Summary
Background
The labile state of short-term memory has been known for more than a century. It has been frequently reported that immediate post-learning intervention can readily disrupt newly formed memories. However, the molecular and cellular mechanisms underlying the labile state of new memory are not understood.
Results
Using a bump-and-hole—based chemical genetic method, we have rapidly and selectively manipulated αCaMKII activity levels in the mouse forebrain during various stages of the short-term memory processes. We find that rapid shift in the αCaMKII activation status within the immediate post-learning 10 minutes severely disrupts short-term memory formation. The same manipulation beyond the postlearning 15 minutes has no effect, suggesting a critical time-window for CaMKII action. We further show that only during this same 10 minute time-window, shifting in CaMKII activation state is capable of altering newly established synaptic weights and/or patterns.
Conclusion
Initial 10 minutes of memory formation and long-term potentiation are sensitive to inducible genetic upregulation of αCaMKII activity. Our results suggest that molecular dynamics of CaMKII plays an important role in underlying synaptic labile state and representation of short-term memory during this critical time window.
Introduction
Short-term memory is known to be in a highly labile state before it is transformed into a longer-lasting, more stable form [1, 2]. Over the past several decades, many experiments show that immediate post-learning electroconvulsion shock (ECS) can induce retrograde amnesia, indicating the existence of a time window during which newly formed memory is in a highly labile state [3]. This labile phase of short-term memory is thought to be mechanistically distinct from the protein synthesis dependent consolidation phase of long-term memory [4, 5]. Currently, little is known regarding the temporal dynamics and molecular basis underlying the labile state of short-term memory.
CaMKII is a major molecule in mediating the NMDA receptor signaling involved in synaptic plasticity and formation of associative memories in the brain [6–15]. It is believed that Ca2+ activates CaMKII through NMDA receptor which in turn causes the translocation of the holoenzyme to post-synaptic density (PSD) and the subsequent retention via physical binding to the NR2B subunit of the NMDA receptor at the synapses [16–20]. Autophosphorylation at the Thr286 site of αCaMKII further enhances its binding affinity to Ca2+/CaM and prolongs the association of CaMKII at PSD [16, 21–23]. It has been suggested that the activated CaMKII at the PSD zone is responsible for potentiating synapses, probably via synaptic insertion and/or increasing channel conductance of the AMPA receptor [24, 25]. Thus, this persistent “On-state” of the CaMKII induced by LTP stimulation may allow the CaMKII to serve as a memory device for information storage [26]. Consistent with this general idea, several genetically modified mice carrying null- or point-mutations in αCaMKII have exhibited deficits in memory tests [27, 28].
Inducible and region-specific gene knockout (third-generation gene knockout) techniques are valuable for molecular and temporal analysis of biological processes [29, 30]. However, because the inactivation event occurs at the DNA level, manifestation of any phenotype depends on the turnover rate of the existing protein, which may require days or even weeks. This inherently slow process has excluded precise investigation of many in vivo biological processes that occur within minutes and hours, such as short-term memory.
Recently, we have reported an inducible protein knockout technology by integrating convergent protein engineering and rational inhibitor design [31, 32]. This method is based on the bump-and-hole approach that creates a specific interaction interface between a modified protein domain and sensitized inhibitors. By introducing this system into genetically modified mice, we can readily and specifically manipulate the activity of a targeted protein, such as CAMKII, in the subregion(s) of the brain in freely behaving mice on the time scale of few minutes [31, 32]. Here, our combined electrophysiological and behavioral analyses suggest that the molecular dynamics of αCaMKII underlies the labile state of short-term memory.
Results
Rapid manipulation of αCaMKII activity in the transgenic mouse forebrain
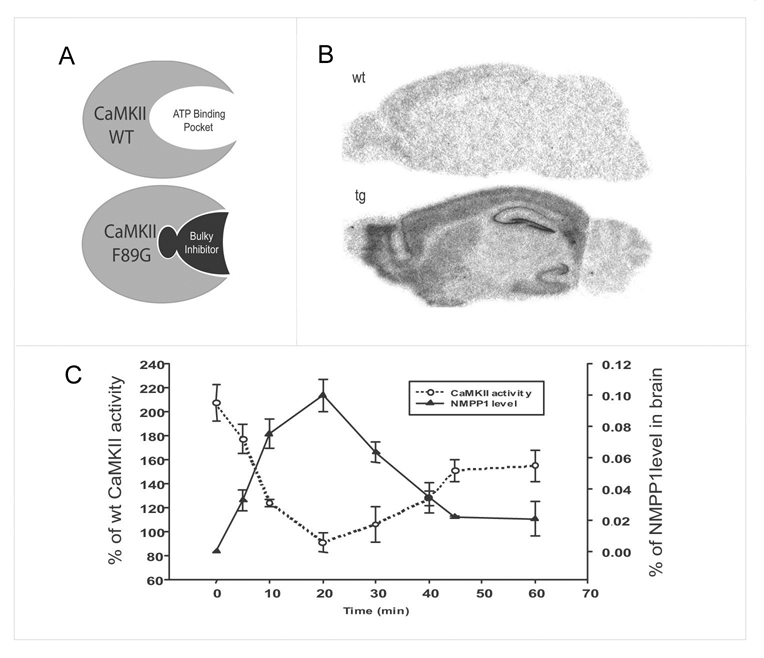
To investigate the role of CaMKII in regulating the temporal labile stage of short-term memory during its initial formation, we use inducible, reversible and forebrain-specific CaMKII transgenic mice generated by a ‘bump-and-hole’ based chemical genetic method [31]. Our overall strategy involved transgenic overexpression of αCaMKII-F89G transgene which encodes αCaMKII with wild-type like catalytic activity, but carrying an extra hidden cavity inside of its ATP-binding pocket (Figure 1A). This “enlarged” mutant αCaMKII can be selectively inhibited by a rationally designed, genetically sensitized small-molecule inhibitor, NM-PP1 [31, 33]. We confirmed the forebrain-specificity of the transgene expression in the offspring of our transgenic mice (Figure 1B).
Figure 1. Chemical-genetic manipulation of CaMKII activity in the mouse forebrain.
(A) The bump-and-hole based chemical genetic strategy for rapid manipulation of CaMKII activity in the specific region(s) of the brain. A genetically sensitized bulky inhibitor can selectively inhibit the mutated CaMKII-F89G which carries a hidden cavity inside of ATP-binding pocket. This transgene is expressed only in the forebrain by using a forebrain-specific αCaMKII promoter. (B) In situ hybridization confirms the forebrain-specific expression of αCaMKII-F89G in the transgenic mouse brain (Tg). A transgene-specific SV40 probe is used. There is no signal from the wild-type brain (WT). (C) Rapid inhibition of αCaMKII-F89G in the transgenic forebrain can be achieved by i.p. injection of the bulky inhibitor, NM-PP1 (5 µM). A single i.p. injection can bring the excessive αCaMKII in the transgenic mice back to a level approximately comparable to wild-type level between 10–35 minutes post-injection. Error bar at 20 min point is small but present. The total CaMKII activity in the transgenic mice is expressed as the percentage of activity in the brains of wild-type littermates.
In these transgenic mice, a single i.p. injection of NM-PP1 (5 µM) is shown to be capable of suppressing CaMKII-F89G activity in the forebrain regions within 8 minutes, consequently, bringing the total amount of CaMKII activity in the transgenic forebrain back to the normal wild-type levels (Figure 1C). This inhibition is readily reversible in about 35 minutes as demonstrated both by pharmacokinetics and enzyme activity assay in Tg-F89G mice. This time course fits well with the measured pharmacokinetics of [3H] labeled NM-PP1 (Figure 1C). Thus, a single i.p. injection of NM-PP1 into freely behaving transgenic mice can completely suppress αCaMKII-F89G activity and consistently maintain total CaMKII activity at the wild-type level between 10–35 minutes after injection. We used this pharmacokinetics as a guide for manipulating CaMKII activity in transgenic mice during our behavioral experiments. Our previous study shows that NM-PP1 has no effect on wild-type mice [31].
Acquisition of short-term memory with normal or elevated CaMKII activity
To determine whether the activation state of CaMKII plays a role in the representation of short-term memory in the brain, we examined individual temporal stages of 1-hr short-term memory processes. We have chosen three different behavioral paradigms, namely novel object recognition memory, contextual fear memory, and cued fear memory. All behavioral experiments were performed on the littermate wild-type and transgenic mice and conducted by the two and/or three experimenters blind to the genotype of each mouse.
We first conducted the novel object recognition short-term memory tests (with 1-hour retention) in transgenic αCaMKII-F89G mice and their littermate controls. Animals were allowed to explore two objects in an open field for 15 minutes. One of the two objects was replaced with a novel one 60 minutes later and the mice were allowed to explore them for 5 minutes. The memory performance was evaluated by the time spent on the novel objects.
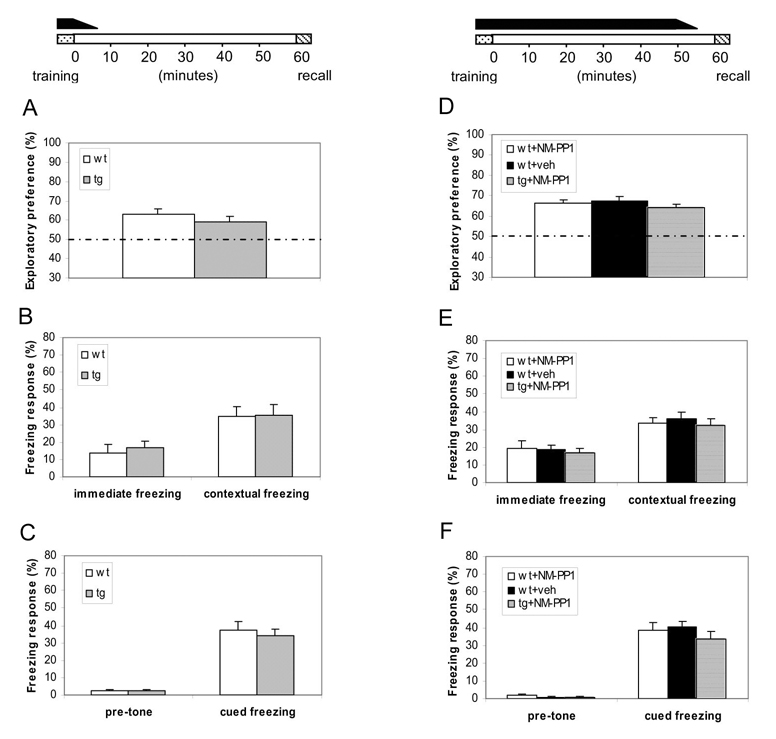
We allowed learning to occur in the temporally restricted presence of elevated CaMKII (no NM-PP1 inhibition), which is known to change plasticity frequency-responses in the transgenic brain [31]. A single I.P.. injection can provide consistent and complete in vivo inhibition of αCaMKII-F89G activity in the transgenic mice between 8–35 minutes post-injection and partial inhibition during the initial 3–8 minutes as well as during the last 35–40 minutes post-injection [31]. Therefore, we designed a two-injection protocol so that we can suppress αCaMKII-F89G activity for more than 60 minutes. Immediately after the training session was completed (within 10–30 seconds), we administered the first IP injection of NM-PP1, and followed by the second injection 30 minutes after the first injection so that we can suppress αCaMKII-F89G in transgenic mice throughout the rest of the experiments (including at the time of recall conducted at the end of one-hour retention). The temporally restricted presence of overexpressed CaMKII-F89G kinase activity in the transgenic mice during learning stage (without NM-PP1 inhibition) is indicated by a black bar above the timeline at the top of Figure 2. Under this “Learning-With-Elevated-CaMKII” paradigm, we found that transgenic mice showed normal acquisition and retention of the memory in comparison to that of wild-type littermate mice (Figure 2A). This suggests that the elevation of forebrain CaMKII activity during learning does not impair the acquisition of short-term recognition memory.
Figure 2. Normal short-term memory formation with elevated CaMKII.
(A–C) Under this “Learning-With-Elevated-CaMKII” paradigm, the temporally restricted presence of αCaMKII-F89G activity during learning did not impair memory acquisition and 1-hr retention as measured by three distinct memory tasks. The black bar on the top indicates the duration for the presence of αCaMKII-F89G activity. Animals were trained without any drug treatment. Immediately after training, they received the 1st i.p. injection of NM-PP1. The 2nd i.p. injection was administered 30 minutes later. The CaMKII activity of the transgenic mice during memory test were measured to be about 98.2 ± 3.8% of that of wild-type mice after receiving two NM-PP1 injections. (A) Indistinguishable performance in the novel object recognition test between Tg (n = 12) and wild-type mice (n = 12) (p > 0.05). (B) Indistinguishable performance in contextual conditioning between wild-type (n = 12) and transgenic mice (n = 12) (p > 0.05). (C) Cued conditioning in the same groups of mice. No significant difference was found between two groups (p > 0.05). All values are mean ± S.E.M. One-way ANOVA-Tukey was used for all the statistical analyses. (D–F) Under this “Learning/retention-With-Elevated-CaMKII” paradigm, transgenic mice with temporally restricted expression of αCaMKII-F89G activity during acquisition and post-training 55 minutes exhibited normal performance in the three tests. The black bar on the top indicates the duration for the presence of elevated αCaMKII activity. NM-PP1 was injected 10 minutes before recall. The CaMKII activity of the transgenic mice at the time of 1-hr-retention tests were estimated to be about 102.5 ± 6.3% of that of wild-type control mice under this injection protocol. (D) Normal performance in novel object recognition test in transgenic mice with NM-PP1 inhibition (n = 10, p > 0.05), compared with wild-type mice receiving vehicle (n = 10, p > 0.05) or wild-type mice with NM-PP1 inhibition (n = 11, p > 0.05). (E) Normal performance in contextual conditioning in transgenic mice with NM-PP1 (n = 10, p > 0.05), compared with wild-type mice with vehicle (n = 10, p > 0.05) or wild-type mice with NM-PP1 (n = 11, p > 0.05). (F) Normal performance in cued conditioning in transgenic mice with NM-PP1 treatment (n = 10, p > 0.05), compared with wild-type mice with vehicle (n = 10, p > 0.05) or wild-type mice with NM-PP1 inhibition (n = 11, p > 0.05). All values are mean ± S.E.M. One-way ANOVA-Tukey was used for all the statistical analyses.
We then conducted the “Learning-With-Elevated-CaMKII”’ experiments using fear conditioning tasks. Mice were given pairing of a tone with a mild foot-shock and memory performance was evaluated as the percentage of time spent on freezing in response to the same training chamber (contextual freezing) or the same tone with a different testing chamber (cued freezing) 60 minutes after training. Again, we found that transgenic mice with the transient CaMKII-F89G expression during learning exhibited normal performance in comparison to their wild-type littermates in either the one-hour short-term contextual memory test (Figure 2B) or cued memory test (Figure 2C). Thus, the transgenic mice can learn normally regardless whether they have the normal (with NM-PP1 treatment) or higher amount (without NM-PP1 treatment) of CaMKII activity during the acquisition phase.
Next, we asked if the transgenic mice can acquire and retain short-term memory when CaMKII-F89G is present not only during learning but also during the following 55 minutes until memory is recalled. This “Learning/Retention-With-Elevated-CaMKII” paradigm would allow further determine whether the continuous presence of CaMKII-F89G kinase activity during this period alters short-term memories. Similar to the “Learning-With-Elevated-CaMKII” paradigm, we found that transgenic mice showed normal acquisition and retention of the visual memory in comparison to that of wild-type littermate mice in the novel object recognition test (Figure 2D). Moreover, behavioral performances of transgenic mice in the 1-hr contextual (Figure 2E) and cued retention tests (Figure 2F) were also indistinguishable from that of their wild-type littermates. Therefore, the persistent presence of CaMKII-F89G in the forebrain during learning and retention did not affect memory formation.
Disruption of short-term memory by shifting learned synaptic states
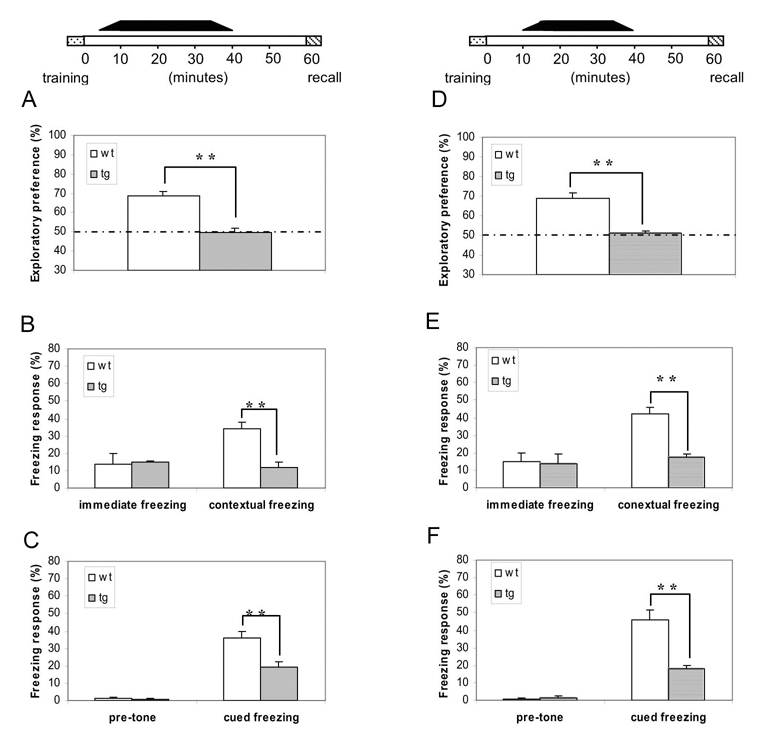
To investigate whether postlearning-shift in CaMKII activity disrupts neural representation of short-term memory, we trained a group of transgenic mice under the conditions as such αCaMKII-F89G activity was unmasked immediately after memory acquisition (<5 minutes) and allowed αCaMKII-F89G enzymatic activity to be expressed between the initial 5–35 minutes (see the black bar on top of Figure 3A–C, which indicates the duration of the availability of αCaMKII-F89G).
Figure 3. Early-stage (within 5–10 minutes after training) unmasking of αCaMKII-F89G activity is capable of disrupting the maintenance of one-hour short-term memory.
(A–C) The black bar on the top indicates the duration for the presence of αCaMKII-F89G activity, unmasking of activated CaMKII activity with 5 minutes after learning. The animals were given first i.p. drug injection 25 minutes before novel object recognition training (which lasts 15 minutes in duration) or 35 minutes before fear conditioning (4 minutes in duration). The 2nd injection was given 30 minutes before retention tests. The CaMKII activity of the transgenic mice at the time of 1-hr-retention tests were estimated to be about 100.7 ± 8.1% of that of wild-type littermates. (A) Novel object recognition memory was severely disrupted by the early-stage unmasking αCaMKII-F89G activity in transgenic mice (10 animals in each group, F(1,9) = 38, p < 0.001). (B) Although there is no significant difference between two groups in immediate freezing, a significantly lower freezing response during the one-hour contextual retention test was found in transgenic mice (n = 10) in comparison to that of wild type mice (n = 10) (F(1,11) = 12.29, p < 0.01). (C) Although there is no significant difference between two groups in pre-tone freezing, a significantly lower freezing response in the one-hour cued retention test was found in transgenic mice (n = 10) in comparison to that of wild-type mice (n = 10) (F(1,11) = 13.95, p < 0.01). All values are mean ± S.E.M. One-way ANOVA-Tukey was used for all the statistical analyses. (D–F) Unmasking of αCaMKII-F89G 10 minutes after learning is capable of disrupting the maintenance of short-term memory. The black bar on the top indicates the duration for the presence of αCaMKII-F89G activity. The animals were given i.p. NM-PP1 injection 20 minutes before novel object recognition training, or 30 minutes before fear conditioning. The 2nd i.p. injection was given 30 minutes before recall. The CaMKII activity of the transgenic mice at the time of 1-hr-retention tests were estimated to be about 101.5 ± 6.2% of that of wild-type mice. (D) Novel object recognition memory was severely disrupted by unmasking αCaMKII-F89G activity 10 minutes after learning in transgenic mice (10 animals in each group, F(1,18) = 31.4, p < 0.01). (E) Disruption of contextual fear memory in transgenic mice by the early-stage unmasking αCaMKII-F89G activity. Although there is no significant difference between two groups in immediate freezing, a significantly lower freezing response during the one-hour contextual retention test was found in transgenic mice (n = 10) in comparison to that of wild type mice (n = 10) (F(1,18) = 39.76, p < 0.01). (F) Disruption of cued fear memory in transgenic mice by the early-stage unmasking αCaMKII-F89G activity. Although there is no significant difference between two groups in pre-tone freezing, a significantly lower freezing response in the one-hour cued retention test was found in transgenic mice (n = 10) in comparison to that of wild-type mice (n = 10) (F(1,18) = 27.03, p < 0.01). All values are mean ± S.E.M. One-way ANOVA-Tukey was used for all the statistical analyses.
We first conducted this “Unmasking-CaMKIIF89G-immediately-After-Learning” protocol using the novel object recognition test. We observed that such an upward shift severely disrupted one-hour retention of recognition memory (Figure 3A; ANOVA F(1,9) = 38, p < 0.001). Similarly, we found the profound retention deficits in the short-term contextual fear memory test (Figure 3B; ANOVA F(1,11) = 12.29, p < 0.01) as well as the short-term cued fear memory test (Figure 3C; ANOVA F(1,11) = 13.95, p < 0.01). The data suggests that short-term memory seems to be maintained in a synaptically labile state that is highly sensitive to post-learning numerical re-adjustment of CaMKII activity.
To further determine the duration of this CaMKII-mediated labile period of short-term memory, we designed the experiments in which αCaMKII-F89G activity was permitted to be active only between post-training 10–40 minutes (see the lack bar on top of Figure 3D–F) so that a normal amount of CaMKII activity was present in the transgenic mice during memory acquisition, the initial post-training 10 minutes, post-training 40–60 minutes and recall. Again, we found that such a “Unmasking-CaMKIIF89G-Within-10 minutes-After-Learning” protocol also caused profound deficits in the retentions of the novel object recognition memory (Figure 3D; ANOVA F(1,18) = 31.4, p < 0.01), contextual fear (Figure 3E ANOVA F(1,18) = 39.76, p < 0.01), and cued fear memories (Figure 3F; ANOVA F(1,18) = 27.03, p < 0.01).
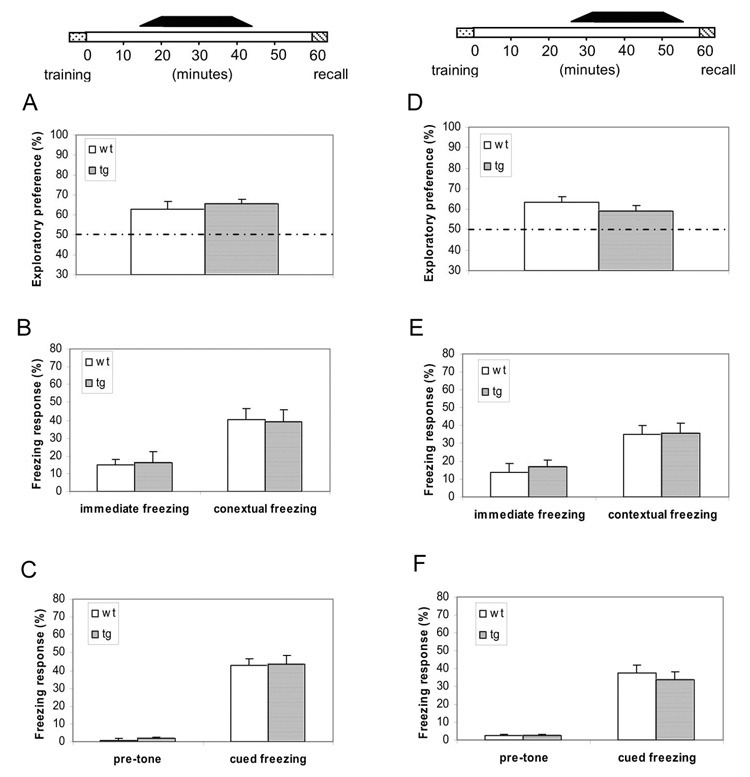
Next, we carried out additional temporal analysis and designed the “Unmasking-CaMKIIF89G-Within-15-minutes-After-Learning” protocol experiments in which αCaMKII-F89G activity would be unmasked only between post-training 15–45 minutes (see the black bar on top of Figure 4A–C). Under this protocol, we observed that the transgenic mice showed normal retentions in the novel object recognition test (Figure 4A), contextual fear (Figure 4B), and cued fear memory tests (Figure 4C). These experiments suggest that there is a critical time-window (<15 minutes) during which short-term memories are sensitive to disruption by upward shift in CaMKII, and that removal of NM-PP1 inhibition of αCaMKII-F89G activity beyond this time-window can no longer disrupt short-term memories.
Figure 4. Unmasking of αCaMKII-F89G 15 minutes and beyond after learning did not disrupt the short-term memory.
(A–C) The black bar on the top indicates the duration for the presence of uninhibited CaMKII-F89G activity, 15 minutes after learning. Mice were given i.p. injection 15 minutes before novel object recognition training or 25 minutes before fear conditioning. The 2nd injection was given 25 minutes before retention tests. The CaMKII activity of the transgenic mice at the time of 1-hr-retention tests were estimated to be about 92.9 ± 5.9% of that of wild-type mice. (A) Normal novel object recognition memory in transgenic mice that unmasking αCaMKII-F89G activity took place 15 minutes after learning (12 animals each group, p > 0.05). (B) Normal contextual memory in both wild-type (n = 10), transgenic mice (n = 12). No significant differences were found in both immediate freezing and contextual conditioning between two groups (p > 0.05). (C) Normal cued fear memory in the same groups of mice. No significant differences were found (p > 0.05). All values are mean ± S.E.M. One-way ANOVA-Tukey was used for all the statistical analyses. (D–F) Unmasking of αCaMKII-F89G 30 minutes after learning did not disrupt the short-term memory. The black bar on the top indicates the duration for the presence of uninhibited αCaMKII-F89G. Mice were given NM-PP1 i.p. injection 5 minutes before object recognition training or 15 minutes before fear conditioning. The 2nd injection was given 10 minutes before retention test. The CaMKII activity of the transgenic mice at the time of 1-hr-retention tests were estimated to be about 95.4 ± 4.5% of that of wild-type mice. (D) Normal novel object recognition memory in transgenic mice after the late-stage (>25 minutes after training) unmasking αCaMKII-F89G activity (12 animals each group, p > 0.05). (E) Normal contextual memory in both wild-type (n = 10), transgenic mice (n = 12). No significant differences were found in both immediate freezing and contextual conditioning between two groups (p > 0.05). (F) Normal cued fear memory in the same groups of mice. No significant differences were found (p > 0.05). All values are mean ± S.E.M. One-way ANOVA-Tukey was used for all the statistical analyses.
Finally, we conduct another set of the “Unmasking-CaMKIIF89G-25-minutes-After-Learning” experiments in which αCaMKII-F89G activity was made available selectively between post-training 25–55 minutes (see the black bar on top of Figure 4D–F). Once again, we found that late-stage removal of αCaMKII-F89G inhibition did not produce detrimental effects on the retentions in the novel object recognition test (Figure 4D), contextual fear (Figure 4E), and cued fear memory tests (Figure 4F). These results demonstrate the critical time-window of the CaMKII-mediated labile period for three types of short-term memory is restricted to the initial 10 minutes after learning.
Temporal dynamics of CaMKII in hippocampal long-term potentiation (LTP)
To determine the effects of molecular dynamics of activated αCaMKII-F89G on synaptic plasticity, we measured LTP in the Schaffer collateral pathway using extracellular field recordings in CA1 regions of hippocampus in both transgenic and control slices. We found that single tetanus (100 Hz, 1s) evoked larger LTP in transgenic slices in comparison to that in control slices (Figure 5A, ANOVA F(1,19) = 5.259, P<0.05). This enhanced LTP can be reduced to the wild-type level by the application of NM-PP1 (5 µM) in the recording chamber throughout the experiments (Figure 5B). Interestingly, post-tetanus application of NM-PP1 had no effect on LTP levels in transgenic hippocampal slices (Figure 5C, ANOVA F(1,26) = 4.692, P<0.05), suggesting that once CaMKII is activated by LTP, inhibition of CaMKII activity could not readily reverse the phosphorylation-triggered downstream events [14, 38].
Figure 5. The presence of over-expressed αCaMKII-F89G leads to larger LTP in transgenic mice.
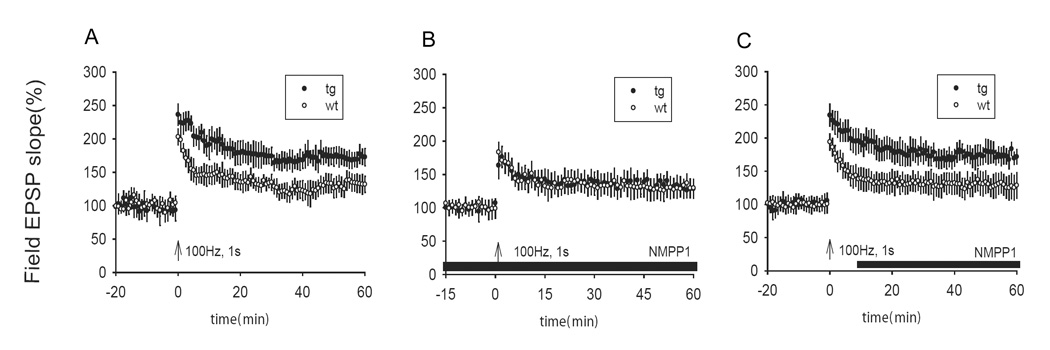
(A) A single tetanus (100 Hz, 1s) evoked large LTP in transgenic slices in comparison to that in control slices (p < 0.001, 8 transgenic mice, 11 slices; 8 control mice, 10 slices). (B) NM-PP1 (5 µM) reduced LTP in transgenic slices back to normal level (p > 0.05, 8 transgenic mice, 11 slices; 8 control mice, 11 slices). (C) Post-tetanus application of NM-PP1 (5 µM) had no effect on the magnitude of LTP in transgenic slices (p < 0.001, 11 transgenic mice, 17 slices; 10 control mice, 16 slices). All values are expressed as mean ± S.E.M. ANOVA-Tukey was used to determine statistical difference in the magnitude of LTP from last 10 minutes block of recordings (51–60 minutes) between transgenic and control slices. The black bars in B–C indicate the duration of NM-PP1 treatment.
We then asked whether αCaMKII-F89G remains in a conformationally active state after tetanic stimulation (e.g. binding with Ca2+/CaM triggers translocation of the CaMKII to the PSD zone) [16, 22, 23]. We reasoned that if αCaMKII-F89G is locked in an active conformation during this transient period, quick removal of NM-PP1 immediately after tetanus would unmask the “conformationally activated” CaMKII-F89G activity, thus may be capable of shifting the established population LTP to a higher level. To test this idea, we designed an experimental protocol in which the hippocampal slices were treated with NM-PP1 for a fixed duration, starting 10 minutes before the tetanus and continued for an additional 5 minutes after the tetanus before washout. Interestingly, we found that such a manipulation shifted the otherwise normal-level LTP to a higher level in transgenic slices (Figure 6A, ANOVA F(1,18) = 6.072, P<0.05).
Figure 6. Temporal dynamics of the activated αCaMKII-F89G during LTP in the Schaffer collateral CA1 pathway in transgenic mice.
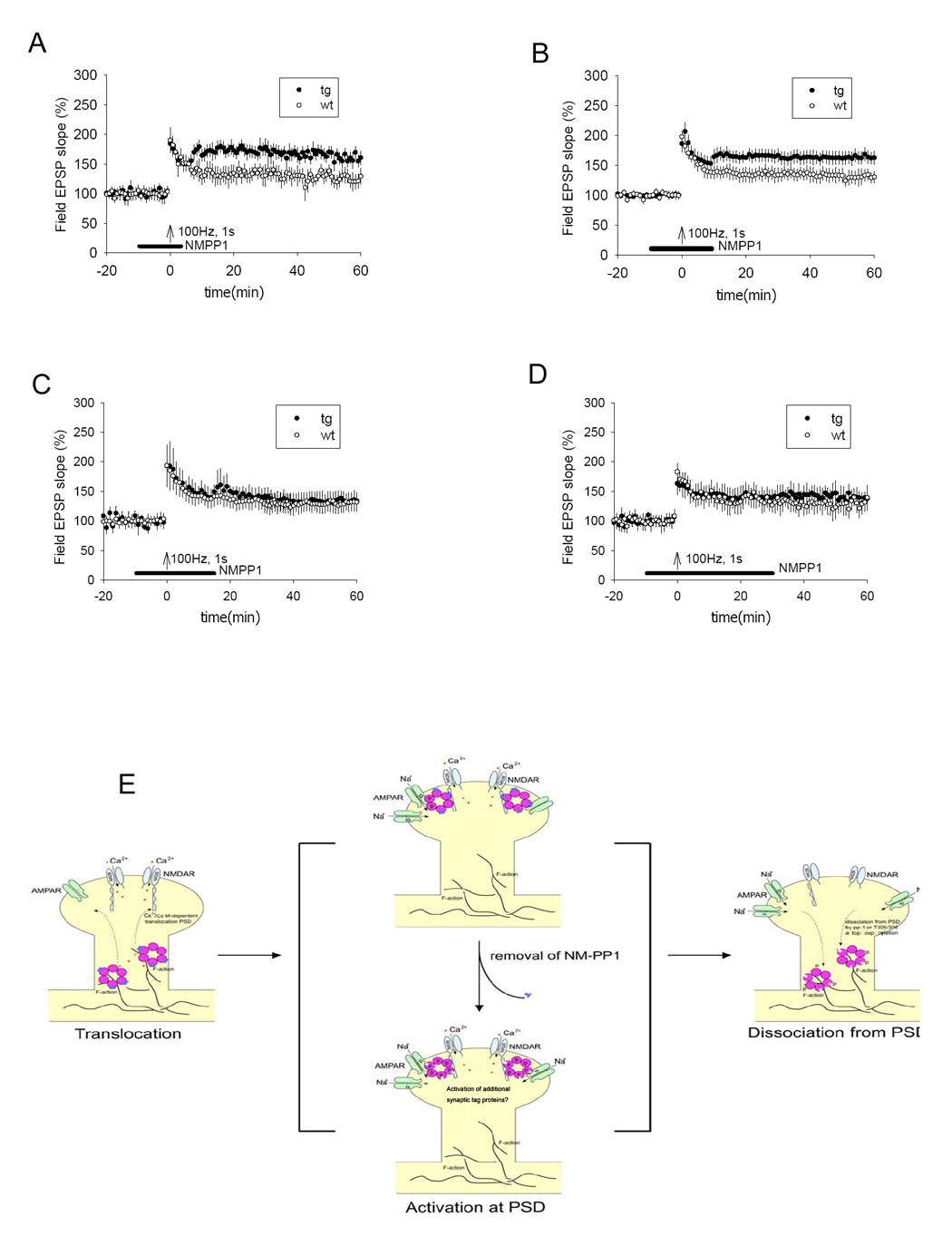
(A) LTP in transgenic slices was shifted to a higher level from the otherwise normal level when NM-PP1 was washed out 5 minutes after tetanus (p < 0.05, 8 transgenic mice, 10 slices; 8 control mice, 10 slices). (B) LTP in transgenic slices was also shifted to a higher level when NM-PP1 was washed out 10 minutes after tetanus (p < 0.05, 8 transgenic mice, 10 slices; 8 control mice, 10 slices). (C) No shift was observed when NM-PP1 was washed out 15 minutes after tetanus (8 transgenic mice, 11 slices; 8 control mice, 11 slices). (D) No shift was observed when NM-PP1 was washed away 30 minutes after tetanus (8 transgenic mice, 11 slices; 8 control mice, 11 slices). All values are expressed as mean ± S.E.M. ANOVA-Tukey was used to determine statistical difference in the magnitude of LTP from last 10 minutes block of recordings (51–60 minutes) between transgenic and control slices. The black bars indicate the duration of NM-PP1 treatment. (E) Schematic drawing illustrates the molecular dynamics of CaMII at activated synapses. During the Initiation phase, learning triggers the activation of the NMDA receptor which permits Ca2+ influx, leading to the binding of Ca2+/calmodulin to CaMKII, which initiates the dissociation of CaMKII from F-actin (through βCaMKII-mediated action) and then translocation of the complex to PSD. The αCaMKII-F89G-containing holoenzyme in the transgenic animals is capable of translocating to PSD in the presence of NM-PP1 inhibition because the translocation is an autophosphorylation-independent step. Once translocated to PSD via binding to NR2B C-terminal, the active CaMKII potentiates synapses and activates ‘synaptic tags’. During this PSD-associated phase, changes in CaMKII activity (such as unmasking the activated αCaMKII-F89G activity) will lead to reset the patterns of potentiated synapses in the network. The dissociation of CaMKII from PSD would make the synapse less vulnerable to the unmasking effect of αCaMKII-F89G activity. The dissociation may be controlled by the dephosphorylation on T286 by phosphatase 1, secondary phosphorylation at inhibitory phosphorylation sites of αCaMKII, and/or βCaMKII activity.
To further examine the duration of this critical time-window, we conducted another set of the recording experiments with the similar protocol except the drug was washed out at 10 minutes after tetanus. Again, we observed the similar effect in shifting LTP to a higher level (Figure 6B ANOVA F(1,17) = 5.166, P<0.05), although we noticed that the level of shift seemed to be smaller than that of the 5-minute-washout experiments. Next, we conducted additional sets of recording experiments in which the washout was carried out 15 minutes after tetanic stimulation. This time no shift could be produced in the transgenic slices (Figure 6C). Furthermore, we observed no effect of unmasking αCaMKII-F89G activity on the established LTP if NM-PP1 was washed out 30 minutes after tetanus stimulation (Figure 6D). Therefore, these results together have demonstrated that the early-stage removal of NM-PP1 within the temporally restricted critical time-window (≤ 10 minutes) after stimulation could readily unmask conformationally activated αCaMKII-F89G activity, leading to upward shifts in the population level or network pattern of synaptic potentiation. This finding suggests that the molecular and temporal dynamics of CaMKII plays a crucial role in controlling the early labile phase of synaptic potentiation.
Discussion
A half century ago, Hebb and Gerard proposed dual-trace theory of memory, suggesting that short-term memory traces are represented in a labile and short-lasting neural state before transforming into long-term memory [1, 2]. Their proposals have led to numerous studies which show that post-learning administration of electroconvulsive shock, brain impact, traumatic injury and seizures appear to preferentially disrupt short-term memories, whereas those same types of interventions leave long-term memory largely intact.
We have chosen three different forms of short-term memory tests for the analysis of the molecular and synaptic mechanism underlying the labile period of newly formed memory. All these behavioral tests involve a single training session and memory acquisitions are dependent on the NMDA receptors [33–37]. More importantly, the novel object recognition memory tends to only last for several days, whereas contextual and cued fear memories can last for months and even years, thereby providing an opportunity to compare the initial stages of these memory processes known to have distinct durations. In addition, these three behavioral tests are known to involve different neuronal circuits. Thus, these different behavioral paradigms have allowed us to measure and directly compare whether the initial labile states of those short-term memories share any commonality.
Using these tasks, we systematically switched on and off the transgenically expressed αCaMKII-F89G activity in freely behaving mice, and asked how the shift of CaMKII activity alters short-term memory. Our experiments demonstrate that the initial 10 minute period represents the critical time-window during which both potentiated synapses and short-term memory are in an especially labile state and are sensitive to numerical increases in CaMKII activity.
What might temporal dynamics of activated CaMKII underlie this critical time-window? Based on the available literature, it seems that dynamics of CaMKII translocation to and disassociation from the postsynaptic density (PSD) could underlie what we observed at the synaptic and behavioral levels (Figure 6E). During this PSD-association period, removal of NM-PP1 inhibitor from transgenic CaMKII-F89G ATP-binding pocket can unmask the activated CaMKII-F89G activity, leading to a rapid shift of synaptic weight from the existing state to a new state. Such a switch of synaptic patterns in a given neural network would then disrupt the on-going memory representation. However, once the CaMKII complex is disassociated from the PSD, unmasking of αCaMKII-F89G activity no longer caused any significant alteration in the patterns of potentiated synapses/neurons, thereby having no disruptive effects on short-term memory representation. There are at least two factors that may control the dissociation of CaMKII from PSD: 1) secondary phosphorylation on Thr305/306 of CaMKII, which is reported to promote disassociation of the enzyme from PSD [20, 22]; and 2) dephosphorylation at the Thr286 site by phosphatase 1 action since both PP1 inhibitor and T286D mutant delayed the dissociation of CaMKII from PSD [22], which suggests the dephosphorylation of the autonomous phosphorylation site T286 is required for dissociation. Consistent with our results, the studies in cultured neurons indicate that the disassociation of CaMKII complex from PSD occurs within minutes [22, 23].
While we have demonstrated that numerical increase in activated CaMKII activity can lead to changes in the established LTP level, we currently do not know whether such changes were due to increased single-channel conductance of AMPA receptors at these synapses [24] or increased insertion of AMPA receptors [25, 39] or activation of other proteins such as those postulated involved in the "synaptic tagging" process (40–42). If potentiation of individual synapses is an “all-or-none” process [43], the upward shift in population LTP by unmasking αCaMKII-F89G at PSD could be a result of an increase in the total numbers of potentiated synapses (network patterns of activated synapses). On the other hand, if potentiation of individual synapses is a graded process, the upward shift in LTP would come from an increase in the degree of potentiation in the same set of synapses.
It is important to point out that while the measurement of experimental form of synaptic plasticity from the hippocampus of CaMKII-F89G transgenic mice is informative, we do not know whether and how such changes in synaptic plasticity measured in hippocampal slices (e.g. at 28°C) reflect in vivo synaptic changes induced by natural learning (at 37°C). Moreover, it would be useful to further examine the role of endogenous αCaMKII function using knock-in method (such as replacing one or both copies of the αCaMKII gene with αCaMKII-F89G). One traditional approach to the study of endogenous gene function is to perform knockout experiments. However, in the case of CaMKII, once activated, this enzyme quickly phoshorylates its downstream substrates. This means that pharmacological inhibition or genetic knockout at this stage will be too late to reverse its consequences which can only be reversed by phosphates over time. On the contrary, the further upregulation of CaMKIIF89G activation can still alter the downstream signaling process in a rapid manner, thereby revealing important insights about CaMKII dynamics and function. The fact that both heterozygous and homozygous αCaMKII knockouts as well as several transgenic overexpressors [15, 27, 28, 44] all produced some types of memory impairment phenotypes further supports the notion that the delicate balance between CaMKII and phosphates is crucial for normal memory function. Our present study provides a novel insight into previously unrecognized role in maintaining short-term memory process.
It is interesting to mention that learning involved in those three types of behavioral tasks is likely to involve many different brain regions, we have not yet examined directly whether the similar changes in the plasticity-frequency responses are also occurred in other forebrain regions (such as the amygdala or cortex) of our transgenic mice. Nonetheless, our data consistently suggest that a switch between the normal state and higher state in terms of CaMKII activity level within certain time-window can severely disrupt memory formation [31, 45]. It is important to point out that our previous study has shown that constitutive presence of αCaMKII-F89G during learning and retention period has no significant effect on memory performances on transgenic mice [31]. This suggests that as long as consistency in CaMKII activity is maintained, learning and consolidation can still occur normally in the brain even with elevated CaMKII activity and heightened LTP levels. However, alteration in CaMKII level during the first post-learning week disrupts long-term memory consolidation. Furthermore, our more recent study also shows that βCaMKII is also involved during the memory consolidation [45]. Taken together with our present findings, we conclude that memory process is sensitive to numerical changes in CaMKII activity at multiple time windows. It would be of great interest to examine how the rapid changes in CaMKII activation affect neural network patterns and dynamics during the encoding of episodic memories (46–48).
In conclusion, using the rapid inducible and reversible chemical genetic technique, we have shown that initial 10 minutes of new memory formation and LTP are sensitive to manipulation of αCaMKII activation state. We conclude that CaMKII activation state plays an important role in regulating synaptic potentiation dynamics and short-term memory representation.
Materials and Methods
Production and biochemical characterizations of transgenic mice
The transgenic mice were produced and maintained on the BCF1 hybrid background [31]. The genotype and the expression pattern of the transgene were determined by in situ hybridization using the same protocol [31]. The NM-PP1 pharmaco-kinetics was determined by using both radioactive tracing method and kinase activity assay [31]. The time course of single NM-PP1 inhibition on CaMKII from pharmacokinetic studies was served as a guideline for the short-term memory analysis. A single i.p. injection of NM-PP1 seems to provide effective inhibition between 10–40 minutes of post-injection. To ensure the valid NM-PP1 inhibition in vivo, we also measured the off-rate of NM-PP1 that may occur during the brain dissection procedure. By carrying out a serial dilution (1:10, 1:100) to the brain homogenates, we found no significant dilution effect on the binding of NM-PP1 on CaMKII in the above assay condition. In addition, we also performed kinase assay to confirm the effective inhibition of CaMKII-F89G activity by NM-PP1 in the transgenic mice receiving the double injection protocols which were designed to compensate for the rebound of CaMKII-F89G activity after 40 minutes of a single injection. The specific suppression of CaMKII-F89G activity in those mice receiving double i.p. NM-PP1 injections was listed in the figure legends of 1-hr short-term memory results.
We also measured NM-PP1 pharmacokinetics synthesizing 3H-labeled NM-PP1 [31]. The injected solution contains the mixture of cold NM-PP1 and [3H]-NM-PP1 at the ration of 1: 100. 10 µl of the body weight of NM-PP1 mixture was i.p. injected into the mice. At each time point, mice were sacrificed and their forebrains were quickly dissected and weighted. The forebrain tissues were homogenized in 1 x ice-cold PBS. 200 µl of the homogenate were put into 4 ml of scintillation solution for measurement of radioactivity. The data presented were the percentage of CPM reading from the total forebrain homogenate compared with that of the total amount of NM-PP1 injected into the body.
Hippocampal slice recording
The recording procedures were the same as previously described [8, 28, 31]. Briefly, transverse slices of the hippocampus from transgenic and wild-type littermate mice (3–4 month old) were rapidly prepared and maintained in a submerge chamber at 28°C. 5 µM of NM-PP1 was perfused in the indicated slices. A bipolar tungsten stimulating electrode was placed in the striatum radiatum in the CA1 region and extracellular field potentials were recorded using a glass microelectrode (3–12 MΩ filled with ACSF) also in the striatum radiatum. Test responses were elicited at 0.017 Hz. In the experiments involving NM-PP1 treatments, the control slices (from wild-type littermates) received the same drug treatment as the transgenic slices during those LTP measurements.
Behavioral Measurements
Mice were maintained in a temperature-controlled environment on a 12hr light/dark cycle. Adult male CaMKII F89G transgenic and wild-type littermate mice (2–4 month old) were used throughout all behavioral tests. For NM-PP1 treatment, the dose of 16.57 ng per gram of body weight per mouse was used via i.p. injection (5 µM). NM-PP1 was dissolved in DMSO with final concentration of 0.02% of DMSO in the solution. All the control animals (wild-type littermates) received the same drug treatment as the transgenic animals during the behavioral experiments.
Novel-object recognition task and fear conditioning
Those behavioral experimental protocols were the same as described previously [8, 29, 35]. For the novel object recognition test, a preference index, a ratio of the time spent exploring any one of the two objects (training session) or the novel one (retention session) over the total time spent exploring both objects, was used to measure recognition memory. For fear conditioning, the freezing responses were recorded in a sampling time of 5 sec. Data were calculated as mean ± SEM. One-way ANOVA-Tukey was used for all the statistical analyses.
Acknowledgements
We thank Eiji Shimizu and Shuqing Zhang for their initial technical assistances and Kevan Shokat for providing NM-PP1 inhibitor. H. W. is supported by a NRSA fellowship. This research was supported by funds from NIH (MH60236, MH61925, MH62632, AG02022), Beckman Foundation, Burroughs Welcome Fund, and W.M. Keck Foundations (all to JZT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gerard RW. Physiology and Psychiatry. Am J Psychiatry. 1949;105:161–173. doi: 10.1176/ajp.106.3.161. [DOI] [PubMed] [Google Scholar]
- 2.Hebb DO. The organization of behavior: a neuropsychological theory. New York: Wiley; 1949. [Google Scholar]
- 3.Squire LR. Memory and Brain. New York: Oxford University Press; 1987. [Google Scholar]
- 4.Davis M, Hitchcock J, Rosen JB. The Psychology of Learning and Memory. New York: Academic Press; 1987. [Google Scholar]
- 5.Goelet P, Castellucci VF, Schacher S, Kandel ER. The long and the short-term memory--a molecular framework. Nature. 1986;322:419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- 6.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 7.Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 8.Tang YP, Shimizu E, Dube GR, Rampon C, Kerchner GA, Zhuo M, Liu G, Tsien JZ. Genetic enhancement of learning and memory in mice. Nature. 1999;401:63–69. doi: 10.1038/43432. [DOI] [PubMed] [Google Scholar]
- 9.Tsien JZ. Building a brainier mouse. Scientific American. 2000;282:62–68. doi: 10.1038/scientificamerican0400-62. [DOI] [PubMed] [Google Scholar]
- 10.Bear MF, Malenka RC. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994;4:389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 11.Lledo PM, Hjelmstad GO, Mukherji S, Soderling TR, Malenka RC, Nicoll RA. Calcium/calmodulin-dependent kinase II and long-term potentiation enhance synaptic transmission by the same mechanism. Proc Natl Acad Sci U S A. 1995;92:11175–11179. doi: 10.1073/pnas.92.24.11175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malinow R, Schulman H, Tsien RW. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- 13.Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986;44:861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- 14.Otmakhov N, Griffith LC, Lisman JE. Postsynaptic inhibitors of calcium/calmodulin-dependent protein kinase type II block induction but not maintenance of pairing-induced long-term potentiation. J Neurosci. 1997;17:5357–5365. doi: 10.1523/JNEUROSCI.17-14-05357.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 16.Strack S, Colbran RJ. Autophosphorylation-dependent targeting of calcium/ calmodulin-dependent protein kinase II by the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1998;273:20689–20692. doi: 10.1074/jbc.273.33.20689. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang Y, Kantor D, Harris KM, Schuman EM, Kennedy MB. Visualization of the distribution of autophosphorylated calcium/calmodulin-dependent protein kinase II after tetanic stimulation in the CA1 area of the hippocampus. J Neurosci. 1997;17:5416–5427. doi: 10.1523/JNEUROSCI.17-14-05416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soderling TR. CaMK-kinase: modulators of synaptic plasticity. Curr Opin Neurobiol. 2000;10:375–380. doi: 10.1016/s0959-4388(00)00090-8. [DOI] [PubMed] [Google Scholar]
- 19.Bayer KU, De Koninck P, Leonard AS, Hell JW, Schulman H. Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature. 2001;411:801–805. doi: 10.1038/35081080. [DOI] [PubMed] [Google Scholar]
- 20.Elgersma Y, Fedorov NB, Ikonen S, Choi ES, Elgersma M, Carvalho OM, Giese KP, Silva AJ. Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning. Neuron. 2002;36:493–505. doi: 10.1016/s0896-6273(02)01007-3. [DOI] [PubMed] [Google Scholar]
- 21.Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaMKII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- 22.Shen K, Meyer T. Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science. 1999;284:162–166. doi: 10.1126/science.284.5411.162. [DOI] [PubMed] [Google Scholar]
- 23.Shen K, Teruel MN, Connor JH, Shenolikar S, Meyer T. Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat Neurosci. 2000;3:881–886. doi: 10.1038/78783. [DOI] [PubMed] [Google Scholar]
- 24.Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 26.Lisman JE, McIntyre CC. Synaptic plasticity: a molecular memory switch. Curr Biol. 2001;11:R788–R791. doi: 10.1016/s0960-9822(01)00472-9. [DOI] [PubMed] [Google Scholar]
- 27.Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- 28.Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- 30.Mark V, et al. Conditional restoration of hippocampal synaptic potentiation in Glur-A-deficient mice. Science. 2001;292:2501–2504. doi: 10.1126/science.1059365. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Shimizu E, Tang YP, Cho M, Kyin M, Zuo W, Robinson DA, Alaimo PJ, Zhang C, Morimoto H, Shokat K, Tsien JZ. Inducible protein knockout reveals temporal requirement of CaMKII reactivation for memory consolidation in the brain. Proc Natl Acad Sci U S A. 2003;100:4287–4292. doi: 10.1073/pnas.0636870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, Wood JL, Morgan DO, Shokat KM. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- 33.Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 34.Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci. 1996;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- 36.Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues SM, Schafe GE, LeDoux JE. Intra-amygdala blockade of the NR2B subunit of the NMDA receptor disrupts the acquisition but not the expression of fear conditioning. J Neurosci. 2001;21:6889–6896. doi: 10.1523/JNEUROSCI.21-17-06889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen HX, Otmakhov N, Strack S, Colbran RJ, Lisman JE. Is persistent activity of calcium/calmodulin-dependent kinase required for the maintenance of LTP? J Neurophysiol. 2001;85:1368–1376. doi: 10.1152/jn.2001.85.4.1368. [DOI] [PubMed] [Google Scholar]
- 39.Rongo C, Kaplan JM. CaMKII regulates the density of central glutamatergic synapses in vivo. Nature. 1999;402:195–199. doi: 10.1038/46065. [DOI] [PubMed] [Google Scholar]
- 40.Sajikumar S, Frey JU. Resetting of 'synaptic tags' is time- and activity-dependent in rat hippocampal CA1 in vitro. Neuroscience. 2004;129:503–507. doi: 10.1016/j.neuroscience.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 41.Sajikumar S, Navakkode S, Frey JU. Identification of compartment- and process-specific molecules required for "synaptic tagging" during long-term potentiation and long-term depression in hippocampal CA1. J Neurosci. 2007;27:5068–5080. doi: 10.1523/JNEUROSCI.4940-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frey S, Frey JU. 'Synaptic tagging' and 'cross-tagging' and related associative reinforcement processes of functional plasticity as the cellular basis for memory formation. Prog Brain Res. 2008;169:117–143. doi: 10.1016/S0079-6123(07)00007-6. [DOI] [PubMed] [Google Scholar]
- 43.Petersen CC, Malenka RC, Nicoll RA, Hopfield JJ. All-or-none potentiation at CA3-CA1 synapses. Proc Natl Acad Sci USA. 1998;95:4086–4088. doi: 10.1073/pnas.95.8.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frankland PW, O'Brien C, Ohno M, Kirkwood A, Silva AJ. Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001;411:309–313. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- 45.Cho MH, Cao X, Wang D, Tsien JZ. Dentate gyrus-specific manipulation of beta-Ca2+/calmodulin-dependent kinase II disrupts memory consolidation. Proc Natl Acad Sci U S A. 2007;104:16317–16322. doi: 10.1073/pnas.0703344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin L, Osan R, Shoham S, Jin W, Zuo W, Tsien JZ. Identification of network-level coding units for real-time representation of episodic experiences in the hippocampus. PNAS USA. 2005;102:6125–6130. doi: 10.1073/pnas.0408233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin L, Osan R, Tsien JZ. Organizing principles of real-time memory encoding: Neural clique assemblies and universal neural codes. Trends in Neurosciences. 2006;29:48–56. doi: 10.1016/j.tins.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Tsien JZ. The memory code. Scientific American. 2007:52–59. doi: 10.1038/scientificamerican0707-52. July issue, [DOI] [PubMed] [Google Scholar]