Abstract
OBJECTIVE—To describe the ability of nonhuman primate endocrine pancreata to reestablish endogenous insulin production after chemical β-cell destruction.
RESEARCH DESIGN AND METHODS—Eleven monkeys (Macaca fascicularis) were rendered diabetic with streptozotocin. Eight diabetic monkeys received intraportal porcine islet transplantation.
RESULTS—Two monkeys transplanted after 75 days of type 1 diabetes showed recovery of endogenous C-peptide production a few weeks after transplantation, concomitant with graft failure. Histological analysis of the pancreas of these monkeys showed insulin-positive cells, single or in small aggregates, scattered in the pancreas and adjacent to ducts. Interestingly, numerous CK19+ cells costained with proinsulin and PDX-1 antibodies. Furthermore, the peculiar double phenotype glucagon-positive/GLUT2+ was observed. In these monkeys as well as in all others, the original islets showed no insulin staining.
CONCLUSIONS—Our data provide evidence that, in nonhuman primates, the pancreas can reestablish endogenous insulin production after chemical β-cell destruction. This seems to be a nongeneralizable event with only 2 out of 11 monkeys recovering β-cell function. In these two monkeys, younger age and islet graft behavior might have played a role in triggering endogenous β-cell recovery.
Until a few years ago, lesions of the endocrine pancreas, as occur in type 1 diabetes, were thought to be permanent and irreversible, since diabetic patients require hormone replacement therapy for life (1). Despite the clinical evolution of the disease, it is still unknown whether the islet β-cells possess, at least in part, the ability to heal from an injury (2).
In mouse models, evidence has accrued that adult animals retain the ability to expand their β-cell mass after stimulation with a variety of triggers (3–8). It remains largely uncertain, however, through which molecular and cellular mechanisms the reparative process works (2).
In humans, the ability of the postnatal pancreas to expand the β-cell mass after injury is still debated (9,10). Spontaneous recovery of β-cell function has been reported in only few patients previously diagnosed with type 1 diabetes (11).
Although nonhuman primates do not develop autoimmune diabetes, a permanent diabetes status can be induced by total pancreatectomy or by chemical destruction of the β-cells with streptozotocin (STZ) (12,13).
In this study, a group of 11 monkeys were rendered diabetic by high STZ doses. Eight of them received intraportal pig islet transplantations under an immunosuppressive regimen based on anti-CD154 antibody and mycophenolate mofetil (14).
In two recipients where pig islet grafts functioned for a few weeks and eventually failed, we observed increasing endogenous C-peptide production paralleled by metabolic improvement. We reviewed all metabolic data, did extensive histological analysis, and here report evidence of recovery of endogenous insulin production in these two monkeys.
RESEARCH DESIGN AND METHODS
A total of 15 male Cynomolgus monkeys (Macaca fascicularis) (Three Spring Scientific, Perkasie, PA), 2–4 years old and 2.6–4.7 kg (median 3.6 kg), were included in the study.
Four wild-type retired breeder pigs (Wally Whippo, Enon Valley, PA) and three GT-DKO pigs (α-1,3-galactosyltransferase KO pigs; Revivicor, Blacksburg, VA) of similar weight were used as sources of pancreata for islet isolation. One wild-type pig was used for two transplants. All animal care procedures were in accordance with the institutional Principles of Laboratory Animal Care.
Induction of diabetes.
Diabetes was induced in 11 monkeys with 125–150 mg/kg i.v. of Zanosar STZ (Sicor Pharmaceuticals, Irvine, CA) in a single dose (12).
Islet preparation and transplantation.
After pancreatectomy in the anesthetized donor pig, islet isolation was carried out according to a modification of the method described for human islets, optimized for pigs (15). Intraportal injection of islets (an average of 60,000 islet equivalents/kg body wt with a range of 40,000–100,000 islet equivalents/kg) resuspended in plain CMRL-1066 with addition of heparin (70–140 units/kg; Baxter, Deerfield, IL) or dextran sulfate (molecular weight ∼5,000 d; 4 mg/kg; Fluka, Buchs, Switzerland) was carried out under general anesthesia of recipients.
Immunosuppressive management in transplanted monkeys.
After induction with anti-thymocyte globulin, immunosuppression was maintained with humanized anti-CD154 monoclonal antibody (ABI 793, generously provided by Novartis Pharma, Basel, Switzerland) and mycophenolate mofetil (Roche, Nutley, NJ) (14).
Anticoagulation/antiaggregation/anti-inflammatory treatment was achieved with heparin or dextran sulfate, prostacyclin (GlaxoSmithKline, Research Triangle Park, NC), and aspirin (14).
Diabetes and graft/endogenous β-cell function monitoring.
Blood glucose (mmol/l) was measured in whole blood with Free Style (Lifescan). Primate C-peptide (nmol/l) levels were measured by radioimmunoassay (Linco Research, St. Charles, MO). Confirmatory post-STZ C-peptide levels were measured using a chemiluminescent-based technology (Bayer Centaur, Tarrytown, NY). Porcine C-peptide (nmol/l) was detected by radioimmunoassay (Linco Research). Intravenous glucose tolerance tests were carried out before and after transplantation, as described (14).
Fixation and immunostaining of specimens.
Monkeys were killed at the time of rejection or when the vascular lines stopped working. Specimens of the pancreatic tissue were obtained, fixed, and analyzed as described in Supplemental Methods (found in an online appendix at http://dx.doi.org/10.2337/db08-1127). Morphometric analysis was conducted as per Bouwens and Pipeleers (16).
RESULTS
Recovery of endogenous C-peptide.
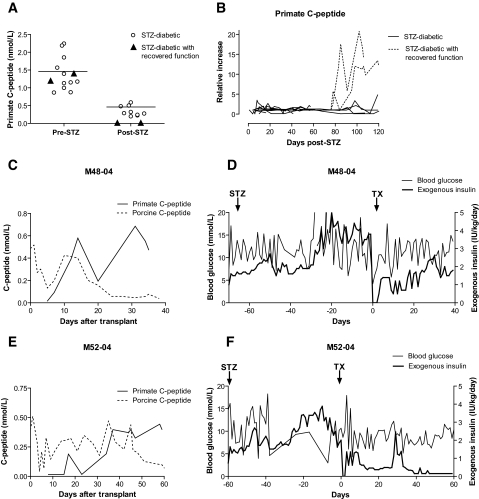
All monkeys treated with STZ became hyperglycemic (blood glucose >15 mmol/l) within 48 h and required exogenous insulin. Fasting C-peptide was under detection levels (<0.16 nmol/l) in all treated animals using a chemiluminescent method. Using commercially available ultra-sensitive radioimmunoassay kits, the monkeys showed an overall reduction in C-peptide of at least 75% compared with the pre-STZ values (Fig. 1A). Lack of C-peptide increased 5 min after glucose infusion during an intravenous glucose tolerance test after STZ treatment characterized all treated monkeys. Diabetic monkeys that did not undergo transplantation (n = 3) and islet recipients with early graft loss (n = 6) (characterized by undetectable or low porcine C-peptide levels for <2 weeks) showed no increase of the autologous C-peptide over the post-STZ basal levels (Fig. 1B). Exogenous insulin requirements after graft failure or in nontransplanted monkeys remained unchanged over time (data not shown). The pancreatic islets of monkeys with STZ-induced diabetes showed complete absence of insulin immunostaining (Supplemental Fig. 1). Monkeys M4804 and M5204, with C-peptide levels below detection using both detection methods for >2 months after STZ treatment, showed substantial recovery of basal endogenous C-peptide after a period of 3 weeks with islet graft function (Fig. 1B). Interestingly, the curves representing endogenous versus porcine C-peptide followed opposite trends (Fig. 1C and E). Glycemia did not worsen after graft failure, exogenous insulin requirement was lower than before transplantation, possibly because of the autologous insulin production (Fig. 1D and F), and body weight increased at the pace of healthy monkeys. Several weeks after transplantation, endogenous C-peptide levels were not only detectable but showed a low but measurable response to glucose stimulation (Supplemental Fig. 2). After islet graft failure in all other recipients, glycemia and insulin requirements returned to pretransplantation levels or worsened (data not shown), while endogenous C-peptide did not increase. In monkeys with long-term graft function (3 and 12 months), recovery of endogenous insulin production was not observed (R.B., A.Cr., A.Ca., J.H., D.J.V.d.W., W.A.R., C.G., M.T., unpublished data).
FIG. 1.
A: Endogenous C-peptide levels before and after STZ treatment. Closed triangles indicate the values of C-peptide in the two monkeys that recovered autologous insulin production. B: Relative increase of endogenous C-peptide over basal post-STZ values. Solid lines indicate STZ-diabetic monkeys that were not transplanted (n = 3) as well as STZ-diabetic recipients that experienced early graft loss (n = 6). Dotted lines represent the monkeys with recovered β-cell function (n = 2). C–F: Metabolic profile of the monkeys with recovered endogenous C-peptide (M4804 and M5204). C and E: Graft (porcine) and endogenous (primate) C-peptide levels. D and F: Blood glucose and exogenous insulin administration.
Histological findings.
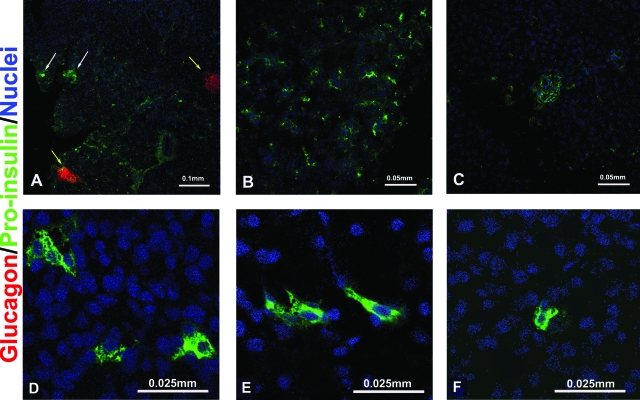
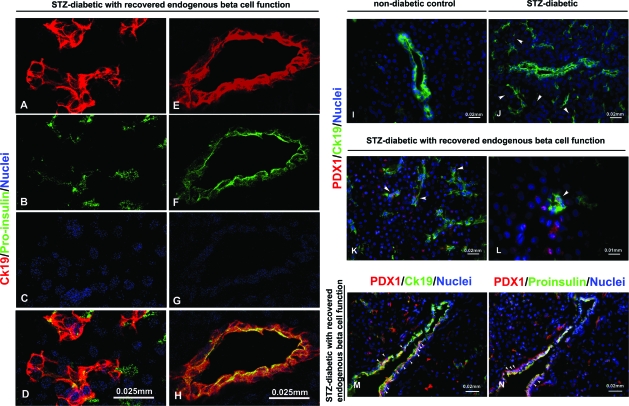
The pancreata of all the monkeys were analyzed for the presence of proinsulin-positive cells. The two monkeys that reestablished endogenous C-peptide production showed several proinsulin-positive cells grouped in small aggregates, or single cells scattered throughout the pancreas but distant from aggregates of glucagon-positive cells (Fig. 2). We quantified the number of proinsulin-positive cell clusters with area ≤30 μm2 (i.e., cell clusters not included in the islets of Langerhans existing before STZ treatment nor in the ducts). Proinsulin-positive cell clusters covered 0.23 and 0.18% of the whole section area in M5204 and M4804, respectively. These values were higher than in STZ-diabetic controls (0.05 ± 0.0004%; n = 4 different monkey pancreata) but lower than nondiabetic monkeys (0.30 ± 0.01%; n = 3 monkey pancreata). Proinsulin-positive cells were co-stained with PDX-1 (Fig. 3N and Supplemental Fig. 3). Interestingly, anti-CK19 antibody, used to identify the ductal/epithelial cells, was costained with proinsulin-positive cells in these monkeys (Fig. 3A–H). The pancreata of these monkeys showed stronger CK19 expression in comparison to nondiabetic and STZ-diabetic, transplanted, and nontransplanted controls (Supplemental Fig. 4).
FIG. 2.
Proinsulin-positive cells in the monkeys with recovered endogenous β-cell function. A–C: Proinsulin-positive cells are organized in small aggregates or scattered as single cells in the pancreas, not associated with glucagon-positive cells. White arrows: proinsulin-positive cells. Yellow arrows: glucagon-positive cells, presumably islets with damaged β-cells. D–F: Higher magnification. Pictures are representative of both monkeys with recovered endogenous β-cell function. (Please see http://dx.doi.org/10.2337/db08-1127 for a high-quality digital representation of this figure.)
FIG. 3.
A–H: Presence of double phenotype CK19/proinsulin in monkeys with recovered endogenous β-cell function. A–D: Monkeys with recovered endogenous β-cell function show co-expression of CK19 with proinsulin (yellow). E–H: Detail of a pancreatic duct. I–N: Double PDX-1+/CK19+ in the pancreas of monkeys with recovered function (K with detail in L, and M). CK19 and PDX-1 do not co-stain in nondiabetic healthy monkeys (I); PDX-1+ cells are found scattered throughout the pancreas of STZ-diabetic monkeys, but do not co-localize with CK19 (J); M and N: consecutive pancreatic sections in M5204 (monkey with recovered β-cell function) showing CK19+/PDX-1+ (M) and CK19+/proinsulin-positive (N) cells, respectively. Arrows show PDX-1+ cells in J; double positive PDX-1+/CK19+ in K, L, and M; and PDX-1+/proinsulin-positive in N. (Please see http://dx.doi.org/10.2337/db08-1127 for a high-quality digital representation of this figure.)
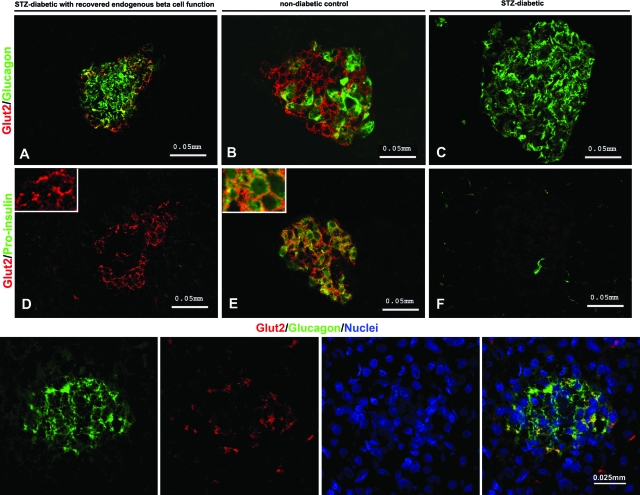
Additionally, the monkeys with recovered β-cell function presented double PDX-1+/CK19+ staining (Fig. 3K–M). PDX-1+ cells were also found in STZ-diabetic control monkeys, but they did not costain with CK19 (Fig. 3J) nor with proinsulin (data not shown). The atypical GLUT2/glucagon phenotype was found in islets devoid of insulin-producing cells in M4804 and M5204 (Fig. 4A and lower panel), whereas it was not observed in STZ-diabetic (Fig. 4C) nor in nondiabetic control monkeys (Fig. 4B).
FIG. 4.
Double GLUT2+/glucagon-positive cells in the pancreas of monkeys with recovered endogenous β-cell function. Co-expression is shown of glucose transporter GLUT2 with glucagon (A) and not with insulin (D) within islets damaged by STZ in M 4804 (monkey with recovered β-cell function). B and E: Nondiabetic monkey islet. C and F: STZ-diabetic monkey islet. Double GLUT2+/glucagon-positive and GLUT2+/proinsulin-positive cells stain in yellow. Lower panel: GLUT2/glucagon-positive cells in monkey M5204 (higher magnification). (Please see http://dx.doi.org/10.2337/db08-1127 for a high-quality digital representation of this figure.)
Anti-Ki67 antibody was used as a nuclear marker of active cell proliferation. Anti-Ki67 stained CK19+ cells and fibroblasts but not proinsulin-positive cells (data not shown).
DISCUSSION
In rodents, regenerative properties of β-cells have been unveiled (6,7). In humans, there is no clear similar indication. The autoimmune process that causes type 1 diabetes in the first place might also be responsible for halting potential attempts at restoring insulin production (2). Nonetheless, it is unclear, even in the absence of the immune attack, if the pancreatic β-cell function can recover efficiently (17). Anecdotic reports describing return to a normoglycemic status in patients diagnosed and treated for type 1 diabetes seem to prove that islet function can be reestablished in humans, concurrent with the disappearance of autoimmunity (11). In nonhuman primates used for preclinical investigation of type 1 diabetes treatments, hyperglycemia can be induced by administration of STZ. Once C-peptide is significantly reduced and exogenous insulin administration begins, in contrast to that observed in rodents, spontaneous normoglycemia is believed to be unrecoverable (18).
However, in our hands, two nonhuman primates rendered diabetic with STZ and with virtually no endogenous residual β-cell function for 2 months regained endogenous insulin production concomitant with pig islet graft failure. At the time they were killed, endogenous C-peptide levels were >10 times higher than after STZ treatment with associated lower insulin requirements, despite C-peptide being below the normal range of a nondiabetic cynomolgous monkey (14).
The posttransplantation clinical course of these two monkeys was characterized by glycemic levels persistently higher than in the normal physiologic range, despite graft insulin production, but well below the range recorded in nontransplanted diabetic monkeys. It is a common notion that chronically elevated blood glucose levels have a negative impact on β-cell function, but it is also known that glucose infusion and mild hyperglycemia may stimulate growth of the β-cell mass. It is unclear whether a threshold for beneficial/toxic effect indeed exists in monkeys and if this effect may have played a role in triggering recovery of insulin production in our monkeys. We observed that in the six monkeys with short graft function and consequent severe hyperglycemia and those that returned to stable normoglycemia after transplantation (R.B., A.Cr., A.Ca., J.H., D.J.V.d.W., W.A.R., C.G., M.T., unpublished data; 19), no recovery of the endogenous function occurred.
Histological examination of the pancreas of these two monkeys showed scattered proinsulin-positive cells, mostly organized as single cells or in small clusters, not associated with glucagon-positive cells, but often to ducts, similar to the ones just recently described (8,20). The frequency of small proinsulin-positive cell aggregates was higher in the monkeys with recovered β-cell function than in diabetic controls but lower than in nondiabetic monkeys. While on one hand we may hypothesize that new cells formed, data do not allow to rule out that proinsulin-positive cells result from a degranulation-regranulation process, as described by Sherry in the autoimmune NOD mouse model (21). However, if regranulation were the mechanism of recovery of the β-cells, proinsulin-positive cells associated with the damaged islets (thus near large glucagon-positive cell aggregates) should have been seen.
Interesting features in the pancreas of these monkeys were the presence of proinsulin-positive cells expressing CK19 within and outside the ducts as well as CK19+/PDX-1–positive/proinsulin-positive cells, suggesting that β-cells may have formed ex novo from duct progenitors, recapitulating pancreatic organogenesis and neogenesis. In agreement with this observation seems to be the molecular analysis of factors physiologically involved in organogenesis, although the modest numbers of monkey tissue for this study limits the soundness of the conclusion (Supplemental Fig. 5).
An additional characteristic of the pancreas of these monkeys was the presence of. glucagon-positive/GLUT2+ cells. A lack of evidence for glucagon-positive/proinsulin-positive cells suggests that glucagon-positive/GLUT2+ cells were unlikely committed to becoming β-cells, recalling embryonic development phases when GLUT2 is expressed temporarily in pancreatic non–β-cells, likely acting as a signal for further development (22).
The histological findings indicate that damage secondary to STZ may be itself a trigger for pancreatic regenerative responses; however, it seems unable to sustain sufficient β-cell recovery. To explain the phenomenon observed in these two monkeys, additional stimuli and peculiar conditions must be contemplated. One factor of interest was age: these monkeys were the youngest in the study (38 and 26 months compared with 49 ± 6 months for all others).
The failure of the graft, i.e., islet β-cell dysfunction and death, may also have fostered regenerating signals, as described in other forms of pancreatic injury (23). This would be in line with reports indicating that patients experiencing islet allograft loss can still exhibit detectable C-peptide levels and a better management of the diabetic status (24,25), even if their immunosuppressive regime included sirolimus and tacrolimus, both known to limit β-cell regeneration (7).
Recovery of the β-cell function can occur in nonhuman primates: the mechanisms that lay behind it, however, remains to be demonstrated.
Acknowledgments
This work was supported by grants from the U.S. Department of Defense (DOD#W81XWH-06-1-0317) and Juvenile Diabetes Research Foundation (JDRF 4-2004-786).
No potential conflicts of interest relevant to this article were reported.
We thank Dr. Yuval Dor and Dr. Pedro Herrera for their meaningful suggestions. We are grateful to Amy Sands and Carmella Knoll for excellent technical assistance.
Published ahead of print at http://diabetes.diabetesjournals.org on 10 November 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bach JF: Insulin-dependent diabetes mellitus as an autoimmune disease. Endocr Rev 15: 516–542 1994 [DOI] [PubMed] [Google Scholar]
- 2.Trucco M: Regeneration of the pancreatic beta cell. J Clin Invest 115: 5–12, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE: A second pathway for regeneration of adult exocrine and endocrine pancreas: a possible recapitulation of embryonic development. Diabetes 42: 1715–1720, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Zorina TD, Subbotin VM, Bertera S, Alexander AM, Haluszczak C, Gambrell B, Bottino R, Styche AJ, Trucco M: Recovery of the endogenous beta cell function in the NOD model of autoimmune diabetes. Stem Cells 21: 377–388, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Bowens L, Rooman I: Regulation of pancreatic beta-cell mass. Physiol Rev 85: 1255–1270, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Xu X, D'Hoker J, Stangé G, Bonné S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, Bowens L, Scharfmann R, Gradwohl G, Heimberg H: Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132: 197–207, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Nir T, Melton DA, Dor Y: Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 117: 2553–2561, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Q, Brown J, Kanarek A, Rajagopal1 J, Melton DA: In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455: 627–632, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler PC, Meier JJ, Butler AE, Bhushan A: The replication of beta cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab 3: 758–768, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza R, Butler P: β-Cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes 57: 1584–94, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karges B, Durinovic-Bello’ I, Heinze E, Boehm B, Debatin K-M, Karges W: Complete long-term recovery of beta-cell function in autoimmune type 1 diabetes after insulin treatment. Diabetes Care 27: 1207–1208, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Rood PP, Bottino R, Balamurugan AN, Smetanka C, Ezzelarab M, Busch J, Hara H, Trucco M, Cooper DKC: Induction of diabetes in cynomolgus monkeys with high-dose streptozotocin: adverse effects and early responses. Pancreas 33: 287–292, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kenyon NS, Chatzipetrou M, Masetti M, Ranuncoli A, Oliveira M, Wagner JL, Kirk AD, Harlan DM, Burkly LC, Ricordi C: Long-term survival and function of intrahepatic islet allografts in rhesus monkeys treated with humanized anti-CD154. Proc Natl Acad Sci U S A 96: 8132–8137, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casu A, Bottino R, Balamurugan AN, Hara H, van der Windt DJ, Campanile N, Smetanka C, Cooper DCK, Trucco M: Metabolic aspects of pig-to-monkey (Macaca fascicularis) islet transplantation: implications for translation into clinical practice. Diabetologia 51: 120–129, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Bottino R, Balamurugan AN, Smetanka C, Bertera S, He J, Rood PP, Cooper DKC, Trucco M: Isolation outcome and functional characteristics of young and adult pig pancreatic islets for transplantation studies. Xenotransplantation 14: 74–82, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Bouwens L, Pipeleers DG: Extra-insular beta cells associated with ductules are frequent in adult human pancreas. Diabetologia 41: 629–633, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Ablamunits V, Sherry NA, Kushner JA, Herold KC: Autoimmunity and beta cell regeneration in mouse and human type 1 diabetes: the peace is not enough. Ann N Y Acad Sci 1103: 19–32, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Dufrane D, van Steenberghe M, Guiot Y, Goebbels RM, Saliez A, Gianello P: Streptozotocin-induced diabetes in large animals (pigs/primates): role of GLUT2 transporter and beta-cell plasticity. Trasnsplantation 81: 36–45, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Hering BJ, Wijkstrom M, Graham ML, Hårdstedt M, Aasheim TC, Jie T, Ansite JD, Nakano M, Cheng J, Li W, Moran K, Christians U, Finnegan C, Mills CD, Sutherland DE, Bansal-Pakala MP, Murtaugh MP, Kirchhof N, Schuurman HJ: Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med 12: 301–303, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Martin-Pagola A, Sisino G, Allende G, Dominguez-Bendala J, Gianani R, Reijonen H, Nepom GT, Ricordi C, Ruiz P, Sageshima J, Ciancio G, Burke GW, Pugliese A: Insulin protein and proliferation in ductal cells in the transplanted pancreas of patients with type 1 diabetes and recurrence of autoimmunity. Diabetologia 51: 1803–1813, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sherry NA, Kushner JA, Glandt M, Kitamura T, Brillantes AM, Herold KC: Effects of autoimmunity and immune therapy on β-cell turnover in type 1 diabetes. Diabetes 55: 3238–3245, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Herrera PL: Adult insulin-and glucagon producing cells differentiate from two independent cell lineages. Development 127: 2317–2322, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Matzinger P: Friendly and dangerous signals: is the tissue in control? Nat Immunol 8: 11–13, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Rother KI, Harlan DM: Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J Clin Invest 114: 877–883, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR: International trial of the Edmonton protocol for islet transplantation. N Engl J Med 355: 1318–1330, 2006 [DOI] [PubMed] [Google Scholar]