Abstract
Background
Neopterin is a well-established marker of macrophage activation. The cerebrospinal fluid (CSF) neopterin levels are elevated in most HIV-1-infected individuals, and decrease significantly after initiation of antiretroviral therapy (ART). Unexpectedly, CSF concentrations often remain mildly abnormal even in patients treated for a long time with suppressive ART. The aims of this study were to analyse if persistently elevated CSF neopterin levels were associated with either the type of antiretroviral regimen or with low-level CSF HIV-1 concentrations, and to evaluate if plasma HIV-1 RNA levels correlated to lingering CSF neopterin concentrations in patients with effective ART.
Methods
One hundred and fifty-seven chronically HIV-1-infected patients with stable ART for ≥ 6 months and no neurological symptoms were included, and 193 HIV-1-infected patients without ART served as controls. Neopterin was analysed with either a radio-immunoassay or an enzyme-linked immunosorbent assay. HIV-1 RNA quantification was performed with the Roche Amplicor assay, version 1.5. Two quantitative HIV-1 RNA assays with sensitivities ≤ 2.5 copies/ml were used in 40 samples.
Results
As anticipated, HIV-1 RNA and CSF neopterin levels were markedly lower in patients on ART compared to untreated controls. No significant difference in CSF neopterin concentrations was found between those treated with protease inhibitor- and non-nuceloside reverse transcriptase-based regimens in combination with 2 nucleoside analogues. Subjects with CSF HIV-1 RNA loads < 2.5 copies/ml had the lowest CSF neopterin levels. Plasma viral load had no impact on intrathecal immune activation in cases with CSF viral loads < 50 copies/ml.
Conclusions
The persistent intrathecal cell-mediated immune response was associated with CSF viral load, but not with treatment regimen in individuals on ART.
Keywords: HIV-1 RNA, cerebrospinal fluid, neopterin, antiretroviral therapy
Introduction
Neopterin is a low molecular weight pteridine, mainly produced by macrophages and dendritic cells stimulated by interferon-gamma (IFN- γ). Its concentrations in body fluids rise when the cellular immune response is activated, for example during infections, autoimmune disorders, tumors, and allograft rejections 1. In cerebrospinal fluid (CSF), increased concentrations of neopterin have been found in several infections of the central nervous system (CNS), including aseptic meningitis, Lyme neuroborreliosis, herpes simplex virus type 1 encephalitis and HIV-1 infection, but also during conditions like multiple sclerosis and several neurodegenerative disorders 2-4.
HIV-1 enters the CNS shortly after transmission, and HIV-1 RNA can be detected in the CSF of nearly all individuals infected by HIV-1, irrespective of disease stage and neurological symptoms. Elevated levels of CSF neopterin have also been found during all stages of infection, with the highest levels in patients with AIDS dementia complex (ADC) and opportunistic infections 5. After initiation of highly active antiretroviral therapy (HAART), concentrations of CSF neopterin decrease rapidly, although nearly half of the patients have slightly elevated levels after two years of successful viral suppression in CSF 6. The reason for this persistent intrathecal immune activation and its clinical significance are unknown. Low level virus replication, autoimmune phenomena, or a slow down-regulation of the immune activity are some pathogenic theories 6.
For many years it was suggested that HIV-1 infection in the CNS might be more difficult to treat than systemic HIV-1, but several studies have indicated that this is not the case. One example is the dramatic decline in the incidence of ADC since the introduction of ART 7. In addition, the CSF viral load decreases markedly in HIV-1-infected patients on effective ART 8. Even patients with incomplete systemic viral suppression, have a pronounced decrease of CSF viral load, as well as of the intrathecal immune activation 9.
Most nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), and some ritonavir-boosted protease inhibitors (PIs) are considered CNS-penetrating 10-14, and lead to suppression of CSF viral load. However, it is not known what impact various combinations of antiretroviral drugs have on the intrathecal immune respons, or if suppressing the CSF viral load to levels less than 2.5 copies/ml leads to decreased immune activation in the CNS.
The aims of this study were to analyse if the persistent CSF neopterin levels in well-treated HIV-1-infected individuals were associated with antiretroviral regimen or with the CSF viral load < 50 copies/ml but detected by more sensitive assays. We also wanted to evaluate whether the plasma HIV-1 RNA levels correlated with elevated CSF neopterin concentrations in patients on HAART having CSF HIV-1 RNA <50 copies/ml, since it has been suggested that the increased intrathecal immune activation may reflect viral replication in the periphery and not in the CNS 9.
Materials and Methods
Patients
Subjects were selected from cohorts in Göteborg, Sweden and San Francisco, USA. All were neurologically asymptomatic and chronically HIV-1-infected maintained on stable ART regimens for ≥ 6 months. In total, 157 HIV-1-infected patients with ART and 193 HIV-1-infected controls without treatment for ≥ 6 months (74 female, 276 male) were retrospectively included in the study (232 from Göteborg and 118 from San Francisco) between November 1985 and August 2006. The subjects were divided into groups according to the number of antiretroviral drugs with which they were treated: (1) 1 NRTI, (2) 2 NRTIs, and (3) HAART, defined as ≥ 3 drugs. If more than one comparable lumbar puncture was available, a single interval was randomly selected. One hundred and thirty-four of the participants were treated with HAART, 11 with 2 NRTIs, and 12 with 1 NRTI. Patient characteristics are shown in Table 1. Among the controls not receiving ART, 151 (78 %) were naive to ART, and the 42 previously treated subjects had been off therapy for a median of 1.9 (IQR 0.7–4.1) years. Study participants were further analysed regarding the effect of CSF viral load on the intrathecal immue activation, and subjects with CSF viral loads < 50 copies/ml were additionally evaluated regarding treatment regimens and the impact of plasma viral load on CSF neopterin levels. This study was approved by the ethics committees of Göteborg University and University of California San Francisco.
Tables.
Characteristics of all HIV-1-infected subjects included in the study divided into groups based on antiretroviral treatment.
| Characteristic | HAART
(n = 134) |
2 NRTIs
(n = 11) |
1 NRTI
(n = 12) |
Untreated controls
(n = 193) |
P-value |
|---|---|---|---|---|---|
| Sex (F:M) | 31:103 | 2:9 | 1:11 | 40:153 | Non-significant |
| Age | 42.1
(36.6–50.2) |
42.0
(35.2–52.0) |
39.1
(33.5–49.6) |
36.8
(30.5–43.7) |
< 0.05a |
| Years since diagnosis | 7.0
(3.4–12.8) |
10.0
(5.9–15.6) |
3.4
(1.6–5.5) |
2.5
(0.4–8.1) |
< 0.05a,b,c,d |
| Years on ART | 2.0
(1.0–3.5) |
2.6
(1.6–4.2) |
1.3
(0.8–2.2) |
– | Non-significant |
| CD4+ T-cell count
(× 106 cells/l) |
370
(249–581) |
328
(170–598) |
150
(76–256) |
308
(120–502) |
< 0.05a,b |
Values shown are median (interquartile range). Controls were untreated for ≥ 6 months. HAART: highly active antiretroviral therapy; NRTI: nucleoside reverse transcriptase inhibitor; F: female; M: male; ART: antiretroviral therapy. Comparisons were made using analysis of variance followed by Tukey test.
HAART vs. untreated controls
HAART vs. 1 NRTI
2 NRTI vs. 1 NRTI
2 NRTI vs. untreated controls
Methods
HIV-1 RNA in CSF and plasma was measured using the Roche Amplicor assay (version 1.5, Hoffman-La Roche, Basel, Switzerland). The assay has a dynamic range down to 50 copies/ml (1.70 log10 copies/ml) and a lower detection limit of 20 copies/ml (1.30 log10 copies/ml) for both plasma and CSF. All HIV-1 RNA values < 20 copies/ml were set at 19 copies/ml. Fourty patients (11 from Sweden and 29 from USA) had their plasma and CSF viral loads determined by modified quantitification assays with detection limits of 2.0 and 2.5 copies/ml respectively, methods described elsewhere 15, 16. Neopterin was measured by a commercially available radio-immunoassay (Henningtest Neopterin, BRAHMS, Berlin, Germany) 17, or enzyme-linked immunosorbent assay (same manufacturer). The same antibodies are used in these two assays, and the results are interchangeable 18. Normal reference values were ≤ 8.8 nmol/l in serum and ≤ 5.8 nmol/l in CSF 19. The CD4+ T-cell count was analysed by flow cytometry.
Statistical analysis
Log10 transformation was applied to all HIV-1 RNA and neopterin data in figures and tables. Nonparametric methods were used for group descriptives (median and interquartile range, IQR). When two groups were being compared, comparisons were done using the Mann-Whitney U-test. Differences among more than two groups were detected using one-way analysis of variance, with multiple-group comparisons done using the Tukey post hoc test.
Results
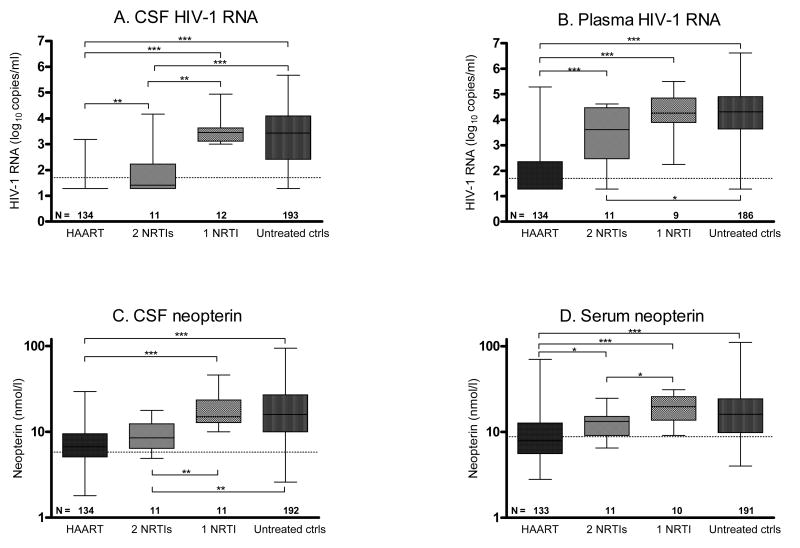
A larger proportion of patients on HAART (88 %, p < 0.001) and 2 NRTIs (64 %, p < 0.001) had CSF viral load < 50 copies/ml than patients treated with 1 NRTI (0 %). Plasma HIV-1 RNA was < 50 copies/ml in 70 % of individuals on HAART, 9 % of individuals on 2 NRTIs, 0 % among subjects treated with 1 NRTI, and 2 % for those without treatment. CSF neopterin levels were significantly lower for individuals on HAART (p < 0.001) or 2 NRTIs (p < 0.01) compared with untreated controls, while patients on 1 NRTI had levels in the same range as the controls (Figure 1). The patients on HAART had significantly higher CD4+ T-cell counts than individuals on 1 NRTI and subjects without ART. Subjects not receiving ART were more recently diagnosed with HIV-1 than treated subjects. It should be kept in mind that the number of subjects receiving 1 NRTI or 2 NRTIs is quite small.
Figure 1.
HIV-1 RNA and neopterin concentrations for the whole study population according to antiretroviral regimen. (A) CSF HIV-1 RNA, (B) plasma HIV-1 RNA, (C) CSF neopterin, and (D) serum neopterin. N denotes the number of patients in each group. Dotted lines indicate upper reference values. Median values, 25th and 75th percentiles, and ranges are shown in the box plots. Ctrls: controls. * = p < 0.05, ** = p < 0.01, and *** = p < 0.001.
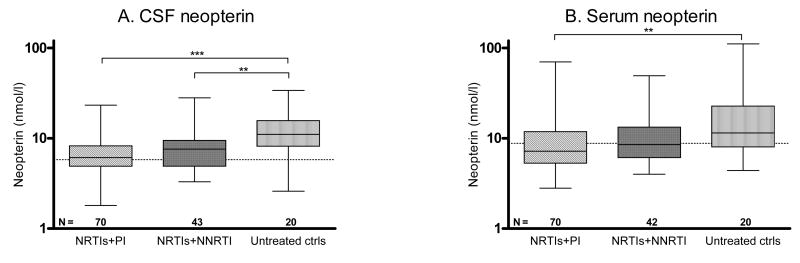
Individuals on PI- or NNRTI-based HAART (defined as ≥ 1 PI or 1 NNRTI in combination with NRTIs) and with CSF viral loads < 50 copies/ml had lower levels of CSF neopterin compared to untreated controls with CSF HIV-RNA <50 copies/ml (p < 0.001 and 0.01, respectively) (Figure 2). The CSF HIV-1 RNA and neopterin for subjects on PI- or NNRTI-based regimens was in the same range. The median (IQR) plasma HIV-1 RNA levels did not differ between subjects on a PI-based regimen; 1.3 (1.3–1.8) log10 copies/ml and a NNRTI-based regimen; 1.3 (1.3–1.3) log10 copies/ml, but was significantly higher in untreated controls; 3.2 (2.1–3.8) log10 copies/ml (p < 0.001). The patients had been on their PI- or NNRTI-based treatment for a median (IQR) of 1.9 (1.0–3.2) and 2.9 (1.1–4.0) years, respectively. Of the 70 patients on PI-based treatment, 21 were on single PI-treatment with indinavir (n = 11), nelfinavir (n = 8), or saquinavir (n = 2); 8 had a regimen including saquinavir and nelfinavir; and 41 had a ritonavir-boosted PI with either lopinavir (n = 22), atazanavir (n = 9), saquinavir (n = 5), and indinavir (n = 5). No differences in CSF neopterin concentrations were found between those groups (data not shown). For the 43 subjects on NNRTI-based regimens, CSF neopterin did not differ between those on efavirenz (n = 24) and those on nevirapine (n = 19).
Figure 2.
Subjects on PI- or NNRTI-based HAART and untreated controls (ctrls) with CSF viral loads < 50 copies/ml. (A) CSF neopterin, (B) serum neopterin. N denotes the number of patients in each group. Dotted lines indicate upper reference values. Median values, 25th and 75th percentiles, and ranges are shown in the box plots. ** = p < 0.01 and *** = p < 0.001.
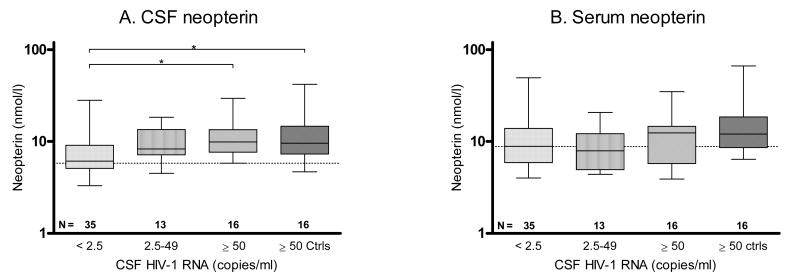
The individulas who had CSF HIV-1 RNA levels < 2.5 copies/ml had significantly lower plasma viral loads than the other groups (p < 0.001). The median (IQR) plasma HIV-1 RNA levels were 0.4 (0.4–0.9) log10 copies/ml for subjects with CSF viral loads < 2.5 copies/ml, 2.6 (1.6–3.5) log10 copies/ml for subjects with CSF HIV-1 RNA 2.5–49 copies/ml, 4.1 (2.7– 4.7) log10 copies/ml for subjects with CSF HIV-1 RNA ≥ 50 copies/ml, and 3.3 (2.3–4.4) log10 copies/ml for untreated controls. The subjects with CSF viral loads < 2.5 copies/ml also had the lowest CSF neopterin concentrations (p < 0.01) (Figure 3). Fifteen out of 35 subjects with CSF viral loads < 2.5 copies/ml had CSF neopterin concentrations within the normal reference value, compared to two of the 29 participants with CSF HIV-1 RNA ≥ 2.5 copies/ml (13 with CSF HIV-1 RNA between 2.5 and 49, and 16 with CSF HIV-1 RNA ≥ 50 copies/ml).
Figure 3.
CSF and serum neopterin levels in HIV-1-infected individuals on HAART, and with different CSF HIV-1 RNA levels. The CSF viral loads for untreated controls (ctrls) were matched to subjects on HAART and with CSF viral loads ≥ 50 copies/ml. (A) CSF neopterin, (B) serum neopterin. N denotes the number of patients in each group. Dotted lines indicate upper reference values. Median values, 25th and 75th percentiles, and ranges are shown in the box plots. * = p < 0.05 and ** = p < 0.01.
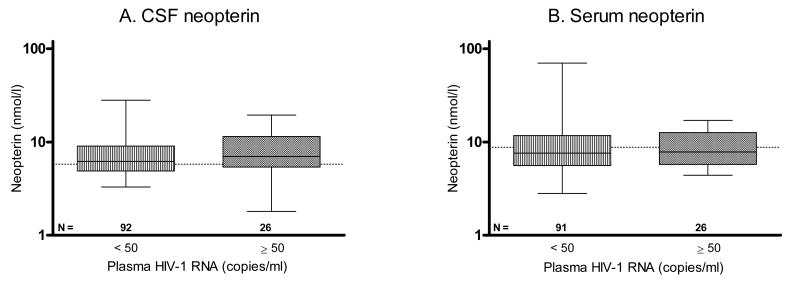
For subjects on HAART and with CSF viral loads < 50 copies/ml, there were no statistically significant differences in CSF and serum neopterin levels, for the 92 individuals with plasma viral loads < 50 copies/ml, and the 26 with plasma viral loads ≥ 50 copies/ml, Figure 4. The median (IQR) plasma HIV-1 RNA level for individuals with detectable viremia was 3.1 (2.4–3.7) log10 copies/ml.
Figure 4.
Subjects on HAART and with CSF viral loads < 50 copies/ml grouped by level of plasma viral load (< 50 or ≥ 50 copies/ml). No significant differences were found in (A) CSF neopterin, or (B) serum neopterin concentrations between groups. N denotes the number of patients in each group. Dotted lines indicate upper reference values. Median values, 25th and 75th percentiles, and ranges are shown in the box plots.
Discussion
As expected, HAART effectively reduces plasma and CSF HIV-1 RNA, as well as the degree of immune activation measured as decreasing concentrations of serum and CSF neopterin. However, despite of virologically succesful ART, many patients demonstrate ongoing intrathecal immune responses, which is in concordance with previous studies 6. The clinical significance of this finding is unknown. Although there have been reports about neurological deteroriation in patients with virologically effective treatment 20, it is a very uncommon clinical development. By analysing various treatment regimens and by using a very sensitive HIV-1 RNA quantification assay we have extended the characterisation of the persisting signs of intrathecal immune activation, but the question whether a low-grade intrathecal immune activation is harmful or not remains.
A weakness with the study is its retrospective approach. CSF analyses are difficult to include in prospective studies because of the reluctance of many to include lumbar punctures in the study protocols comparing treatments. Therefore retrospective studies in well categorised patient materials are, as yet, the main means of gathering more information.
Current treatment guidelines recommend a combination of 2 NRTIs plus either a NNRTI or a PI when initiating antiretroviral treatment 21, 22. In this study, we compared patients treated with NRTIs + PI and NRTIs + NNRTI. The CSF neopterin levels were similar in these two groups which indicates that there are no large differences between these HAART regimens on the persistent low-level intrathecal immune activation. It should be noticed, however, that NNRTI-treated patients had been somewhat longer on their treatment than PI-treated patients. Another finding demonstrating that many treatment regimens are effective in the CSF is that the nowadays non-accepted treatment with only 2 NRTIs leads to a much more pronounced reduction of viral load in CSF than in plasma 23, which was confirmed in the present study.
There were previously concerns that the CNS could be a sanctuary site that is difficult to reach with antiretroviral drugs. Theoretically, the lower drug concentrations in CSF, and the large mass of infected cells in the CNS being long-lived macrophages and microglia, might lead to a slower and less impressive decline in CSF than in plasma. This type of viral kinetics has been observed in patients with ADC 24, where virus in CSF mostly emanates from local production within the CNS, as opposed to a larger ratio of virus trafficking from the periphery in neurologically asymptomatic individuals, as in this study.
Beneficial virological treatment results in CSF in individuals without neurocognitive symptoms could, at least in part, be due to the fact that the plasma viral load in most cases exceeds the CSF viral load 25, or that some ART regimens more effectively reduce viral replication in CSF than in blood. In vitro experiments have demonstrated a superior antiviral effect for NRTIs in macrophages than in activated lymphocytes, since cells like macrophages have much lower levels of 2′-deoxy-nucleoside triphosphates (dNTPs), thereby enhancing the chain-terminating activity of NRTIs 26. Antiretroviral drugs are required to penetrate into the CNS in order to achieve a substantial decline of the CSF viral load, but the number of CSF-penetrating drugs needed to do this is not established. Monotherapy with zidovudine, reduces the viral burden and the cell-mediated immune response in CSF more than in plasma, and even led to a decrease in the incidence of ADC in the late 1980s 27-29. Drugs that are undetectable or barely detectable in CSF, such as saquinavir/ritonavir, didanosine, or nelfinavir, do not lead to viral suppression in CSF when used on their own 27, 30, 31. Lopinavir/ritonavir, on the other hand, reaches CSF concentrations exceeding the 50 % inhibitory concentration (IC50) 13, and suppresses the CSF viral load succesfully for a median of 38 weeks when used as monotherapy 32. Thus one potent antiretroviral drug could suffice, as long as it is has the capacity to cross the BBB. How long the effect of a single agent will last in the CSF, and whether this in the long run can lead to increased development of resistance needs further evaluation.
Among treated subjects, the group with CSF HIV-1 RNA < 2.5 copies/ml had significantly lower CSF neopterin levels than subjects with higher CSF viral loads. It has previously been observed that higher CSF viral loads are associated with higher CSF neopterin concentrations, but this has not been extended to CSF HIV-1 RNA levels < 50 copies/ml. We found that also a very low viral activity stimulates the immune activation albeit at much lower levels than in untreated patients. If the intrathecal immune activation is thought to be harmful a conclusion drawn from these results would be to include more CSF monitoring analysis and to lower the threshold for viral detection and goal for treatment.
Interestingly, in another study, subjects who exhibited systemic virological failure (defined as HIV-1 RNA > 500 copies/ml), still had beneficiary results of their ART with a decreased systemic immune activation, and a larger reduction of CSF than plasma viral load 9. The failing patients in that study had lower CSF neopterin than untreated subjects, but higher than the successes. It was hypothesised that the reduced activity of the peripheral immune system might lead to fewer T-cells trafficking through the BBB, and reduced CSF viral load as a consequence, since short-lived cells from outside the CNS have been shown to sustain a large proportion of the CSF viral load in neurologically asymptomatic subjects 33. Bearing this in mind, we expected the individuals in our study with systemic virological failures (plasma HIV-1 RNA ≥ 50 copies/ml) to have higher CSF neopterin levels than the treatment successes (plasma HIV-1 RNA < 50 copies/ml). However, this was only true for subjects who also had CSF viral loads ≥ 50 copies/ml. Individuals with plasma viral loads ≥ 50 copies/ml (failures), but with undetectable CSF viral loads did not have higher CSF neopterin levels than the group with undetectable plasma HIV-1 RNA as well. This again points towards the viral burden in CSF as an important factor behind increased CSF neopterin concentrations. Furthermore, no difference in CSF neopterin was found between patients on HAART and CSF HIV-1 RNA ≥ 50 copies/ml and matched untreated controls. Our findings thus may argue against the prevalent role of T-cell trafficking through the BBB even in individuals without neurological symptoms.
Although ART reduces the CSF viral load dramatically, decreases the incidence of neurological complications, and even improves symptoms in patients with ADC, it does not have the same impressive effect on the intrathecal cell-mediated immune activation, as measured by neopterin. Even though the individuals with CSF viral loads < 2.5 copies/ml had the lowest CSF neopterin levels, they were still slightly elevated in many (57%) of these virologically well-suppressed patients. We do not yet know the clinical consequences of having an active immune system in the CNS for a prolonged time, and if this could lead to neurological injuries in the future.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NINDS R01 NS43103-01), the Medical Faculty of Göteborg University (ALFGBG-2874), the Göteborg Medical Society, the Swedish Physicians Against AIDS Research Foundation, and the government of the State of the Austrian Tyrol.
References
- 1.Wirleitner B, Schroecksnadel K, Winkler C, et al. Neopterin in HIV-1 infection. Mol Immunol. 2005 Feb;42(2):183–194. doi: 10.1016/j.molimm.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Furukawa Y, Nishi K, Kondo T, et al. Significance of CSF total neopterin and biopterin in inflammatory neurological diseases. J Neurol Sci. 1992 Aug;111(1):65–72. doi: 10.1016/0022-510x(92)90113-y. [DOI] [PubMed] [Google Scholar]
- 3.Fredrikson S, Link H, Eneroth P. CSF neopterin as marker of disease activity in multiple sclerosis. Acta Neurol Scand. 1987 May;75(5):352–355. doi: 10.1111/j.1600-0404.1987.tb05458.x. [DOI] [PubMed] [Google Scholar]
- 4.Hagberg L, Dotevall L, Norkrans G, et al. Cerebrospinal fluid neopterin concentrations in central nervous system infection. J Infect Dis. 1993;168:1285–1288. doi: 10.1093/infdis/168.5.1285. [DOI] [PubMed] [Google Scholar]
- 5.Griffin DE, McArthur JC, Cornblath DR. Neopterin and interferon-gamma in serum and cerebrospinal fluid of patients with HIV-associated neurologic disease. Neurology. 1991 Jan;41(1):69–74. doi: 10.1212/wnl.41.1.69. [DOI] [PubMed] [Google Scholar]
- 6.Abdulle S, Hagberg L, Svennerholm B, et al. Continuing intrathecal immunoactivation despite two years of effective antiretroviral therapy against HIV-1 infection. Aids. 2002 Nov 8;16(16):2145–2149. doi: 10.1097/00002030-200211080-00006. [DOI] [PubMed] [Google Scholar]
- 7.d'Arminio Monforte A, Cinque P, Mocroft A, et al. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann Neurol. 2004 Mar;55(3):320–328. doi: 10.1002/ana.10827. [DOI] [PubMed] [Google Scholar]
- 8.Mellgren Å, Antinori A, Cinque P, et al. Cerebrospinal fluid HIV-1 infection usually responds well to antiretroviral treatment. Antivir Ther. 2005;10(6):701–707. [PubMed] [Google Scholar]
- 9.Spudich S, Lollo N, Liegler T, et al. Treatment benefit on cerebrospinal fluid HIV-1 levels in the setting of systemic virological suppression and failure. J Infect Dis. 2006 Dec 15;194(12):1686–1696. doi: 10.1086/508750. [DOI] [PubMed] [Google Scholar]
- 10.Burger DM, Kraaijeveld CL, Meenhorst PL, et al. Penetration of zidovudine into the cerebrospinal fluid of patients infected with HIV. Aids. 1993 Dec;7(12):1581–1587. doi: 10.1097/00002030-199312000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Haas D, Stone J, Clough LA, et al. Steady-state pharmacokinetics of indinavir in cerebrospinal fluid and plasma among adults with human immunodeficiency virus type 1 infection. Clin Pharmacol Ther. 2000;68:367–374. doi: 10.1067/mcp.2000.109391. [DOI] [PubMed] [Google Scholar]
- 12.Haworth SJ, Christofalo B, Anderson RD, et al. A single-dose study to assess the penetration of stavudine into human cerebrospinal fluid in adults. J Acquir Immune Defic Syndr. 1998;17:235–238. doi: 10.1097/00042560-199803010-00008. [DOI] [PubMed] [Google Scholar]
- 13.Yilmaz A, Ståhle L, Hagberg L, et al. Cerebrospinal fluid and plasma HIV-1 RNA levels and lopinavir concentrations following lopinavir/ritonavir regimen. Scand J Infect Dis. 2004;36(1112):823–828. doi: 10.1080/00365540410025320. [DOI] [PubMed] [Google Scholar]
- 14.Antinori A, Perno CF, Giancola ML, et al. Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin Infect Dis. 2005 Dec 15;41(12):1787–1793. doi: 10.1086/498310. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz A, Svennerholm B, Hagberg L, et al. Cerebrospinal fluid viral loads reach less than 2 copies/ml in HIV-1-infected patients with effective antiretroviral therapy. Antivir Ther. 2006;11(7):833–837. [PubMed] [Google Scholar]
- 16.Havlir DV, Bassett R, Levitan D, et al. Prevalence and predictive value of intermittent viremia with combination HIV therapy. JAMA. 2001 Jul 11;286(2):171–179. doi: 10.1001/jama.286.2.171. [DOI] [PubMed] [Google Scholar]
- 17.Werner E, Bichler A, Daxenbichler G, et al. Determination of neopterin in serum and urine. Clin Chem. 1987;33:62–66. [PubMed] [Google Scholar]
- 18.Mayersbach P, Augustin R, Schennach H, et al. Commercial enzyme-linked immunosorbent assay for neopterin detection in blood donations compared with RIA and HPLC. Clin Chem. 1994 Feb;40(2):265–266. [PubMed] [Google Scholar]
- 19.Hagberg L, Andersson L, Abdulle S, et al. Clinical application of cerebrospinal fluid neopterin concentrations in HIV infection. Pteridines. 2004;15:102–106. [Google Scholar]
- 20.Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004 Dec;10(6):350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- 21.Gissln M, Ahlqvist-Rastad J, Albert J, et al. Antiretroviral treatment of HIV infection: Swedish recommendations 2005. Scand J Infect Dis. 2006;38(2):86–103. doi: 10.1080/00365540500388834. [DOI] [PubMed] [Google Scholar]
- 22.Hammer SM, Saag MS, Schechter M, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006 Aug 16;296(7):827–843. doi: 10.1001/jama.296.7.827. [DOI] [PubMed] [Google Scholar]
- 23.Gisslén M, Svennerholm B, Norkrans G, et al. Cerebrospinal fluid and plasma viral load in HIV-1-infected patients with various anti-retroviral treatment regimens. Scand J Infect Dis. 2000;32(4):365–369. doi: 10.1080/003655400750044926. [DOI] [PubMed] [Google Scholar]
- 24.Ellis RJ, Gamst AC, Capparelli E, et al. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000 Feb 22;54(4):927–936. doi: 10.1212/wnl.54.4.927. [DOI] [PubMed] [Google Scholar]
- 25.Gisslén M, Fuchs D, Svennerholm B, et al. Cerebrospinal fluid viral load, intrathecal immunoactivation, and cerebrospinal fluid monocytic cell count in HIV-1 infection. J Acquir Immune Defic Syndr. 1999 Aug 1;21(4):271–276. doi: 10.1097/00126334-199908010-00003. [DOI] [PubMed] [Google Scholar]
- 26.Aquaro S, Perno CF, Balestra E, et al. Inhibition of replication of HIV in primary monocyte/macrophages by different antiviral drugs and comparative efficacy in lymphocytes. J Leukoc Biol. 1997 Jul;62(1):138–143. doi: 10.1002/jlb.62.1.138. [DOI] [PubMed] [Google Scholar]
- 27.Gisslén M, Norkrans G, Svennerholm B, et al. The effect on human immunodeficiency virus type 1 RNA levels in cerebrospinal fluid after initiation of zidovudine or didanosine. J Infect Dis. 1997 Feb;175(2):434–437. doi: 10.1093/infdis/175.2.434. [DOI] [PubMed] [Google Scholar]
- 28.Portegies P, de Gans J, Lange JM, et al. Declining incidence of AIDS dementia complex after introduction of zidovudine treatment. BMJ. 1989 Sep 30;299(6703):819–821. doi: 10.1136/bmj.299.6703.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hagberg L, Norkrans G, Gisslén M, et al. Intrathecal immunoactivation in patients with HIV-1 infection is reduced by zidovudine but not by didanosine. Scand J Infect Dis. 1996;28(4):329–333. doi: 10.3109/00365549609037914. [DOI] [PubMed] [Google Scholar]
- 30.Gisolf EH, Enting RH, Jurriaans S, et al. Cerebrospinal fluid HIV-1 RNA during treatment with ritonavir/saquinavir or ritonavir/saquinavir/stavudine. Aids. 2000 Jul 28;14(11):1583–1589. doi: 10.1097/00002030-200007280-00014. [DOI] [PubMed] [Google Scholar]
- 31.Lafeuillade A, Solas C, Halfon P, et al. Differences in the detection of three HIV-1 protease inhibitors in non-blood compartments: clinical correlations. HIV Clin Trials. 2002 Jan-Feb;3(1):27–35. doi: 10.1310/WMWL-6W9Y-PXV2-X148. [DOI] [PubMed] [Google Scholar]
- 32.Yeh R, Letendre S, Novak I, et al. Single agent therapy with lopinavir/ritonavir controls HIV-1 replication in the central nervous system [381]. Presented at:14th Conference on Retroviruses and Opportunistic Infections; 2007; Los Angeles. 2007. [Google Scholar]
- 33.Harrington PR, Haas DW, Ritola K, et al. Compartmentalized human immunodeficiency virus type 1 present in cerebrospinal fluid is produced by shortlived cells. J Virol. 2005 Jul;79(13):7959–7966. doi: 10.1128/JVI.79.13.7959-7966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]