Abstract
OBJECTIVE—To determine the mortality rate after diabetes-related lower-extremity amputation (LEA) in an African-descent Caribbean population.
RESEARCH DESIGN AND METHODS—We conducted a prospective case-control study. We recruited case subjects (with diabetes and LEA) and age-matched control subjects (with diabetes and no LEA) between 1999 and 2001. We followed these groups for 5 years to assess mortality risk and causes.
RESULTS—There were 205 amputations (123 minor and 82 major). The 1-year and 5-year survival rates were 69 and 44% among case subjects and 97 and 82% among control subjects (case-control difference, P < 0.001). The mortality rates (per 1,000 person-years) were 273.9 (95% CI 207.1–362.3) after a major amputation, 113.4 (85.2–150.9) after a minor amputation, and 36.4 (25.6–51.8) among control subjects. Sepsis and cardiac disease were the most common causes of death.
CONCLUSIONS—These mortality rates are the highest reported worldwide. Interventions to limit sepsis and complications from cardiac disease offer a huge potential for improving post-LEA survival in this vulnerable group.
The diabetic foot syndrome, including lower-extremity amputation (LEA), is a major contributor to morbidity and mortality from diabetes in developed countries (1). There are limited outcome data from the developing world (2). High incidence of type 2 diabetes is reported in the Caribbean (3), and the incidence of diabetes-related LEA in Barbados ranks among the highest reported in the world (4).
RESEARCH DESIGN AND METHODS
The Barbados studies of amputation among people with diabetes proceeded in three stages. An initial amputation incidence study recruited participants undergoing LEA in the 12-month period starting in November 1999. We defined an amputation as minor if it involved the toes or foot and as major if it was through the tibia or femur. We further divided major amputations into below-the-knee amputations (BKAs) or above-the-knee amputations (AKAs). We then recruited one randomly selected population-based control group for every case to initiate a case-control study of risk factors for diabetes-related LEA. Control subjects had diabetes, had never had an amputation, and were age matched in 5-year age-groups. We restricted analyses to participants who reported their race as black (205 case and 194 control subjects). A detailed description of methods, along with LEA incidence and risk factors, has been previously published (4). The follow-up via telephone interviews continued until July 2007 to assess 5-year survival. We confirmed all reported deaths using official records. For those who had died, we collected cause of death from death certificates to report all-cause mortality. The study received ethics approval, and all participants (or their proxies) gave informed consent for baseline and follow-up studies.
Statistical methods
We calculated survival using the Kaplan-Meier analysis with stratification by amputation status (AKA, BKA, minor amputation, and control subjects). We calculated all-cause mortality rates per 1,000 person-years using the same group stratification and adjusting for age using a Poisson regression model accounting for censored data. We compared these rates using mortality rate ratios from our regression model, with all analyses performed using Stata (Release 10; StataCorp, College Station, TX).
RESULTS
There were 123 (60%) minor amputations, 47 (23%) BKAs, and 35 (17%) AKAs. Case and control subjects were similarly age matched as a result of the study design (case subjects 70.5 years [interquartile range 12.8], control subjects 69.3 years [12.2]; P = 0.34). Case subjects with a minor amputation were younger than those with a major amputation (minor LEA 67.1 years [13.2], major LEA 75.7 years [10.3]; P < 0.001), and we controlled for this age difference by presenting age-adjusted mortality rates. The average diabetes duration for case and control subjects was 17.8 years (interquartile range 11.2) and 11.8 years (9.8), respectively (P < 0.001), with no difference between those with minor and major LEAs (P = 0.35). There were marginally more male case subjects than control subjects (44.9 vs. 34.5%; P = 0.04). Case subjects were more likely than control subjects to have poorer glycemic control (GHb 11.1 vs. 9.1%, respectively; P < 0.001).
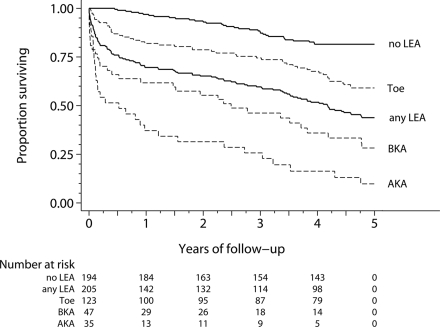
During the 5 years after the index LEA, there were 145 deaths (112 amputees and 33 control subjects). Figure 1 presents the survival experience among case and control subjects and highlights the low survival rate after a major amputation. Survival rates at 3 months, 6 months, 1 year, and 5 years after an AKA were 51, 49, 34, and 10%, respectively, and after a BKA were 68, 64, 60, and 28%. Survival rates after a minor amputation were 92, 86, 81, and 59%. Overall survival rates among case subjects were 80, 75, 69, and 44% and among control subjects were 100, 99, 97, and 82%.
Figure 1.
•••.
Age-adjusted all-cause mortality rates per 1,000 person-years were 432.0 (95% CI 294.7–633.1) after an AKA, 205.9 (142.8–296.8) after a BKA, 273.9 (207.1–362.3) after a major amputation, 113.4 (85.2–150.9) after a minor amputation, and 36.4 (25.6–51.8) among control subjects. All-cause mortality after any amputation was 162.6/1,000 person-years (132.1–200.1). Mortality after a major LEA was over seven times higher than that in control subjects (rate ratio 7.59 [4.97–11.58]; P < 0.001) and over twice that for minor LEAs (2.43 [1.66–3.55]; P < 0.001). The minor LEA rate was over three times higher than that for control subjects (3.12 [2.01–4.86]; P < 0.001). Overall, mortality among case subjects was almost five times that of control subjects (4.68 [3.17–6.90]; P < 0.001).
We could not ascertain cause of death in 5 of 145 (3%) subjects (3 with major amputations and 2 control subjects). Among 181 recorded causes of death, the 5 principal causes in case and control subjects, respectively, were sepsis (49 and 27%), cardiac disease (45 and 25%), stroke (18 and 10%), pneumonia (17 and 9%), and renal problems (14 and 8%). There were no statistically significant differences in the proportion of case and control subjects dying from each cause (P > 0.05 for each cause of death).
CONCLUSIONS
This study documents, for the first time, mortality associated with diabetes-related LEA in the Caribbean. Mortality rates were above those from other studied populations (2,5). In particular, the all-cause mortality rate after a BKA among another high-risk group, American Indians in North America, was reported as 143.7/1,000 person-years—compared with 205.9/1,000 person-years following a BKA in our population.
Cardiovascular disease is the principal cause of death following diabetes-related amputation in the developed world (5). In this population, sepsis and cardiac disease contributed to a similar number of deaths, as they did in a single study from West Africa (6), which suggests the possibility of a different hierarchy of postamputation complications in the developing world. Such differences may be underpinned by health care access and social influences, including footwear choices (4,7).
Patient factors and health care system inadequacies may contribute to the burden of diabetes-related complications. Effective solutions to reduce the burden of amputation have been successfully implemented in other settings (8). Improvement is required in simple techniques for optimizing glycemic control, preventing and detecting foot complications, managing wounds and ulcers, and providing postamputation support services.
Acknowledgments
This work was supported by a Collaborative Research Initiative Grant awarded by the Wellcome Trust.
No other potential conflicts of interest relevant to this article were reported.
Published ahead of print at http://care.diabetesjournals.org on 4 November 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Bild DE, Selby JV, Sinnock P, Browner WS, Braveman P, Showstack JA: Lower-extremity amputation in people with diabetes: epidemiology and prevention. Diabetes Care 12:24–31, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi N, Stevens LK, Fuller JH, Lee ET, Lu M: Risk factors, ethnic differences and mortality associated with lower-extremity gangrene and amputation in diabetes. The WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 44 (Suppl. 2):S65–S71, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Hennis A, Wu SY, Nemesure B, Li X, Leske MC: Diabetes in a Caribbean population: epidemiological profile and implications. Int J Epidemiol 31:234–239, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Hennis AJ, Fraser HS, Jonnalagadda R, Fuller J, Chaturvedi N: Explanations for the high risk of diabetes-related amputation in a Caribbean population of black African descent and potential for prevention. Diabetes Care 27:2636–2641, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Resnick HE, Carter EA, Lindsay R, Henly SJ, Ness FK, Welty TK, Lee ET, Howard BV: Relation of lower-extremity amputation to all-cause and cardiovascular disease mortality in American Indians: the Strong Heart Study. Diabetes Care 27:1286–1293, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Kidmas AT, Nwadiaro CH, Igun GO: Lower limb amputation in Jos, Nigeria. East Afr Med J 81:427–429, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Gulliford MC, Mahabir D: Diabetic foot disease and foot care in a Caribbean community. Diabetes Res Clin Pract 56:35–40, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Nelson EA, O'Meara S, Craig D, Iglesias C, Golder S, Dalton J, Claxton K, Bell-Syer SE, Jude E, Dowson C, Gadsby R, O'Hare P, Powell J: A series of systematic reviews to inform a decision analysis for sampling and treating infected diabetic foot ulcers. Health Technol Assess 10:1–221, 2006 [DOI] [PubMed] [Google Scholar]