Abstract
OBJECTIVE—The purpose of this study was to assess the efficacy of the postload plasma glucose concentration in predicting future risk of type 2 diabetes, compared with prediction models based on measurement of the fasting plasma glucose (FPG) concentration.
RESEARCH DESIGN AND METHODS—A total of 2,442 subjects from the Botnia Study, who were free of type 2 diabetes at baseline, received an oral glucose tolerance test (OGTT) at baseline and after 7–8 years of follow-up. Future risk for type 2 diabetes was assessed with area under the receiver-operating characteristic curve for prediction models based up measurement of the FPG concentration 1) with or without a 1-h plasma glucose concentration during the OGTT and 2) with or without the metabolic syndrome.
RESULTS—Prediction models based on measurement of the FPG concentration were weak predictors for the risk of future type 2 diabetes. Addition of a 1-h plasma glucose concentration markedly enhanced prediction of the risk of future type 2 diabetes. A cut point of 155 mg/dl for the 1-h plasma glucose concentration during the OGTT and presence of the metabolic syndrome were used to stratify subjects in each glucose tolerance group into low, intermediate, and high risk for future type 2 diabetes.
CONCLUSIONS—The plasma glucose concentration at 1 h during the OGTT is a strong predictor of future risk for type 2 diabetes and adds to the prediction power of models based on measurements made during the fasting state. A plasma glucose cut point of 155 mg/dl plus the Adult Treatment Panel III criteria for the metabolic syndrome can be used to stratify nondiabetic subjects into low-, intermediate-, and high-risk groups.
Reliable models for identification of individuals at high risk for future type 2 diabetes are essential and have important clinical implications for intervention programs. Because subjects with impaired glucose tolerance (IGT) have an increased risk for future type 2 diabetes (1), the oral glucose tolerance test (OGTT) has become the standard method for identifying individuals at risk for developing type 2 diabetes. Indeed, all clinical trials that have assessed strategies for type 2 diabetes prevention have recruited subjects with IGT (2). However, performance of an OGTT is time consuming, and models based on measurement of the fasting plasma glucose (FPG) concentration and plasma lipid profile, in addition to medical history and anthropometric measurements, have been developed to predict the future risk of type 2 diabetes (3–8). Of note, IGT converts to type 2 diabetes in only ∼50% of subjects within 10 years of follow-up (1). Moreover, in longitudinal epidemiological studies ∼40% of subjects who develop type 2 diabetes have normal glucose tolerance (NGT) at baseline, indicating that there is a population of subjects with NGT who are at risk for future type 2 diabetes (1).
We have demonstrated that, although subjects with NGT have a relatively low risk for the future development of type 2 diabetes, a group of subjects with NGT with an increased risk for diabetes can be identified on the basis of the relationship between their postload and FPG concentrations (9) or on the 1-h plasma glucose concentration and presence of the metabolic syndrome (10).
Insulin resistance and impaired insulin secretion represent the characteristic pathophysiologic disturbances responsible for development of type 2 diabetes (11,12). Although both insulin resistance and β-cell dysfunction are present long before the onset of diabetes, progressive β-cell failure is the principal factor responsible for the development of overt hyperglycemia (13). Prediction models for type 2 diabetes that have been developed based on measurement of the fasting state include FPG and lipid concentrations, waist circumference, and blood pressure. All of these risk factors are components of the metabolic (insulin resistance) syndrome, which itself is a predictor of future type 2 diabetes in nondiabetic individuals (14). Thus, these risk factors would be expected to correlate strongly with the presence of insulin resistance but less well with impairment in β-cell function. In a recent publication, we demonstrated that the 1-h plasma glucose concentration correlates strongly with indexes of both insulin resistance and insulin secretion and is a better predictor for future type 2 diabetes than either the FPG concentration or the 2-h plasma glucose concentration in Mexican-American individuals (15). Further, we demonstrated that addition of the 1-h plasma glucose concentration to a prediction model based on clinical parameters significantly improved the ability of the model to predict future type 2 diabetes in Mexican Americans (15) and was able to stratify subjects with NGT and IGT into low-, intermediate-, and high-risk groups (10).
Because the relative contributions of insulin resistance and impaired β-cell function may vary among various ethnic groups (16), the aim of this study was to assess the ability of the1-h plasma glucose concentration during an OGTT to predict future risk of type 2 diabetes compared with the fasting and 2-h plasma glucose concentrations in a European Caucasian population. We also examined whether addition of the 1-h plasma glucose concentration to models based on fasting measurements would enhance their predictive value for development of future type 2 diabetes.
RESEARCH DESIGN AND METHODS
The participants in this study were subjects who participated in the Botnia Study (17), were free of diabetes at baseline, had their plasma glucose and insulin concentrations measured during an OGTT, and had a repeat OGTT after 7–8 years. Subjects were classified into various categories of glucose tolerance based on their fasting and 2-h plasma glucose concentrations during an OGTT, according to the American Diabetes Association (ADA) criteria (18).
All subjects received a standard 75-g OGTT after a 12-h overnight fast. Plasma glucose and serum insulin concentrations were measured at 0, 30, 60, and 120 min. Anthropometric and lipid profiles were also obtained at baseline. Subjects were followed for 7–8 years, and glucose tolerance status was determined at follow-up with a repeat OGTT according to the ADA criteria (18). A detailed description of the study design was previously published elsewhere (17).
Analytical methods
Plasma glucose was measured with the glucose oxidation method using a Beckman glucose analyzer (Beckman Instruments, Fullerton, CA). Serum insulin was measured in duplicate by radioimmunoassay (Pharmacia, Uppsala, Sweden).
Calculations
The diagnosis of diabetes was based on ADA criteria (18). The metabolic syndrome was diagnosed according to Adult Treatment Panel III criteria (19).
Areas under the glucose and insulin curves were calculated by the trapezoid rule. The Matsuda index of insulin sensitivity was calculated as reported previously (20). The insulinogenic index was calculated by dividing the increment in serum insulin by the increment in plasma glucose from 0 to 30 min of the OGTT (I0–30/G0–30). The insulin secretion/insulin resistance (disposition) index was calculated as the product of the insulinogenic index across 120 min (I0–120/G0–120) and the Matsuda index of insulin sensitivity. We tested the following prediction models that rely on fasting measurements: 1) a previously described, multivariate model (San Antonio Diabetes Prediction Model [SADPM]) for predicting future type 2 diabetes (3), which includes age, sex, ethnicity, BMI, blood pressure, and FPG, triglyceride, and HDL concentrations; 2) the ATP III criteria for the metabolic syndrome (19); 3) a risk score index (Score Model I) based on age, obesity measurements, use of hypertensive medications, and family history of diabetes (6); and 4) a diabetes risk score based on sex, age, and measurement of FPG and triglyceride concentrations (8). The predictive value of these four models also was evaluated after addition of the 1-h plasma glucose concentration during the OGTT. We also evaluated the risk of future diabetes using a tree model analysis.
Tree model analysis
Recursively partitioned classification trees (21) were used to model the relationship between the future risk of type 2 diabetes and 1) the 1-h plasma glucose concentration during the OGTT and 2) the presence or absence of the metabolic syndrome. Subjects were classified into groups with NGT, IGT, impaired fasting glucose (IFG), or combined glucose intolerance (CGI) (IGT + IFG) according to ADA criteria (18). Sequential partitioning of individuals within each glucose tolerance group (NGT, IGT, IFG, and CGI) based on a 1-h plasma glucose concentration > or <155 mg/dl and the presence or absence of the metabolic syndrome produced subgroups of individuals with a homogeneous risk for future type 2 diabetes. Subgroups with a risk for future type 2 diabetes that was <2.5% over 7–8 years were considered to have a low risk for future type 2 diabetes. A risk between 5 and 10% over 7–8 years was considered to represent intermediate risk. A risk >15% over 7–8 years was considered to represent high risk.
Statistical methods
Variables are presented as means ± SD. The significance of the mean differences was tested with ANOVA. Statistical significance was considered at the level of P < 0.05. Assessment of the predictive discrimination of the various models was made using the receiver operating characteristic (ROC) curve by plotting the sensitivity against the corresponding false-positive rate. The area under the ROC (aROC) curve was used as a measure of how well a continuous variable predicts the development of type 2 diabetes. To examine whether differences between two aROC curves were statistically different, the algorithm developed by DeLong et al. (22) was used. Statistical analyses were performed with the SPSS statistical software system.
RESULTS
Table 1 presents the anthropometric, laboratory, and clinical characteristics of the study population. Of the 2,442 study participants, 1,110 had NGT, 949 had IFG, 123 had IGT, and 260 had CGI at baseline. A total of 124 subjects (5.1%) developed type 2 diabetes over the 7–8 years of follow-up. The conversion rates to type 2 diabetes were 2.4, 5.1, 11.5, and 13.5% for subjects with NGT, IFG, IGT, and CGI, respectively.
Table 1.
Anthropometric, clinical, and laboratory characteristics of the study population
| NGT | IFG | IGT | CGI | Total population | ANOVA | |
|---|---|---|---|---|---|---|
| n | 1,110 | 949 | 123 | 260 | 2,442 | |
| Sex (female/male) | 661/449 | 427/522 | 72/51 | 155/105 | 1,315/1,127 | <0.0001 |
| Age (years) | 45 ± 1 | 46 ± 1 | 50 ± 2 | 52 ± 1 | 46 ± 0.3 | <0.0001 |
| BMI (kg/m2) | 24.1 ± 0.7 | 23.5 ± 0.3 | 24.9 ± 0.2 | 25.6 ± 0.2 | 25.8 ± 0.08 | <0.0001 |
| Waist (cm) | 84.5 ± 0.4 | 88.4 ± 0.4 | 90.1 ± 1.1 | 92.7 ± 0.8 | 87.2 ± 0.24 | <0.0001 |
| FPG (mg/dl) | 92 ± 1 | 108 ± 1 | 93 ± 1 | 110 ± 1 | 100 ± 0.2 | <0.0001 |
| 2-h plasma glucose (mg/dl) | 99 ± 1 | 109 ± 1 | 158 ± 2 | 158 ± 1 | 112 ± 0.6 | <0.0001 |
| Total cholesterol (mmol/l) | 5.4 ± 0.03 | 5.6 ± 0.04 | 5.6 ± 0.09 | 5.8 ± 0.07 | 5.6 ± 0.02 | <0.01 |
| HDL cholesterol (mmol/l) | 1.42 ± 0.01 | 1.37 ± 0.03 | 1.33 ± 0.03 | 1.29 ± 0.02 | 1.38 ± 0.01 | <0.05 |
| Triglycerides (mmol/l) | 1.15 ± 0.02 | 1.31 ± 0.03 | 1.51 ± 0.08 | 1.65 ± 0.06 | 1.28 ± 0.02 | <0.05 |
| Blood pressure (mmHg) | 125/77 | 129/78 | 132/81 | 139/83 | 128/78 | <0.001 |
| Metabolic syndrome (%) | 8.1 | 30.5 | 22.8 | 55.4 | 22.6 | <0.0001 |
| No. converted to diabetes | 27 | 48 | 14 | 35 | 124 | <0.0001 |
| % converted to diabetes | 2.4 | 5.1 | 11.5 | 13.5 | 5.1 | <0.0001 |
Data are means ± SD unless indicated otherwise.
The aROC curve was used to evaluate the predictive power of the various prediction models. All plasma glucose concentrations (0, 30, 60, and 120 min) during the OGTT were significant predictors for future risk of type 2 diabetes (Table 2). However, the plasma glucose concentration at 60 min was the strongest predictor of future risk for type 2 diabetes. The aROC curve for the FPG concentration in this population was significantly less than the aROC curve for the 30- and 60-min plasma glucose concentrations during the OGTT. The aROC curve for the 120-min plasma glucose concentration was smaller (0.688) than the aROC curve for both the 30- and 60-min plasma glucose concentrations during the OGTT (Table 2).
Table 2.
aROC curve and simple correlation (Pearson) between plasma glucose concentration during the OGTT and log transformation of insulin secretion and insulin sensitivity indices
| aROC curve | Matsuda index | ΔI0–30/ΔG0–30 | ΔI0–120/ΔG0–120 | ΔI0–30/ΔG0–30 × Matsuda | ΔI0–120/ΔG0–120 × Matsuda | |
|---|---|---|---|---|---|---|
| FPG | 0.672* | 0.38§ | 0.06‖ | 0.02 | 0.33§ | 0.24§ |
| PG at 30 min | 0.735* | 0.45§ | 0.42§ | 0.39§ | 0.73§ | 0.62§ |
| PG at 60 min | 0.795 | 0.50§ | 0.40§ | 0.55§ | 0.73§ | 0.72§ |
| PG at 120 min | 0.688* | 0.46§ | 0.11§ | 0.34§ | 0.42§ | 0.58§ |
| A1C | 0.679* | 0.18§ | 0.06§ | 0.1‖ | 0.19§ | 0.19§ |
| ΔG0–120 | 0.77† | 0.48§ | 0.42§ | 0.6§ | 0.73§ | 0.83§ |
| Score Model I | 0.646* | 0.36§ | 0.02 | 0.015 | 0.05‖ | 0.07‖ |
| Score Model II | 0.74* | 0.40§ | 0.04 | 0.07‖ | 0.01 | 0.07‖ |
| SADPM | 0.743* | 0.51§ | 0.025 | 0.042 | 0.333§ | 0.351§ |
| MS | 0.72* | 0.56§ | 0.017 | 0.015 | 0.032 | 0.316§ |
| MS + G60 | 0.813‡ | 0.53§ | 0.23§ | 0.272§ | 0.53§ | 0.53§ |
| Model I + G60 | 0.805‡ | 0.38§ | 0.13§ | 0.09‖ | 0.11§ | 0.22§ |
| Model II + G60 | 0.822‡ | 0.43§ | 0.11§ | 0.09‖ | 0.11§ | 0.22§ |
| SADPM + G60 | 0.832‡ | 0.52§ | 0.214§ | 0.256§ | 0.52§ | 0.51§ |
P < 0.001 compared with the aROC curve for plasma glucose concentration at 60 min.
P < 0.05 versus the aROC curve for plasma glucose concentration 60 min.
P < 0.0001 compared with the aROC curve of the same model without G60.
P < 0.0001.
P < 0.05. G60, plasma glucose concentration at 60 min during the OGTT; PG, plasma glucose; MS, metabolic syndrome; SADPM, San Antonio Diabetes Prediction Model.
The aROC curve for A1C (0.697) was similar to that for FPG (0.672). The incremental area under the glucose curve during the OGTT (ΔG0–120) was a strong predictor for the future risk of type 2 diabetes and had an aROC curve (0.770) comparable with that for the 1-h plasma glucose concentration (0.795).
Because the 1-h plasma glucose concentration is the strongest predictor for future risk of type 2 diabetes, we tested whether addition of the 1-h plasma glucose concentration to prediction models (SADPM, ATP III criteria, Score Model I, and Score Model II) based on measurements made during the fasting state improves their predictive power. All four models performed well in predicting the future risk for type 2 diabetes, and the aROC curve ranged from 0.64 to 0.74. However, addition of the 1-h plasma glucose concentration markedly enhanced the predictive power for each of the four models, resulting in an aROC curve >0.8 (Table 2).
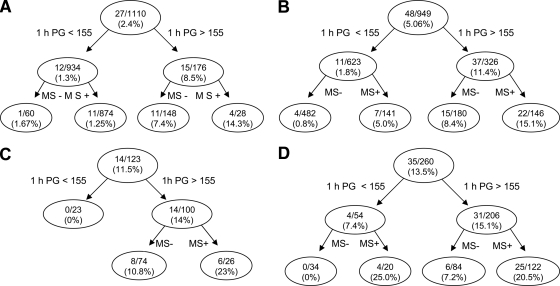
We demonstrated previously that a 1-h plasma glucose cut point of 155 mg/dl during the OGTT and the presence of the metabolic syndrome could classify nondiabetic subjects into three risk groups: low, intermediate, and high (10). In the present study, we constructed a tree model on the basis of glucose intolerance status, the 1-h plasma glucose concentration, and the presence of the metabolic syndrome to classify the risk for future type 2 diabetes. The ROC curve for this model was 0.92. In this model, individuals were divided, according to the ADA criteria, into four groups (NGT, IFG, IGT, and CGI) based on their fasting and 2-h plasma glucose concentrations. Individuals in each group were further divided into two subgroups based on their 1-h plasma glucose concentration (> or <155 mg/dl). Figure 1 depicts the incidence of type 2 diabetes based on the 1-h plasma glucose concentration and the presence or absence of the metabolic syndrome in each glucose tolerance group. Although, as a whole, subjects with NGT had a low risk for developing type 2 diabetes (2.4%), subjects with NGT with a 1-h plasma glucose concentration >155 mg/dl had a significantly increased risk (8.5%) for future type 2 diabetes compared with subjects with NGT with a 1-h plasma glucose concentration <155 mg/dl (1.3%) (P < 0.0001). Further division of this group based on the presence or absence of the metabolic syndrome demonstrated that subjects with NGT with a 1-h plasma glucose concentration >155 mg/dl and the metabolic syndrome had a 14.3% incidence rate of type 2 diabetes compared with a 7.4% incidence rate for subjects without the metabolic syndrome.
Figure 1.
Tree model based on the glucose tolerance status ([A]NGT, [B]IFG, [C]IGT, or [D]CGI [IFG + IGT]) of the subjects, 1-h plasma glucose concentration > or <155 mg/dl, and the presence or absence of the metabolic syndrome. The numbers in each nodule represent the number of subjects converting to diabetes/total number of subjects in each particular group and the incidence rate of conversion to diabetes over 8 years. 1 h PG, 1-h plasma glucose concentration during the OGTT; MS+, metabolic syndrome present; MS−, metabolic syndrome absent.
Subjects with both IFG and IGT with a 1-h plasma glucose concentration >155 mg/dl and the metabolic syndrome had a high risk for future type 2 diabetes (15.1 and 23%, respectively), whereas subjects with IFG and IGT with a 1-h plasma glucose concentration <155 mg/dl without the metabolic syndrome had a very low risk for future type 2 diabetes (0.8 and 0%, respectively). Subjects with a 1-h plasma glucose concentration <155 mg/dl with the metabolic syndrome or a 1-h plasma glucose concentration >155 mg/dl without the metabolic syndrome had an intermediate risk for future type 2 diabetes (5–10%).
Subjects with CGI and the metabolic syndrome had a very high risk for future type 2 diabetes (>20%), whereas none of the subjects with a 1-h plasma glucose concentration <155 mg/dl without the metabolic syndrome developed type 2 diabetes. Subjects with a 1-h plasma glucose concentration >155 mg/dl without the metabolic syndrome had an intermediate (7.2%) risk for future type 2 diabetes.
CONCLUSIONS
Clinical trials have demonstrated that lifestyle intervention and pharmacological therapy can reduce the incidence rate of type 2 diabetes among high-risk individuals (2), and an ADA consensus statement has recommended treatment with metformin, in addition to diet and exercise, in high-risk individuals with IGT or IFG (23). This recommendation for pharmacologic intervention in “pre-diabetic” individuals underscores the need for models that reliably identify subjects at increased risk for future development of type 2 diabetes. The results of this study demonstrate that in a Scandinavian Caucasian population, the predictive value for future type 2 diabetes using the 1-h plasma glucose concentration is superior to that for the 2-h plasma glucose concentration and models based only on measurements taken during the fasting state. In this Scandinavian Caucasian population, as previously demonstrated in a Mexican-American population, the plasma glucose concentration at 1 h during the OGTT is a useful tool that can be used to stratify the risk of future type 2 diabetes.
Subjects with IGT (2-h plasma glucose concentration = 140–199 mg/dl) have an increased risk for future type 2 diabetes, and intervention studies systematically have recruited subjects with IGT to test the efficacy of interventions aimed to reduce the conversion rate of IGT to type 2 diabetes. Because performance of an OGTT in routine clinical practice is complicated and time consuming, investigators have developed prediction models based on measurements made during the fasting state to predict the risk of future type 2 diabetes (3–8). These models perform equally well in predicting future type 2 diabetes compared with the 2-h plasma glucose concentration during the OGTT. Furthermore, addition of the 2-h plasma glucose value to these models did not improve their predictive power (3). In this study we demonstrated that the 1-h plasma glucose concentration is superior to both the 2-h plasma glucose concentration and predictive models based only on fasting measurements in predicting the future risk for type 2 diabetes. Furthermore, addition of the 1-h plasma glucose concentration to models based only on fasting measurements markedly improved their predictive power (Table 2).
A1C, which reflects the mean plasma glucose concentration over the prior 3 months, has predictive power similar to that of the FPG value, and it was much weaker than the 1-h plasma glucose concentration in predicting the future risk for type 2 diabetes. Of note, the optimal A1C cut point for predicting future type 2 diabetes was 5.6%, which is well within the range considered normal.
The parameters used in the fasting models (BMI, waist circumference, lipid profile, fasting glucose, and blood pressure) are components of the metabolic or insulin resistance syndrome, and, as such, they have greater sensitivity in assessing insulin resistance than β-cell function. Similarly, A1C correlated poorly with indexes of β-cell function (Table 2). Conversely, measurement of the postload plasma glucose concentrations (e.g., 1-h plasma glucose concentration and ΔG0–120) correlated well with indexes of β-cell function (Table 2). Thus, it should be emphasized that progressive β-cell failure is the principal factor responsible for the progressive decline in glycemic control as individuals progress from NGT to IGT to type 2 diabetes (11,16,24,25). In this study, we have shown that in a Caucasian population, the 1-h plasma glucose concentration correlates better with OGTT-derived indexes of β-cell function than the 2-h plasma glucose concentration or prediction models based on fasting measurements; these results are similar to our previous results in Mexican Americans. The strong correlation between the 1-h plasma glucose concentration and β-cell function could explain the superior performance of the 1-h plasma glucose concentration in predicting the risk of future type 2 diabetes compared with 2-h plasma glucose concentration and especially compared with models based only on fasting measurements. Similarly, ΔG0–120, which is highly dependent on β-cell function, also is a strong predictor for future risk of type 2 diabetes. These results indicate that assessment of future risk for type 2 diabetes requires that the β-cell be “stressed” to accurately assess its function. In contrast, prediction models based on measurements made during fasting states do not have the ability to assess β-cell function and, therefore, have modest predictive power for future diabetes risk compared with the 1-h plasma glucose concentration after a glucose challenge.
In this study, we also demonstrated that the 1-h plasma glucose concentration is a useful measure to stratify Caucasian subjects into low, intermediate, and high risk categories. In general, subjects with NGT have low risk for progression to type 2 diabetes (<1% annual rate) (2). However, ∼30–40% of individuals who develop type 2 diabetes have NGT at baseline (2) and, in the present study, 22% of subjects who developed type 2 diabetes had NGT at baseline. In this study we demonstrate that subjects with NGT with a 1-h plasma glucose concentration >155 mg/dl have a greater risk for future type 2 diabetes (8.5%) than subjects with NGT with a 1-h plasma glucose concentration <155 mg/dl (1.3%) (P < 0.00001). Further, subjects with NGT with a 1-h plasma glucose concentration >155 mg/dl, who also fulfill the ATP III criteria for the metabolic syndrome, had a 15% risk for future type 2 diabetes. Thus, the group of subjects with NGT with a 1-h plasma glucose concentration >155 mg/dl and the metabolic syndrome have a high risk for the development of type 2 diabetes, and their risk exceeds that of subjects with IFG or IGT. Consistent with an ADA consensus statement (23), this group of high-risk individuals with NGT could benefit from an intervention program including diet and exercise and possibly pharmacotherapy to reduce their future risk for diabetes.
Subjects with CGI have the greatest risk (13.5%) for future type 2 diabetes, whereas subjects with isolated IFG or IGT have an intermediate risk between those with CGI and NGT. However, within the IFG and IGT groups, the 1-h plasma glucose concentration during the OGTT also identifies high-risk individuals. Thus, subjects with IFG and IGT with a 1-h plasma glucose concentration <155 mg/dl have a <2% risk compared with a >10% risk for those with a 1-h plasma glucose concentration >155 mg/dl. Thus, the plasma glucose concentration at 1 h during the OGTT, independent of glucose tolerance status, is a strong predictor for future type 2 diabetes. Subjects with NGT, IFG, and IGT who fulfill the ATP III criteria for metabolic syndrome and have a 1-h plasma glucose concentration >155 mg/dl have the greatest risk (>15%) for future type 2 diabetes, and, in addition to lifestyle intervention, pharmacological therapy should be considered in these subjects. Conversely, subjects with a 1-h plasma glucose concentration <155 mg/dl without the metabolic syndrome have a very low risk for future diabetes (<1%) and, therefore, no intervention is necessary in this group.
In summary, measurement of the postload plasma glucose concentration has additive value to models based only on fasting measurements in predicting the future risk for type 2 diabetes. Similar to results in Mexican Americans, the plasma glucose concentration at 1 h during the OGTT is a strong predictor of future risk for type 2 diabetes in Caucasians, and a 1-h plasma glucose cut point of 155 mg/dl plus the ATP III criteria for the metabolic syndrome can be used to stratify nondiabetic subjects into low-, intermediate-, and high-risk categories.
Acknowledgments
The Botnia Study was funded by grants from the Sigrid Juselius Foundation, the Folkhälsan Research Foundation, the Finnish Diabetes Research Foundation, and the Swedish Research Council.
No potential conflicts of interest relevant to this article were reported.
Published ahead of print at http://care.diabetesjournals.org on 18 November 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Unwin N, Shaw J, Zimmet P, Alberti KGMM: Impaired glucose tolerance and impaired fasting glycemia: the current status on definition and intervention. Diabet Med 19:708–723, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Diabetes Prevention Research Group: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stern MP, Williams K, Haffner SM: Identification of persons at high risk for type 2 diabetes mellitus: do we need the oral glucose tolerance test? Ann Intern Med 136:575–581, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Aekplakorn W, Bunnag P, Woodward M, Sritara P, Cheepudomwit S, Yamwong S, Yipintsoi T, Rajatanavin R: A risk score for predicting incident diabetes in the Thai population. Diabetes Care 29:1872–1877, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Wannamethee SG, Shaper AG, Lennon L, Morris RW: Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med 165:2644–2650, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Kanaya AM, Wassel Fyr CL, de Rekeneire N, Shorr RI, Schwartz AV, Goodpaster BH, Newman AB, Harris T, Barrett-Connor E: Predicting the development of diabetes in older adults: the derivation and validation of a prediction rule. Diabetes Care 28:404–408, 2005 [DOI] [PubMed] [Google Scholar]
- 7.McNeely MJ, Boyko EJ, Leonetti DL, Kahn SE, Fujimoto WY: Comparison of a clinical model, the oral glucose tolerance test, and fasting glucose for prediction of type 2 diabetes risk in Japanese Americans. Diabetes Care 26:758–763, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Lindström J, Tuomilehto J: The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 26:725–731, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Abdul-Ghani MA, Williams K, DeFronzo R, Stern M: Risk of progression to type 2 diabetes based on relationship between postload plasma glucose and fasting plasma glucose. Diabetes Care 29:1613–1618, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Abdul-Ghani MA, Abdul-Ghani T, Ali N, DeFronzo RA: One hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for type 2 diabetes. Diabetes Care 31:1650–1655, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdul-Ghani MA, Tripathy D, DeFronzo RA: Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care 29:1130–1139, 2006 [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA: Lilly Lecture 1987: The triumvirate: β-cell, muscle, liver: a collusion responsible for NIDDM. Diabetes 37:667–687, 1988 [DOI] [PubMed] [Google Scholar]
- 13.DeFronzo RA: Pathogenesis of type 2 diabetes. Med Clin North Am 88:787–835, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Lorenzo C, Okoloise M, Williams K, Stern MP, Haffner SM, San Antonio Heart Study. The metabolic syndrome as predictor of type 2 diabetes: the San Antonio Heart Study. Diabetes Care 26:3153–3159, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M: What is the best predictor of future type 2 diabetes? Diabetes Care 30:1544–1548, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Abdul-Ghani MA, Matsuda M, Sabbah M, Jenkinson C, Richardson D, Kaku K, DeFronzo RA: The relative contributions of insulin resistance and β cell failure to the transition from normal to impaired glucose tolerance varies in different ethnic groups. Diabetes Metab Syn Res Rev 1:105–112, 2007 [Google Scholar]
- 17.Groop L, Forsblom C, Lehtovirta M, Tuomi T, Karanko S, Nissen M, Ehrnstrom BO, Forsen B, Isomaa B, Snickars B, Taskinen MR: Metabolic consequences of a family history of NIDDM (the Botnia study): evidence for sex-specific parental effects. Diabetes 45:1585–1593, 1996 [DOI] [PubMed] [Google Scholar]
- 18.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus: Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20:1183–1197, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F: Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, DeFronzo RA: Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic glucose clamp. Diabetes Care 22:1462–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Clark LA, Pregibon D: Tree model analysis. In Statistical Models. Chambers JM, Hastie TJ, Eds. Pacific Grove, CA, Wadsworth & Brooks, 1992, p. 377–420
- 22.DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845, 1988 [PubMed] [Google Scholar]
- 23.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B, American Diabetes Association: Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care 30:753–759, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA: Insulin secretion and insulin action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study (VEGAS). Diabetes 55:1430–1435, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Abdul-Ghani MA, Matsuda M, Jani R, Jenkinson CP, Coletta DK, Kaku K, DeFronzo RA: The relationship between fasting hyperglycemia and insulin secretion in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab 295:E401–E406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]