Abstract
OBJECTIVE—The purpose of this study was to examine the effect of type of insulin analog and age of insertion site on the pharmacodynamic characteristics of a standard insulin bolus in youth with type 1 diabetes receiving insulin pump therapy.
RESEARCH DESIGN AND METHODS—Seventeen insulin pump–treated adolescents with type 1 diabetes underwent two euglycemic clamp procedures after a 0.2 unit/kg bolus of either insulin aspart or lispro on day 1 and day 4 of insulin pump site insertion. The glucose infusion rate (GIR) required to maintain euglycemia was the primary pharmacodynamic measure.
RESULTS—There were no statistically significant differences in any of the pharmacodynamic parameters between aspart and lispro during day 1 and day 4. However, when the two groups were combined, time to discontinuation of exogenous glucose infusion, and time to half-maximal onset and offset of insulin action were observed significantly earlier during day 4 compared with day 1 (P = 0.03–0.0004), but the overall area under the GIR curve was similar on day 1 and day 4.
CONCLUSIONS—With both insulin aspart and lispro, there is an earlier peak and shorter duration of action with increasing duration of infusion site use, but overall insulin action is not affected.
As a result of the Diabetes Control and Complications Trial and its follow-up Epidemiology of Diabetes Interventions and Complications study, current recommendations mandate that youth with type 1 diabetes should aim to achieve metabolic control as close to normal as possible and as early in the course of the disease as possible. Although such strict treatment goals are particularly difficult to achieve in adolescents with type 1 diabetes (1–3), advances in insulin pump technology and the development of rapid-acting insulin analogs have provided clinicians who treat adolescents with type 1 diabetes with new management tools.
With continuous subcutaneous insulin infusion (CSII) pump therapy, bolus doses of rapid-acting insulin analogs provide better control of postprandial hyperglycemia without increasing the risk of hypoglycemia in comparison to what can be achieved with regular insulin (4–9). In addition, the newest insulin pumps account for residual insulin action in their bolus calculator software to prevent hypoglycemia related to multiple bolus doses given over a short interval. However, the duration and decay curves of insulin action programmed into these systems are based on small numbers of adult patients, because the data regarding the pharmacodynamic properties of rapid-acting insulin analogs in CSII-treated youth with type 1 diabetes were not available.
Using the euglycemic glucose clamp technique to describe the time-action profile of insulin in adolescents with type 1 diabetes, we recently demonstrated that the peak action of insulin aspart is not observed until 90 min after a standard bolus dose of 0.2 unit/kg, a full 40 min after the peak plasma insulin concentrations are achieved (10). It is important to note that all of these studies were performed in insulin pump–treated patients ∼12 h after insertion of a new infusion set catheter. Surprisingly, the effects of duration of infusion set use on the pharmacodynamic properties of rapid-acting insulin analogs in youth with type 1 diabetes have not been studied. Consequently, the recommendations that patients change their infusion sites every 2–4 days are based primarily on anecdotal reports of increasing glucose variability as the infusion site ages.
In this study, we used the glucose clamp technique to determine whether the pharmacodynamic profiles of aspart insulin differed when the same bolus dose of insulin was delivered through the infusion set after 12 h (day 1) and 84 h (day 4) of use. We also examined whether the pharmacodynamic profiles differed whether lispro or aspart was used in CSII.
RESEARCH DESIGN AND METHODS
Seventeen subjects with type 1 diabetes (8 boys and 9 girls) who attended the Yale Children's Type 1 Diabetes Clinic were studied. Eligibility criteria included a clinical diagnosis of type 1 diabetes for at least 1 year's duration, age ranging from 11 to 17 years, CSII therapy for at least 3 months, A1C <9.0%, BMI <95% for age and sex, and the ability to comprehend written and spoken English. Subjects were excluded for any other medical disease aside from type 1 diabetes or treated hypothyroidism, use of medications besides insulin and levothyroxine, pregnancy, breast-feeding, or not consistently using barrier methods or abstinence as contraception or any other condition that in the judgment of the investigators would interfere with the subject's or parent's ability to provide informed consent or the investigator's ability to perform the study. The Yale University Human Investigation Committee approved the study.
At the initial enrollment visit, the risks and benefits of the study were explained, informed consent from the parents and informed assent from the subjects were obtained, history and physical examinations were performed, and A1C was measured by DCA 2000 analyzer (Bayer, Tarrytown, NY). The subjects were then randomly assigned to either the aspart (n = 8) or lispro (n = 9) groups. There were no significant differences between the aspart and lispro groups with respect to age (14.3 ± 2.8 vs. 14.5 ± 1.8 years), BMI percentile (66 ± 24 vs. 72 ± 14), A1C (6.7 ± 0.5 vs. 7.1 ± 0.8%), or duration of diabetes (5.2 ± 4 vs. 5.5 ± 2.6 years), respectively.
Preparation for glucose clamp procedure
Each subject enrolled in the study underwent two euglycemic clamp procedures on day 1 (∼12 h after infusion set insertion) and day 4 (∼84 h after infusion set insertion). Subjects were admitted on the evening before both euglycemic clamp studies to the Yale Center for Clinical Investigation's Clinical Research Unit. Girls of child-bearing potential were screened with a urine pregnancy test. For the first study, a new subcutaneous insulin infusion set was placed at the time of admission in a gluteal location, and they were readmitted for the second clamp study after 3 days’ use of the same infusion set. Subjects continued to use their own insulin pump and type of infusion set and either aspart or lispro insulin depending on random assignment. An intravenous catheter was inserted in the forearm for overnight blood sampling. Plasma glucose levels were checked every hour overnight, and the insulin infusion via CSII was adjusted to achieve target fasting glucose levels between 80 and 120 mg/dl at the start of the clamp the next morning.
Glucose clamp procedure
The following morning a second intravenous catheter was inserted in the contralateral forearm, and baseline samples were obtained for plasma glucose. The subjects then received a 0.2 unit/kg bolus of aspart or lispro insulin through the insulin pump, and the pump was suspended. A variable rate of 20% dextrose solution was used to clamp the plasma glucose at the desired target of 80–90 mg/dl for 5 h, as described previously (10,11). Plasma glucose levels were measured at the bedside every 5 min using the YSI 2300 glucose analyzer (YSI Life Sciences, Yellow Springs, OH). The original intent was to analyze the pharmacokinetic data; however, because of sample handling problems in several studies, insulin levels were only available for four aspart and six lispro subjects, therefore preventing this analysis.
Changes in the rates of exogenous glucose infusion were adjusted, as needed, every 5 min throughout the study. The study was terminated at 5 h or 20 min after the infusion of exogenous glucose was discontinued. At the completion of the clamp, the subjects received a meal, and the insulin pump was restarted. Subjects were instructed to preserve the insertion catheter currently in use until readmission to the Clinical Research Unit 3 days later. Some subjects, in whom an immediate readmission could not be arranged, were restudied with an infusion set that they had inserted at home 84 h before study. The subjects then underwent a second clamp procedure identical to the first.
Calculations and statistical methods
The pharmacodynamic parameters evaluated were area under the curve of the glucose infusion rate (AUCGIR), maximum glucose infusion rate (GIRmax), time to maximum glucose infusion rate (TmaxGIR), time to discontinuation of exogenous glucose infusion, time to half-maximal increase of peak action (Ti50), and time to half-maximal decrease from peak action (Td50).
Fisher's exact test and unpaired t tests were used where applicable to compare the clinical data, initial blood glucose values, and mean clamp blood glucose values, which are reported as means ± SD. The t test is fairly robust to the assumption of normality. Nevertheless, we performed Wilcoxon rank-sum tests and Wilcoxon signed-rank tests. The results of these nonparametric tests did not alter the conclusions that were apparent from parametric testing. We therefore present means, P values, and 95% CIs based on the assumptions of the parametric model to compare pharmacodynamic outcome measures for both between- and within-subject comparisons where applicable.
RESULTS
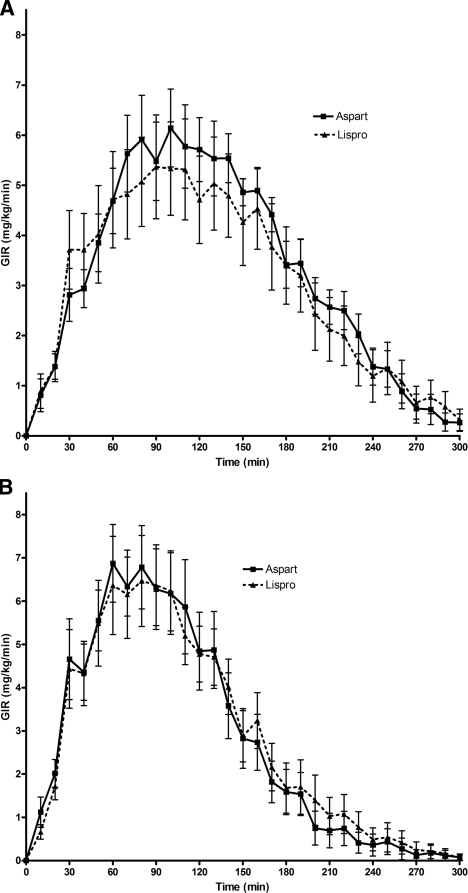
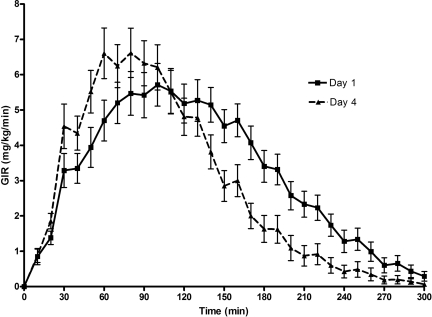
Except for higher baseline plasma glucose levels with lispro than with aspart on day 4, there were no other statistically significant differences in the baseline or mean plasma glucose values during the euglycemic clamp studies, both within and between groups (Table 1). As shown in Fig. 1, GIR curves for aspart and lispro were nearly identical in both day 1 and day 4 studies. As shown in Table 2, there were no statistically significant differences in AUCGIR, TmaxGIR, GIRmax, time to discontinuation of exogenous glucose infusion, Ti50, or Td50 between subjects treated with aspart and lispro when they were compared with each other during day 1 and day 4. Consequently, aspart and lispro results were combined to examine the effect of duration of infusion set use on the time-action profiles of these rapid-acting insulins. As shown in Fig. 2, prolonged use of an insulin infusion site had a substantial effect on the pharmacodynamic profile of the same dose of rapid-acting insulin. The TmaxGIR, time to discontinuation of exogenous glucose infusion, Ti50, and Td50 were observed significantly earlier during day 4 compared with day 1 of infusion site use (Table 3), whereas there were no statistically significant differences in total AUCGIR or GIRmax at day 1 and day 4.
Table 1.
Mean plasma glucose levels at baseline and during the clamp procedure
| Day 1 |
Day 4 |
|||||
|---|---|---|---|---|---|---|
| Aspart | Lispro | P | Aspart | Lispro | P | |
| Baseline plasma glucose (mg/dl) | 128 ± 41 | 145 ± 44 | 0.42 | 108 ± 21 | 142 ± 41 | 0.05 |
| Plasma glucose during clamp (mg/dl) | 99 ± 12 | 102 ± 11 | 0.65 | 98 ± 7 | 103 ± 11 | 0.27 |
Data are means ± SD.
Figure 1.
Pharmacodynamic profiles. Insulin action, as expressed as GIR, required to maintain euglycemia after a standard bolus of 0.2 unit/kg insulin aspart or lispro. Data are presented as means ± SEM. A: Day 1 of catheter site insertion. B: Day 4 of catheter site insertion.
Table 2.
Comparison of pharmacodynamic parameters for subjects using aspart and lispro
| Pharmacodynamic parameters | Day 1 |
P | Day 4 |
P | ||||
|---|---|---|---|---|---|---|---|---|
| Aspart | Lispro | Difference (95% CI) | Aspart | Lispro | Difference (95% CI) | |||
| AUCGIR (mg/kg) | 982 ± 83 | 919 ± 175 | 63 (−341 to 467) | 0.76 | 839 ± 124 | 854 ± 116 | 15 (−354 to 325) | 0.93 |
| GIRmax (mg · kg−1 · min−1) | 7.3 ± 0.6 | 6.4 ± 1.1 | 0.96 (−1.57 to 3.49) | 0.46 | 7.7 ± 0.9 | 7.3 ± 1.0 | 0.39 (−2.38 to 3.16) | 0.78 |
| TmaxGIR (min) | 101 ± 9 | 92 ± 11 | 9 (−19 to 37) | 0.53 | 79 ± 9 | 79 ± 7 | 0.14 (−23 to 22) | 0.99 |
| Time to discontinuation of exogenous glucose (min) | 263 ± 10 | 246 ± 15 | 16 (−21 to 54) | 0.40 | 208 ± 20 | 214 ± 17 | 6 (−58 to 45) | 0.81 |
| Ti50 (min) | 58 ± 6 | 46 ± 5 | 12 (−3 to 27) | 0.14 | 38 ± 4 | 39 ± 4 | 1 (−12 to 9) | 0.79 |
| Td50 (min) | 199 ± 13 | 191 ± 12 | 8 (−27 to 42) | 0.66 | 136 ± 10 | 168 ± 13 | 32 (−64 to 0.58) | 0.07 |
Data are means ± SE unless indicated otherwise.
Figure 2.
Pharmacodynamic profiles for all subjects on day 1 versus day 4 of catheter site insertion. Insulin action, as expressed as GIR, required to maintain euglycemia after a standard bolus of 0.2 unit/kg insulin aspart or lispro. Data are presented as means ± SEM.
Table 3.
Comparison of pharmacodynamic parameters between day 1 and day 4
| Pharmacodynamic parameters | All Subjects |
P | ||
|---|---|---|---|---|
| Day 1 | Day 4 | Difference (95%CI) | ||
| AUCGIR (mg/kg) | 948 ± 98 | 847 ± 82 | 101 (−151 to 353) | 0.42 |
| GIRmax (mg/kg/min) | 6.8 ± 0.6 | 7.5 ± 0.7 | −0.69 (−2.49 to 1.12) | 0.39 |
| TmaxGIR (min) | 97 ± 7 | 79 ± 5 | 18 (0.38 to 35) | 0.03 |
| Time to discontinuation of exogenous glucose (min) | 254 ± 9 | 211 ± 12 | 43 (12 to 74) | 0.004 |
| Ti50 (min) | 51 ± 4 | 38 ± 3 | 13 (4 to 22) | 0.004 |
| Td50 (min) | 195 ± 8 | 153 ± 9 | 42 (18 to 66) | 0.0004 |
Data are means ± SE.
CONCLUSIONS
This study was undertaken to examine whether there was any effect of increasing duration of infusion site use on the absorption and time-action profile of a standard 0.2 unit/kg body weight dose of rapid-acting insulin analogs in CSII-treated adolescents. We also examined whether and to what extent the effect of infusion site age differed between aspart and lispro insulin. The first clamp study was performed 12 h after the placement of a new infusion set (day 1) to allow the infusion site time to recover from the trauma of catheter insertion. The second study was performed after 84 h, because in clinical practice patients routinely use the same infusion site for 3–4 days.
The most important findings of this study are that more prolonged use of an infusion site resulted in earlier peak action and shorter duration of action of a standard bolus dose, whereas the overall area under the curve of insulin action did not differ. Moreover, a similar change in pharmacodynamic characteristics is seen with both aspart and lispro insulin. Our data indicating that there is earlier peak action of insulin with increasing duration of infusion site use could be the result of increased blood flow around the infusion site due to changes in the vascular microenvironment. Some loss of insulin due to precipitation in the set or partial occlusion of the infusion set by insulin cannot be excluded as contributing factors. However, precipitation and occlusion alone would not explain the earlier peak action or the similar total AUCGIR values on day 4 versus day 1.
It is important to note that the earlier onset and shorter duration of bolus doses on day 4 do not, in themselves, have negative clinical implications. Indeed, it could be argued that the responses on day 4 are more favorable than those on day 1 with respect to control of meal-related glucose excursions. Earlier onset and shorter duration are reasons that rapid-acting insulin analogs are felt to be superior to regular insulin in this regard. The negative implication of this study for clinical practice is that the duration of infusion site use is yet another factor, like puberty (10), that may contribute to day-to-day variability and plasma glucose lability in adolescents with type 1 diabetes. Conversely, more frequent site changes could lead to more consistent pharmacodynamic profiles. These findings also raise the question whether duration of infusion site use affects the consistency and reproducibility of pharmacodynamic responses to the same bolus dose in the same subject and whether similar changes in pharmacodynamic properties are seen in other body regions (e.g., abdominal sites).
Acknowledgments
This work was supported by grants from the Juvenile Diabetes Research Foundation, Novo Nordisk Pharmaceuticals, and the Stephen I. Morse Pediatric Diabetes Research Fund and by Clinical and Translational Science Award U54 RR 023423 from the National Institutes of Health.
W.V.T. has received honoraria as a member of the speaker's bureau of Lilly and the speaker's bureau and advisory board of Novo Nordisk. S.A.W. has received honoraria as a member of the speaker's bureau of Lilly and grant support from Novo Nordisk. G.M.S. is a former employee of and currently holds stock in Medtronic MiniMed. G.R.V. is employed by Medtronic Minimed. No other potential conflicts of interest relevant to this article were reported.
We thank the nurses and staff of the Yale Center for Clinical Investigation Clinical Research Unit.
Published ahead of print at http://care.diabetesjournals.org on 18 November 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.DCCT Research Group: The effects of intensive diabetes treatment on the development and progression of long-term complications in insulin-dependent diabetes mellitus: the Diabetes Control and Complications Trial. N Engl J Med 329:977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 2.The DCCT Research Group: The effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: the Diabetes Control and Complications Trial. J Pediatr 125:177–188, 1994 [DOI] [PubMed] [Google Scholar]
- 3.DCCT/EDIC Research Group: Prolonged beneficial effects of intensive therapy of diabetes during adolescence: microvascular outcomes four years after conclusion of the Diabetes Control and Complications Trial. Pediatrics 139:804–812, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Zinman B, Tildesley H, Chiasson TJ, Tsue E, Strack T: Insulin lispro in CSII: results of a double-blind crossover study. Diabetes 46:440–443, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Howey DC, Bowsher RR, Brunelle RL, Woodworth JR: [Lys(B28), Pro(B29)] human insulin: a rapidly absorbed analogue of human insulin. Diabetes 43:396–402, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Mudaliar S, Lindberg FA, Joyce M, Beerdsen P, Strange P, Lin A, Henry RR: Insulin aspart (B28 Asp-insulin): a fast acting analog of human insulin: absorption kinetics and action profile compared with regular human insulin in healthy nondiabetic subjects. Diabetes Care 22:1501–1506, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Becker RHA, Frick A, Wessels D, Scholtz H: Evaluation of the pharmacodynamic and pharmacokinetic profiles of insulin glulisine—a novel, rapid-acting, human insulin analogue (Abstract). Diabetologia 46:775, 2003 [Google Scholar]
- 8.Becker RHA, Frick AD, Kapitza C, Heise T, Rave K: Pharmacodynamics (PD) and pharmacokinetics (PK) of insulin glulisine (GLU) versus insulin lispro (IL) and regular human insulin (RHI) in patients with type 2 diabetes. Diabetes 53(Suppl. 2):503, 2004 [Google Scholar]
- 9.Danne T, Becker RHA, Heise T, Bittner C, Frick AD, Rave K: Pharmacokinetics, prandial glucose control, and safety of insulin glulisine in children and adolescents with type 1 diabetes. Diabetes Care 28:2100–2105, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Swan KL, Weinzimer SA, Steil G, Voskanyan G, Steffen A, Martin M, Tamborlane WV: Effect of puberty on the pharmacodynamic and pharmacokinetic properties of insulin pump therapy in youth with TIDM. Diabetes Care 31:44–46, 2008 [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223, 1979 [DOI] [PubMed] [Google Scholar]