Abstract
OBJECTIVE—Markers of hemostasis and inflammation such as plasminogen activator inhibitor-1 (PAI-1) and fibrinogen have been associated with risk of type 2 diabetes. We aimed to identify food intake patterns influencing this pathway and evaluate their association with incident diabetes.
RESEARCH DESIGN AND METHODS—The Insulin Resistance Atherosclerosis Study cohort included 880 middle-aged adults initially free of diabetes. At the 5-year follow-up, 144 individuals had developed diabetes. Usual dietary intake was ascertained with a 114-item food frequency questionnaire. Using reduced rank regression, we identified a food pattern maximizing the explained variation in PAI-1 and fibrinogen. Subsequently, the food pattern–diabetes association was evaluated using logistic regression.
RESULTS—High intake of the food groups red meat, low-fiber bread and cereal, dried beans, fried potatoes, tomato vegetables, eggs, cheese, and cottage cheese and low intake of wine characterized the pattern, which was positively associated with both biomarkers. With increasing pattern score, the odds of diabetes increased significantly (Ptrend < 0.01). After multivariate adjustment, the odds ratio comparing extreme quartiles was 4.3 (95% CI 1.7–10.8). Adjustment for insulin sensitivity and secretion and other metabolic factors had little impact (4.9, 1.8–13.7).
CONCLUSIONS—Our findings provide support for potential behavioral prevention strategies, as we identified a food intake pattern that was strongly related to PAI-1 and fibrinogen and independently predicted type 2 diabetes.
Markers of hemostasis and inflammation are considered risk factors in the pathogenesis of type 2 diabetes (1,2). Data from the Insulin Resistance Atherosclerosis Study (IRAS) indicate that the effect of plasminogen activator inhibitor-1 (PAI-1) on diabetes risk is independent not only of adiposity but also of insulin sensitivity (1). In the context of diabetes prevention, these findings challenge us to explore the determinants of the prothrombotic and inflammatory state, particularly modifiable risk factors such as dietary intake.
The importance of food and nutrient intake in the development of diabetes and its precursors is well recognized (3). The Diabetes Prevention Program trial provides evidence for the effectiveness of dietary and lifestyle modification approaches in the prevention of type 2 diabetes among high-risk individuals (4). The focus of the lifestyle intervention in the Diabetes Prevention Program was weight loss via modification of energy and fat intake and physical activity. To date, very few intervention trials have evaluated the impact of larger dietary patterns on health outcomes (5–7).
Two general approaches have dominated the field of observational research on dietary patterns: the a priori approach uses prior knowledge such as dietary recommendations to create quality indexes (8); and the exploratory approaches such as principal components, factor analysis, or cluster analysis are entirely empirical, data-driven methods. Recently, reduced rank regression (RRR) has been introduced as a method that combines the strengths of both approaches (9) because it identifies patterns among food groups by concurrently using data on a set of response variables (ideally biomarkers) selected because of known associations with the disease of interest. Two recent studies using RRR have revealed strong associations between food intake patterns and risk of diabetes (10,11). We aimed to identify RRR-determined food intake patterns that affect diabetes-related inflammatory biomarkers and to evaluate their association with incident type 2 diabetes, taking into account measures of insulin sensitivity and secretion in the IRAS population.
RESEARCH DESIGN AND METHODS
IRAS is a multicenter, observational study evaluating the relations between insulin resistance, cardiovascular risk factors, and disease in a multiethnic cohort (12). The study was designed to obtain nearly equal representation of participants across age, sex, three race/ethnic groups, and glucose tolerance status sampled from four centers.
A total of 1,624 participants aged 40–69 years were recruited for the baseline examination (1992–1994), which included a baseline history, physical examination, and laboratory measures. In 1997–1999, the cohort was invited again (average follow-up 5.2 years), and 81% returned. All participants provided written informed consent as approved by their center's institutional review board.
Each examination required a two-visit protocol. Participants fasted for 12 h, abstained from heavy exercise and alcohol for 24 h, and abstained from smoking in the morning. At the first visit, after an initial blood sample, a 2-h, 75-g oral glucose tolerance test (OGTT) (Orangedex; Custom Laboratories, Baltimore, MD) was performed. The second visit included a 12-sample, insulin-enhanced, frequently sampled intravenous glucose tolerance test (13,14). Insulin sensitivity (SI) and acute insulin response (AIR) were assessed using minimal model analysis (15,16). AIR was calculated from insulin levels through the 8-minute blood samples before insulin infusion.
Diabetes at both baseline and follow-up was defined as 2-h glucose ≥200 mg/dl according to the 1985 World Health Organization criteria (17). Participants who reported taking hypoglycemic medication were assumed to have type 2 diabetes regardless of OGTT results. Individuals with diabetes at baseline were excluded.
PAI-1 was measured in citrated plasma with a two-site immunoassay sensitive to free active and latent PAI-1 but not to PAI-1 complexed with tissue plasminogen activator (1). The coefficient of variation was 14%. Fibrinogen was measured in citrated plasma with a modified clot-rate assay, using a Diagnostica STAGO ST4 instrument (Diagnostica, Parsippany, NJ) with a coefficient of variation of 3.0%. Plasma lipid concentrations were determined at the central laboratory following Lipid Research Clinics methodology. Height, waist circumference, and weight were measured in duplicate and recorded to the nearest 0.5 cm and 0.1 kg, respectively. BMI was calculated as weight in kilograms divided by the square of height in meters.
Usual dietary intake was assessed by centrally trained interviewers using a 1-year semiquantitative 114-item food frequency questionnaire (18) ascertaining both frequency and serving size. Servings per day were converted to the medium size (19). We created 33 food groups on the basis of similarities in food and nutrient composition (19). Alcoholic beverage consumption was queried separately. Daily nutrient and energy intake was estimated from the food frequency questionnaire and the alcohol questionnaire using an expanded nutrient database (HHHQ-DIETSYS analysis software, version 3.0; National Cancer Institute, Bethesda, MD). A structured interview was used to collect 1-year recall of physical activity from which total energy expenditure was estimated. Family history of diabetes, smoking, and race/ethnicity were self-reported.
Statistical analysis
Of the 1,071 adults with normal glucose tolerance or impaired glucose tolerance (IGT) at baseline, 906 returned for follow-up. From these, we excluded 26 participants missing baseline PAI-1 and fibrinogen values, leaving a total of 880 observations. Statistical analyses were conducted in two steps. Using RRR, we identified a food pattern maximizing the explained variation in PAI-1 and fibrinogen. Subsequently, the food pattern–diabetes association was evaluated using multivariate logistic regression.
RRR is a factor analysis technique from which one or more factors may be determined. We used the SAS PLS procedure with the method = RRR option (9). Because of the specificity of the RRR terminology, we first distinguish two types of observed variables: the food group data used as predictor variables and the PAI-1 and fibrinogen biomarker data used as response variables. Similar to principal components analysis (PCA), RRR uses observed data to determine a set of unobserved factors (scores). Although PCA produces one set of scores, RRR determines two distinct sets, X scores and Y scores. Here, the X scores were based on food groups (hence called food pattern scores), and the Y scores were based on PAI-1 and fibrinogen (hence called response scores).
In both RRR and PCA, the food pattern scores (X scores) are linear functions of predictor variables (here, 33 food groups described in ref. 19); however, their determination differs between the methods. In technical terms, in PCA, the coefficient vectors of the extracted linear function are eigenvectors of the covariance matrix of predictors. RRR, however, starts from the covariance matrix of responses. A response score (Y score) is created with weights from eigenvectors of the covariance matrix of responses predicted by ordinary least squares regression. The Y scores are then projected onto the space of predictors (food groups), forming an X score (food pattern score). In simpler terms, RRR extracts food pattern scores while concurrently maximizing the explained variation in a set of response variables.
Initially, we identified two food pattern scores because the number of extracted X scores is always equal to the number of response variables. Only the first score was retained for subsequent analyses because the second score was not associated with diabetes (P = 0.51). To reduce the dependency of the score from the data, we simplified the score by including only food groups with high factor loadings ≥ 0.2 and then summing the standardized food group intake while retaining the direction of the factor loading (9). The simplified pattern score was subsequently categorized into quartiles.
Odds ratios (ORs) and 95% CIs were obtained from multivariate logistic regression models adjusting for established confounders. The test for trend across quartiles used the P value from the type III analysis of effects based on the Wald χ2 test. Based on previous work, sex, race/ethnicity, IGT status, and baseline obesity status were considered a priori as potential effect modifiers of the pattern score–diabetes association and evaluated by stratification and by including pattern score by covariate interaction terms. Only the interaction with obesity was of borderline statistical significance (P = 0.08); hence, we also show our results in obese versus nonobese individuals. All analyses were performed in SAS (version 8.2; SAS Institute, Cary, NC).
RESULTS
Table 1 illustrates characteristics of the food pattern score and its relation with the nine food groups constituting the simplified food pattern score. The factor loadings were estimated within the RRR procedure and represent the correlation of the food group with the original (nonsimplified) score. Initially, all 33 food groups were ranked by decreasing absolute factor loadings. Only those with a loading ≥0.2 were retained for the simplified score and are shown here. Red meat and low-fiber bread and cereal explained 19.3 and 18.1%, respectively, of the variation in the pattern score. Taken together, all nine food groups explained 72.8% of food pattern score variation.
Table 1.
Food groups strongly associated with food pattern score and their characteristics (n = 880)
| Food groups | Score characteristics |
Quartiles of simplified food pattern score (servings/day) |
Ptrend | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor loading* | Standard score parameter† | Pearson's correlation coefficient | Explained variation in score‡ | 1 | 2 | 3 | 4 | ||
| Red meats§ | 0.42 | 0.30 | 0.64 | 19.29 | 0.3 ± 0.3 | 0.7 ± 0.4 | 1.1 ± 0.5 | 1.8 ± 0.8 | <0.0001 |
| Low-fiber bread and cereal‖ | 0.41 | 0.29 | 0.62 | 18.12 | 0.5 ± 0.4 | 0.9 ± 0.6 | 1.4 ± 0.8 | 2.3 ± 1.2 | <0.0001 |
| Dried beans¶ | 0.29 | 0.05 | 0.43 | 2.35 | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.3 ± 0.2 | 0.6 ± 0.5 | <0.0001 |
| Fried potatoes# | 0.25 | 0.04 | 0.38 | 1.52 | 0.0 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.3 ± 0.3 | <0.0001 |
| Tomato vegetables** | 0.23 | 0.21 | 0.35 | 7.43 | 0.3 ± 0.3 | 0.6 ± 0.4 | 0.8 ± 0.5 | 1.1 ± 0.6 | <0.0001 |
| Eggs†† | 0.23 | 0.03 | 0.35 | 1.16 | 0.1 ± 0.1 | 0.1 ± 0.2 | 0.2 ± 0.2 | 0.4 ± 0.4 | <0.0001 |
| Cheese‡‡ | 0.23 | 0.11 | 0.34 | 3.65 | 0.1 ± 0.2 | 0.3 ± 0.3 | 0.5 ± 0.4 | 0.7 ± 0.5 | <0.0001 |
| Cottage cheese§§ | 0.22 | 0.30 | 0.34 | 10.11 | 0.0 ± 0.1 | 0.0 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.2 | <0.0001 |
| Wine‖‖ | −0.21 | −0.28 | −0.33 | 9.16 | 0.4 ± 0.7 | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.1 ± 0.2 | <0.0001 |
Data are means ± SD unless indicated otherwise.
Factor loading obtained directly from reduced rank regression procedure.
Standardized parameters obtained from multiple linear regression of food groups on original food pattern score.
Explained variation is calculated by multiplication of the standardized parameters with the empirically calculated Pearson's correlation coefficient × 100.
Red meat: hamburgers, cheeseburgers, meat loaf, picadillo, carne guisada (asada); beef (steaks, roasts, etc., including on sandwiches); beef stew or pot pie with carrots or other vegetables; pork, including chops, roasts, or ribs; ham, ham hocks (including ham on sandwiches); game, including venison, rabbit; liver, including chicken livers; burritos, including breakfast burritos, soft taco with flour tortillas; green chili con carne; Asian food; liverwurst; hot dogs (include pork, beef, turkey); bologna, salami, Spam, other lunch meats (excluding ham); bacon; sausage, chorizo; veal, lamb; Italian sausage; pate.
Low-fiber bread and cereal: white bread, biscuits, flour and corn tortilla, corn bread, fortified cereal, cold cereal, sweetened cereal, cooked cereal, pizza, burritos, enchiladas, tacos.
Dried beans: refried beans (as side dish, not including those in burritos, etc.); other beans such as pintos, black beans, garbanzo beans, baked beans, or lentils; burritos, including breakfast burritos, soft taco with flour tortillas; chili with beans.
Fried potatoes: french fries, fried potatoes.
Tomato vegetables: tomatoes, tomato juice; salsa picante, taco sauce; spaghetti, lasagna, other pasta or mixed dishes with tomatoes or tomato sauce; pizza; enchiladas, tamales, tacos, tostades, chalupas, other Mexican dishes with corn tortillas; vegetable and tomato soup.
Eggs: eggs, omelets, frittata.
Cheese: pizza; mixed dish with cheese (including macaroni and cheese, chili rellenos, cheese quesadillas quiche); enchiladas, tamales, tacos, tostades, chalupas, other Mexican dishes with corn tortillas, including nachos with chili and cheese (0.5); cream soups (0.5); cheese (cheddar, American, cream cheese, parmesan, Velveeta, other cheeses or cheese spreads; including on sandwiches or as snacks).
Cottage cheese: cottage cheese, ricotta cheese.
Wine includes both red and white wine.
With increasing quartile of the simplified pattern score, the quality of the food intake pattern tended to worsen, i.e., a higher score reflected higher intakes of red meat, low-fiber bread and cereal, dried beans, fried potatoes, tomato vegetables, eggs, cheese, and cottage cheese and lower intake of wine. Compared with those in the lowest pattern quartile, individuals in the highest quartile consumed on average 1.5 servings more of the red meat food group and 1.8 servings more of the low-fiber bread and cereal food group per day.
Both PAI-1 and fibrinogen exhibited a positive association with the food pattern score (response score coefficients PAI-1 = 0.964; fibrinogen = 0.265). The derived pattern score explained 8% of the variation in the inflammatory response variables and was largely driven by the explained variation in PAI-1 (15%) and marginally by fibrinogen (1%).
At baseline, marked associations between food pattern score and age and race/ethnicity were observed (Table 2). By design of the RRR method, concentrations of PAI-1 and fibrinogen increased systematically with increasing food pattern score. Furthermore, BMI, waist circumference, fasting insulin, AIR, and triglycerides increased with increasing score quartile, whereas SI and HDL cholesterol were inversely related. A total of 144 incident cases of type 2 diabetes developed over 5 years, yielding a crude incidence of type 2 diabetes of 163 per 1,000.
Table 2.
Study population characteristics by quartiles of the simplified food pattern score at baseline (n = 880)
| Quartiles of simplified food pattern score |
P (χ2 test for trend) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Demographics and design characteristics | |||||
| Age (years) | 56.4 ± 8.4 | 55.4 ± 8.3 | 53.7 ± 8.2 | 53.2 ± 8.6 | 0.0001 |
| Male sex (%) | 42.3 | 38.6 | 42.3 | 49.1 | 0.1625 |
| Female sex (%) | 57.7 | 61.4 | 57.7 | 50.9 | 0.1625 |
| Non-Hispanic white (%) | 41.4 | 47.7 | 38.2 | 33.6 | 0.0221 |
| Non-Hispanic black (%) | 51.3 | 31.4 | 16.4 | 4.6 | <0.0001 |
| Hispanic (%) | 7.3 | 20.9 | 45.4 | 61.8 | <0.0001 |
| Normal glucose tolerance (%) | 66.8 | 62.7 | 68.6 | 67.7 | 0.5695 |
| IGT (%) | 33.2 | 37.3 | 31.4 | 32.3 | 0.5695 |
| Biomarkers | |||||
| PAI-1 (ng/ml) | 15 ± 15 | 22 ± 28 | 24 ± 18 | 28 ± 20 | <0.0001 |
| Fibrinogen (mg/dl) | 269 ± 52 | 277 ± 60 | 276 ± 56 | 280 ± 56 | 0.2127 |
| BMI (kg/m2)* | 27.2 ± 4.3 | 28.6 ± 6.0 | 28.7 ± 5.8 | 29.3 ± 6.3 | 0.0025 |
| Waist circumference (cm) | 87.6 ± 11.4 | 90.3 ± 13.2 | 90.5 ± 12.3 | 92.8 ± 12.8 | 0.0004 |
| Fasting insulin (μU/ml)† | 14 ± 14 | 15 ± 13 | 16 ± 12 | 18 ± 20 | <0.0001 |
| SI (min−1)(μU−1)(ml−1)(10−4)† | 2.5 ± 2.2 | 2.1 ± 1.8 | 2.2 ± 1.9 | 2.0 ± 1.8 | 0.0261 |
| AIR (μU/ml/min)† | 57.5 ± 47.2 | 64.7 ± 52.6 | 70.1 ± 61.1 | 72.8 ± 64.8 | 0.0041 |
| Triglycerides (mmol/l)† | 1.3 ± 0.8 | 1.5 ± 0.9 | 1.6 ± 1.1 | 1.6 ± 1.1 | 0.0006 |
| HDL cholesterol (mmol/l)† | 1.3 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.1 ± 0.3 | <0.0001 |
Data are % or means ± SD.
Sample size is n = 878.
Sample size is n = 831.
A positive association between the simplified dietary pattern score and risk of diabetes was observed (Table 3), independent of age, sex, race/ethnicity/clinic, family history of diabetes, glucose tolerance status at baseline, energy expenditure, smoking, and energy intake. The odds of incident diabetes increased significantly with increasing score from a threefold risk associated with the second quartile to a more than fourfold risk for the fourth quartile of the dietary pattern score. Adjustment for baseline levels of SI and AIR, both of which were significant independent predictors of diabetes, further strengthened the association of the food pattern score with incident diabetes (model 2). Adjustment for BMI (model 3) had little impact. Further adjustment for baseline hypertension, triglycerides, or HDL cholesterol did not alter results nor did adjustment for a change in BMI, total energy expenditure, vigorous physical activity, antihypertensive drugs, lipid-lowering drugs, or antidepressant medications (data not shown). We found no evidence for effect modification by sex, race/ethnicity, or baseline IGT status.
Table 3.
Risk of type 2 diabetes according to quartile of the simplified food pattern score at baseline
| Quartiles of simplified food pattern score |
P (test for trend) | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Cases/population at risk | 23/220 | 46/220 | 34/220 | 41/220 | |
| Crude incidence per 1,000 | 104.5 | 209.1 | 154.5 | 186.4 | |
| Model 1† | 1.00 | 3.02 (1.60–5.68) | 2.76 (1.29–5.91) | 4.27 (1.69–10.82) | 0.0042 |
| Model 2‡ | 1.00 | 3.03 (1.49–6.18) | 2.78 (1.17–6.57) | 4.88 (1.75–13.65) | 0.0099 |
| Model 3§ | 1.00 | 2.79 (1.36–5.73) | 2.47 (1.03–5.92) | 4.51 (1.60–12.69) | 0.0173 |
Data are ORs (95% CI) unless indicated otherwise.
*P value from the type III analysis.
Adjusted for age, sex, race/clinic, family history of diabetes, glucose tolerance status at baseline, energy expenditure, smoking, and energy intake (n = 862).
Same as model 1 plus SI and AIR (n = 822).
Same as model 2 plus BMI (n = 822).
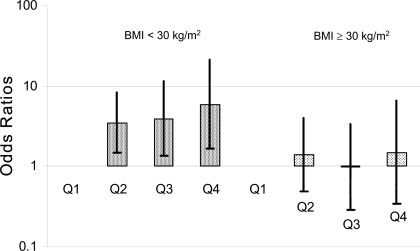
Figure 1 illustrates the positive, graded association between food pattern score quartile and risk of type 2 diabetes, stratified by obesity status. Although the association was strongly present in nonobese individuals (628 at risk, 72 cases; Ptrend = 0.02), it did not seem present in obese individuals (250 at risk, 72 cases; Ptrend = 0.77).
Figure 1.
Risk of type 2 diabetes by quartile of simplified food pattern score and obesity status at baseline. Adjusted for age, sex, race/clinic, family history of diabetes, glucose tolerance status at baseline, energy expenditure, smoking, and energy intake (n = 878). All ORs for BMI <30 kg/m2 are statistically significant.
We also evaluated to what extent the choice of response variables would affect our findings. The food pattern scores resulting from the addition of waist circumference, SI, and HDL cholesterol to our primary response variables were not associated with diabetes in a consistent manner, substantiating the critical role of PAI-1 and fibrinogen in this pathway (data not shown).
CONCLUSIONS
We identified a food pattern that was strongly predictive of type 2 diabetes in a cohort of middle-aged adults using PAI-1 and fibrinogen as response variables and the RRR method. Previous work in the IRAS has shown that elevated PAI-1 levels and progression of PAI-1 levels and, to a lesser extent, fibrinogen predict type 2 diabetes independent of other known factors including insulin sensitivity (1,20). The present study expands these findings, since we have identified a dietary pattern that is simultaneously associated with PAI-1 and fibrinogen and the development of diabetes.
Dietary predictors of type 2 diabetes have been studied at a number of levels including nutrients, foods, and food groups (21). The magnitude of their effect has generally been small to moderate relative risks ranging from 0.4 to 0.9 and 1.1 to 1.6 when extreme intake levels were compared. More recently, dietary pattern approaches such as the more exploratory factor and cluster analyses (19,22) and a priori methods such as dietary indexes (23,24) have attempted to capture the effect of total dietary behavior. In U.S. populations, a “prudent” and a “western” dietary pattern have been identified (25), but associations with diabetes have again been rather modest (26,27).
In contrast, the present study and two others using pattern scores obtained from RRR and markers of hemostasis and inflammation as response variables have reported very strong associations with type 2 diabetes (10,11). We observed a threefold to more than fourfold increased odds of diabetes associated with a food intake pattern high in red meats, low-fiber bread and cereal, dried beans, fried potatoes, tomato vegetables, eggs, cheese, and cottage cheese and low in wine. Schulze et al. (10) found a two- to three-fold increase in the risk of diabetes associated with a similar dietary pattern high in processed meats, refined grains, and sugar-sweetened and diet soft drinks and low in wine, coffee, cruciferous vegetables, and yellow vegetables. Heidemann et al. (11) reported a two- to fivefold increase in risk associated with a pattern high in red meat, processed meat, refined- grain bread, beer, poultry, legumes, and high-calorie soft drinks and low in fresh fruit.
The RRR method combines the strengths of a priori and exploratory approaches (9) by using prior knowledge in the selection of response variables, which is a clear advantage. RRR shares a number of limitations with the exploratory approaches, including the facts that the identified food intake patterns are specific to the population under study, quantitative differences in food intake between populations are lost due to the standardization inherent in the method, and the pattern score does not have an immediate clinical interpretation. These issues can be partially addressed by validation efforts in differing populations or split-sample approaches, derivation of simplified patterns, and thorough descriptive analyses.
RRR can be particularly powerful if the response variables are biomarkers and are known to be strongly associated with the disease of interest, such as markers of hemostasis or inflammation or insulin resistance. A recent study used the homeostasis model assessment of insulin resistance as a response variable (28). It too showed a strong and graded association with diabetes risk. In our study, none of the variations of the PAI-1- and fibrinogen-based response sets, including the addition of SI, showed any improvement over the original. It is possible that the RRR method allows the identification of very specific pathways by which dietary intake may affect diabetes risk. To the extent that different biomarker response variables reflect different stages of pathogenesis, it is likely that distinct food intake patterns can be identified.
A number of limitations are worth mentioning. Our study included fewer cases and a shorter follow-up than other studies (10,28). This limitation became particularly apparent when we evaluated the effect-modifying role of obesity. Although the formal test of interaction was of borderline significance (P = 0.08), stratification on obesity revealed that the positive, graded association of the pattern score seemed to be limited to the nonobese population. It is conceivable that food intake may have an impact on diabetes development via food quality and composition (i.e., nutrients and other constituents), separately from energy intake and that these effects differ during different phases of diabetes pathogenesis. It is also possible that nonbiological reasons explain the differences, such as the small sample of obese patients, recent dietary changes, and differences in the accuracy of recall. Finally, even though the dietary intake reflects the usual intake over the past year, its assessment occurred contemporaneously with the collection of baseline levels of PAI-1 and fibrinogen. Therefore, we are unable to conclusively disentangle the temporal relationship between food intake and biomarkers. However, when we adjusted our final model additionally for baseline levels of PAI-1 and fibrinogen, the results were not attenuated.
A unique strength of our study is that we were able to account for two strong risk factors for diabetes, SI and AIR. Furthermore, unlike previous research (10,11), glucose tolerance/diabetes status was verified by OGTT at baseline and follow-up visit.
In summary, we identified a food pattern that simultaneously affected PAI-1 and fibrinogen and the development of type 2 diabetes. The pattern was characterized by higher intakes of the food groups red meat, low-fiber bread and cereal, dried beans, fried potatoes, tomato vegetables, eggs, cheese, and cottage cheese and a low intake of wine. Our findings provide support for further exploration of food group–based dietary behavior strategies for the prevention of type 2 diabetes.
Acknowledgments
This study was supported by an American Heart Association grant-in-aid to A.D.L. The IRAS study was supported by National Institutes of Health National Heart, Lung, and Blood Institute Grants UO1 HL/17887, UO1 HL/17889, UO1 HL/17890, UO1 HL/17892, UO1 HL/17902, and DK29867.
No potential conflicts of interest relevant to this article were reported.
We thank Theodosha Gilliard and Michele Nichols for assistance in data management and statistical analysis.
Published ahead of print at http://care.diabetesjournals.org on 25 November 2008.
We dedicate this manuscript to our recently deceased friend and colleague Dr. Kurt Hoffmann from the German Institute of Human Nutrition, Potsdam-Rehbrücke, Germany, who introduced RRR to nutritional epidemiology and spent countless hours educating us on this method with patience and humor.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Festa A, D'Agostino R Jr, Tracy RP, Haffner SM: Elevated levels of acute phase proteins and plasminogen activator inhibitor-1 (PAI-1) predict the development of type 2 diabetes mellitus: the Insulin Resistance Atherosclerosis Study (IRAS). Diabetes 51:1131–1137, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Meigs JB, O'Donnell CJ, Tofler GH, Benjamin EJ, Fox CS, Lipinska I, Nathan DM, Sullivan LM, D'Agostino RB, Wilson PW: Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes 55:530–537, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, van Dam RM, Liu S: Diet and risk of type II diabetes: the role of types of fat and carbohydrate. Diabetologia 44:805–817, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV: Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and Diabetes Study. Diabetes Care 20:537–544, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N: A clinical trial of the effects of dietary patterns on blood pressure: DASH Collaborative Research Group. N Engl J Med 336:1117–1124, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M: Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Hu FB: Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol 13:3–9, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann K, Schulze MB, Schienkiewitz A, Nothlings U, Boeing H: Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am J Epidemiol 159:935–944, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Schulze MB, Hoffmann K, Manson JE, Willett WC, Meigs JB, Weikert C, Heidemann C, Colditz GA, Hu FB: Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr 82:675–684, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heidemann C, Hoffmann K, Spranger J, Klipstein-Grobusch K, Mohlig M, Pfeiffer AF, Boeing H: A dietary pattern protective against type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study cohort. Diabetologia 48:1126–1134, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Wagenknecht LE, Mayer EJ, Rewers M, Haffner S, Selby J, Borok GM, Henkin L, Howard G, Savage PJ, Saad MF: The Insulin Resistance Atherosclerosis Study (IRAS) objectives, design, and recruitment results. Ann Epidemiol 5:464–472, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Bergman RN, Finegood DT, Ader M: Assessment of insulin sensitivity in vivo. Endocr Rev 6:45–86, 1985 [DOI] [PubMed] [Google Scholar]
- 14.Yang YJ, Youn JH, Bergman RN: Modified protocols improve insulin sensitivity estimation using the minimal model. Am J Physiol 253:E595–E602, 1987 [DOI] [PubMed] [Google Scholar]
- 15.Pacini G, Bergman RN: MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed 23:113–122, 1986 [DOI] [PubMed] [Google Scholar]
- 16.Saad MF, Anderson RL, Laws A, Watanabe RM, Kades WW, Chen YD, Sands RE, Pei D, Savage PJ, Bergman RN: A comparison between the minimal model and the glucose clamp in the assessment of insulin sensitivity across the spectrum of glucose tolerance: Insulin Resistance Atherosclerosis Study. Diabetes 43:1114–1121, 1994 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization: Diabetes Mellitus: Report of a WHO Study Group. Geneva, World Health Org., 1985. (Tech. Rep. Ser., no. 727) [PubMed]
- 18.Mayer-Davis EJ, Vitolins MZ, Carmichael SL, Hemphill S, Tsaroucha G, Rushing J, Levin S: Validity and reproducibility of a food frequency interview in a Multi-Cultural Epidemiology Study. Ann Epidemiol 9:314–324, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Liese AD, Schulz M, Moore CG, Mayer-Davis EJ: Dietary patterns, insulin sensitivity, and adiposity in the multi-ethnic Insulin Resistance Atherosclerosis Study population. Br J Nutr 92:973–984, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Festa A, Williams K, Tracy RP, Wagenknecht LE, Haffner SM: Progression of plasminogen activator inhibitor-1 and fibrinogen levels in relation to incident type 2 diabetes. Circulation 113:1753–1759, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Schulze MB, Hu FB: Dietary patterns and risk of hypertension, type 2 diabetes mellitus, and coronary heart disease. Curr Atheroscler Rep 4:462–467, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Togo P, Osler M, Sorensen TI, Heitmann BL: Food intake patterns and body mass index in observational studies. Int J Obes Relat Metab Disord 25:1741–1751, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Jacobson HN, Stanton JL: Pattern analysis in nutrition. Clin Nutr 5:249–253, 1986 [Google Scholar]
- 24.McCullough ML, Feskanich D, Rimm EB, Giovannucci EL, Ascherio A, Variyam JN, Spiegelman D, Stampfer MJ, Willett WC: Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in men. Am J Clin Nutr 72:1223–1231, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Slattery ML, Boucher KM, Caan BJ, Potter JD, Ma KN: Eating patterns and risk of colon cancer. Am J Epidemiol 148:4–16, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Fung TT, Schulze M, Manson JE, Willett WC, Hu FB: Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med 164:2235–2240, 2004 [DOI] [PubMed] [Google Scholar]
- 27.van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB: Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med 136:201–209, 2002 [DOI] [PubMed] [Google Scholar]
- 28.McNaughton SA, Mishra GD, Brunner EJ: Dietary patterns, insulin resistance and incidence of type 2 diabetes in the Whitehall II study. Diabetes Care 31:1343–1348, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]