Abstract
OBJECTIVE—The International Diabetes Mellitus Practice Study is a 5-year survey documenting changes in diabetes treatment practice in developing regions.
RESEARCH DESIGN AND METHODS—Logistic regression analysis was used to identify factors for achieving A1C <7% in 11,799 patients (1,898 type 1 diabetic and 9,901 type 2 diabetic) recruited by 937 physicians from 17 countries in Eastern Europe (n = 3,519), Asia (n = 5,888), Latin America (n = 2,116), and Africa (n = 276).
RESULTS—Twenty-two percent of type 1 diabetic and 36% of type 2 diabetic patients never had A1C measurements. In those with values for A1C, blood pressure, and LDL cholesterol, 7.5% of type 1 diabetic (n = 696) and 3.6% of type 2 diabetic (n = 3,896) patients attained all three recommended targets (blood pressure <130/80 mmHg, LDL cholesterol <100 mg/dl, and A1C <7%). Self-monitoring of blood glucose was the only predictor for achieving the A1C goal in type 1 diabetes (odds ratios: Asia 2.24, Latin America 3.55, and Eastern Europe 2.42). In type 2 diabetes, short disease duration (Asia 0.97, Latin America 0.97, and Eastern Europe 0.82) and treatment with few oral glucose–lowering drugs (Asia 0.64, Latin America 0.76, and Eastern Europe 0.62) were predictors. Other region-specific factors included lack of microvascular complications and old age in Latin America and Asia; health insurance coverage and specialist care in Latin America; lack of obesity and self-adjustment of insulin dosages in Asia; and training by a diabetes educator, self-monitoring of blood glucose in patients who self-adjusted insulin, and lack of macrovascular complications in Eastern Europe.
CONCLUSIONS—In developing countries, factors pertinent to patients, doctors, and health care systems all impact on glycemic control.
Although optimizing diabetes care reduces death and complication rates (1–3), multiple barriers hinder turning evidence into practice (4,5). Most diabetic patients reside in developing countries (6) where standardized data on quality of care is relatively scarce. The International Diabetes Management Practices Study (IDMPS) is an ongoing observational survey to collect, analyze, and disseminate data in a standardized manner. By documenting changes in practices over time in a broad range of health care settings, we aim to raise awareness and identify barriers to quality diabetes care. Other objectives include evaluation of clinical progress, levels of compliance, attainment of treatment targets, and rates of hospitalization and work absenteeism. This analysis of the first-year survey examines factors predictive of glycemic control.
RESEARCH DESIGN AND METHODS
There are five waves in this 5-year study, each consisting of a 2-week cross-sectional and a 9-month longitudinal survey. A 3-month interval separates the end of the longitudinal survey and the start of the next wave. Study design and reporting format are in accordance with the recommended STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines (7).
During the first wave, 18 countries recruited participants between 5 May and 28 November 2005. These were Korea, China, Indonesia, India, Hong Kong, Taiwan, Malaysia, and Thailand from Asia (n = 5,888); Romania, Bulgaria, Turkey, Tunisia, and Bosnia from Eastern Europe (n = 3,519); Argentina, Ecuador, Venezuela, and Colombia from Latin America (n = 2,116); and Tunisia from Africa (n = 276). Physicians enrolled the first five type 1 diabetic and first 10 type 2 diabetic patients aged ≥18 years who attended their clinics over a 2-week period. Exclusion criteria included active participation in a clinical study or recent short-term insulin treatment. Diabetes was defined by the 2002 World Health Organization criteria (8).
Data collection and outcome measures
Data were collected on case report forms for demographic and socioeconomic profile, medical history, medications, glycemic control, blood pressure and lipid status, self-care, access to patient education, mode of follow-up, work absenteeism, and hospitalization. Outcome measures included attainment of treatment goals defined as A1C <7%, blood pressure <130/80 mmHg, and LDL cholesterol <100 mg/dl (8).
Selection of physicians and sample size estimation
A main objective of the IDMPS is to document the pattern of insulin usage. Thus, endocrinologists, diabetologists, and general practitioners with experience in initiation and titration of insulin therapy were invited to participate. The number of participating physicians in each country was calculated according to estimated percentages of insulin-treated type 2 diabetic patients in the country. A total of 937 physicians participated, with the highest enrollment in India (1,825 patients, 183 physicians) and the lowest enrollment in Tunisia (361 patients, 37 physicians).
Study implementation
A steering committee advised the project team on study design and registry structure, monitored study progress, reviewed and validated all study-related documents, and proposed and approved decisions on protocol amendments, analyses, and publications. The study was coordinated by sanofi-aventis Intercontinental. In each country, the study was championed by a leading diabetologist who compiled and endorsed the list of investigators. The latter were assisted by local sanofi-aventis staff in collecting relevant information including clinical and laboratory parameters. Ethics approval was obtained from institutional boards of each country. All participants provided written informed consent.
Statistical analysis
All data were transferred from study centers to Mapi-Naxis, France, for quality control and analysis using SAS (version 8.02; SAS Institute, Cary, NC). Descriptive analysis, ANOVA, χ2 test, and Fisher's exact test were used as appropriate. Univariate and logistic regression analyses were performed to identify predictive factors for A1C <7%. Age, duration of disease, and number of oral glucose-lowering drugs (OGLDs) were considered continuous variables. Due to regional heterogeneity, a logistic regression model per region was performed entering factors significant at the 10% level from the univariate analysis. Odds ratios (ORs) with 95% CIs were estimated for each significant predictor. A backward selection procedure identified predictive factors that were significant at the 5% level. Relevant interactions (age × time since diagnosis, age × microvascular complications, age × macrovascular complications, time since diagnosis × microvascular complications, time since diagnosis × macrovascular complications, BMI × microvascular complications, BMI × macrovascular complications, and self-monitoring of blood glucose [SMBG] × self-adjustment of insulin) were tested, and those significant at the 10% level were added to the final model. A P value <0.05 (two-tailed) was considered significant. Because of the low number of patients recruited in Tunisia, the data from this country were not included in the analysis.
RESULTS
Type 1 diabetes
Of the 1,898 type 1 diabetic patients recruited by 937 physicians (Eastern Europe n = 914, Asia n = 512, Latin America n = 404, and Africa n = 68), 20% reported diabetes-related hospitalization or work absenteeism in the last 6 months, 22% never had A1C measured, and 10–30% were not screened for complications in the last 24 months. Eastern Europe had the lowest proportion of screened patients. Clinical profiles were similar in all three regions (50% female, mean age 36 years, mean disease duration 11.5 years), with the lowest BMI in Asia (Table 1).
Table 1.
Regional distribution of clinical profile and care practices in patients with type 1 diabetes or type 2 diabetes
| Asia |
Eastern Europe |
Latin America |
All type 1 diabetic patients | All type 2 diabetic patients | ||||
|---|---|---|---|---|---|---|---|---|
| Type 1 diabetes | Type 2 diabetes | Type 1 diabetes | Type 2 diabetes | Type 1 diabetes | Type 2 diabetes | |||
| n | 510 | 5,376 | 914 | 2,605 | 404 | 1,712 | 1,898 | 9,901 |
| Clinical profile | ||||||||
| Age (years) | 35.0 ± 13.5 | 57.1 ± 11.7 | 35.2 ± 12.5 | 59.0 ± 10.0 | 40.8 ± 16.8 | 61.6 ± 11.4 | 36.4 ± 14.0 | 58.4 ± 11.30 |
| BMI (kg/m2) | ||||||||
| Men | 22.0 ± 3.7 | 25.5 ± 3.9 | 24.0 ± 3.4 | 28.4 ± 4.2 | 24.6 ± 3.7 | 28.1 ± 4.4 | 23.6 ± 3.7 | 26.6 ± 4.3 |
| Women | 22.1 ± 3.3 | 25.9 ± 4.4 | 23.4 ± 3.6 | 30.1 ± 5.0 | 24.2 ± 4.3 | 29.0 ± 5.30 | 23.3 ± 3.8 | 27.8 ± 5.2 |
| Waist circumference (cm) | ||||||||
| Men | 81 ± 10.1 | 92.2 ± 11.5 | 86.7 ± 10.8 | 101.5 ± 12.7 | 87.9 ± 12.6 | 100.8 ± 12.6 | 85.4 ± 11.3 | 95.9 ± 12.7 |
| Women | 75.9 ± 10.1 | 88.6 ± 12.5 | 79.1 ± 11.3 | 99.4 ± 14.1 | 83.0 ± 15.1 | 95.6 ± 13.6 | 79.4 ± 12.6 | 93.2 ± 14.1 |
| Time since diagnosis (years) | 9.7 ± 7.7 | 8.1 ± 6.8 | 11.0 ± 8.8 | 8.0 ± 7.0 | 14.4 ± 10.8 | 10.0 ± 8.3 | 11.5 ± 9.2 | 8.4 ± 7.2 |
| A1C (%) | 8.6 ± 2.1 | 7.7 ± 1.7 | 8.0 ± 1.9 | 7.9 ± 1.9 | 8.3 ± 1.9 | 7.9 ± 1.8 | 8.3 ± 2.0 | 7.8 ± 1.8 |
| Care practices | ||||||||
| Has health coverage | 322 (63.3) | 3,038 (58.2) | 354 (99.2) | 911 (99.3) | 314 (80.1) | 1,235 (75.8) | 1,045 (79.0) | 5,355 (67.3) |
| >6 physician visits per year | 178 (43.2) | 1,697 (38.7) | 242 (28.2) | 441 (19.1) | 86 (27.2) | 304 (24.3) | 506 (30.7) | 2,442 (30.0) |
| Trained by diabetes educator | 386 (76.6) | 2,927 (55.8) | 837 (92.6) | 1,782 (70.2) | 261 (66.4) | 806 (48.0) | 1,515 (81.1) | 5,624 (58.2) |
| Member of diabetes association | 58 (12.0) | 336 (6.5) | 179 (20.0) | 217 (8.5) | 60 (15.2) | 165 (10.0) | 299 (16.3) | 723 (7.6) |
| Regularly self-monitors blood glucose | 327 (63.9) | 1,597 (29.7) | 737 (81.4) | 926 (35.7) | 299 (74.0) | 659 (38.5) | 1,385 (73.3) | 3,205 (32.4) |
| Has never been screened for Cardiovascular disease | 136 (26.9) | 1,051 (19.8) | 99 (11.0) | 222 (8.7) | 112 (28.4) | 326 (19.5) | 369 (19.8) | 1,618 (16.6) |
| Retinopathy | 74 (14.7) | 1,314 (24.9) | 43 (4.8) | 274 (10.7) | 55 (14.0) | 388 (23.3) | 176 (9.5) | 1,991 (20.5) |
| Neuropathy | 117 (23.3) | 1,423 (27.0) | 86 (9.6) | 468 (18.3) | 107 (27.2) | 549 (33.0) | 330 (17.8) | 2,495 (25.8) |
| Microalbuminuria | 113 (22.5) | 1,883 (35.8) | 213 (24.0) | 985 (38.9) | 78 (19.8) | 535 (32.1) | 430 (23.2) | 3,478 (36.0) |
| Diabetic foot ulcer | 114 (22.7) | 1,406 (26.7) | 112 (12.5) | 443 (17.3) | 87 (22.1) | 398 (24.0) | 328 (17.7) | 2,288 (23.6) |
| Lipid abnormalities | 57 (11.3) | 686 (13.0) | 54 (6.0) | 155 (6.1) | 44 (11.2) | 166 (9.9) | 171 (9.2) | 1,024 (10.5) |
| A1C monitored ever | 415 (81.1) | 3,438 (64.0) | 666 (73.1) | 1,444 (55.8) | 328 (81.4) | 1,292 (75.5) | 1,469 (77.6) | 6,346 (64.2) |
| A1C <7% | 85 (21.0) | 1,268 (37.3) | 201 (31.3) | 510 (36.0) | 65 (21.1) | 453 (36.0) | 358 (25.3) | 2,272 (36.4) |
| Blood pressure <130/80 mmHg | 236 (47.4) | 1,150 (21.8) | 395 (43.8) | 313 (12·0.1) | 174 (44.1) | 374 (22.1) | 835 (44.9) | 1,873 (19.2) |
| LDL cholesterol <100 mg/dl | 96 (39.0) | 1,018 (37.0) | 136 (41.5) | 294 (25.5) | 80 (37.4) | 330 (31.6) | 317 (39.5) | 1,661 (33.2) |
| HDL cholesterol >40 mg/dl | 228 (84.1) | 1,878 (64.4) | 370 (78.4) | 944 (64.5) | 190 (82.3) | 701 (63.4) | 799 (80.7) | 3,585 (64.4) |
| Triglycerides <150 mg/dl | 213 (73.2) | 1,754 (51.4) | 511 (70.5) | 977 (44.6) | 204 (77.9) | 643 (48.2) | 966 (73.1) | 3,488 (49.0) |
| A1C <7%, blood pressure <130/80 mmHg, and LDL cholesterol <100 mg/dl | 14 (6.8) | 96 (4.7) | 28 (9.6) | 12 (1.3) | 10 (5.4) | 34 (3.8) | 52 (7.5) | 142 (3.6) |
Data are means ± SD or n (%).
Attainment of targets, treatment, and self care
Among patients with available A1C, 25% had a value <7 and 45% had attained blood pressure and LDL cholesterol goals. In patients with all three measurements (n = 696), 7.5% reached all three goals. Over 70% of patients performed SMBG, with the lowest percentage in Asia (Table 1). Approximately 80% had health coverage and access to diabetes educators, whereas 16% belonged to a diabetes association. When stratified by SMBG and access to diabetes educators, 29% of patients with both factors reached the A1C goal, compared with 21% with SMBG only, 14% with education only, and 8% with neither (P < 0.001).
The most popular insulin regimens were basal plus bolus in Latin America and Eastern Europe and a premix regimen in Asia. The mean insulin dose ranged from 0.5 to 0.8 IU/kg, with the highest dose used in the basal plus bolus regimen. Irrespective of treatment regimens or insulin dose, 20–30% reached the A1C goal (data not shown).
Physicians’ perceptions and risk factor control
Physicians were to tick yes/no checkboxes for three questions: “Is the patient at target for glycemic control?,” “Is the patient suffering from hypertension?,” and “Is the patient suffering from dyslipidemia?” For glycemic control, they answered “yes” for 717 (38%) patients, although 25% did not have an A1C value. For 483 patients without A1C, 38% were considered to be at target. For patients with A1C ≥7%, 21% were considered to be at target, and for those with fasting blood glucose >100 mg/dl, 33% were considered to be at target. Similarly, 46% of patients untreated for hypertension had a blood pressure ≥130/80 mmHg, and 40% untreated for dyslipidemia had a LDL cholesterol level ≥100 mg/dl.
Predictors for glycemic control
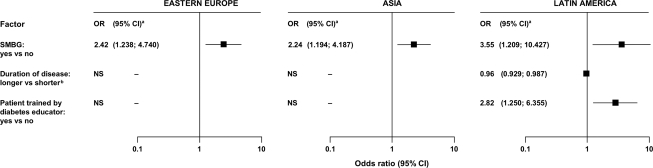
There were few differences between patients with A1C <7% and ≥7% (Table 2). Only SMBG was associated with two- to threefold increased odds of reaching the A1C goal in all three regions. Short disease duration and training by diabetes educators were predictors of glycemic control in Latin America (Fig. 1).
Table 2.
Univariate analysis of potential predictive factors for A1C <7% in type 1 diabetic or type 2 diabetic patients by region
| Eastern Europe |
Asia |
Latin America |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| A1C <7% | A1C ≥7% | P* | A1C <7% | A1C ≥7% | P* | A1C <7% | A1C ≥7% | P* | |
| Type 1 diabetes | |||||||||
| Clinical profile | |||||||||
| n | 201 | 441 | 85 | 320 | 65 | 243 | |||
| Age (years) | 33.8 ± 12 | 34 ± 11.96 | 0.887 | 33.6 ± 12.69 | 35.3 ± 13.45 | 0.287 | 39.0 ± 15.53 | 41.3 ± 16.13 | 0.309 |
| Mean disease duration (years) | 10.9 ± 9.10 | 11.4 ± 8.43 | 0.529 | 9.6 ± 8.99 | 10.1 ± 7.21 | 0.582 | 12.6 ± 9.51 | 16.1 ± 11.30 | 0.022 |
| BMI by categories (kg/m2) | — | — | 0.252 | — | — | 0.126 | — | — | 0.516 |
| <18.5 | 9 (4.5) | 12 (2.7) | 6 (7.2) | 29 (9.2) | 2 (3.1) | 11 (4.6) | |||
| 18.5 to <25 | 140 (70.0) | 290 (66.1) | 71 (85.5) | 228 (72.2) | 33 (50.8) | 134 (55.8) | |||
| 25 to <30 | 41 (20.5) | 122 (27.8) | 5 (6.0) | 48 (15.2) | 27 (41.5) | 79 (32.9) | |||
| 30 to <35 | 8 (4.0) | 12 (2.7) | 1 (1.2) | 10 (3.2) | 3 (4.6) | 10 (4.2) | |||
| ≥35 | 2 (1.0) | 3 (0.7) | 0 (0) | 1 (0.3) | 0 (0) | 6 (2.5) | |||
| ≥1 microvascular complication | 102 (50.7) | 253 (57.4) | 0.293 | 38 (44.7) | 155 (48.4) | 0.610 | 26 (40.0) | 135 (55.6) | 0.052 |
| ≥1 macrovascular complication | 18 (9.0) | 45 (10.3) | 0.462 | 7 (8.2) | 26 (8.1) | 0.225 | 7 (10.8) | 48 (19.8) | 0.047 |
| Self-management | |||||||||
| SMBG (fasting blood glucose or postprandial glucose) | 189 (94.5) | 383 (87.6) | 0.008 | 71 (83.5) | 225 (70.3) | 0.015 | 60 (92.3) | 195 (80.2) | 0.022 |
| Patient self-adjusts insulin | 177 (90.3) | 378 (86.5) | 0.178 | 53 (64.6) | 175 (55.7) | 0.146 | 49 (75.4) | 161 (67.4) | 0.215 |
| Care processes | |||||||||
| ≤6 consultations per year | 147 (74.2) | 309 (71.5) | 0.745 | 35 (41.7) | 157 (49.4) | 0.362 | 37 (57.8) | 145 (59.7) | 0.962 |
| Trained by diabetes educator | 189 (95.0) | 404 (92.2) | 0.207 | 65 (79.3) | 254 (80.1) | 0.863 | 55 (87.3) | 168 (70.6) | 0.007 |
| Seen by endocrinologists or diabetologists | 177 (88.1) | 378 (85.7) | 0.421 | 76 (89.4) | 273 (85.3) | 0.330 | 48 (73.8) | 186 (76.5) | 0.651 |
| Health insurance coverage | 96 (99.0) | 179 (100) | 0.174 | 54 (63.5) | 217 (68.5) | 0.390 | 53 (84.1) | 198 (83.2) | 0.859 |
| Type 2 diabetes | |||||||||
| Clinical profile | |||||||||
| n | 510 | 904 | 1268 | 2131 | 453 | 807 | |||
| Age (years) | 58.8 ± 10.69 | 57.9 ± 10.25 | 0.137 | 58.4 ± 11.53 | 57.2 ± 11.74 | 0.002 | 63.3 ± 11.74 | 61.9 ± 10.84 | 0.035 |
| Mean disease duration (years) | 7.5 ± 6.70 | 9.3 ± 7.37 | <0.001 | 7.5 ± 6.73 | 9.4 ± 7.02 | <0.001 | 8.6 ± 7.89 | 11.4 ± 8.54 | <0.001 |
| BMI by categories (kg/m2) | — | — | 0.430 | — | — | 0.005 | — | — | 0.595 |
| <18.5 | 2 (0.4) | 1 (0.1) | 19 (1.5) | 34 (1.6) | 1 (0.2) | 3 (0.4) | |||
| 18.5 to <25 | 88 (17.3) | 162 (18.0) | 594 (47.7) | 960 (45.7) | 102 (22.8) | 167 (21.2) | |||
| 25 to <30 | 229 (45.0) | 368 (41.0) | 505 (40.5) | 797 (37.9) | 185 (41.3) | 360 (45.7) | |||
| 30 to <35 | 138 (27.1) | 259 (28.8) | 104 (8.3) | 239 (11.4) | 108 (24.1) | 180 (22.8) | |||
| ≥35 | 52 (10.2) | 108 (12.0) | 24 (1.9) | 71 (3.4) | 52 (11.6) | 78 (9.9) | |||
| ≥1 microvascular complications | 267 (52.5) | 585 (64.6) | <0.001 | 579 (45.7) | 1,186 (55.9) | <0.001 | 220 (49.0%) | 524 (65.1%) | <0.001 |
| ≥1 macrovascular complication | 120 (23.6) | 346 (38.2) | <0.001 | 261 (20.6) | 479 (22.5) | 0.374 | 116 (25.6) | 264 (32.8) | 0.004 |
| Treatment | |||||||||
| OGLD | 417 | 633 | 1115 | 1863 | 369 | 607 | |||
| OGLD only | 369 (88.5) | 446 (70.5) | 982 (88.1) | 1,344 (72.1) | 309 (83.7) | 406 (66.9) | |||
| OGLD + insulin | 48 (11.5) | 187 (29.5) | 133 (11.9) | 519 (27.9) | 60 (16.3) | 201 (33.1) | |||
| Mean number of OGLDs† | 1.2 ± 0.81 | 1.1 ± 0.92 | 0.028 | 1.5 ± 0.86 | 1.7 ± 0.91 | <0.001 | 1.3 ± 0.82 | 1.2 ± 0.87 | 0.208 |
| Self-management | |||||||||
| SMBG (FBG or PPG) | 218 (42.7) | 420 (46.4) | 0.184 | 502 (39.6) | 857 (40.3) | 0.703 | 209 (46.1) | 374 (46.4) | 0.928 |
| Patient self-adjusts insulin‡ | 72 (14.3) | 237 (26.5) | <0.001 | 93 (7.4) | 215 (10.1) | <0.001 | 32 (7.1) | 113 (14.1) | <0.001 |
| Care processes | |||||||||
| ≤6 consultations per year | 408 (85.2) | 653 (77.7) | 0.003 | 659 (52.4) | 1,099 (52.2) | 0.722 | 288 (64.0) | 484 (60.2) | 0.006 |
| Trained by diabetes educator | 359 (71.5) | 585 (65.7) | 0.027 | 785 (63.7) | 1,383 (66.3) | 0.136 | 263 (59.0) | 434 (54.4) | 0.118 |
| Seen by endocrinologists or diabetologists | 417 (81.8) | 720 (79.4) | 0.280 | 950 (74.9) | 1,616 (75.8) | 0.550 | 285 (62.9) | 428 (53.0) | <0.001 |
| Health insurance coverage | 142 (100) | 271 (98.9) | 0.211 | 788 (63.6) | 1,287 (61.9) | 0.338 | 384 (87.7) | 589 (75.6) | <0.001 |
Data are means ± SD or n (%) unless otherwise indicated.
Student's t test for continuous variables and χ2 test for categorical variables.
Calculated for all patients, including those not taking OGLDs.
Those patients without self-adjustment of insulin comprise insulin-treated patients not self-adjusting and patients not treated with insulin. FBG, fasting blood glucose; PPG, postprandial glucose.
Figure 1.
Predictive factors for attaining A1C <7% in patients with type 1 diabetes, divided by regions.
Type 2 diabetes
Of 9,901 type 2 diabetic patients recruited by 937 physicians (Eastern Europe n = 2,605, Asia n = 5,376, Latin America n = 1,712, and Africa n = 208), 10% reported diabetes-associated hospitalization or absenteeism from work in the last 6 months, 33% did not have health coverage, 36% never had A1C measured, and 11–36% were not screened for complications in the last 2 years. There was marked regional heterogeneity for performance indexes, but for all three regions 20–40% of patients were at target for A1C, blood pressure, or lipids. In patients with all three risk factors measured (n = 3,896), 3.6% attained all three targets. Clinical profiles were similar in the three regions (mean age 58 years and mean disease duration 8 years), with the lowest BMI and waist circumferences in Asia (Table 1).
Attainment of targets, treatment, and self-care
Insulin doses and regimens were similar in all three regions (data not shown). Overall, 3% of patients were treated with diet and exercise alone, 66% with OGLDs alone, and 31% with insulin, with or without OGLDs (details by country available in the online appendix at http://dx.doi.org/10.2337/dc08-0435). Patients treated with insulin only had mean ± SD insulin doses ranging from 0.70 ± 0.35 IU/kg in those using basal plus prandial insulin to 0.45 ± 0.21 IU/kg in those using basal insulin alone. Patients treated with OGLDs plus insulin had mean doses of 0.68 ± 0.29 IU/kg (basal plus prandial) and 0.35 ± 0.20 IU/kg (basal alone). Irrespective of region or insulin regimen, 18–35% had A1C <7%. Overall, 42% never received diabetes education, 32% performed SMBG, and 8% belonged to a diabetes association.
Physician perception versus reality
Of patients considered by physicians to be at goal, 34% did not have an A1C value available. Of patients with A1C ≥7%, 27% were considered to be at target, and of those whose fasting blood glucose was >100 mg/dl, 41% were considered to be at target. Among insulin-treated patients, 22% with A1C ≥7% were considered to be at target. Among patients untreated for hypertension or dyslipidemia, 63% had a blood pressure ≥130/80 mmHg and 35% had LDL cholesterol ≥100 mg/dl.
Predictors for glycemic control
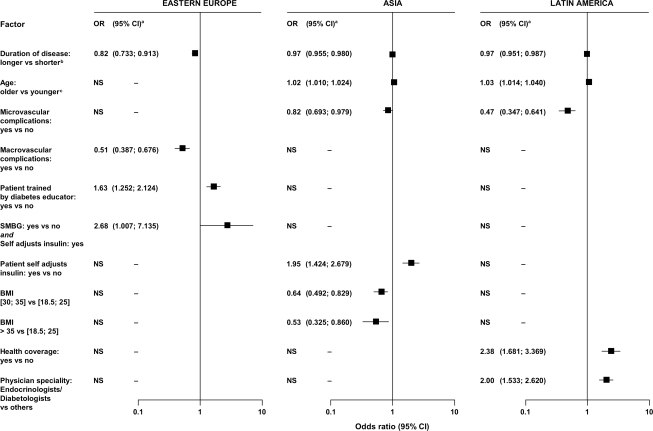
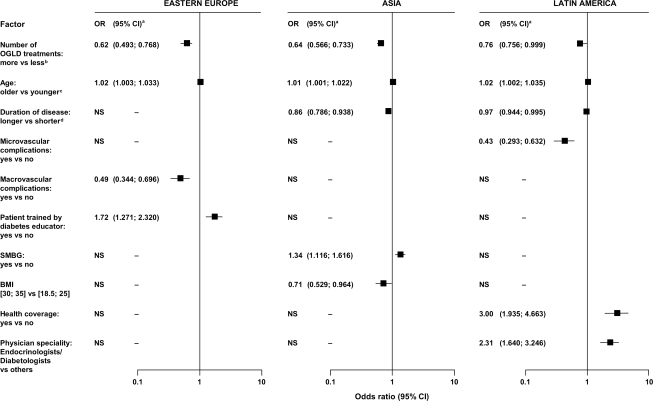
In all three regions, short disease duration was a predictor for A1C <7%. Region-specific predictors were lack of microvascular complications and old age in Latin America and Asia; training by a diabetes educator, SMBG in patients who self-adjusted insulin dosages, and lack of macrovascular complications in Eastern Europe; BMI <30 kg/m2 and self-adjustment of insulin dosages in Asia; and management by specialists and having health insurance coverage in Latin America (Table 2, Fig. 2). In patients treated with OGLDs only, use of fewer OGLDs was a predictor for reaching target in all three regions (Fig. 3).
Figure 2.
Predictive factors for attaining A1C <7% in patients with type 2 diabetes, divided by regions.
Figure 3.
Predictive factors for attaining A1C <7% in patients with type 2 diabetes treated only with OGLDs, divided by regions.
CONCLUSIONS
This multinational survey confirms the chasm between guidelines and practice in Asia, Eastern Europe, and Latin America. Based on case records, 10–40% of patients were not screened for risk factors or complications in the last 24 months. Only 20–30% of patients were at the A1C goal, whereas 7.5% of type 1 and 3.6% of type 2 diabetic patients attained three treatment goals. Furthermore, 20% of type 1 and 10% of type 2 diabetic patients reported hospitalization or work absenteeism in the past 6 months. Such suboptimal performance indexes call for closer surveillance to improve control.
Greater education needed
Whether managed by specialists or general practitioners, patients had similar patterns of care and levels of control. In both types of diabetes, there was significant mismatch between patient risk factor control and physician perception. Many physicians noted adequate glycemic control despite nonavailability of A1C measurements, whereas others overestimated the proportions of patients at goal. This agrees with other reports of delayed escalation in therapy (clinical inertia) and clinical assessments not translated into actions to improve control (9). Our findings are also consistent with those reported in Europe and the U.S., although reports from these regions show a slow trend of improvement in practice (10–12).
Predictors of glycemic control
In both diabetes types, although body weight–adjusted insulin doses were within recommended guidelines, neither doses nor regimens predicted glycemic control. SMBG was the only predictor for glycemic control in all three regions for type 1 diabetes. In Latin America, short disease duration and training by diabetes educators were also predictors: patients who had diabetes education and performed SMBG were fourfold more likely to be at target than those with neither (29 vs. 7%).
In type 2 diabetes, despite regional heterogeneity, short disease duration and use of few OGLDs were predictive factors in all three regions. In Asia and South America, absence of microvascular complications was an additional predictor. These findings suggest that early diagnosis and prompt initiation of insulin therapy in patients treated with multiple OGLDs may increase the likelihood of attaining glycemic targets, although definitive studies are required. In Asia and South America, old age was a predictor, which agrees with data from the U.S. Diabetes Prevention Program, showing lifestyle modification was more effective in elderly than young people (13), who may be less compliant because of competing priorities (14). In Asia, lack of obesity and self-adjustment of insulin dosages were predictors, emphasizing the double hit of obesity and β-cell insufficiency in Asian populations (15). Other region-specific factors relevant to self-care (e.g., SMBG and self-adjustment of insulin) and health care systems (e.g., health insurance coverage and access to specialists and diabetes educators) highlight the multiple challenges in optimizing diabetes care.
Strengths and weaknesses of our study
Whereas standardized methods used in the IDMPS allowed regional comparisons of diabetes practices, there are potential limitations including nonstandardized laboratory assays and assessments of complications. Selection of physicians experienced with insulin may introduce bias by overrepresenting patients with advanced diseases or complex regimens. However, the majority of patients were treated with OGLDs, and our analyses show that factors pertaining to the health care system, including access to educators, laboratory tests, and medications, are important barriers to achieving glycemic control. Inferring from these findings, we speculate that physicians with less system support and experience may face even greater challenges in managing these patients with multiple needs. Despite its cross-sectional nature, our data strongly suggest that prompt diagnosis, early intervention, and self-management are important determinants for glycemic control.
In conclusion, apart from contributing to the global landscape of diabetes practice, our data enable us to track performance indexes over time and generate a hypothesis to explain suboptimal diabetes care. Our findings have quantified factors pertinent to patients, care providers, and the health care system, all of which impact on the quality of diabetes care. There is an urgent need for the public, policy makers, and care providers to develop a strategy encompassing education, audits, mandates, and incentives to make multidisciplinary care and self-management more accessible, sustainable, and affordable (16).
Supplementary Material
Acknowledgments
The IDMPS is an epidemiological survey entirely funded by sanofi-aventis.
J.-M. C. is an employee of sanofi-aventis, which is a sponsor of the IDMPS. All of the other authors are members of the IDMPS Steering Committee and have received honoraria and traveling sponsorships in relation to the IDMPS. No other potential conflicts of interest relevant to this article were reported.
We thank the staff at all physicians’ offices for their excellent efforts. We also thank our friends and colleagues for various forms of assistance that led to the successful completion of the first wave of this global study.
Published ahead of print at http://care.diabetesjournals.org on 25 November 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Gaede P, Lund-Andersen H, Parving HH, et al.: Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358:580–591, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study: Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853, 1998 [PubMed] [Google Scholar]
- 4.Grol R, Grimshaw J: From best evidence to best practice: effective implementation of change in patients’ care. Lancet 362:1225–1230, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Narayan KMV, Gregg EW, Engelgau MM, et al.: Translation research for chronic diseases: the case for diabetes. Diabetes Care 23:1794–1798, 2000 [DOI] [PubMed] [Google Scholar]
- 6.International Diabetes Federation: Diabetes Atlas. 3rd ed.. Brussels, Belgium, International Diabetes Federation, 2006
- 7.von Elm E, Altman DG, Egger M, et al.: Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 335:806–808, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Diabetes Association: Summary of revisions for the 2007 clinical practice recommendations. Diabetes Care 30(Suppl. 1):S3–S4, 2007 [Google Scholar]
- 9.Grant RW, Buse JB, Meigs JB: Quality of diabetes care in U.S. academic medical centers: low rates of medical regimen change. Diabetes Care 28:337–442, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eeg-Olofsson K, Cederholm J, Nilsson PM, et al.: Glycemic and risk factor control in type 1 diabetes: results from 13,612 patients in a national diabetes register. Diabetes Care 30:496–502, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Eliasson B, Eeg-Olofsson K, Cederholm J, et al.: Antihyperglycaemic treatment of type 2 diabetes: results from a national diabetes register. Diabete Metab 33:269–276, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Saaddine JB, Cadwell B, Gregg EW, et al.: Improvements in diabetes processes of care and intermediate outcomes: United States, 1988–2002. Ann Intern Med 144:465–474, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Knowler WC, Barrett-Connor E, Fowler SE, et al.: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarzer R: Social-cognitive factors in changing health-related behaviors. Curr Dir Psychol Sci 10:47–51, 2001 [Google Scholar]
- 15.Boyko EJ, Fujimoto WY, Leonetti DL, et al.: Visceral adiposity and risk of type 2 diabetes: a prospective study among Japanese Americans. Diabetes Care 23:465–471, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Farrell D, Henke NP, Mango PD: Universal principles for health care reform. The McKinsey Quarterly 87–97, 2007
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.