05, **P < 0.01 vs. 1 mmol/l glucose alone.
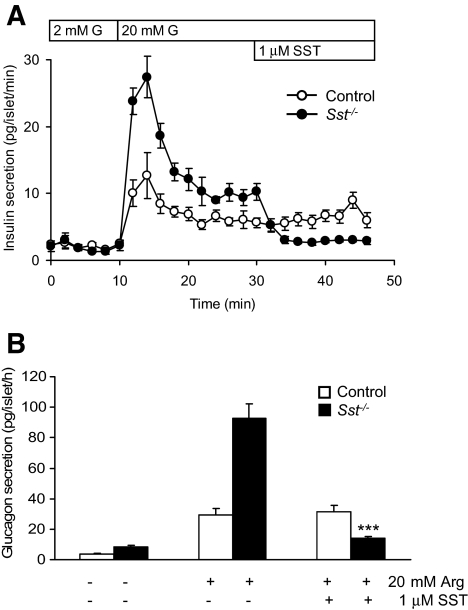
RT-PCR measurements confirmed the expression of mRNAs for SST receptor subtypes 1–5 in both control and Sst−/− islets (Table 1). Exogenous SST (1 μmol/l) had no significant effect in vitro on stimulus-dependent insulin (Fig. 4A) or glucagon (Fig. 4B) secretion from control islets (P > 0.2) but exerted a marked inhibition of secretion of both hormones from Sst−/− islets (Fig. 4A and B; P < 0.001).
FIG. 4.
Inhibition of insulin and glucagon secretion by exogenous SST. Effect of exogenous SST (1 μmol/l) on dynamic glucose-induced insulin secretion (20 mmol/l G, bar) (A) and static arginine-induced (20 mmol/l) glucagon secretion (B) from control islets and Sst−/− islets. Points show means ± SE, n = 4 separate perifusion channels in each experiment, typical of six separate experiments. Bars represent means ± SE, n = 6. ***P < 0.001 vs. arginine (Arg) alone.
Insulin secretion from control and Sst−/− islets after removal of glucose stimulus.
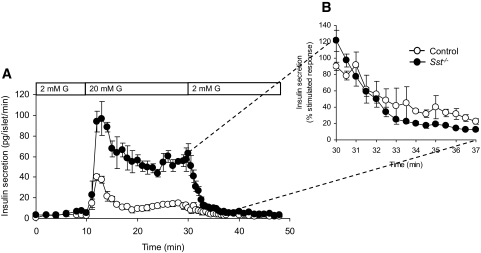
To investigate whether δ-cell SST facilitates the rapid cessation of insulin secretion when normoglycemia is achieved, we measured the rate of insulin secretion from perifused islets in vitro. As shown in Fig. 5, the rate at which insulin secretion declined to basal levels on removal of glucose (20 mmol/l) was not decreased in Sst−/− islets compared with control islets (Fig. 5A). Similarly, no differences in the switch-off rate of insulin secretion were detected when insulin secretion was expressed as a percentage of stimulated response, to take into account the different magnitude of glucose-induced stimulation in the two genotypes (Fig. 5B).
FIG. 5.
Rate of decline in insulin secretion from control and Sst−/− islets after removal of glucose stimulus. A: Glucose-induced (20 mmol/l G, bar) insulin secretion from Sst−/− islets and control islets is reversed to basal levels upon removal of stimulus. B: The decline in insulin secretion from stimulated to basal levels is expressed as percent stimulated insulin secretion before removal of glucose. Points show means ± SE, n = 4 separate perifusion channels.
Parasympathetic stimulation of insulin secretion from control and Sst−/− islets.
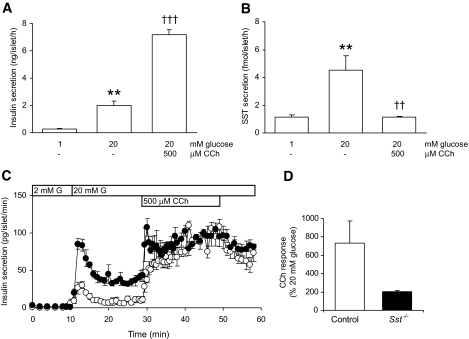
Figure 3E shows that glucose, arginine, and tolbutamide all stimulated SST secretion from δ-cells, consistent with enhanced insulin and glucagon release in response to these stimuli in the absence of δ-cell SST. However, as shown in Fig. 6A and B, activation of islet cholinergic receptors had opposite effects on insulin and SST secretion, inducing the expected enhancement of glucose-induced insulin secretion (P < 0.001; Fig. 6A) while causing a concomitant inhibition of glucose-induced SST secretion (P < 0.01; Fig. 6B). These observations are consistent with the effects of carbamylcholine (CCh), an acetylcholine analog, on insulin secretion from control and Sst−/− islets, as shown in Fig. 6C. Thus, both control and Sst−/− islets showed an amplification of glucose-induced insulin secretion in response to CCh (Fig. 6C) with no detectable differences in the rate of onset of CCh-induced insulin secretion. However, the extra magnitude of the secretory response of Sst−/− islets to CCh was less than that of control islets, when compared with the glucose-induced secretory response alone (Fig. 6D).
FIG. 6.
Parasympathetic stimulation of insulin secretion from control and Sst−/− islets. Static insulin (A) and SST secretion (B) from control islets in response to glucose (20 mmol/l) and the cholinergic agonist CCh (500 μmol/l). Bars represent means ± SE, n = 8–9 in one experiment typical of three separate experiments. **P < 0.01 vs. 1 mmol/l glucose alone, ††P < 0.01, †††P > 0.001 vs. 20 mmol/l glucose. Data in A and B are from the same experiment. C: Effect of CCh (500 μmol/l) on dynamic glucose-induced (20 mmol/l G, bar) insulin secretion from control (○) and Sst−/− (•) islets. Fractions were collected every 1 min between 10 and 30 min and 50 and 60 min and every 30 s between 30 and 50 min. Points show means ± SE, n = 3 perifusion channels in one experiment representative of three separate experiments. D: The effect of CCh is expressed as a percentage stimulation of the glucose-induced (20 mmol/l) secretory response (mean amplitude of secretion at time points 31–40 min expressed as a percentage of mean amplitude at 21–30 min).
Lack of glucose-induced suppression of glucagon secretion in Sst−/− islets.
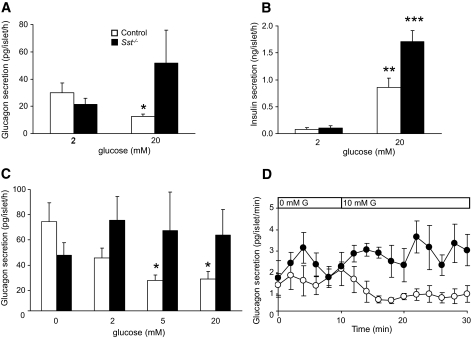
Our studies using Sst−/− islets also revealed an important role for δ-cell SST in the inhibition of glucagon secretion in response to increases in plasma glucose. Thus, glucose-induced suppression of glucagon secretion in vitro was not detected in Sst−/− islets (Fig. 7), although it was present in control islets at both high (Fig. 7A and C) and intermediate (Fig. 7C and D) glucose concentrations in both static and dynamic secretion experiments. Figure 7B shows the release of insulin in response to glucose in the same experiment as Fig. 7A.
FIG. 7.
Glucose-induced suppression of glucagon secretion requires SST. A: Effect of high glucose concentration (20 mmol/l) on glucagon secretion from control islets (□) and Sst−/− islets (▪) in static incubations. Bars represent means ± SE, n = 5–7. *P < 0.05 vs. 2 mmol/l glucose. B: Glucose-induced insulin secretion from the same experiment. **P < 0.01, ***P < 0.001 vs. 2 mmol/l glucose. There was no significant increase in glucagon secretion from Sst−/− islets in response to glucose in seven separate experiments, although in three of these, secretion appeared higher (see A). C: Glucagon secretion from control islets (□) and Sst−/− islets (▪) in response to increasing glucose concentrations. Islets were preincubated in the absence of glucose for 1 h before exposure to the glucose concentrations shown in the figure. Bars represent means ± SE, n = 5–6. *P < 0.05 vs. 0 mmol/l glucose. D: Effect of 10 mmol/l glucose (G, bar) on dynamic glucagon secretion from control islets (○) and Sst−/− islets (•) preincubated in the absence of glucose for 20 min. Points show means ± SE, n = 4 perifusion channels. Controls, P < 0.001 for 0 vs. 10 mmol/l glucose mean values.
DISCUSSION
Although SST is acknowledged as an inhibitor of insulin and glucagon secretion, the physiological relevance of δ-cell SST remains unknown. Our in vivo data showing that the global absence of SST in Sst−/− mice resulted in increased glucagon and insulin secretion in response to nutrient stimuli confirmed the importance of SST as a negative regulator of islet α- and β-cell function but did not identify δ-cells as the source of that SST. However, in our in vitro studies, the isolated islets were removed from neural, neuroendocrine, and gastrointestinal sources of SST, and this enabled us to focus on the functional consequences of an absence of islet SST. Overall, the lack of any significant differences in islet size, hormone content, or morphology between Sst−/− and control islets suggested that the observed differences in hormone secretion are most likely due to the absence of SST. Consequently, our in vitro studies demonstrated that the lack of locally released δ-cell SST had a profound effect on both insulin and glucagon secretion, leading to enhanced levels of stimulus-induced release of both hormones from Sst−/− islets compared with control islets without affecting basal levels of hormone secretion. Thus, although we cannot rule out an effect of circulating SST of neuroendocrine and/or gastrointestinal origin on islet function in vivo, our in vivo and in vitro studies are consistent with an important role for islet SST in the regulation of β- and α-cell secretory function. Our direct measurements of SST secretion in vitro also confirmed previous reports that some insulin and/or glucagon secretagogues, including glucose, arginine, and tolbutamide, also stimulate the secretion of SST from δ-cells (1,33,34). Taken together, our data are consistent with a model in which SST released from islet δ-cells exerts direct, tonic inhibitory effects on glucagon and insulin secretion from neighboring α- and β-cells. Under resting conditions, the quiescent δ-cells exert little or no influence on the neighboring α- and β-cells, and no difference is observed between control and Sst−/− islets with regard to basal secretion nor in the basal levels of plasma insulin and glucagon between control and Sst−/− mice. However, exposure of islets to glucose or sulfonylureas activates both β-cells and δ-cells (1,34), which initiates an insulin secretory response and simultaneously causes the local release of SST to limit that response. Similarly, the stimulation of glucagon secretion by arginine is accompanied by both the activation of δ-cells (33) and the intraislet release of SST to limit the α-cell secretory response. In this way, both in vitro and in vivo results are consistent with δ-cell SST exerting an inhibitory paracrine influence on islet α- and β-cells. The route through which δ-cell SST reaches α- and β-cells is uncertain; early reports on islet blood flow do not support a vascular route from δ-cells to α- and β-cells (35,36), although other data do imply a direct vascular route from δ- to β-cells (37). Alternatively, SST delivery may be mediated by diffusion via interstitial compartments (38,39) independent of islet vasculature. An extensive exploration of the mechanism of action of islet SST was beyond the scope of this study, but it is of interest that the first phase of insulin release was enhanced in Sst−/− islets, perhaps suggesting a direct or indirect effect via the ATP-sensitive K+ channels.
The proposed model of paracrine regulation of islet function by δ-cell SST is further supported by our observations that exogenous SST had no significant effect in vitro on stimulus-dependent insulin or glucagon secretion from control islets but exerted a marked inhibition of secretion of both hormones from Sst−/− islets. The lack of effect of exogenous SST on hormone secretion from control islets is consistent with a tonic inhibition by endogenous δ-cell SST of islet hormone secretion that cannot be further repressed by the provision of exogenous SST. In contrast, the absence of this inhibitory input by endogenous SST in Sst−/− islets reveals the ability of exogenous SST to inhibit both insulin and glucagon secretion. An experimental model in which δ-cell SST prevents further inhibition by exogenous SST would account for the reported (22) and anecdotal variability of the effects of SST on islet hormone secretion in studies using isolated islets, because the inhibition of hormone secretion by exogenous SST will be inversely related to the content of endogenous SST in islet δ-cells, which are easily lost or damaged during islet isolation because of their location on the periphery of the islets, particularly in rodents (40).
The intraislet inhibitory input from δ-cells is likely to be involved in fine-tuning β- and α-cell responses to external signals: The regulation of homeostatic responses through a balance between excitatory and inhibitory inputs has many parallels in physiology and would enable the precise degree of control required for the endocrine regulation of plasma glucose within strict limits. We considered first whether δ-cell SST was used to ensure the rapid cessation of insulin secretion on achieving normoglycemia (41). However, our in vitro measurements demonstrated that the rate of decline of insulin secretion on removal of glucose was not decreased in Sst−/− islets compared with control islets, suggesting that δ-cell SST does not regulate the termination of insulin secretory responses to glucose.
Although many insulin secretagogues also stimulate SST release from δ-cells, we found that the receptor-operated cholinergic agonist CCh enhanced glucose-induced insulin secretion but caused a marked inhibition of glucose-induced SST secretion from control islets, as has been suggested by some previous studies (42,43). The proposed model of paracrine regulation of insulin secretion by δ-cell SST would therefore predict that activation of islet cholinergic receptors in the presence of stimulatory concentrations of glucose enhances insulin secretion both by direct stimulatory effects on β-cells and by relieving the inhibitory δ-cell input as a consequence of the CCh-mediated inhibition of glucose-induced SST secretion. In this model, the effect of cholinergic activation to enhance a maximum glucose-induced insulin secretory response will be reduced in the absence of SST because the tonic inhibitory effect of SST on glucose-induced insulin secretion will be absent and will therefore not be alleviated by CCh. Our in vitro studies using Sst−/− islets concur with this prediction. Thus, both control and Sst−/− islets showed an amplification of glucose-induced insulin secretion in response to CCh to achieve similar maximum rates of secretion. However, the extent of the CCh-induced component of the secretory response in Sst−/− islets was much less than that of control islets when compared with their maximum responses to glucose alone. The overall effect of the presence of δ-cell SST was therefore to enhance the amplitude of the CCh-induced component of the secretory response. We propose that one physiological function of the tonic inhibitory input of δ-cell SST on islet β-cells is to ensure that a relatively transient stimulatory input via parasympathetic activation results in large changes in the overall mass of insulin secreted for the duration of the cholinergic activation.
Finally, our in vitro experiments using Sst−/− islets revealed a further role for δ-cell SST in the inhibition of glucagon secretion in response to increases in extracellular glucose. Various mechanisms have been proposed for the inhibitory effects of glucose on α-cell secretory function, including direct effects of glucose on α-cells (1,44) or paracrine inhibitory effects of β-cell secretory products such as insulin (14), Zn2+ (13), and γ-aminobutyric acid (45). In our experiments using Sst−/− islets, glucose-induced suppression of glucagon secretion was not detected, although it was observed in control islets over a range of glucose concentrations, suggesting that glucose-induced SST release is a major contributor to the inhibitory effects of glucose on islet α-cells. Consistent with this, it has been reported recently that the concentration dependency of glucose-induced inhibition of glucagon secretion correlates closely to that for the stimulation of SST secretion but is dissociated from that for β-cell activation (1). Our results therefore suggest an important role for δ-cell SST in the regulation of glucagon secretion by glucose but do not rule out the involvement of other regulatory mechanisms.
In summary, our studies using Sst−/− mice support important intraislet paracrine roles for δ-cell–derived SST in the regulation of islet hormone secretion. Intraislet SST exerts tonic inhibitory effects on stimulus-induced insulin and glucagon secretion, which may be important in regulating responses to receptor-operated stimuli, such as cholinergic agonists. Our studies also suggest that δ-cell SST also plays a significant role in the suppression of glucagon secretion by glucose. These observations highlight the importance of δ-cells in the normal function of the endocrine pancreas and suggest that δ-cell dysfunction in diabetes (46) may have important consequences for hormone secretion from islet α- and β-cells.
Acknowledgments
A.C.H.-E. and C.C.R. were funded by Diabetes UK. A.J.K. is a Research Council UK Research Fellow. D.C. has received MRC core funding. I.C.A.F.R. has received MRC core funding.
No potential conflicts of interest relevant to this article were reported.
We gratefully acknowledge the assistance of William Jefferson and Dr. Henry Asare-Anane with hormone measurements.
Published ahead of print at http://diabetes.diabetesjournals.org on 4 November 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Vieira E, Salehi A, Gylfe E: Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia 50: 370–379, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Gopel SO, Kanno T, Barg S, Rorsman P: Patch-clamp characterisation of somatostatin-secreting δ-cells in intact mouse pancreatic islets. J Physiol 528: 497–507, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishop AE, Polak JM: The anatomy, organization and ultrastructure of the islets of Langerhans. In Textbook of Diabetes. Pickup JC, Williams G, Eds. Oxford, U.K., Blackwell Science, 2002, p. 10.1–10.6
- 4.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A: The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A 103: 2334–2339, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halban PA, Wollheim CB, Blondel B, Meda P, Niesor EN, Mintz DH: The possible importance of contact between pancreatic islet cells for the control of insulin release. Endocrinology 111: 86–94, 1982 [DOI] [PubMed] [Google Scholar]
- 6.Halban PA, Powers SL, George KL, Bonner-Weir S: Spontaneous reassociation of dispersed adult rat pancreatic islet cells into aggregates with three-dimensional architecture typical of native islets. Diabetes 36: 783–790, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Hopcroft DW, Mason DR, Scott RS: Insulin secretion from perifused rat pancreatic pseudoislets. In Vitro Cell Dev Biol 21: 421–427, 1985 [DOI] [PubMed] [Google Scholar]
- 8.Montesano R, Mouron P, Amherdt M, Orci L: Collagen matrix promotes reorganization of pancreatic endocrine cell monolayers into islet-like organoids. J Cell Biol 97: 935–939, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosco D, Orci L, Meda P: Homologous but not heterologous contact increases the insulin secretion of individual pancreatic B-cells. Exp Cell Res 184: 72–80, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Hauge-Evans AC, Squires PE, Persaud SJ, Jones PM: Pancreatic β-cell–to–β-cell interactions are required for integrated responses to nutrient stimuli: enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes 48: 1402–1408, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Luther MJ, Hauge-Evans A, Souza KL, Jorns A, Lenzen S, Persaud SJ, Jones PM: MIN6 beta-cell-beta-cell interactions influence insulin secretory responses to nutrients and non-nutrients. Biochem Biophys Res Commun 343: 99–104, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Brereton H, Carvell MJ, Persaud SJ, Jones PM: Islet alpha-cells do not influence insulin secretion from beta-cells through cell-cell contact. Endocrine 31: 61–65, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB: Islet beta-cell secretion determines glucagon release from neighbouring alpha-cells. Nat Cell Biol 5: 330–335, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Leung YM, Ahmed I, Sheu L, Gao X, Hara M, Tsushima RG, Diamant NE, Gaisano HY: Insulin regulates islet alpha-cell function by reducing KATP channel sensitivity to adenosine 5′-triphosphate inhibition. Endocrinology 147: 2155–2162, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Ravier MA, Rutter GA: Glucose or insulin, but not zinc ions, inhibit glucagon secretion from mouse pancreatic α-cells. Diabetes 54: 1789–1797, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Reichlin S: Somatostatin. N Engl J Med 309: 1495–1501, 1983 [DOI] [PubMed] [Google Scholar]
- 17.Reichlin S: Somatostatin (second of two parts). N Engl J Med 309: 1556–1563, 1983 [DOI] [PubMed] [Google Scholar]
- 18.Bertherat J, Bluet-Pajot MT, Epelbaum J: Neuroendocrine regulation of growth hormone. Eur J Endocrinol 132: 12–24, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Low MJ: Clinical endocrinology and metabolism: the somatostatin neuroendocrine system: physiology and clinical relevance in gastrointestinal and pancreatic disorders. Best Pract Res Clin Endocrinol Metab 18: 607–622, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Ludvigsen E, Olsson R, Stridsberg M, Janson ET, Sandler S: Expression and distribution of somatostatin receptor subtypes in the pancreatic islets of mice and rats. J Histochem Cytochem 52: 391–400, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Strowski MZ, Parmar RM, Blake AD, Schaeffer JM: Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology 141: 111–117, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Cejvan K, Coy DH, Efendic S: Intra-islet somatostatin regulates glucagon release via type 2 somatostatin receptors in rats. Diabetes 52: 1176–1181, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Schuit FC, Derde MP, Pipeleers DG: Sensitivity of rat pancreatic A and B cells to somatostatin. Diabetologia 32: 207–212, 1989 [DOI] [PubMed] [Google Scholar]
- 24.Lins PE, Efendic S, Meyers CA, Coy DH, Schally A, Luft R: Selective effect of some somatostatin analogs on glucagon as opposed to insulin release in rats in vivo. Metabolism 29: 728–731, 1980 [DOI] [PubMed] [Google Scholar]
- 25.Wang XP, Norman M, Yang J, Liu SH, Magnusson J, DeMayo FJ, Brunicardi FC: The effect of global SSTR5 gene ablation on the endocrine pancreas and glucose regulation in aging mice. J Surg Res 129: 64–72, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Wang XP, Norman M, Yang J, Magnusson J, Kreienkamp HJ, Richter D, DeMayo FJ, Brunicardi FC: Alterations in glucose homeostasis in SSTR1 gene-ablated mice. Mol Cell Endocrinol 247: 82–90, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Low MJ, Otero-Corchon V, Parlow AF, Ramirez JL, Kumar U, Patel YC, Rubinstein M: Somatostatin is required for masculinization of growth hormone-regulated hepatic gene expression but not of somatic growth. J Clin Invest 107: 1571–1580, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hauge-Evans AC, Squires PE, Belin VD, Roderigo-Milne H, Ramracheya RD, Persaud SJ, Jones PM: Role of adenine nucleotides in insulin secretion from MIN6 pseudoislets. Mol Cell Endocrinol 191: 167–176, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Jones PM, Persaud SJ, Howell SL: Time-course of Ca2+-induced insulin secretion from perifused, electrically permeabilised islets of Langerhans: effects of cAMP and a phorbol ester. Biochem Biophys Res Commun 162: 998–1003, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Gey GO, Gey MK: Maintenance of human normal cells in continuous culture, preliminary report: cultivation of mesoblastic tumours and normal cells and notes on methods of cultivation. Am J Cancer 27: 45–76, 2008 [Google Scholar]
- 31.Bjaaland T, Hii CS, Jones PM, Howell SL: Role of protein kinase C in arginine-induced glucagon secretion from isolated rat islets of Langerhans. J Mol Endocrinol 1: 105–110, 1988 [DOI] [PubMed] [Google Scholar]
- 32.Jones PM, Salmon DM, Howell SL: Protein phosphorylation in electrically permeabilized islets of Langerhans: effects of Ca2+, cyclic AMP, a phorbol ester and noradrenaline. Biochem J 254: 397–403, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cejvan K, Coy DH, Holst JJ, Cerasi E, Efendic S: Gliclazide directly inhibits arginine-induced glucagon release. Diabetes 51 (Suppl. 3): S381–S384, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Sako Y, Wasada T, Umeda F, Ibayashi H: Effect of glibenclamide on pancreatic hormone release from isolated perifused islets of normal and cysteamine-treated rats. Metabolism 35: 944–949, 1986 [DOI] [PubMed] [Google Scholar]
- 35.Bonner-Weir S, Orci L: New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes 31: 883–889, 1982 [DOI] [PubMed] [Google Scholar]
- 36.Samols E, Stagner JI, Ewart RB, Marks V: The order of islet microvascular cellular perfusion is B—-A—-D in the perfused rat pancreas. J Clin Invest 82: 350–353, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murakami T, Fujita T: Microcirculation of the rat pancreas, with special reference to the insulo-acinar portal and insulo-venous drainage systems: a further scanning electron microscope study of corrosion casts. Arch Histol Cytol 55: 453–476, 1992 [DOI] [PubMed] [Google Scholar]
- 38.Kawai K, Ipp E, Orci L, Perrelet A, Unger RH: Circulating somatostatin acts on the islets of Langerhans by way of a somatostatin-poor compartment. Science 218: 477–478, 1982 [DOI] [PubMed] [Google Scholar]
- 39.Weir GC, Bonner-Weir S: Islets of Langerhans: the puzzle of intraislet interactions and their relevance to diabetes. J Clin Invest 85: 983–987, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Orci L: The microanatomy of the islets of Langerhans. Metabolism 25: 1303–1313, 1976 [DOI] [PubMed] [Google Scholar]
- 41.Otonkoski T, Andersson S, Simell O: Somatostatin regulation of beta-cell function in the normal human fetuses and in neonates with persistent hyperinsulinemic hypoglycemia. J Clin Endocrinol Metab 76: 184–188, 1993 [DOI] [PubMed] [Google Scholar]
- 42.Vonen B, Florholmen J, Giaever AK, Burhol P: Somatostatin secretion from isolated rat pancreatic islets. Scand J Clin Lab Invest 49: 139–143, 1989 [DOI] [PubMed] [Google Scholar]
- 43.Samols E, Weir GC, Ramseur R, Day JA, Patel YC: Modulation of pancreatic somatostatin by adrenergic and cholinergic agonism and by hyper- and hypoglycemic sulfonamides. Metabolism 27: 1219–1221, 1978 [DOI] [PubMed] [Google Scholar]
- 44.MacDonald PE, De Marinis YZ, Ramracheya R, Salehi A, Ma X, Johnson PR, Cox R, Eliasson L, Rorsman P: A K ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol 5: e143, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bailey SJ, Ravier MA, Rutter GA: Glucose-dependent regulation of γ-aminobutyric acid (GABA A) receptor expression in mouse pancreatic islet α-cells. Diabetes 56: 320–327, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Gerich JE: Role of somatostatin and its analogues in the pathogenesis and treatment of diabetes mellitus. Metabolism 39: 52–54, 1990 [DOI] [PubMed] [Google Scholar]