Abstract
OBJECTIVE—We sought to determine whether an oral disposition index (DIO) predicts the development of diabetes over a 10-year period. First, we assessed the validity of the DIO by demonstrating that a hyperbolic relationship exists between oral indexes of insulin sensitivity and β-cell function.
RESEARCH DESIGN AND METHODS—A total of 613 Japanese-American subjects (322 men and 291 women) underwent a 75-g oral glucose tolerance test (OGTT) at baseline, 5 years, and 10 years. Insulin sensitivity was estimated as 1/fasting insulin or homeostasis model assessment of insulin sensitivity (HOMA-S). Insulin response was estimated as the change in insulin divided by change in glucose from 0 to 30 min (ΔI0–30/ΔG0–30).
RESULTS—ΔI0–30/ΔG0–30 demonstrated a curvilinear relationship with 1/fasting insulin and HOMA-S with a left and downward shift as glucose tolerance deteriorated. The confidence limits for the slope of the loge-transformed estimates included −1 for ΔI0–30/ΔG0–30 versus 1/fasting insulin for all glucose tolerance groups, consistent with a hyperbolic relationship. When HOMA-S was used as the insulin sensitivity measure, the confidence limits for the slope included −1 only for subjects with normal glucose tolerance (NGT) or impaired fasting glucose (IFG)/impaired glucose tolerance (IGT) but not diabetes. On the basis of this hyperbolic relationship, the product of ΔI0–30/ΔG0–30 and 1/fasting insulin was calculated (DIO) and decreased from NGT to IFG/IGT to diabetes (P < 0.001). Among nondiabetic subjects at baseline, baseline DIO predicted cumulative diabetes at 10 years (P < 0.001) independent of age, sex, BMI, family history of diabetes, and baseline fasting and 2-h glucose concentrations.
CONCLUSIONS—The DIO provides a measure of β-cell function adjusted for insulin sensitivity and is predictive of development of diabetes over 10 years.
Type 2 diabetes is characterized by both insulin resistance and β-cell dysfunction (1). Abnormalities in β-cell function are present in high-risk individuals long before they develop hyperglycemia (1). This recognition has occurred in part because of a better understanding of the ability of the β-cell to regulate its insulin response to stimuli based on differences in insulin sensitivity.
Using intravenous testing, subjects with normal β-cell function demonstrate a hyperbolic relationship between insulin sensitivity and insulin responses (2,3), consistent with a classic feedback loop. On the basis of this hyperbolic relationship, the product of these two variables, referred to as the disposition index, can be calculated and has highlighted the inability of the β-cell to compensate for insulin resistance in subjects at risk for diabetes (4,5) and with higher fasting glucose levels (6–8). In prospective studies, the disposition index declines well before glucose levels rise into the diabetic range (9). Thus, a low disposition index is an early marker of inadequate β-cell compensation.
This hyperbolic relationship has been demonstrated between measures of insulin sensitivity and response derived from intravenous tests (3) as well as between the early insulin response during an oral glucose tolerance test (OGTT) and insulin sensitivity derived from intravenous testing (10). However, intravenous tests are time-consuming, expensive, and not practical for large studies. The OGTT is less precise (11) but simpler to perform and is often used in large epidemiological or intervention studies. Recently, a nonlinear function describing the relationship between the oral glucose–induced early insulin response and insulin sensitivity has been used to assess β-cell function in both observational (12) and interventional studies (13). Using OGTT measures, Retnakaran et al. (14) were able to show a hyperbolic relationship between insulin sensitivity (Matsuda index) and the incremental area under the curve insulin/glucose (incAUCins/glu) response but not the early insulin response. Sakaue et al. (15) also failed to demonstrate a hyperbolic relationship between OGTT-derived insulin sensitivity and the early insulin response. However, the latter regression analysis failed to account for measurement error in the independent variable, which leads to an underestimation of the slope (16).
We first tested whether the relationship between the early insulin response or the incAUCins/glu response after oral glucose and surrogate measures of insulin sensitivity were related in a hyperbolic manner using a regression technique that takes measurement error in both variables into account. We then tested whether this relationship exists for different glucose tolerance categories. Finally, as we found the relationship to be hyperbolic, we examined whether a composite measure (oral disposition index) is associated with the development of diabetes.
RESEARCH DESIGN AND METHODS
The Japanese American Community Diabetes Study was conducted in King County, Washington, with baseline testing performed between 1983 and 1988 and follow-up examinations ∼5 and 10 years later. The design and methods used in this study have been described previously (17). The study was approved by the local institutional review board, and written informed consent was obtained from each participant.
Baseline oral glucose tolerance testing was performed in 658 subjects; 640 subjects had complete data for insulin and glucose values at basal, 30 min, and 120 min. Ten subjects were excluded because of negative or zero ΔI0–30/ΔG0–30 (n = 7) or incAUCins/glu responses (n = 3). Seventeen subjects were excluded as outliers (see statistical methods). Subjects who did not have diabetes at baseline (n = 498) had follow-up examinations and OGTTs at 5 or 6 years (5 year, n = 448) and at 10 or 11 years (10 year, n = 398).
Study procedures and assays
A standard 75-g OGTT was performed in the morning after a 10-h overnight fast. Samples were drawn just before and at 30, 60, and 120 min after ingestion of glucose. Samples were collected in EDTA, separated, and stored at −20°C before being assayed. Plasma glucose was measured by the glucose oxidase method. Plasma insulin was measured using a modified double-antibody radioimmunoassay as described previously (17). Height, weight, and abdominal circumference (umbilicus) were measured three times at each visit, and the average for each visit was used.
Classification of glucose tolerance
Using the 2003 American Diabetes Association criteria (18), subjects were categorized as having normal glucose tolerance (NGT) (fasting plasma glucose [FPG] <5.56 mmol/l and 2-h plasma glucose <7.78 mmol/l), impaired glucose metabolism (IGM) (either impaired fasting glucose [IFG]: FPG 5.56–6.99 mmol/l and/or impaired glucose tolerance [IGT]: 2-h plasma glucose 7.78–11.10 mmol/l) or diabetes (FPG ≥7.0 mmol/l and/or 2-h plasma glucose ≥11.11 mmol/l).
Calculations
Insulin sensitivity was estimated by two methods: 1) 1/fasting insulin or 2) homeostasis model assessment (HOMA) of insulin sensitivity (HOMA-S) using the Web-based HOMA calculator for nonspecific insulin (http://www.dtu.ox.ac.uk) (19). The early insulin response was calculated as the ratio of the change in insulin to the change in glucose from 0 to 30 min (ΔI0–30/ΔG0–30). The incAUCins/glu response was calculated by the trapezoidal method from 0 to 120 min. The composite measure of β-cell function, which we have termed the oral disposition index (DIO), was calculated as ΔI0–30/ΔG0–30 × 1/fasting insulin.
Statistical analysis
To determine whether the relationships between the dependent (ΔI0–30/ΔG0–30 or incAUCins/glu) and independent (1/fasting insulin or HOMA-S) variables were consistent with a rectangular hyperbola (x × y = constant), we estimated ln(ΔI0–30/ΔG0–30 or incAUCins/glu) as a linear function of ln(1/fasting insulin or HOMA-S) using regression analysis. If the hyperbolic relationship exists, the slope of the regression line should not be significantly different from −1. When error is present in both x and y variables, the slope that is determined by ordinary least-squares regression is underestimated because it assumes all error in the y variable. The regression method we used corrects this bias by incorporation of a factor computed as the ratio of the variances of the error in the y to x variables (16). The error estimates for these measurements (57.1% for ΔI0–30/ΔG0–30, 24.9% for incAUCins/glu, 16.6% for 1/fasting insulin, and 16.4% for HOMA-S) were based on the day-to-day coefficients of variation in a group of subjects with various glucose tolerance (11). A hyperbolic relationship was presumed if the 95% CI of the slope included −1. The 95% CI was calculated using the bootstrap method. Subjects were subdivided for analysis by glucose tolerance category. The y intercept of the regression line of the ln-transformed variables was calculated, assuming a slope of −1.
Because outlying values can have marked adverse effects on regression parameters, the data were subjected to a series of tests for influential values, specifically, Cooks distance, DFBETA, DFFIT, COVRatio, and HATvalue using the R statistical procedure “influence measures” (20). Because of the high number of data points, the critical value for each test was set such that the α (type 1 error) was <0.002. A data point that was identified as an influential point by any of these tests was reviewed graphically, and the subject was eliminated from further analysis.
To exclude the possibility that the regression is artifactual—driven by fasting insulin and glucose appearing in both the insulin sensitivity and ΔI0–30/ΔG0–30 equations—the same regression procedures were performed on the data except that the 30-min insulin values were shuffled using the Fisher-Yates algorithm to remove any physiological relationship. This shuffle-regression procedure was repeated 100,000 times, and the median and 95% CIs were determined. These simulations showed that only a weak, positive slope resulted with wide confidence limits that did not include −1 [subjects with NGT: ln(HOMA-S) vs. ln(ΔI0–30/ΔG0–30) = +0.53 (0.13–0.94); ln(1/fasting insulin) vs. ln(ΔI0–30/ΔG0–30) = +0.46 (0.11–0.82)].
Statistical analysis was performed using STATA (version 9.0; StataCorp, College Station, TX). Variables that were not normally distributed were loge transformed to achieve a normal distribution. ANOVA with a post hoc Scheffe correction was performed to compare variables between different glucose tolerance categories. Multiple logistic regression analysis was performed to determine whether DIO was independently associated with cumulative diabetes (yes/no) at 10 years. Only subjects without diabetes at baseline were included in this analysis. A diagnosis of diabetes at 5 years was carried forward and included in the cumulative 10-year incidence. The model included ln(DIO), ln(age), sex, ln(BMI), ln(fasting glucose), and ln(2-h glucose). In addition, subjects without diabetes at baseline were divided into quintiles of baseline DIO, and the multiple logistic regression analysis was rerun.
To determine whether DIO was a better predictor of diabetes than 1/fasting insulin or ΔI0–30/ΔG0–30 alone, nonparametric receiver operating characteristic (ROC) curve analysis was performed with cumulative diabetes at 10 years (no) as the outcome variable. An optimal cut point for DIO was obtained using the Youden index (maximum [sensitivity + specificity –1]). P < 0.05 was considered statistically significant.
RESULTS
The 613 subjects were categorized by glucose tolerance on the basis of their fasting and 2-h plasma glucose measurements (Table 1). Fasting insulin, HOMA-S, and ΔI0–30/ΔG0–30 did not differ between isolated IFG (iIFG), isolated IGT (iIGT), and IFG + IGT groups. Thus, these groups were combined for analysis into the IGM group. Age, BMI, and abdominal circumference increased progressively with deteriorating glucose metabolism, whereas insulin sensitivity and insulin responses decreased progressively from NGT to IGM to diabetes (Table 1).
Table 1.
Subject characteristics by glucose tolerance category
| NGT | iIFG | iIGT | IFG + IGT | IGM | Diabetes | |
|---|---|---|---|---|---|---|
| n | 244 | 60 | 118 | 76 | 254 | 115 |
| Age (years) (mean ± SD) | 48.6 ± 11.9 | 56.4 ± 10.7 | 54.0 ± 11.5 | 59.1 ± 9.5§ | 56.1 ± 10.9* | 61.1 ± 7.4* |
| Sex (female/male) | 120/124 | 20/40 | 79/39† | 23/53§ | 122/132 | 49/66 |
| BMI (kg/m2) | 23.5 ± 0.2 | 24.7 ± 0.3 | 23.7 ± 0.3 | 25.8 ± 0.4§ | 24.6 ± 0.2* | 25.7 ± 0.3* |
| Abdominal circumference (cm) | 84.0 ± 0.51 | 87.7 ± 0.8 | 85.5 ± 0.9 | 90.0 ± 0.9§ | 87.6 ± 0.5* | 91.0 ± 0.8* |
| FPG (mmol/l) | 4.89 (0.58) | 5.72 (0.39) | 5.06 (0.50)† | 5.89 (0.39)§ | 5.56 (0.72)* | 7.56 (3.33)* |
| 2-h plasma glucose (mmol/l) | 6.22 (1.44) | 6.94 (1.08) | 8.69 (1.11)† | 9.19 (1.78)‡§ | 8.47 (1.56)* | 15.17 (8.56)* |
| Fasting insulin (pmol/l) | 66 (36) | 81 (48) | 69 (48) | 78 (51) | 78 (54)* | 102 (96)* |
| HOMA-S | 81.9 (45.2) | 63.9 (36.4) | 79.2 (50.2) | 65.8 (38.8) | 70.7 (46.9)* | 47.0 (44.7)* |
| ΔI0–30/ΔG0–30 (pmol/mmol) | 105.7 (102.5) | 100.2 (101.9) | 81.7 (92.6) | 77.9 (65.0) | 80.9 (86.5)* | 29.9 (41.4)* |
| incAUCins/glu (pmol/mmol) | 148.6 (138.6) | 138.5 (146.5) | 104.3 (83.8)† | 105.6 (77.8)‡ | 113.3 (90.8)* | 33.8 (49.0)* |
Data are reported as mean ± SEM or median (interquartile range) unless otherwise specified.
P < 0.001 NGT versus IGM versus diabetes;
iIFG versus iIGT,
iIFG versus IFG + IGT,
iIGT versus IFG + IGT, P < 0.05.
Hyperbolic relationship between insulin sensitivity and insulin responses in subjects with NGT
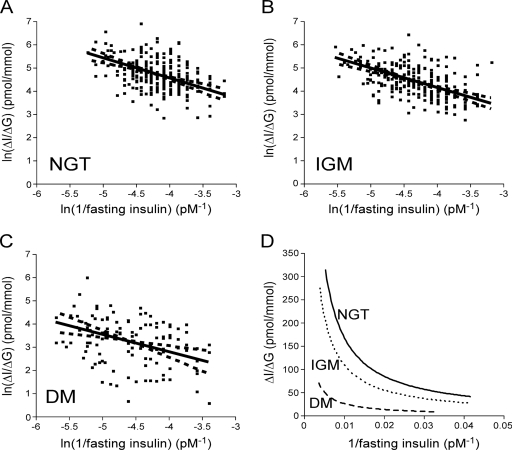
The 95% CI for the regression slopes included −1 for the relationship between ln(1/fasting insulin) and both ln(ΔI0–30/ΔG0–30) (slope −0.87 [95% CI −1.13 to −0.61]) (Fig. 1A) and ln(incAUCins/glu) (−1.82 [−0.97 to −0.66]). The same analyses were performed substituting HOMA-S for fasting insulin with similar results [ln(ΔI0–30/ΔG0–30) vs. ln(HOMA-S): −0.91 (−1.12 to −0.62]); ln(incAUCins/glu) vs. ln(HOMA-S) −2.06 (−3.38 to −0.75)]. For all regressions in subjects with NGT, the slopes included −1 in keeping with a hyperbolic relationship.
Figure 1.
The computed slopes (——) and 95% CIs for the slopes (– – –) for ln(ΔI0–30/ΔG0–30) versus ln(1/fasting insulin) are plotted for subjects with NGT (slope −0.87 [95% CI −1.13 to −0.61]) (A), IGM (−0.84 [−1.05 to −0.63]) (B), and diabetes (DM) (−0.76 [−1.16 to −0.35]) (C). D: The hyperbolic curves for NGT, IGM, and diabetes, assuming a slope of −1, are plotted for ΔI0–30/ΔG0–30 versus 1/fasting insulin.
Hyperbolic relationship between insulin sensitivity and insulin responses in subjects with IGM and diabetes
In subjects with IGM, the corrected slopes included −1 for both the relationship between ln(ΔI0–30/ΔG0–30) and ln(1/fasting insulin) (−0.84 [95% CI −1.05 to −0.63]) (Fig. 1B) and between ln(incAUCins/glu) and ln(1/fasting insulin) (−1.27 [−1.68 to −0.86]). Similar results were found when HOMA-S was substituted for 1/fasting insulin (data not shown). When analyzed for each individual IGM group, the relationship between ln(ΔI0–30/ΔG0–30) and ln(1/fasting insulin) was hyperbolic for the iIGT group (−1.15 [−1.43 to −0.87]) and the IFG + IGT group (−0.76 [−1.08 to −0.44]) but not for the iIFG group (−0.47 [−0.89 to −0.06]). Similar results were found when HOMA-S was substituted for 1/fasting insulin (data not shown). The slopes for the relationship with ln(incAUCins/glu) included −1 for the iIFG and IFG + IGT groups but not for the iIGT group using either 1/fasting insulin or HOMA-S (data not shown).
In those with diabetes, the corrected slopes included −1 for the relationship between ln(ΔI0–30/ΔG0–30) and ln(1/fasting insulin) (−0.76 [−1.16 to −0.35]) (Fig. 1C) and between ln(incAUCins/glu) and ln(1/fasting insulin) (−2.33 [−3.97 to −0.69]). Use of ln(HOMA-S) in place of ln(1/fasting insulin) resulted in a flatter slope that did not include −1 for ln(ΔI0–30/ΔG0–30) (−0.55 [−0.97 to −0.12]) and a slope that did include −1 but had a very wide CI for ln(incAUCins/glu) (−2.74 [−5.82 to 0.35]).
The hyperbolic curves demonstrated a shift to the left and downward from NGT to IGM to diabetes (ΔI0–30/ΔG0–30 versus 1/fasting insulin) (Fig. 1D). Similar shifts were seen when HOMA-S or incAUCins/glu was used. This decrease in β-cell function is best evaluated by examination of the y intercepts for the ln-ln relationships. These intercepts decreased from NGT (mean ± SD 0.53 ± 0.63) to IGM (0.09 ± 0.61) to diabetes (−1.36 ± 0.99) for ln(ΔI0–30/ΔG0–30) versus ln(1/fasting insulin). Similar decreases were seen for ln(ΔI0–30/ΔG0–30) versus ln(HOMA-S) (NGT 9.12 ± 0.63, IGM 8.66 ± 0.61, and diabetes 7.13 ± 1.04), ln(incAUCins/glu) versus ln(1/fasting insulin) (NGT 0.82 ± 0.62, IGM 0.39 ± 0.52, and diabetes −1.15 ± 0.98), and ln(incAUCins/glu) and ln(HOMA-S) (NGT 9.42 ± 0.62, IGM 8.97 ± 0.52, and diabetes 7.34 ± 1.05) (P < 0.005 for all comparisons).
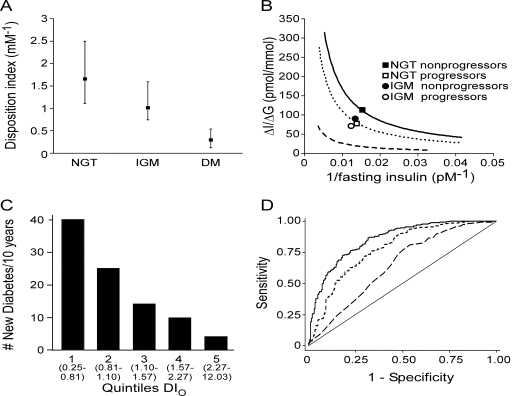
On the basis of the hyperbolic relationship between ΔI0–30/ΔG0–30 and 1/fasting insulin, the product of these two variables (DIO) was computed as a composite measure of β-cell function. DIO decreased progressively from NGT to IGM to diabetes (P < 0.001 for all comparisons) (Fig. 2A). Similar results were obtained when DIO was calculated using HOMA-S instead of 1/fasting insulin (data not shown). When DIO was compared among the three IGM groups (iIFG median 1.15 [interquartile range 0.96], iIGT 1.07 [0.82], and IFG + IGT 0.89[0.77] mM−1), DIO was lower in the IFG + IGT group than in the iIFG group (P = 0.01) or iIGT group (P = 0.051) but did not differ significantly between the iIFG and iIGT groups.
Figure 2.
A: The DIO (ΔI0–30/ΔG0–30 × 1/fasting insulin) decreases from NGT to IGM to diabetes (DM) (median [interquartile range]). B: The logarithmic means for baseline ΔI0–30/ΔG0–30 and 1/fasting insulin values are plotted for subjects with NGT (▪ and □) and IGM (• and ○) as nonprogressors (▪ and •) and progressors (□ and ○) relative to the hyperbolic curves. Progressors had lower β-cell function at baseline. C: The number of subjects who developed diabetes over the 10-year follow-up period by quintiles of baseline DIO. D: ROC curves comparing ability of baseline ΔI0–30/ΔG0–30 (dotted line), 1/fasting insulin (dashed line), and DIO (solid line) to predict cumulative diabetes at 10 years.
DIO was associated with the development of diabetes over 10 years
Subjects who progressed to diabetes over the 10-year follow-up period (“progressors”: n = 9 with baseline NGT and n = 84 with baseline IGM) were compared with those who did not progress (“nonprogressor”: n = 235 with baseline NGT and n = 170 with baseline IGM). Baseline DIO was significantly lower in the progressors versus the nonprogressors (NGT median 0.90 [interquartile range 0.40] vs. 1.72 [1.42] mM−1, P < 0.05; IGM 0.85 [0.57] vs. 1.12 [0.94] mM−1, P < 0.001). The relationship between insulin sensitivity and insulin response at baseline was shifted downward and to the left in the progressors compared with the nonprogressors (Fig. 2B).
We examined whether DIO was an independent predictor of the development of diabetes over time. In subjects who did not have diabetes at baseline, a higher DIO was associated with a decreased risk of diabetes at 10 years (odds ratio [OR] 0.40 [95% CI 0.25–0.66], P < 0.001) after adjustment for ln(age), sex, ln(BMI), ln(fasting glucose), and ln(2-h glucose). Family history added to the model was a weak independent predictor of diabetes (1.79 [1.04–3.09], P = 0.03) but did not change the significance of DIO (0.41 [0.25–0.68], P < 0.001). Similar results were obtained when both family history and ln(triglycerides) were added to the model. When ln(1/fasting insulin) and ln(ΔI0–30/ΔG0–30) were included in the basic model in place of ln(DIO), both were independent predictors of diabetes at the 10-year follow-up: ln(1/fasting insulin) 0.32 [0.14–0.73] and ln(ΔI0–30/ΔG0–30) 0.41 [0.25–0.68]. Similar results were obtained when HOMA-S was used to compute DIO (data not shown). When the analysis was restricted to subjects with NGT at baseline, DIO was still an independent predictor of diabetes (0.24 [0.06–0.88], P = 0.03).
When the multiple logistic regression analysis was rerun using quintiles of DIO (categorical variables coded as dummy variables), the risk for diabetes decreased as DIO increased (versus quintile 1 [lowest DIO]: quintile 2 OR 0.63 [95% CI 0.32–1.25], P = 0.19; quintile 3 0.40 [0.18–0.90], P = 0.03; quintile 4 0.39 [0.17–0.94], P = 0.04; and quintile 5 [highest DIO] 0.14[0.05–0.45], P = 0.001). The number of subjects who developed diabetes over the 10 years decreased as the DIO quintile increased (Fig. 2C).
ROC curve analysis was performed to determine whether the composite measure DIO was better at predicting protection from diabetes compared with ΔI0–30/ΔG0–30 or 1/fasting insulin alone. The area under the ROC curve was highest using DIO (0.86 [95% CI 0.82–0.89]), intermediate for ΔI0–30/ΔG0–30 (0.78 [0.74–0.82]), and lowest for 1/fasting insulin (0.65 [0.60–0.70]). The area under the ROC curve for DIO was significantly higher than that for the other two variables (P < 0.001 for each) (Fig. 2D). The best predictor for remaining without diabetes was DIO ≥1.24 (sensitivity of 59.3% and specificity of 79.6%).
CONCLUSIONS
A hyperbolic relationship between insulin sensitivity and responses using intravenous measures (3) has been widely accepted. This study demonstrates that measures of insulin sensitivity and response derived from an OGTT are also compatible with a hyperbolic association and that this relationship is present not only in subjects with NGT but also in subjects with IGM and diabetes. Importantly, the existence of this relationship allows calculation of a DIO, which was predictive of future development of diabetes above and beyond traditional risk factors, such as family history and fasting and 2-h glucose levels. The DIO as a composite measure was a better predictor for diabetes than either ΔI0–30/ΔG0–30 or 1/fasting insulin alone.
Our finding that the relationship between insulin sensitivity and insulin response based on OGTT measures is shifted downward and to the left as glucose tolerance deteriorates is consistent with previous work using intravenous tests (6,21). With the use of intravenous measures, the disposition index was lower at baseline and declined further in those whose glucose metabolism deteriorated over time (9,21,22). Our current findings extend use of this approach to measure β-cell function using OGTT-derived measures. Importantly, we have shown that the DIO was inversely correlated with the risk of future diabetes. Thus, the DIO may be useful to help identify subjects in large epidemiological studies who have an increased risk of developing diabetes.
The statistical methodology for assessment of this hyperbolic relationship is critical when both the x and y variables are measured with error, as the slope will be underestimated if measurement error in the independent variable is not accounted for (23). The absence of a hyperbolic relationship between these same OGTT measures in a previously published study (15) may have been due to this issue. Although Retnekaran et al. (14) did account for error estimates in both variables, the slopes for the relationships between ΔI0–30/ΔG0–30 and measures of insulin sensitivity (1/HOMA for insulin resistance and the Matsuda index) were <−1 (−1.61 and −1.60, respectively) and because of the wide CIs that included 0 were not considered to be hyperbolic. In contrast, we found that the slopes for subjects with NGT for the log-relationships between ΔI0–30/ΔG0–30 and 1/fasting insulin (slope = −0.87) or HOMA-S (slope = −0.91) were slightly >−1, but the CI still included −1, consistent with a hyperbolic relationship. Differences in results between our study and that by Retnakaran et al. could be due to differences in error estimates, as the slopes are quite sensitive to changes in error estimates. Of note, our results are consistent with the slope estimates for the log-relationship between the acute insulin response to glucose and the insulin sensitivity index (SI) derived from a frequently sampled intravenous glucose tolerance test (slope = −0.97) (3) or between ΔI0–30/ΔG0–30 and SI (slope = −0.86) (10).
The strengths of this study include the large number of subjects and the longitudinal study design. However, we failed to show a hyperbolic relationship for some regressions when the iIFG and iIGT groups were examined separately. It is possible that glucose tolerance in these subpopulations fails to follow a hyperbolic relationship, although it seems unlikely because when they are combined the hyperbolic relationship is present. In the group with diabetes, the log-relationships between ΔI0–30/ΔG0–30 and insulin sensitivity measures were flatter and did not include −1 when HOMA-S was used. This may reflect the much broader range of glucose tolerance (and fasting glucose levels) in this group. Finally, the CIs for the slopes using the incAUCins/glu ratio, although including −1, were much wider and thus less reliable. Other limitations include the fact that we excluded 17 subjects (2.7%) with outlier data from this analysis, as outliers can have a disproportionate effect on regression analysis. Finally, this study was performed in Japanese Americans, and, thus, we cannot generalize the conclusions to other ethnic populations, although it is likely that the same physiological feedback processes would occur in other ethnic groups.
There are limitations to application of the DIO that need to be kept in mind. In particular, because of the increased variability of OGTT measures compared with intravenous testing, the DIO will be more variable and, hence, appropriately large sample sizes will be needed. Second, it cannot be assumed that all measures of insulin sensitivity or response will follow a hyperbolic pattern and thus simply multiplying any two measures together without first demonstrating a hyperbolic function is not appropriate. Also, it should be kept in mind that the compensatory insulin response includes both changes in insulin secretion as well as adaptations in hepatic insulin extraction (24) and changes in incretin hormone responses that may modulate both insulin secretion and hepatic insulin extraction (25).
In summary, we have demonstrated that use of OGTT-derived measures of insulin response and insulin sensitivity can delineate differences in β-cell function between glucose tolerance categories. Furthermore, the composite measure DIO can be used to assess β-cell function and was independently associated with future diabetes risk. Thus, the disposition index approach using these specific measures offers a way to assess β-cell function using an OGTT and could be used to identify subjects with poor β-cell function for intervention trials and to assess the impact of interventions in large clinical studies.
Acknowledgments
This work was supported by the Department of Veteran Affairs and the Veteran Affairs Geriatrics Research, Education, and Clinical Center, National Institutes of Health grants DK-02654, DK-31170, HL-49293, RR-00037, DK-35816, and P30 DK-17047.
No potential conflicts of interest relevant to this article were reported.
We thank Brian Fish for his help with the database.
Parts of this study were presented in poster form at the 68th Scientific Sessions of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
Published ahead of print at http://care.diabetesjournals.org on 28 October 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Kahn SE: The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia 46:3–19, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bergman RN, Phillips LS, Cobelli C: Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and β-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68:1456–1467, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, Neifing JL, Ward WK, Beard JC, Palmer JP, et al.: Quantification of the relationship between insulin sensitivity and β-cell function in human subjects: evidence for a hyperbolic function. Diabetes 42:1663–1672, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP: Preservation of pancreatic β-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes 51:2796–2803, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS: Insulin secretory defects in polycystic ovary syndrome: relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest 96:520–527, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Utzschneider KM, Prigeon RL, Carr DB, Hull RL, Tong J, Shofer JB, Retzlaff BM, Knopp RH, Kahn SE: Impact of differences in fasting glucose and glucose tolerance on the hyperbolic relationship between insulin sensitivity and insulin responses. Diabetes Care 29:356–362, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Tripathy D, Eriksson KF, Orho-Melander M, Fredriksson J, Ahlqvist G, Groop L: Parallel manifestation of insulin resistance and β-cell decompensation is compatible with a common defect in type 2 diabetes. Diabetologia 47:782–793, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Stumvoll M, Tataranni PA, Stefan N, Vozarova B, Bogardus C: Glucose allostasis. Diabetes 52:903–909, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Xiang AH, Wang C, Peters RK, Trigo E, Kjos SL, Buchanan TA: Coordinate changes in plasma glucose and pancreatic β-cell function in Latino women at high risk for type 2 diabetes. Diabetes 55:1074–1079, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Berkowitz K, Marroquin A, Goico J, Ochoa C, Azen SP: Response of pancreatic β-cells to improved insulin sensitivity in women at high risk for type 2 diabetes. Diabetes 49:782–788, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Utzschneider KM, Prigeon RL, Tong J, Gerchman F, Carr DB, Zraika S, Udayasankar J, Montgomery B, Mari A, Kahn SE: Within-subject variability of measures of β-cell function derived from a 2 h OGTT: implications for research studies. Diabetologia 50:2516–2525, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Florez JC, Jablonski KA, Bayley N, Pollin TI, de Bakker PI, Shuldiner AR, Knowler WC, Nathan DM, Altshuler D: TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med 355:241–250, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kitabchi AE, Temprosa M, Knowler WC, Kahn SE, Fowler SE, Haffner SM, Andres R, Saudek C, Edelstein SL, Arakaki R, Murphy MB, Shamoon H: Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: effects of lifestyle intervention and metformin. Diabetes 54:2404–2414, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Retnakaran R, Shen S, Hanley AJ, Vuksan V, Hamilton JK, Zinman B: Hyperbolic relationship between insulin secretion and sensitivity on oral glucose tolerance test. Obesity (Silver Spring) 16:1901–1907, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Sakaue S, Ishimaru S, Ikeda D, Ohtsuka Y, Honda T, Suzuki J, Kawakami Y, Ishii J, Nishimura M: Estimation of β-cell function from the data of the oral glucose tolerance test. Am J Physiol Endocrinol Metab 292:E1575–E1580, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Riggs DS, Guarnieri JA, Addeman S: Fitting straight lines when both variables are subject to error. Life Sci 22:1305–1360, 1978 [DOI] [PubMed] [Google Scholar]
- 17.Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, Fujimoto WY: Visceral adiposity and the risk of impaired glucose tolerance: a prospective study among Japanese Americans. Diabetes Care 26:650–655, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P: Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26:3160–3167, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Levy JC, Matthews DR, Hermans MP: Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21:2191–2192, 1998 [DOI] [PubMed] [Google Scholar]
- 20.The R Development Core Team: A Language and Environment for Statistical Computing, Reference Index [Internet], 2005. version 2.1.1. Vienna, Austria, R Foundation for Statistical Computing. Available from http://www.R-project.org. Accessed 15 September 2007
- 21.Weyer C, Bogardus C, Mott DM, Pratley RE: The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest 104:787–794, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cnop M, Vidal J, Hull RL, Utzschneider KM, Carr DB, Schraw T, Scherer PE, Boyko EJ, Fujimoto WY, Kahn SE: Progressive loss of β-cell function leads to worsening glucose tolerance in first-degree relatives of subjects with type 2 diabetes. Diabetes Care 30:677–682, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Armitage P, Berry G, Matthews J: Statistical Methods in Medical Research. Oxford, Blackwell Science, 2002
- 24.Kautzky-Willer A, Thomaseth K, Clodi M, Ludvik B, Waldhausl W, Prager R, Pacini G: β-cell activity and hepatic insulin extraction following dexamethasone administration in healthy subjects. Metabolism 45:486–491, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Rudovich NN, Rochlitz HJ, Pfeiffer AF: Reduced hepatic insulin extraction in response to gastric inhibitory polypeptide compensates for reduced insulin secretion in normal-weight and normal glucose tolerant first-degree relatives of type 2 diabetic patients. Diabetes 53:2359–2365, 2004 [DOI] [PubMed] [Google Scholar]