Abstract
OBJECTIVE—The G6PC2 gene encoding islet-specific glucose-6-phosphatase related protein (IGRP) has a common promoter variant, rs573225 (−231G/A), located within a Foxa binding site. We tested the cis-regulatory effects of rs573225 on promoter activity and its association with insulin response to oral glucose.
RESEARCH DESIGN AND METHODS—Functional effects of rs573225 were explored in transfected INS-1 and HIT-T β-cell lines. A total of 734 young obese subjects of European ancestry were genotyped for rs573225. Insulin and glucose levels were measured in response to oral glucose, and the insulinogenic index (IGI) of insulin secretion was calculated.
RESULTS—In vitro, the G allele showed a higher affinity for binding Foxa2 transcription factor and increased G6PC2 promoter activity. Foxa2 binding is modified if the C adjacent to the G allele is methylated. IGI was associated with rs573225 by linear regression analysis and was 30% greater in AA or AG than in GG obese children. rs573225 was also associated with fasting glucose.
CONCLUSIONS—rs573225 is a functional cis-regulatory (epi)-single-nucleotide polymorphism (SNP) of G6PC2 associated with glucose-insulin homeostasis in obese children, likely to explain the results of recent genome-wide association studies in nondiabetic adults.
The flux through glycolysis is the sensed process that makes β-cells glucose responsive. Thus, altered kinetics of glucose-induced insulin secretion could result from a changed intrinsic activity, cellular level, or allosteric regulation of several enzymes including glucokinase, phospho-fructokinase, G6PC2 (glucose-6-phosphatase, catalytic, 2), or others involved in the regulation of cellular glucose-6-phosphate (G6P) content. Whereas glucokinase, a high Km enzyme, phosphorylates glucose into G6P in β-cells, G6PC2, a low Km enzyme, catalyzes G6P dephosphorylation (1), which could antagonize insulin secretion (2). In mice, G6PC2 has only ∼5% of the activity of liver G6Pase (1), sufficient however for the knockout of G6PC2 to result in a small decrease in glycemia (3). The human G6PC2 gene is located in 2q24 in a region that we found linked to glycemia (4). The G6PC2 promoter is inactive in HepG2 cells but has 150-fold more activity in HIT-T15 β-cells, due to the region located between −306 and +3 (5). This proximal promoter region regulates G6PC2 expression in transiently transfected βTC-3, HIT-T15, and Min6 cells through the binding of Foxa2 and MafA transcription factors (6). We found three single-nucleotide polymorphisms (SNPs) in the G6PC2 promoter in public banks, among which we selected rs573225, a G/A variant located at position −231. Our choice was based on the fact that this variant is common among European populations and belongs to a binding site for Foxa transcription factors (5,7) that carries a potentially methylatable CG motif created by the G allele. The Foxa proteins, previously designated HNF-3, are known to modulate the expression of pancreatic genes involved in glucose homeostasis (8–11).
We performed a functional analysis of rs573225 in β-cell lines and found that this promoter SNP binds Foxa2 with variable affinity and can act as a transcriptional regulator and then explored whether rs573225 genotypes were associated with insulin responses to oral glucose in 734 obese children of European ancestry. Recently, two independent genome-wide association studies revealed that SNPs in linkage disequilibrium with rs573225, but with no known function, were associated with fasting glucose levels in nondiabetic adults (12,13). We thus suspect rs573225 to be the causative variant for the observed associations.
RESEARCH DESIGN AND METHODS
Methylation-specific PCR.
Human pancreatic DNAs were obtained from human islets provided by T. Berney; 1 μg was fragmented with XbaI and denaturated by incubation in 3 mol/l NaOH for 15 min at 37°C. Bisulfite conversion was carried out in a solution of sodium bisulfite and hydroquinone at 55°C overnight. After desalting, DNAs were desulfonated by incubation in NaOH at 37°C for 15 min, the solution was neutralized with Na-acetate, and DNAs were ethanol precipitated. Pellets were dissolved in distilled H2O, and DNA aliquots were used for PCR amplifications by TaqDNA polymerase (Invitrogen) under the following conditions: 94°C, 5 min; 45 amplification cycles (94°C, 30 s; 50°C, 30 s; 72°C, 30 s); and finally at 72°C, 10 min. The PCR products were purified using QIAquick kit (Qiagen) and used for automated sequencing (DNA Sequencer, Roche).
Electrophoretic mobility shift assay.
Nuclear extracts were prepared from HepG2 cells using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce). Binding reactions used binding buffer with 1 μl dIdC (Pierce). A total of 4 μg of the protein extracts was used per gel mobility shift sample. Double-stranded oligonucleotides used to detect protein binding on the G6PC2-231 element were end-labeled with biotin (Operon). The double-strand oligonucleotides were “G” probe, 5′-biotin-AATTTCAAGCAAACATGATCCAACTGTT-3′; “A” probe, 5′-biotin-AATTTCAAACAAACATGATCCAACTGTT-3′; and “GCm” probe, 5′-biotin-AATTTCAAGCMeAAACATGATCCAACTGTT-3′. Samples were subjected to electrophoretic separation on a 6% nondenaturating polyacrylamide gel. The gel was transferred to a Nylon membrane (Pierce), which was cross-linked using a UV-Light cross linker instrument (Bio-Rad) and incubated with Nucleic Acid Detection buffer (Pierce). Signals were visualized using Chemiluminescent Substrate.
For supershift assays, binding reactions were incubated with 1 μl of antibodies (Foxa 1, 2, or 3; Santa Cruz) before addition of biotin probes. DNA protein complexes were electrophoresed through 6% nondenaturing polyacrylamide gel and revealed with the Nucleic Acid Detection Buffer.
Cell cultures.
INS-1 cells derived from a rat insulinoma cell line developed and propagated at the Division of Biochimie Clinique (C.B. Wollheim) were cultured in RPMI-1640 media (GIBCO) with 10% heat-inactivated fetal bovine serum (Dainippon Pharmaceutical), 50 μmol/l 2-mercaptoethanol, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. HIT-T15 cells were cultured in the same media.
Transfections.
We amplified the proximal promoter (−306 + 14 bp) segment of the human G6PC2 carrying either −231G or −231A allele. We inserted each form into the pGl3 basic vector plasmid (Promega). Sequencing confirmed that constructs differed uniquely at the −231 position. A total of 100,000 INS-1 or HIT-T15 cells were plated on 24-well plates in RPMI-1640 (GIBCO) and cotransfected with vector plasmids: 200 ng pGl3–231G or −231A per well and 70 ng pRL-TK plasmid (Promega) per well using Fugene HD (Roche). Luciferase activities were determined 24 h after transfection using dual-luciferase reporter assays (Promega) using a Centro LB 960 luminometer (Berthold).
Cohort.
The 734 studied obese children were recruited in the pediatric endocrinology department of Saint Vincent de Paul Hospital as reported (4). All were of Western European ancestry assessed by family history and grandparents’ birthplace. Inclusion criteria were a BMI reaching the 95th percentile at time of study, exceeding the 85th percentile before 6 years of age, and a monotonic weight curve from birth to study time. After 12 h of overnight fasting after 3 days of a standardized isocaloric diet, the oral glucose tolerance test (OGTT) consisted of 1.75 g/kg glucose in 200 ml lemon-flavored water at 10°C. Plasma insulin concentrations were measured in duplicate (14) at 0, 30, 60, 90, and 120 min. The insulinogenic index (IGI) was calculated as [Ins30 − Ins0 (pmol/l)]/[Gly30 − Gly0 (mmol/l)], and homeostasis model assessment (HOMA)-β was calculated as [20 × Ins0 (pmol/l)]/[Gly0 (mmol/l)] (14).
Genotyping.
The rs573225 polymorphism was detected by fluorescent hybridization probe melting curves using real-time PCR. Samples were run on a Roche LightCycler380; data were analyzed using LightCycler Software 3.5 (10 min denaturation; 40 cycles at 95°C, 1 s; 50°C, 5 s; 72°C, 15 s). Primer sequences are available on request. Three internal controls made of samples that were previously genotyped and sequenced were systematically introduced into each 96-well plate.
Statistical analysis.
Values are reported as means ± SD. For all tests, a P value <0.05 was considered significant. General linear regression models were used to assess the relation between rs573225 and glucose or insulin values. Regression model coefficients were determined to be significant with the use of the standard t test. For glycemia and IGI analyses, the covariates included age and BMI (15). Normality of residuals was systematically verified, and robustness analyses were performed by dropping outliers from analyses. For in vitro analyses, a t test was used to compare luciferase activity in the genotypic groups for each cell type. Data analyses used the R statistical software.
RESULTS
In vitro.
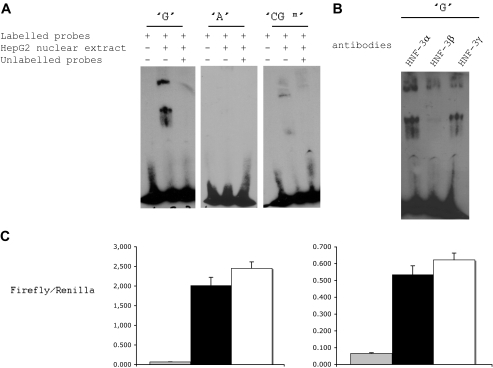
We found that rs573225 alleles modified the binding of Foxa to G6PC2 promoter by comparing the binding of nuclear proteins from HepG2 cells to probes carrying the “G” or the “A” allele or to a “GCm” probe (Figs. 1 and 2). Electrophoretic mobility shift assay experiments revealed that the G probe showed a specific binding to HepG2 cell nuclear protein complex, whereas the A probe could not bind this complex and the GCm probe bound this complex with a lower affinity (Fig. 2A). The presence of Foxa2 proteins in the DNA-protein complex was confirmed by gel supershift assay experiments (Fig. 2B). These observations indicate that rs573225 alleles create a functional variation in a Foxa2 binding motif, with an added effect of the methylation of the cytosine residue adjacent to the G allele of rs573225. When the proximal segment of G6PC2 gene promoter bearing rs573225 and placed upstream of the luciferase gene in the pGl3 basic vector was transfected into INS-1 or HIT-T15 cells, the G allele was consistently associated with a higher promoter activity than the A allele (Fig. 2C).
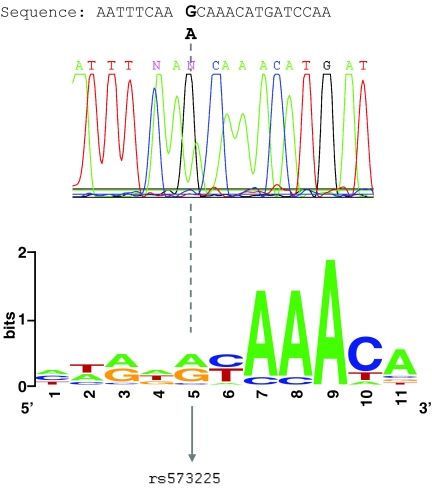
FIG. 1.
Representative standard sequencing diagram for bisulfite direct sequencing of G6PC2 gene promoter. Human pancreatic DNA provided from a rs573225 heterozygote is shown. Signals of false conversion were not observed. All cytosines in this sequence were not converted to uracil. The G6PC2 gene promoter region was methylated. On the bottom, the Foxa consensus site in humans is shown. (Please see http://dx.doi.org/10.2337/db08-0587 for a high-quality digital representation of this image.)
FIG. 2.
The G allele of the rs573225 (−231G/A) binds Foxa2 and is associated with increased transcriptional activity of the G6PC2 gene. A: Electrophoretic mobility shift assay (EMSA) carried out with HepG2 nuclear extracts and unmethylated G probe or A probe and methylated CGm probe. The oligonucleotide sequence includes the G6PC2 promoter region −239 to −267. B: Specific antibodies was added in the gel supershift assay and carried out with the G probe only. C: Mutation of the rs573225 G6PC2 promoter variant reduces G6PC2 transcriptional activity in HIT-T15 cells (left) and in INS-1 cells (right). For each cell type, the following is shown: in gray, luciferase activity in pGl3 basic vector without G6PC2 promoter (n = 24); in black, luciferase activity in pGl3 basic vector with G6PC2 promoter bearing the −231A allele (n = 24); and in white, luciferase activity in pGl3 basic vector with G6PC2 promoter bearing the −231G allele (n = 24). Data are means ± SD. P = 2.55 × 10−7 −231G allele vs. −231A allele, after Bonferroni correction.
In vivo.
Our hypothesis that the candidate rs573225 variant was associated with parameters of insulin-glucose homeostasis was supported by several findings (Table 1). Linear regression analysis found that rs573225 was associated with IGI (P = 0.0191 under a recessive model and P = 0.0102 under an additive model). Indeed, IGI was ∼30% greater in these obese children with AA (36.8 ± 38.1 mmol/l) or AG (33.5 ± 24.2 mmol/l) genotypes compared with GG homozygotes (21 ± 16.9 mmol/l). Insulin levels showed no significant differences sensu stricto, but showed a trend for higher insulin levels in obese children of AA or AG genotype (P = 0.058 for Ins0 and P = 0.059 for Ins30). In addition, linear regression analysis found that rs573225 was associated with fasting glucose (P = 0.032 under a recessive model and P = 0.037 under an additive model) with higher glucose levels in obese children with AA or AG alleles.
TABLE 1.
Clinical and biological characteristics of insulin secretion in our young obese Caucasian cohort, according to rs573225 variant
| G/G | G/A | A/A | |
|---|---|---|---|
| n | 60 | 293 | 381 |
| Age (years) | 12.4 ± 2.8 | 11.8 ± 3 | 11.7 ± 2.8 |
| BMI (kg/m2) | 30.7 ± 5.6 | 29.7 ± 5.3 | 29.6 ± 5.5 |
| Fasting glucose (mmol/l) | 4.41 ± 0.51* | 4.53 ± 0.53 | 4.56 ± 0.57 |
| Glycemia at 30 min (mmol/l) | 7.47 ± 1.22 | 7.67 ± 1.23 | 7.53 ± 1.3 |
| Fasting insulin (μU/ml) | 15 ± 7.7 | 15.7 ± 8.7 | 15.9 ± 9.9 |
| Insulin at 30 min (μU/ml) | 87 ± 52 | 109 ± 77 | 106 ± 85 |
| IGI | 21 ± 16.9† | 33.5 ± 24.2 | 36.8 ± 38.1 |
| HOMA-β | 68.5 ± 34.1 | 69.6 ± 38.8 | 69.9 ± 41.4 |
Data are means ± SD. P values under a recessive model:
P = 0.032 and P = 0.037 under an additive model,
P = 0.0191 under a recessive model, and P = 0.0102 under an additive model.
DISCUSSION
When studied during their dynamic period of fat accumulation, obese children have glucose-insulin homeostasis yet are unaltered by dietary restriction or drugs, thus allowing bona fide genotype-phenotype studies. This population has the additional advantage of having normal fasting glucose levels, which avoids the interference of glucotoxicity with the studied β-cell parameters. Obese children oversecrete insulin in response to mounting insulin resistance; thus, we could explore genotypic effects over a wide range of insulin levels. Being aware that insulin and glucose levels collected during OGTTs are not entirely replicable in the same obese child (16), we minimized stress and diet variations in controlled in-hospital conditions. These strict criteria do not allow the study of the thousands of subjects necessary to well-powered association studies. We therefore consider our results as tentative and needing further replication. Already, two genome-wide association studies have reported the association of G6PC2 variants and fasting glycemia. In 9,353 normoglycemic subjects, Bouatia-Naji et al. (13) found an association between fasting glycemia and rs560887. The magnitude of the genotypic effect on glycemia was only 0.09 mmol/l, explaining why thousands of subjects were necessary to reach significance. Chen et al. (12) found a significant association between rs563694 and fasting glucose in 5,088 nondiabetic individuals from Finland and Sardinia and a P value of 5.7 × 10−7 for rs573225. Because our rs573225 is in almost complete linkage disequilibrium with rs560887 (r2 = 0.96) and with rs563694 (r2 = 0.95) (according to the HapMap CEU reference panel), it is likely that these three SNPs represent the same association signal. Bouatia-Naji et al. (13) found an association with the HOMA-β index of insulin secretion derived from fasting insulin and glucose values, whereas our study found only an association of rs573225 with IGI, an index derived from OGTT responses, not with HOMA-β. IGI is an index of insulin secretion that is based on the first-phase response to oral glucose and possibly more sensitive to acute changes in β-cell G6P level modulated by G6PC2 activity than is HOMA-β. We suspect that these apparent differences between results in large genome-wide association studies and our candidate variant study may be due to differences in sample size and nature of proxies used to estimate insulin secretion.
Our functional analysis of the rs573225 variant in β-cell lines found that the G allele was able to bind Foxa2 factor more efficiently than the A allele and was associated with a higher G6PC2 promoter activity. Previous studies have found that several mutated transcription factor sites located in G6PC2 promoter could modify its expression (6,17). Studies reviewed in Martin et al. (6) support a positive role for Foxa2 in G6PC2 expression and a negative impact on insulin secretion in vitro (18–20). G6PC2 appears to negatively affect stimulus-secretion coupling (3). Based on these studies and our own observations, we postulate that the higher affinity of Foxa factor for the G allelic form of the binding promoter motif could result in 1) a higher expression of G6PC2, 2) a diminution of β-cell G6P content, and 3) a diminution of insulin secretion in GG carriers. The cytosine adjacent to the G allele creates a CG/CGm epi-allele that showed variable Foxa2 binding capacity, which could possibly add allele-specific epigenetic effects to the Foxa2-dependent transcriptional activity of the promoter. Rs573225 can thus be called an epiSNP.
In summary, the present study reports that the G6PC2 promoter carries an rs573225 variant that modulates Foxa2 binding and promoter activity and could influence insulin secretion consistently with the known role of G6PC2 in β-cell physiology.
Acknowledgments
This work was supported by a grant from Agence Nationale de la Recherche (ANR). D.F. was supported by a doctoral fellowship grant from Ministère de la recherché, the Alfred Jost Research Fellowship sponsored by Merck Serono Company, and the Association Française des Diabétiques.
C.D.S. and P.B. report no other dualities of interest.
C.B. Wollheim and T. Berney provided INS-1 cells and isolated human islets. We thank Deci Delà for daily support and discussion and C. Le Stunff and A. Dermane for collecting DNA and OGTT data.
Published ahead of print at http://diabetes.diabetesjournals.org on 4 November 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Petrolonis AJ, Yang Q, Tummino PJ, Fish SM, Prack AE, Jain S, Parsons TF, Li P, Dales NA, Ge L, Langston SP, Schuller AG, An WF, Tartaglia LA, Chen H, Hong SB: Enzymatic characterization of the pancreatic islet-specific glucose-6-phosphatase-related protein (IGRP). J Biol Chem 279: 13976–13983, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Iizuka K, Nakajima H, Ono A, Okita K, Miyazaki J, Miyagawa J, Namba M, Hanafusa T, Matsuzawa Y: Stable overexpression of the glucose-6-phosphatase catalytic subunit attenuates glucose sensitivity of insulin secretion from a mouse pancreatic beta-cell line. J Endocrinol 164: 307–314, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Martin CC, Oeser JK, Sarkar S, McGuinness OP, Hutton JC, O'Brien RM: Deletion of the gene encoding the islet-specific glucose-6-phosphatase catalytic subunit-related protein autoantigen results in a mild metabolic phenotype. Diabetologia 50: 774–778, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Fradin D, Heath S, Lathrop M, Bougneres P: QTLs for fasting glucose in young Europeans replicate previous findings for type 2 diabetes in 2q23–24 and other locations. Diabetes 56: 1742–1745, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Ebert DH, Bischof LJ, Streeper RS, Chapman SC, Svitek CA, Goldman JK, Mathews CE, Leiter EH, Hutton JC, O'Brien RM: Structure and promoter activity of an islet-specific glucose-6-phosphatase catalytic subunit-related gene. Diabetes 48: 543–551, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Martin C, Flemming B, Wang Y, Oeser J, O'Brien R: Foxa2 and MafA regulate islet-specific glucose-6-phosphatase catalytic subunit-related protein (IGRP/G6PC2) gene expression. J Mol Endocrinol 41: 315–328, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bischof LJ, Martin CC, Svitek CA, Stadelmaier BT, Hornbuckle LA, Goldman JK, Oeser JK, Hutton JC, O'Brien RM: Characterization of the mouse islet-specific glucose-6-phosphatase catalytic subunit-related protein gene promoter by in situ footprinting: correlation with fusion gene expression in the islet-derived βTC-3 and hamster insulinoma tumor cell lines. Diabetes 50: 502–514, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Cha JY, Kim H, Kim KS, Hur MW, Ahn Y: Identification of transacting factors responsible for the tissue-specific expression of human glucose transporter type 2 isoform gene: cooperative role of hepatocyte nuclear factors 1alpha and 3beta. J Biol Chem 275: 18358–18365, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Gerrish K, Gannon M, Shih D, Henderson E, Stoffel M, Wright CV, Stein R: Pancreatic beta cell-specific transcription of the pdx-1 gene: the role of conserved upstream control regions and their hepatic nuclear factor 3beta sites. J Biol Chem 275: 3485–3492, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Kaestner KH, Katz J, Liu Y, Drucker DJ, Schutz G: Inactivation of the winged helix transcription factor HNF3alpha affects glucose homeostasis and islet glucagon gene expression in vivo. Genes Dev 13: 495–504, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philippe J: Hepatocyte-nuclear factor 3 beta gene transcripts generate protein isoforms with different transactivation properties on the glucagon gene. Mol Endocrinol 9: 368–374, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Chen WM, Erdos MR, Jackson AU, Saxena R, Sanna S, Silver KD, Timpson NJ, Hansen T, Orru M, Grazia Piras M, Bonnycastle LL, Willer CJ, Lyssenko V, Shen H, Kuusisto J, Ebrahim S, Sestu N, Duren WL, Spada MC, Stringham HM, Scott LJ, Olla N, Swift AJ, Najjar S, Mitchell BD, Lawlor DA, Smith GD, Ben-Shlomo Y, Andersen G, Borch-Johnsen K, Jorgensen T, Saramies J, Valle TT, Buchanan TA, Shuldiner AR, Lakatta E, Bergman RN, Uda M, Tuomilehto J, Pedersen O, Cao A, Groop L, Mohlke KL, Laakso M, Schlessinger D, Collins FS, Altshuler D, Abecasis GR, Boehnke M, Scuteri A, Watanabe RM: Variations in the G6PC2/ABCB11 genomic region are associated with fasting glucose levels. J Clin Invest 118: 2620–2628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouatia-Naji N, Rocheleau G, Van Lommel L, Lemaire K, Schuit F, Cavalcanti-Proenca C, Marchand M, Hartikainen AL, Sovio U, De Graeve F, Rung J, Vaxillaire M, Tichet J, Marre M, Balkau B, Weill J, Elliott P, Jarvelin MR, Meyre D, Polychronakos C, Dina C, Sladek R, Froguel P: A polymorphism within the G6PC2 gene is associated with fasting plasma glucose levels. Science 320: 1085–1088, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Le Stunff C, Fallin D, Schork NJ, Bougneres P: The insulin gene VNTR is associated with fasting insulin levels and development of juvenile obesity. Nat Genet 26: 444–446, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Yeckel CW, Taksali SE, Dziura J, Weiss R, Burgert TS, Sherwin RS, Tamborlane WV, Caprio S: The normal glucose tolerance continuum in obese youth: evidence for impairment in beta-cell function independent of insulin resistance. J Clin Endocrinol Metab 90: 747–754, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S: Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab 93: 4231–4237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin CC, Oeser JK, O'Brien RM: Differential regulation of islet-specific glucose-6-phosphatase catalytic subunit-related protein gene transcription by Pax-6 and Pdx-1. J Biol Chem 279: 34277–34289, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Lee CS, Sund NJ, Vatamaniuk MZ, Matschinsky FM, Stoffers DA, Kaestner KH: Foxa2 controls Pdx1 gene expression in pancreatic β-cells in vivo. Diabetes 51: 2546–2551, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Sund NJ, Vatamaniuk MZ, Casey M, Ang SL, Magnuson MA, Stoffers DA, Matschinsky FM, Kaestner KH: Tissue-specific deletion of Foxa2 in pancreatic beta cells results in hyperinsulinemic hypoglycemia. Genes Dev 15: 1706–1715, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Brautigan DL: A novel transmembrane Ser/Thr kinase complexes with protein phosphatase-1 and inhibitor-2. J Biol Chem 277: 49605–49612, 2002 [DOI] [PubMed] [Google Scholar]