Abstract
OBJECTIVE—Glomerular mesangial expansion and podocyte loss are important early features of diabetic nephropathy, whereas tubulointerstitial injury and fibrosis are critical for progression of diabetic nephropathy to kidney failure. Therefore, we analyzed the expression of genes in glomeruli and tubulointerstitium in kidney biopsies from diabetic nephropathy patients to identify pathways that may be activated in humans but not in murine models of diabetic nephropathy that fail to progress to glomerulosclerosis, tubulointerstitial fibrosis, and kidney failure.
RESEARCH DESIGN AND METHODS—Kidney biopsies were obtained from 74 patients (control subjects, early and progressive type 2 diabetic nephropathy). Glomerular and tubulointerstitial mRNAs were microarrayed, followed by bioinformatics analyses. Gene expression changes were confirmed by real-time RT-PCR and immunohistological staining. Samples from db/db C57BLKS and streptozotocin-induced DBA/2J mice, commonly studied murine models of diabetic nephropathy, were analyzed.
RESULTS—In human glomeruli and tubulointerstitial samples, the Janus kinase (Jak)-signal transducer and activator of transcription (Stat) pathway was highly and significantly regulated. Jak-1, -2, and -3 as well as Stat-1 and -3 were expressed at higher levels in patients with diabetic nephropathy than in control subjects. The estimated glomerular filtration rate significantly correlated with tubulointerstitial Jak-1, -2, and -3 and Stat-1 expression (R2 = 0.30–0.44). Immunohistochemistry found strong Jak-2 staining in glomerular and tubulointerstitial compartments in diabetic nephropathy compared with control subjects. In contrast, there was little or no increase in expression of Jak/Stat genes in the db/db C57BLKS or diabetic DBA/2J mice.
CONCLUSIONS—These data suggest a direct relationship between tubulointerstitial Jak/Stat expression and progression of kidney failure in patients with type 2 diabetic nephropathy and distinguish progressive human diabetic nephropathy from nonprogressive murine diabetic nephropathy.
Early clinical diabetic nephropathy is characterized by progressive increases in albuminuria that are associated with the development of characteristic histopathologic features including thickening of the glomerular basement membrane and mesangial expansion due to accumulation of extracellular matrix proteins (1). As albuminuria progresses and renal insufficiency ensues, glomerulosclerosis, arteriolar hyalinosis, and tubulointerstitial fibrosis develop (2). Mesangial expansion and the degree of tubulointerstitial fibrosis correlate inversely with glomerular filtration rate (GFR) in humans with diabetic kidney disease and appear to be critical steps in the progression of diabetic nephropathy to end-stage renal disease (1,3,4). Over 20 years ago, Mauer et al. (1) established the clear link between mesangial matrix expansion and progression of diabetic kidney disease by demonstrating that measures of mesangial expansion strongly predicted the clinical manifestations of diabetic nephropathy. The critical role of tubulointerstitial expansion and fibrosis in the progression of diabetic nephropathy has also been recognized for at least two decades (1,5). Tubulointerstitial changes, including fibrosis, appear to be critical for final progression of diabetic nephropathy to kidney failure in type 1 diabetic patients and may play an even more important, though heterogeneous, role in type 2 diabetic patients (6). Despite agreement about these pathologic predictors, there is no consensus about the processes that lead to progressive glomerulosclerosis and tubulointerstitial fibrosis.
While hypothesis-driven studies have led to many insights about the development and progression of diabetic nephropathy, there is recent interest in using more broad-based approaches to understanding the pathogenesis of human renal disease (7–9). Moreover, while current animal models of diabetic nephropathy replicate well the early stages of human disease, virtually all fail to develop the severe glomerulosclerosis, progressive tubulointerstitial fibrosis, and gradual decline in GFR that characterize human diabetic nephropathy (10). Therefore, use of a broad-based approach with tissues from human patients with progressive diabetic nephropathy might allow elucidation of pathways that would not be observed in hypothesis-based studies of animal or cell models (7–9). In this report, we show that a transcriptomic analysis of glomerular and tubulointerstitial tissues from patients with early and progressive diabetic nephropathy reveals regulation of the expression of multiple members of the Jak/Stat pathway.
We focused on these results because the activation of Jak/Stat signaling pathways can be implicated in both tubulointerstitial fibrosis and epithelial to mesenchymal transition in several conditions, including diabetes, in animal models (11–13). Similarly, Jak/Stat activation is reported in rat glomerular cells exposed to high glucose (14,15) and may be important in the glomerular transforming growth factor-β activation and fibronectin accumulation critical for extracellular matrix deposition in early diabetic nephropathy (15). Moreover, ACE inhibitors and angiotensin receptor blockers, which prevent the progression of diabetic nephropathy, also prevent Jak/Stat activation in glomerular cells from diabetic rats (14). Therefore, we studied expression and effects of Jak/Stat members in human and mouse diabetic nephropathy.
RESEARCH DESIGN AND METHODS
Human renal biopsy samples for genome-wide expression profiling and real-time RT-PCR.
Gene expression profiling of 74 kidney biopsies was performed essentially as reported by Cohen et al. (16) and Lindenmeyer et al. (17). For a detailed description, see the online appendix (available at http://dx.doi.org/10.2337/db08-1328). Clinical data for these patients are provided in the online appendix Tables 1 and 2.
Microarray target preparation, data processing, analysis, and pathway mapping.
Microarrays from extracted microdissected renal compartment RNAs were processed following a previously published protocol (18). The CEL files were processed by the ChipInspector software (Genomatix Software, http://www.genomatix.de). Briefly, and as described in the user manual, ChipInspector extracted the single probes significantly differentially expressed in Affymetrix GeneChip microarrays (false discovery rate [fdr] = 0). In the designed analysis treatment/control experiment, living donor and minimal change disease patients were the control group and the early diabetic nephropathy (Pima Indians) or progressive diabetic nephropathy (European cohort) subjects were the experiment group. The ChipInspector analysis steps were as follows: normalization, log22 transformation, statistical analysis, and mapping to transcripts. The resulting lists were uploaded into the Ingenuity Pathway Analysis software (IPA, www.ingenuity.com). Differential regulation was displayed in a hierarchical manner with color-coded expression levels. All the genes of a family were combined and represented by the generic family name.
Histology and immunohistochemistry.
Following the previously described protocol (19), immunohistochemistry studies were performed from an independent cohort of control subjects (histologically verified unaffected regions from tumor nephrectomies), type 2 progressive diabetic nephropathy case subjects, and other kidney disease biopsies (online appendix Table 3). Jak2 staining was performed using a primary monoclonal antibody at 1:100 (AHO1352; Biosource, Camarillo, CA). A biotinylated goat anti-mouse antibody (HistoLine; Zymed, Milan, Italy) was used as secondary antibody (1:200).
Morphometric analysis of the progressive diabetic nephropathy patients included analysis of the numbers of glomeruli that had segmental or global glomerulosclerosis or were sclerotic. The presence of tubulointerstitial damage was determined by the presence of tubular atrophy, presence of myofibroblasts, and/or accumulation of extracellular matrix proteins (online appendix Table 4).
Animals.
Mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Db/db and db/m mice on a C57BKLS background (BKS.Cg-m +/+ Leprdb/J) became obese around 3–4 weeks and developed hyperglycemia between 4 and 8 weeks of age. Male 10-week-old DBA/2J mice were allowed to acclimate to their environment for at least 3 days before injection. Mice were fasted for 4 h and then given intraperitoneal injections of 40 mg/kg streptozotocin (STZ) or the vehicle (control mice) daily for 5 consecutive days as previously reported (10,20). Mice were subcutaneously implanted with pellets impregnated with bovine insulin (LinBit tablets; LinShin Canada, Toronto, ON, Canada) at 10 weeks, 15 weeks, and 20 weeks post-STZ. The administered dose of insulin was ∼0.1 unit/day. These animals were killed at 24 weeks after completion of STZ injections.
Phenotypic evaluation of the DBA/2J diabetic and control mice was reported previously (21). Albuminuria was increased threefold, mesangial matrix was increased ∼65%, and podocyte number was decreased ∼30% in the diabetic DBA/2J mice compared with controls (21). The db/db C57BLKS mice were not phenotyped in this study. However, this model has been extensively phenotyped by others in previous studies and has very similar changes of early diabetic nephropathy (22).
The procedures used in this study were in accordance with the guidelines of the University of Michigan Committee on the Use and Care of Animals and conformed to “The Guide for the Care and Use of Laboratory Animals” (Department of Health, Education, and Welfare, publication no. NIH 86-23). Veterinary care was provided by the University of Michigan Unit for Laboratory Animal Medicine. The University of Michigan is accredited by the American Association of Laboratory Animal Care.
Mouse kidney samples.
Kidney cortex and glomeruli were harvested from 32-week-old diabetic db/db C57BLKS mice and their control db/m littermates and from STZ-induced diabetic and control DBA/2J mice 24 weeks after STZ or vehicle injection. Mice were anesthetized by intraperitoneal pentobarbital (0.06 mg/g body wt). Briefly, blood was flushed from the mice with 0.1 mol/l PBS (sterile, pH 7.4) via a catheter inserted into the abdominal aorta. A 20-ml iron oxide bolus (5 mg/ml in 0.9% NaCl) followed. The right kidney was then removed, minced, and pushed through a 90-μm nylon mesh (cat. no. 03-90/49; Sefar America, Kansas City, MO) into a beaker over a magnet (grade 8), used to retain the iron-perfused glomeruli, then washed two times with sterile PBS (pH 7.4). One-fifth of the glomeruli harvested were then stored in RNA Later (Ambion, Austin-TX) for RNA analysis; the remaining glomeruli were lysed and stored at −80°C for protein analysis.
Mouse RNA and protein extraction.
Total RNA was isolated from the microdissected tissues using the RNeasy Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. Harvested mouse kidney samples were lysed in a buffer containing 10 mmol/l Tris-HCl, pH7.4; 150 mmol/l NaCl; 1% Triton X-100; 0.1% SDS; 1 mmol/l Na3VO4; 50 mmol/l NaF; 1 mmol/l phenylmethylsulphonyl fluoride; and complete protease inhibitor cocktail (Roche, Indianapolis, IN) and were sonicated and centrifuged 20 min at 15,000g and 4°C. The supernatant was obtained and stored at −80°C until analyzed.
Human and mouse real-time RT-PCR.
Reverse transcription of RNA and amplification was performed as described previously (7) using commercially available predeveloped TaqMan reagents for Jak-2, Stat-3, and 18S ribosomal RNA for result normalization (Assay-on-Demand; Applied Biosystems, Darmstadt, Germany). Quantification of the given templates was performed according to the standard curve method for the human samples and the ΔCt value for the mouse samples. Serial dilutions of kidney cDNA were included in all PCR runs and served as standard curve. All measurements were performed in duplicate. Controls consisting of double distilled H2O were negative in all runs.
Mesangial cell culture and transfection.
Murine mesangial cells obtained from the American Type Cell Collection (MES-13, cloned from mice transgenic for the early region of the SV-40 virus) were grown in low glucose DMEM (Life Technologies, BRL) containing 5% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin in a humidified air/5% CO2 atmosphere at 37°C. Nucleofection of mesangial cells was performed according to the optimized protocols provided by the manufacturer (Amaxa, Gaithersburg, MD). Briefly, 1.5 × 106 cells were gently resuspended in 100 μl of Amaxa Nucleofector Solution (cat. no. VCA 1003) and mixed with 1.5 μg Jak-2 prk-5 or vector plasmid (23). Following transfection using the l-29 program on the Amaxa Nucleofector, cells were cultured for 12 h and incubated in 5.5 mmol/l d-glucose + 24.5 mmol/l mannitol or 30 mmol/l d-glucose serum-free DMEM. Cells were assayed 24 h later for Western blotting or quantification of intracellular reactive oxygen species (ROS). Transfection efficiency as quantified by nucleofection of the pMAX-GFP construct (included with the Amaxa kit) was ∼60% (not shown).
Western blot analysis.
Equal amounts of microdissected cortex and glomerular or cultured mesangial cell protein samples were loaded onto 7.5% SDS-PAGE gels, electrophoresed, and transferred onto nitrocellulose membranes (Schleicher & Schuell, Keene, NH). The blots were then incubated overnight at 4°C with a monoclonal mouse anti–Jak-2 antibody (AHO1352, dilutions: 1:1,000 [glomeruli], 1:2000 [cortex]; Biosource), STAT-3 (dilutions: 1:1,000 [glomeruli], 1:2,000 [cortex]; Cell Signaling Technology, Beverly, MA), phosphor–STAT-3 (tyrosine 705) (dilutions: 1:1,000 [glomeruli], 1:2,000 [cortex]; Cell Signaling Technology); or 1 h at room temperature with an anti–β-tubulin antibody (dilution: 1:6,000; Millipore, Lake Placid, NY). Subsequently, the blots were washed and incubated for 1 h at room temperature with a horseradish peroxidase–conjugated goat anti-mouse antibody (dilution: 1:1,000 [glomeruli], SuperSignal West Dura Extended Duration Substrate kit; Pierce, Rockford, IL; or 1:10000 [cortex], SC-2031; Santa Cruz Biotechnology). The peroxidase luminescence intensity was measured using NIH ImageJ software.
Detection of intracellular ROS.
Intracellular ROS generation was monitored by confocal microscopy using the fluoroprobe carboxymethyl-H2-dichlorofluorescein diacetate (CM-H2DCFDA; Molecular Probes, Eugene, OR). Transfected cells were grown on glass Lab-Tek Chamber Slides (Nunc, Naperville, IL) in serum-free DMEM for 24 h with either 5.5 mmol/l d-glucose + 24.5 mmol/l mannitol, or 30 mmol/l d-glucose. They were washed with PBS and incubated in the dark for 30 min in phenol red-free DMEM containing 5 μmol/l CM-H2DCFDA. CM-H2DCFDA passively diffuses into cells, where its acetate groups are cleaved by intracellular esterases and its thiol-reactive chloromethyl group reacts with intracellular glutathione and other thiols. Subsequent oxidation yields a fluorescent adduct that is trapped inside the cell. After incubation, images were captured with an Olympus FluoView 500 Laser Scanning Confocal Microscope. Three fields from each of five separate slide wells were assessed for each condition. Fluorescence was quantified from 50 randomly chosen cells from each slide well using NIH ImageJ software.
Statistics.
A t test was carried out between the groups of patients. A Bonferroni correction was performed when multiple samples were being compared. For protein analyses, a one-way ANOVA followed by Tukey-Kramer post hoc analysis was performed. P values <0.05 were considered statistically significant. All data are presented as mean ± SD or SE, as noted.
RESULTS
Jak/Stat pathway in human diabetic nephropathy.
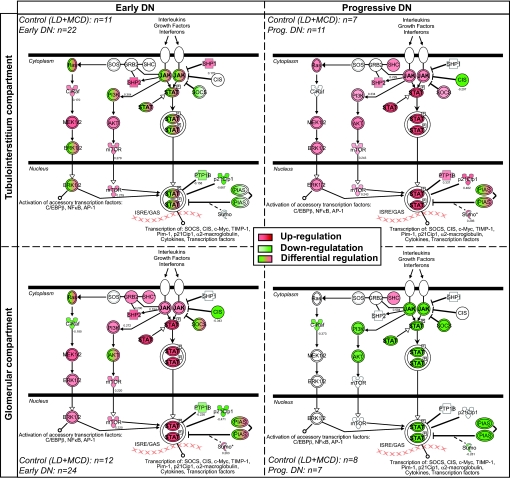
Pathway mapping from Affymetrix microarray analysis identified Jak/Stat signaling as one of the highly regulated pathways in the glomerular and tubulointerstitial compartments of patients with early and progressive diabetic nephropathy compared with control subjects (Fig. 1). In the tubulointerstitial compartment, several Jak/Stat family members were downregulated in early diabetic nephropathy patients, whereas most were expressed at higher levels in progressive diabetic nephropathy patients compared with control subjects (Fig. 1, upper panel). These included Jak-1, -2, and -3 and Stat-1, -3, -4, and -5B. Conversely, in the glomerular compartment, Jak/Stat pathway members were mainly increased in early diabetic nephropathy compared with control subjects then downregulated in progressive diabetic nephropathy (Fig. 1, lower panel).
FIG. 1.
Jak/Stat canonical pathway in human diabetic nephropathy as assessed by Ingenuity Pathway Analysis software. The Jak/Stat family members represented include Jak-1, -2, and -3 and Stat-1, -2, -3, -4, -5A, -5B, and -6. The mRNA expression changes for Jak/Stat family members in early (PIMA Indians, n = 22–24) (left panel) and progressive (European, n = 7–11) (right panel) human diabetic nephropathy (DN) are indicated in red for increased expression and green for decreased expression, compared with the corresponding control group (LD + MCD, n = 7–12). 2000–2008 Ingenuity Systems. All rights reserved. LD, living donor; MCD, minimal change disease.
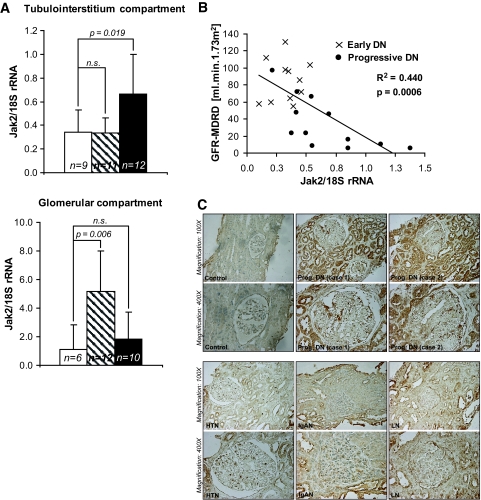
Real-time RT-PCR analysis of RNA from control and early and progressive diabetic nephropathy microdissected biopsies was performed to confirm the mRNA changes of Jak/Stat pathway members. As suggested by the microarray data, a significant regulation of Jak-1, -2, and -3 and Stat-1 and -3 mRNAs was demonstrated in glomeruli from patients with early and progressive diabetic nephropathy and in the tubulointerstitium of patients with progressive diabetic nephropathy. In contrast to the microarray data, real-time RT-PCR found that mRNA expression of most Jak/Stat family members remained elevated in glomeruli from progressive diabetic nephropathy patients compared with control samples (Supplementary Table 5A). In the remainder of our study, we focused on Jak-2 since it appears to play a key role in proinflammatory responses in both glomerular and tubular cells (24). Jak-2 mRNA levels were significantly increased in the glomeruli of early diabetic nephropathy patients and in the tubulointerstitium of patients with progressive diabetic nephropathy compared with those of control subjects (Fig. 2A). Jak-2 mRNA levels were not increased in glomeruli or tubulointerstitium from patients with other progressive kidney diseases (Supplementary Table 5B).
FIG. 2.
Jak-2 mRNA expression levels in human diabetic nephropathy: correlation with renal function. A: Increased expression of Jak-2 mRNA in the glomeruli of early diabetic nephropathy patients and in the tubulointerstitium of progressive diabetic nephropathy patients compared with control subjects (n = 6–9 in the control group, n = 11–12 in the early diabetic nephropathy group, and n = 10–12 in the progressive diabetic nephropathy group). □, control;  , early diabetic nephropathy; ▪, progressive diabetic nephropathy. B: Correlation of tubulointerstitial Jak-2 mRNA expression (as measured by real-time RT-PCR) with eGFR in patients with early (×, n = 11) and progressive (•, n = 12) diabetic nephropathy. C: Representative Jak-2 immunohistographs of kidney biopsies from patients with no kidney disease (Control), progressive diabetic nephropathy (Prog. DN), hypertensive nephropathy (HTN), IgA nephropathy (IgAN), or lupus nephritis (LN). Jak-2 expression was substantially and significantly increased in the proximal tubular cells and glomeruli of the patients with diabetic nephropathy compared with the control and not in other progressive kidney diseases. (Please see http://dx.doi.org/10.2337/db08-1328 for a high-quality digital representation of this figure.)
, early diabetic nephropathy; ▪, progressive diabetic nephropathy. B: Correlation of tubulointerstitial Jak-2 mRNA expression (as measured by real-time RT-PCR) with eGFR in patients with early (×, n = 11) and progressive (•, n = 12) diabetic nephropathy. C: Representative Jak-2 immunohistographs of kidney biopsies from patients with no kidney disease (Control), progressive diabetic nephropathy (Prog. DN), hypertensive nephropathy (HTN), IgA nephropathy (IgAN), or lupus nephritis (LN). Jak-2 expression was substantially and significantly increased in the proximal tubular cells and glomeruli of the patients with diabetic nephropathy compared with the control and not in other progressive kidney diseases. (Please see http://dx.doi.org/10.2337/db08-1328 for a high-quality digital representation of this figure.)
The estimated GFR (eGFR) of patients with diabetic nephropathy (early and progressive), as calculated by the Modification of Diet in Renal Disease Study formula (25), was strongly and inversely correlated with Jak-2 tubulointerstitial (Fig. 2B) but not glomerular mRNA levels, as determined by quantitative RT-PCR; this is also true with 1/serum creatinine (Supplementary Table 5C). Tubulointerstitial, but not glomerular, mRNA expression of Jak-1 and -3 and Stat-1 were also inversely and significantly correlated with eGFR and 1/serum creatinine (Supplementary Table 5C). Mean blood pressure also inversely correlated with tubulointerstitial Jak-3 mRNA expression (Supplementary Table 5C).
Jak-2 immunohistochemistry showed a robust increase in Jak-2 protein expression in proximal tubular epithelia and glomerular cells in diabetic nephropathy compared with those in control subjects and other progressive kidney diseases (Fig. 2C), indicating that protein expression corresponded with mRNA levels. In the tubulointerstitial regions, contribution from cellular elements other than tubular cells was minimal (data not shown).
Jak-2 expression in db/db C57BLKS and STZ-induced DBA/2J diabetic mice.
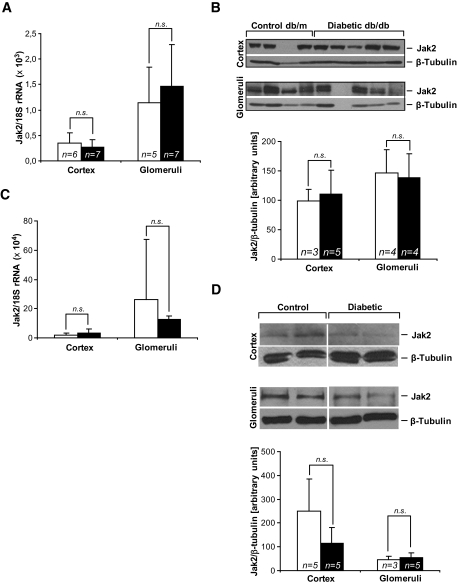
Since common murine diabetic nephropathy models fail to develop severe glomerulosclerosis or tubulointerstitial fibrosis and kidney failure, we hypothesized that these models would also fail to manifest critical gene expression changes that occur in humans with progressive diabetic nephropathy. Db/db C57BLKS mice and STZ-induced diabetic DBA/2J mice develop robust changes of early diabetic nephropathy (albuminuria, mesangial expansion, and podocyte loss) but do not develop the significant tubulointerstitial fibrosis and decline in kidney function that characterize progressive diabetic nephropathy in humans (10,23,24). Therefore, we specifically hypothesized that Jak-2 expression would be unchanged in the glomeruli and renal cortex (largely tubulointerstitial tissue) of these two mouse models. Indeed, Jak-2 mRNA and protein levels were similar in diabetic mice and their nondiabetic controls (Fig. 3).
FIG. 3.
Jak-2 in the C57BLKS type 2 diabetic nephropathy and STZ-induced DBA/2J type 1 diabetic nephropathy mouse models. A: Jak-2 real-time RT-PCR mRNA expression in cortex (n = 6 control, n = 7 diabetic) and glomeruli (n = 5 control, n = 7 diabetic) was not altered in db/db mice (▪) compared with db/m control animals (□). B: Jak-2 immunoblot (upper panel) and densitometry (lower panel). Jak-2 protein levels were unchanged in the cortex (n = 3 control, n = 5 diabetic) and glomeruli (n = 4 in both groups) of db/db mice (▪) versus db/m mice (□). C: Jak-2 qRT-PCR mRNA expression in cortex and glomeruli was not altered in DBA/2J diabetic mice (▪) compared with control animals (□) (n = 5 in each group). D: Jak2 immunoblot (upper panel) and densitometry (lower panel) in DBA/2J mice. ▪, diabetic; □, control. Jak-2 protein levels were not significantly different in the cortex or glomeruli of DBA/2J diabetic mice. n.s., statistically nonsignificant.
Effects of increased Jak-2 expression in murine mesangial cells.
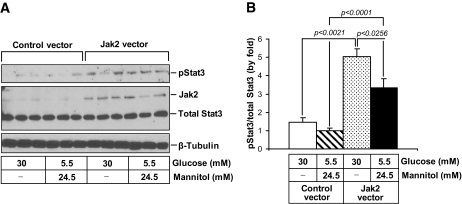
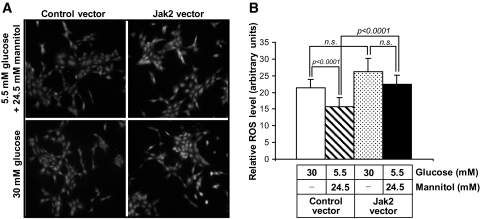
To determine whether increases in Jak-2 expression alone could induce downstream signaling, we analyzed the overexpression of Jak-2 in cultured mouse mesangial cells for 36 h. Jak-2 overexpression in cells incubated in normal (5.5 mmol/l) glucose resulted in a threefold increase in phosphorylation of Stat-3 on tyrosine 705, consistent with Stat-3 activation (Fig. 4). There was no significant change in the level of total Stat-3 protein. Incubation in high (30 mmol/l) glucose medium alone did not significantly enhance Stat-3 phosphorylation compared with normal (5.5 mmol/l glucose) but did lead to an additive increase in Stat-3 phosphorylation in the Jak-2–transfected cells. We also determined whether increased Jak-2 expression could enhance ROS species, since enhanced ROS generation appears to be a hallmark of progressive diabetic nephropathy in humans (26). As determined by CM-H2DCF fluorescence, a significant increase in ROS was observed in Jak-2–overexpressing cells compared with control vector cells when both cell types were grown in 5.5 mmol/l glucose (Fig. 5). A similar increase in ROS generation was demonstrated in control vector cells incubated in 30 mmol/l glucose media compared with cells grown in 5.5 mmol/l glucose median. There was a further, but nonsignificant, increase in ROS in Jak-2–transfected cells incubated in high glucose medium compared with Jak-2–transfected cells grown in normal glucose medium.
FIG. 4.
Effects of Jak-2 overexpression and high glucose on Stat-3 phosphorylation in Jak-2 mesangial cells. A: Jak-2 (130 kDa), total Stat-3 (79 kDa), phosphorylated Stat-3 (79 kDa), and β-tubulin (50 kDa) immunoblots of murine mesangial cells transfected with a Jak-2 prk-5 plasmid or a control vector (n = 3 per group). B: Phosphorylated Stat-3 levels normalized to total Stat-3 levels.
FIG. 5.
Effects of Jak-2 overexpression and high glucose on ROS generation in mesangial cells. A: DCF fluorescence in murine mesangial cells transfected with either Jak-2 prk-5 or control vectors exposed to 5.5 mmol/l d-glucose + 24.5 mmol/l mannitol or 30 mmol/l d-glucose for 24 h. Image exposure times were identical and are representative of those from three separate fields on five separate slides for each condition. B: ROS quantitation from the DCF fluorescence experiments. Fluorescence intensity was determined using NIH ImageJ. (n = 5 per group; 50 cells were assessed for each slide).
DISCUSSION
Diabetic nephropathy in humans requires activation of multiple molecular programs to become manifest. The current pathophysiological understanding of diabetic nephropathy is derived largely from analysis of animal and cellular models. Several pathways were identified by these approaches and were confirmed to play critical roles in the evolution of diabetic nephropathy in humans (27,28). However, despite pathogenic changes in a variety of different signaling pathways, current murine models of diabetic nephropathy fail to completely replicate progressive human diabetic nephropathy (glomerulosclerosis, tubulointerstitital fibrosis, and decline in GFR) (10). This discrepancy could be due to partial, incomplete, or temporary activation of critical pathogenic responses and/or distinctive protective responses that counteract or prevent nephropathy in mice that are not manifest in humans. To identify those human-specific pathogenic or murine protective pathways, we used a comparative transcriptomic approach to study diabetic nephropathy in both species.
In human diabetes, as assessed by microarray analysis and confirmed by RT-PCR and immunohistochemistry, the Jak/Stat signaling pathway appeared to be one of the top regulated pathways and was consistently altered in both glomeruli and tubulointerstitium from patients with diabetic nephropathy. Marrero's group previously has identified Jak/Stat activation as potentially pathogenic in mediating angiotenisin II signaling, in inducing transforming growth factor-β expression, and in stimulating extracellular matrix protein production in cultured mesangial cells and animal models of diabetic nephropathy (14,15,24,29,30). However, to our knowledge the current report is the first study to demonstrate enhanced Jak/Stat expression in human diabetic nephropathy. While multiple members of the Jak/Stat family showed increased mRNA expression in microarrays and RT-PCR expression studies, we focused on the expression patterns of Jak-2, since it is a critical upstream regulator of many Jak/Stat signaling events and was already implicated in processes that enhance fibrosis and epithelial mesenchymal transition in diabetic nephropathy (11,13–15,24,29,30).
Our findings suggest an interesting compartmental and temporal association of enhanced Jak-2 expression. Glomerular Jak-2 mRNA levels increase several-fold in diabetic patients with early diabetic nephropathy and then decline in later stages as tubulointerstitial Jak-2 increases along with progressive tubulointerstitial fibrosis and reduction in kidney function. Thus, enhanced Jak-2 expression temporally corresponds to the evolution of human diabetic nephropathy, with glomerulopathy followed by tubulointerstitial fibrosis. The impressive inverse correlation between tubulointerstitial with eGFR suggests a potential causal relationship between enhanced Jak/Stat expression and progressive tubulointerstitial fibrosis and renal failure. While some of this apparent correlation could be due to population differences between the early diabetic nephropathy group, who are all Pima Indians, and the mostly European progressive diabetic nephropathy group, a strong correlation between tubulointerstitial Jak-2 expression and eGFR remained in the progressive diabetic nephropathy group alone, suggesting that this association was not due to population differences.
Jak-2 mRNA induction in diabetic nephropathy was confirmed by immunochemistry and appears to be diabetic nephropathy specific, as there was no induction of Jak-2 mRNA in glomeruli or tubulointerstitum in lupus nephritis, IgA nephropathy, or hypertensive nephrosclerosis. Jak-2 protein expression was increased in both glomeruli and proximal tubular cells in humans with diabetic nephropathy, but there was variable expression in other nephropathies with tubular damage and proteinuria, consistent with the notion that Jak-2 activation is not generic to all progressive renal diseases. This observation confirmed the results obtained by Affymetrix GeneChip compared with those for control patients (living donors, n = 21), where Jak-2 mRNA showed no consistent regulation in patients with hypertensive nephropathy (n = 20), IgA nephropathy (n = 27), and lupus nephritis (n = 32), with fold changes between 0.93 and 1.10 in the glomerular and tubulointerstitial compartments.
Similarly, there was no correlation between gene expression of Jak-2 or other Jak/Stat members in diabetic nephropathy samples with blood pressure, duration of diabetes, glycosylated hemoglobin, or BMI (with the sole exception of Jak-3 and blood pressure), suggesting that the correlation with eGFR was specific and not due to some confounding factor. Nonetheless, a significant correlation between Jak-2 and eGFR does not necessarily imply a causal connection between the two parameters, nor does it imply directionality if a causal relationship exists. It is imperative to follow up these studies with prospective analysis of patients with diabetic nephropathy to determine whether glomerular or tubulointerstitial Jak-2 expression truly predicts progression of diabetic nephropathy in humans. Additionally, tissue-specific Jak-2–overexpression studies in animal models of diabetic nephropathy will be important.
The absence of enhanced Jak-2 expression in the db/db C57BLKS and STZ-induced diabetic DBA/2J mouse diabetic nephropathy models is interesting since these models, frequently used for studies of diabetic nephropathy (10,22,31,32), develop high levels of albuminuria and undergo extensive early glomerular changes of diabetic nephropathy (22,31,32) but rarely progress to severe glomerulosclerosis and do not develop significant tubulointerstitial fibrosis and progressive kidney failure (10,31,32). No increases in Jak-2 mRNA or protein expression were found in either glomerular or tubulointerstitial compartments, suggesting one possible reason for the lack of progressive diabetic nephropathy induction in these mouse models. It is certainly likely that additional responses critical for progression of human diabetic nephropathy are missing in conventional murine models of diabetic nephropathy and that protective responses in mouse models may be absent from humans with progressive diabetic nephropathy. Thus, the strategy of uncovering divergent responses between human progressive diabetic nephropathy and murine models of early diabetic nephropathy may reveal other pathways that are essential for the pathogenesis of diabetic nephropathy and that are potential targets for therapeutic or prevention strategies.
Since Jak/Stat pathways are activated by growth factors, cytokines, or other upstream signals, and because Jak-2 protein activation is via autophosphorylation, enhanced Jak-2 expression should result in Stat tyrosine phosphorylation and activation only with Jak-2 activation. Thus, it was important to test whether Jak-2 overexpression alone could induce Stat phosphorylation and other downstream responses. While in vivo confirmation of such a response necessarily awaits the generation and testing of tissue-specific Jak-2 transgenic mice, we have confirmed that Jak-2 overexpression alone, without additional growth factors or cytokines, substantially increases Stat-3 phosphorylation on tyrosine 705. This observation confirms activation of Jak-2/Stat-3 signaling simply by overexpressing Jak-2 in mouse mesangial cells. Interestingly, high glucose had only a modest and statistically insignificant effect on Stat-3 phosphorylation, implying that high glucose alone is not sufficient to trigger Jak-2 signaling in our system. However, high glucose had an additive effect on Jak-2 overexpression on Stat-3 phosphorylation. The molecular mechanism by which Jak-2 overexpression induces Stat-3 phosphorylation remains to be determined.
Enhanced production of ROS has been described as a potential major activator of Jak/Stat signaling (33) and can occur independently of the addition of exogenous cytokines (34). This phenomenon appears to be relatively general since ROS mediate induction of Jak-2 activation in tissues such as cardiac myocytes and vascular smooth muscle cells (34–36) as well as kidney cells (12,14,15,32). We did not test whether ROS could further augment the signaling to Stat-3 in our Jak-2–overexpressing cells but we did show that Jak-2 overexpression induced ROS production in mesangial cells, especially those cultured in high glucose medium. This observation suggests the presence of an amplification loop in diabetic glomerular and proximal tubular cells in which there is both enhanced Jak-2 expression, as shown in this study, as well as enhanced ROS production, due to metabolic alterations resulting from enhanced glucose flux (26,37). These two independent processes can each enhance the other, leading to progressive downstream signaling from both pathways and inexorable fibrosis and kidney failure.
Enhanced Jak-2 expression in both glomerular and proximal tubule cells may stimulate a host of downstream processes that lead to the glomerulosclerosis, tubulointerstitial fibrosis, and kidney failure that affects up to 40% of diabetic patients. The absence of this response may protect mice from these late, but deadly, aspects of progressive diabetic nephropathy. Further exploration of Jak/Stat pathways in diabetic nephropathy as well as additional systems analysis of the differences between mice and humans may elucidate targets for therapies that could help humans with diabetic nephropathy be more like mice.
Supplementary Material
Acknowledgments
These studies were supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (U01 DK60994 to F.C.B., U01 DK076139 to F.C.B. and M.K., and R21 DK079441 to M.K.) and the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. This research used the Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center supported by National Institutes of Health Grant DK20572.
No potential conflicts of interest relevant to this article were reported.
We are indebted to Drs. Almut Nitsche and Bodo Brunner (sanofi-aventis Deutschland, Frankfurt, Germany) for DNA chip hybridizations and delivery of the dataset of hybridization results.
APPENDIX
Members of the European Renal cDNA Bank-Kroener-Fresenius biopsy bank at the time of the study: C.D. Cohen, M. Fischereder, H. Schmid, P.J. Nelson, M. Kretzler, D. Schloendorff, Munich; J.D. Sraer, P. Ronco, Paris; M.P. Rastaldi, G. D'Amico, Milan; F. Mampaso, Madrid; P. Doran, H.R. Brady, Dublin; D. Moenks, Goettingen; P. Mertens, J. Floege, Aachen; N. Braun, T. Risler, Tuebingen; L. Gesualdo, F.P. Schena, Bari; J. Gerth, G. Wolf, Jena; R. Oberbauer, D. Kerjaschki, Vienna; B. Banas, B.K. Kraemer, Regensburg; W. Samtleben, Munich; H. Peters, H.H. Neumayer, Berlin; K. Ivens, B. Grabensee, Dueseldorf; R.P. Wuethrich, Zurich; V. Tesar, Prague.
Published ahead of print at http://diabetes.diabetesjournals.org on 18 November 2008.
A.B. is currently affiliated with sanofi-aventis Deutschland, Frankfurt, Germany.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC: Structural-functional relationships in diabetic nephropathy. J Clin Invest 74: 1143–1155, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mauer SM, Lane P, Zhu D, Fioretto P, Steffes MW: Renal structure and function in insulin-dependent diabetes mellitus in man. J Hypertens Suppl 10: S17–20, 1992 [DOI] [PubMed] [Google Scholar]
- 3.Chavers BM, Bilous RW, Ellis EN, Steffes MW, Mauer SM: Glomerular lesions and urinary albumin excretion in type I diabetes without overt proteinuria. N Engl J Med 320: 966–970, 1989 [DOI] [PubMed] [Google Scholar]
- 4.Nangaku M: Mechanisms of tubulointerstitial injury in the kidney: final common pathways to end-stage renal failure. J Int Med 43: 9–17, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Bader R, Bader H, Grund KE, Mackensen-Haen S, Christ H, Bohle A: Structure and function of the kidney in diabetic glomerulosclerosis: correlations between morphological and functional parameters. Pathol Res Pract 167: 204–216, 1980 [DOI] [PubMed] [Google Scholar]
- 6.Fioretto P, Mauer M: Histopathology of diabetic nephropathy. Semin Nephrol 27: 195–207, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henger A, Kretzler M, Doran P, Bonrouhi M, Schmid H, Kiss E, Cohen CD, Madden S, Porubsky S, Grone EF, Schlondorff D, Nelson PJ, Grone HJ: Gene expression fingerprints in human tubulointerstitial inflammation and fibrosis as prognostic markers of disease progression. Kidney Int 65: 904–917, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Kretzler M, Cohen CD, Doran P, Henger A, Madden S, Grone EF, Nelson PJ, Schlondorff D, Grone HJ: Repuncturing the renal biopsy: strategies for molecular diagnosis in nephrology. J Am Soc Nephrol 13: 1961–1972, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Susztak K, Bottinger EP: Diabetic nephropathy: a frontier for personalized medicine. J Am Soc Nephrol 17: 361–367, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Breyer MD, Boettinger E, Brosius FC, Coffman TM, Harris RC, Heilig CW, Sharma K: AMDCC: mouse models of diabetic nephropathy. J Am Soc Nephrol 16: 27–45, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Huang JS, Chuang LY, Guh JY, Chen CJ, Yang YL, Chiang TA, Hung MY, Liao TN: Effect of nitric oxide-cGMP-dependent protein kinase activation on advanced glycation end-product-induced proliferation in renal fibroblasts. J Am Soc Nephrol 16: 2318–2329, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Nakajima H, Takenaka M, Kaimori JY, Hamano T, Iwatani H, Sugaya T, Ito T, Hori M, Imai E: Activation of the signal transducer and activator of transcription signaling pathway in renal proximal tubular cells by albumin. J Am Soc Nephrol 15: 276–285, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Nightingale J, Patel S, Suzuki N, Buxton R, Takagi KI, Suzuki J, Sumi Y, Imaizumi A, Mason RM, Zhang Z: Oncostatin M, a cytokine released by activated mononuclear cells, induces epithelial cell-myofibroblast transdifferentiation via Jak/Stat pathway activation. J Am Soc Nephrol 15: 21–32, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Banes AK, Shaw S, Jenkins J, Redd H, Amiri F, Pollock DM, Marrero MB: Angiotensin II blockade prevents hyperglycemia-induced activation of JAK and STAT proteins in diabetic rat kidney glomeruli. Am J Physiol Renal Physiol 286: F653–F659, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Shaw S, Amiri F, Eaton DC, Marrero MB: Inhibition of the Jak/STAT signaling pathway prevents the high glucose-induced increase in TGF-β and fibronectin synthesis in mesangial cells. Diabetes 51: 3505–3509, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Cohen CD, Frach K, Schlondorff D, Kretzler M: Quantitative gene expression analysis in renal biopsies: a novel protocol for a high-throughput multicenter application. Kidney Int 61: 133–140, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Lindenmeyer MT, Kretzler M, Boucherot A, Berra S, Yasuda Y, Henger A, Eichinger F, Gaiser S, Schmid H, Rastaldi MP, Schrier RW, Schlondorff D, Cohen CD: Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. J Am Soc Nephrol 18: 1765–1776, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Schmid H, Boucherot A, Yasuda Y, Henger A, Brunner B, Eichinger F, Nitsche A, Kiss E, Bleich M, Grone HJ, Nelson PJ, Schlondorff D, Cohen CD, Kretzler M: European Renal c DNABC: modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes 55: 2993–3003, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Lorz C, Benito-Martin A, Boucherot A, Ucero AC, Rastaldi MP, Henger A, Armelloni S, Santamaria B, Berthier CC, Kretzler M, Egido J, Ortiz A: The death ligand TRAIL in diabetic nephropathy. J Am Soc Nephrol 19: 904–914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siu B, Saha J, Smoyer WE, Sullivan KA, Brosius FC III: Reduction in podocyte density as a pathologic feature in early diabetic nephropathy in rodents: prevention by lipoic acid treatment. BMC Nephrol 7: 6, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Saha J, Byun J, Schin M, Lorenz M, Kennedy RT, Kretzler M, Feldman EL, Pennathur S, Brosius 3rd FC: Rosiglitazone reduces renal and plasma markers of oxidative injury and reverses urinary metabolite abnormalities in the amelioration of diabetic nephropathy. Am J Physiol Renal Physiol 295: F1071–F1081, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma K, McCue P, Dunn SR: Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol 284: F1138–F1144, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Argetsinger LS, Kouadio JL, Steen H, Stensballe A, Jensen ON, Carter-Su C: Autophosphorylation of JAK2 on tyrosines 221 and 570 regulates its activity. Mol Cell Biol 24: 4955–4967, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marrero MB, Banes-Berceli AK, Stern DM, Eaton DC: Role of the JAK/STAT signaling pathway in diabetic nephropathy. Am J Physiol Renal Physiol 290: F762–F768, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G: Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G: The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease: Modification of Diet in Renal Disease Study Group. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Beisswenger PJ, Drummond KS, Nelson RG, Howell SK, Szwergold BS, Mauer M: Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes 54: 3274–3281, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Brosius FC, Heilig CW: Glucose transporters in diabetic nephropathy. Pediatr Nephrol 20: 447–451, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Tuttle KR, Anderson PW: A novel potential therapy for diabetic nephropathy and vascular complications: protein kinase C beta inhibition. Am J Kidney Dis 42: 456–465, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Amiri F, Shaw S, Wang X, Tang J, Waller JL, Eaton DC, Marrero MB: Angiotensin II activation of the JAK/STAT pathway in mesangial cells is altered by high glucose. Kidney Int 61: 1605–1616, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Banes-Berceli AK, Shaw S, Ma G, Brands M, Eaton DC, Stern DM, Fulton D, Caldwell RW, Marrero MB: Effect of simvastatin on high glucose- and angiotensin II-induced activation of the JAK/STAT pathway in mesangial cells. Am J Physiol Renal Physiol 291: F116–F121, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD: Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes 54: 2628–2637, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Gurley SB, Clare SE, Snow KP, Hu A, Meyer TW, Coffman TM: Impact of genetic background on nephropaty in diabetic mice. Am J Physiol Renal Physiol 290: F214–F222, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Simon AR, Rai U, Fanburg BL, Cochran BH: Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol 275: C1640–C1652, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Madamanchi NR, Moon SK, Hakim ZS, Clark S, Mehrizi A, Patterson C, Runge MS: Differential activation of mitogenic signaling pathways in aortic smooth muscle cells deficient in superoxide dismutase isoforms. Arterioscler Thromb Vasc Biol 25: 950–956, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Modesti A, Bertolozzi I, Gamberi T, Marchetta M, Lumachi C, Coppo M, Moroni F, Toscano T, Lucchese G, Gensini GF, Modesti PA: Hyperglycemia activates JAK2 signaling pathway in human failing myocytes via angiotensin II-mediated oxidative stress. Diabetes 54: 394–401, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Madamanchi NR, Li S, Patterson C, Runge MS: Reactive oxygen species regulate heat-shock protein 70 via the JAK/STAT pathway. Arterioscler Throm Vasc Biol 21: 321–326, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.