Abstract
Aims
To develop a novel nanomedicine approach for the treatment of multidrug-resistant (MDR) cancer by combining an anticancer drug and suppressors of cellular resistance within one multifunctional nanocarrier-based delivery system (NDS).
Materials & methods
The NDS consisted of cationic liposomes (carrier, 100–140 nm), doxorubicin (DOX, anticancer drug), siRNA targeted to MRP1 and BCL2 mRNA (suppressors of pump and nonpump cellular-resistance, respectively). The resulting approximately 500 nm complex has a zeta potential of +4 mV.
Results & discussion
The NDS provides an effective co-delivery of DOX and siRNA as well as cell-death induction and suppression of cellular resistance in MDR lung cancer cells.
Conclusion
We demonstrate NDS-enhanced efficiency of chemotherapy to a level that cannot be achieved by applying its components separately.
Keywords: apoptosis, BCL2, cationic liposomes, chemotherapy, cytotoxicity, lung cancer, MRP, multidrug resistance, nanoscale-based intracellular delivery
Lung cancer is the leading cause of cancer death in the USA. Small-cell lung carcinoma (SCLC) is the most aggressive type of lung cancer and is responsible for high mortality. Owing to the size and distribution of SCLC, cytoreductive surgery is not very effective for this disease and, therefore, chemotherapy and/or radiation are the treatment of choice. However, the efficacy of chemotherapy in lung cancer is limited by the development of cancer-cell resistance during treatment. To overcome this resistance, higher doses of the toxic anticancer drug is administered, thus resulting in adverse side effects on healthy organs. Two main mechanisms are responsible for the observed resistance: pump and nonpump resistance (FIGURE 1) [1,2]. Pump resistance is caused by membrane efflux pumps that decrease the anticancer-drug concentration inside the cells [3]. Preliminary studies showed that, in contrast to other multidrug-resistant (MDR) cancer cells, the main transporters responsible for the pump resistance in SCLC cells are the members of the so-called ‘multi-drug resistance-associated proteins’ (MRPs) (FIGURE 2) [2,4,5]. The P-glycoprotein efflux pump has a less important role in drug resistance of SCLC. Nonpump drug resistance is attributed primarily to the mechanisms responsible for the activation of anti-apoptotic cellular defense and the BCL2 protein is a key player in this defense [6–11]. It appears that the expression of genes and proteins responsible for pump and nonpump resistance increase in different cancer cells after treatment with an anticancer drug (FIGURE 2). Therefore, many widely spread types of human MDR cancers (lung, breast, colon and ovarian cancer) activate both pump and non-pump resistance in response to treatment with an anticancer drug. Consequently, simultaneous suppression of both types of cellular resistance is required to substantially enhance the efficacy of the treatment.
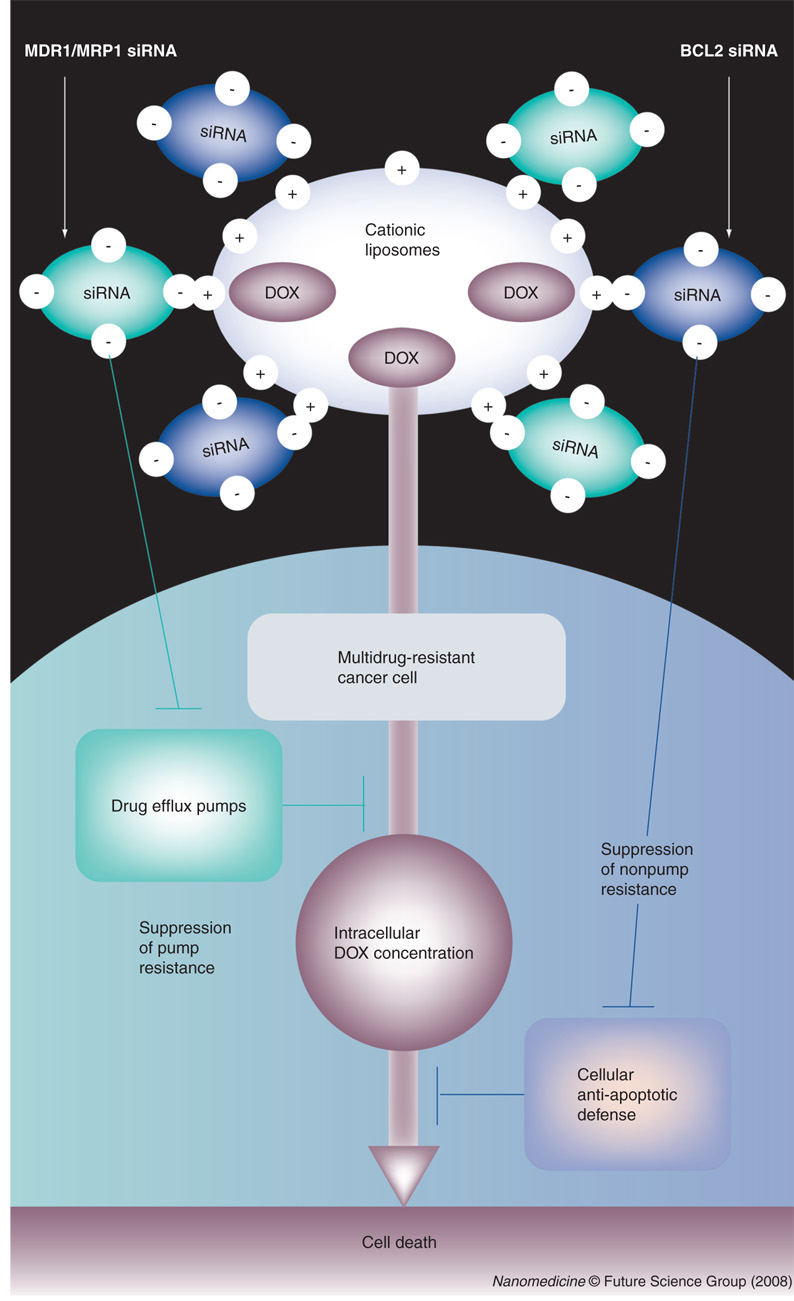
Figure 1. Co-delivery in one liposomal drug delivery system of doxorubicin simultaneously with siRNA targeted to MDR1/MRP1 and BCL2 mRNA enhances cell-death induction by increasing intracellular doxorubicin concentration and suppression of cellular anti-apoptotic defense.
DOX: Doxorubicin.
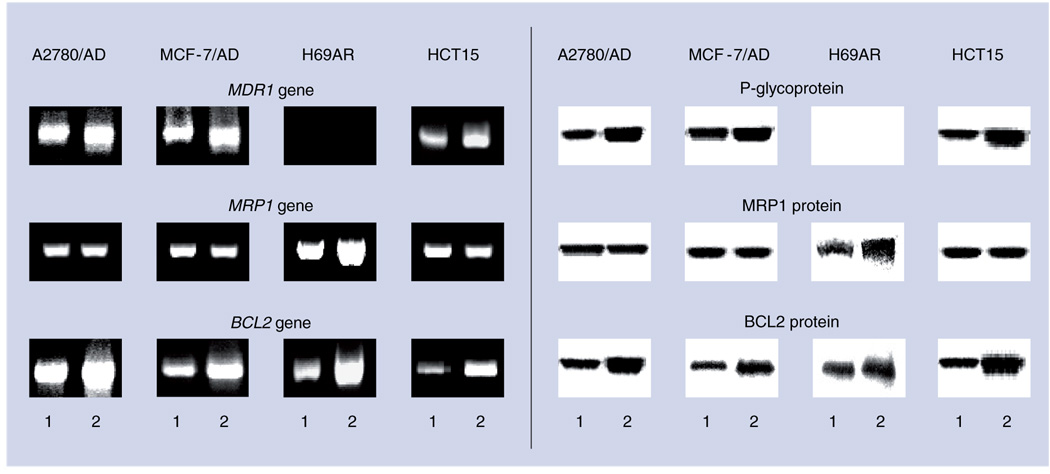
Figure 2. Simultaneous activation of pump and nonpump resistance in different multidrug-resistant human cancer cells.
Typical images of reverse transcription-PCR products (left panel) and corresponding proteins (western blotting, right panel) responsible for pump (MDR1 and MRP1 genes, p-glycoprotein and MRP1 protein) and nonpump (BCL2 gene and protein) resistance in human multidrug-resistant ovarian (A2780/AD), breast (MCF-7/AD), lung (H69AR) and colon (HCT15) cancer cells.
Considerable efforts have been made recently to suppress multidrug resistance and/or anti-apoptotic cellular defense [12–14]. These include: synthetic analogs of the BCL2 homology 3 (BH3) domain of the pro-apoptotic members of the BCL2 protein family, including BAK, BAX and BAD [7,10,15–20]; antisense oligonucleotides and small-interfering RNA (siRNA) targeted to BCL2, MDR1 and MRP mRNA [21–23], c-Jun NH2-terminal kinase [24]; ribozymetraditional drugs [25]; several drug groups from a traditional mitomycin C [26]; and the exotic plant stress hormones family of jasmonates [27]. However, these attempts have not demonstrated a high efficiency in terms of their anticancer effect. In our opinion, three main deficiencies in the previous approaches are primarily responsible for their relatively low efficacy in treating MDR cancers in general and SCLC in particular. First, in most cases, drug efflux pumps and anti-apoptotic cellular defense are suppressed separately [14,16–21,23–26]. However, the inhibition of only one contributor to cellular resistance is usually not sufficient for overcoming all mechanisms of cancer-cell resistance to chemotherapy. For instance, we found that an increase in intracellular drug concentration as a result of the suppression of drug efflux pumps usually leads to almost proportionate activation of anti-apoptotic cellular defense [2,28]. As a result, such an increase in the concentration does not result in a proportionate increase in cell death. Similarly, a suppression of only anti-apoptotic cell death is not sufficient for overcoming multidrug resistance [2,15,29,30]. Second, suppression of drug-efflux pumps alone without simultaneous induction of cell death signal is not sufficient for the effective killing of resistant cancer cells [1,14,21,23,24,26]. Third, even when suppressors of one or both types of resistance are used in combination with an anticancer drug, these components are usually delivered separately to cancer cells [26,31,32]. However, we believe that, to maximize the efficacy of the treatment, all cell-death inducer(s) and suppressor(s) of both types of cellular-drug resistance should be delivered simultaneously inside the cancer cell and active components should be released with a comparable profile [3,29,33]. Such spatial–temporal synchronization requires one complex system simultaneously encapsulating all the aforementioned active components.
Based on this analysis, we hypothesize that only simultaneous suppression of both pump and nonpump cellular resistance in combination with cell-death induction by an anticancer drug is able to significantly increase the efficacy of chemotherapy against potentially resistant lung cancers. Such an objective can only be achieved if an anticancer agent is delivered simultaneously in one multifunctional nanocarrier-based system in combination with other active ingredients that perform different specific functions for enhancing cellular uptake and efficiency of the main drug specifically in cancer cells and preventing the development and/or suppression of the existent drug resistance. In this study, we apply nanotechnology approaches to the development and evaluation of such multifunctional nano-therapeutics. We constructed a novel multifunctional nanocarrier-based delivery system (NDS), which provides co-delivery of an anticancer drug simultaneously with suppressors of pump and nonpump cellular resistance. The NDS contains the following components (FIGURE 1):
Cationic liposomes as a carrier
Doxorubicin (DOX) as a cell-death inducer/anticancer agent
siRNA targeted to MRP1 mRNA as a suppressor of drug-efflux pump (pump resistance) in SCLC
siRNA targeted to BCL2 mRNA as a suppressor of cellular anti-apoptotic defense (nonpump resistance).
This paper describes the results of the evaluation of the efficacy of the proposed drug-delivery system.
Material & methods
Cell line
Human MDR H69AR lung cancer, MCF-7/ AD breast cancer and HCT15 colon cancer cell lines were obtained from ATCC (Manassas, VA, USA). Human MDR A2780/AD ovarian cancer cells were obtained from Dr TC Hamilton (Fox Chase Cancer Center). Cells were cultured in RPMI 1640 medium (Sigma, St Louis, MO, USA) supplemented with 20% fetal bovine serum (Fisher Chemicals, Fairlawn, NJ, USA). Cells were grown at 37°C in a humidified atmosphere of 5% CO2 (v/v) in air. All experiments were performed on cells in the exponential growth phase.
Drug & siRNA
DOX was obtained from Sigma (St Louis). The sequences of the siRNA targeted to BCL2, MRP1 and MDR1 mRNA were: 5′-GUGAAGUCAACAUGCCUGCTT-3′, 5′-GGCUACAUUCAGAUGACACTT-3′ and 5’-AAAAUGUUGUCUGGACAAGCATT-3’, respectively. siRNA was synthesized by Applied Biosystems/Ambion (Austin, TX, USA). Mock nontargeting control siRNA with limited sequence similarity to known genes (Silencer® Negative Control) was obtained from Applied Biosystems/Ambion.
Multifunctional NDS
Cationic liposomes were prepared from positively charged 1,2-dioleoyl-3-trimethylammonium– propane (DOTAP; Avanti Polar Lipids, Alabaster, AL, USA) using the ethanol-injection method [34]. The formation of liposomes from DOTAP without a helper lipid has been documented in several studies [35–37]. Briefly, dry lipid was dissolved in 98% ethanol (10% from final volume) at room temperature and the dissolved mixture was added to 0.9% NaCl to a final lipid concentration of 10 mg/ml. Obtained liposomes were extruded gradually using 200- and 100-nm polycarbonate membranes at room temperature using an extruder device from Northern Lipids Inc. (Vancouver, BC, Canada) and were loaded passively with DOX (5 mg/ml) by mixing liposomes and DOX at 37°C for 40 min. Liposomes were separated from free drug by dialysis against 100 volumes 0.9% NaCl overnight at 4 °C. The encapsulation efficacy of DOX was approximately 70%. The average diameter of obtained particles was 100–140 nm. The aliquots of liposomes were destroyed in isopropanol in a ratio of 10:90 (liposomes/isopropanol) and the concentration of DOX in liposomes was determined by high-performance liquid chromatography using a symmetry C18 column (150 mm × 4.6 mm, Waters Corporation, Milford, MA, USA) operated at room temperature. The mobile phase consisted of 0.1% trifluoroacetic acid in water/ acetonitrile 25:75 v/v; the flow rate was set to 1.0 ml/min, wavelength 480 nm. The chromatographic instillation consisted of a Model 1525 pump (Waters Corporation, Milford, MA, USA), a Model 717 Plus auto-injector (Waters Corporation) and a Model 2487 variable wavelength UV detector (Waters Corporation) connected to the Millennium software. The siRNA (stock concentration, 370 µM) was prepared by being dissolved in RNAse-free water at room temperature. Cationic liposome:siRNA complexes with ± charge ratio 4:1 were formed by mixing appropriate amounts of siRNA and cationic liposomes in 0.9% NaCl [38,39]. This mixture was vortexed and incubated at room temperature for 30 min. The ± charge ratio is calculated as the total number of positive charges on the DOTAP molecules, divided by the total number of negative charges on the siRNA (with one siRNA molecule containing 42 negatively charged phosphate groups). The final siRNA concentration in the delivery system was 123 µM when MRP1 and BCL2 siRNA were used separately and 61.6 µM of each siRNA when they were used in combination.
Liposome size & zeta potential
Particle size was measured by dynamic-light scattering using a 90 Plus Particle Sizer Analyzer (Brookhaven Instruments Corp., New York, NY, USA). An aliquot of 40 µl of each sample was diluted in 2 ml of its external buffer. Zeta potential was measured on PALS Zeta Potential Analyzer (Brookhaven Instruments Corp.). Samples were taken as is and their volume was 1.5 ml. All measurements were carried out at room temperature. Each parameter was measured in triplicate and average values were calculated.
Intracellular localization of liposomes, siRNA & DOX
To analyze cellular internalization of liposomes, in aliquots of liposomal formulations, DOTAP was mixed with the fluorescent NBD-DOTAP (1-oleoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol- 4-yl)amino]hexanoyl]-3-trimethylammonium propane chloride salt, Avanti Polar Lipids) in a mole ratio of 100:1 DOTAP:NBD-DOTAP. NBD-DOTAP has maximums of excitation and emission of 460 and 534 nm, respectively (green fluorescence). A siGLO red transfection indicator (RNA duplex labeled with Pierce NuLight DY-547 fluorophore) was purchased from Dharmacon Inc. (Chicago, IL, USA) and was used to study siRNA delivery and cellular internalization. Cell nuclei were stained by Hoechst 33258 nuclear dye (Sigma). The fluorescent labels were visualized using a fluorescent microscope (Olympus, Center Valley, PA, USA).
Cytotoxicity
The cellular cytotoxicity of all studied formulations was assessed using a modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as described previously [2,30,40]. To measure cytotoxicity, H69AR human lung cancer cells were incubated separately in 96-well microtiter plate with different concentrations of all possible combinations of our NDS components, which resulted in a total of 15 separate series of experiments:
Control (fresh media)
Empty cationic liposomes
Free BCL2 siRNA
Free MRP1 siRNA
Free BCL2 siRNA–MRP1 siRNA
Free DOX
Cationic liposomes–DOX
Cationic liposomes–mock nontargeting control siRNA
Cationic liposomes–BCL2 siRNA
Cationic liposomes–MRP1 siRNA
Cationic liposomes–MRP1 siRNA–BCL2 siRNA
Cationic liposomes–DOX–BCL2 siRNA
Cationic liposomes–DOX–MRP1 siRNA
Cationic liposomes–BCL2 siRNA–MRP1 siRNA mixed with free DOX
Cationic liposomes–DOX–BCL2 siRNA– MRP1 siRNA in the cell growth medium
Control cells received an equivalent volume of fresh medium. The duration of incubation was 48 h. Based on these measurements, IC50 doses of free and liposomal formulations of all delivery systems (the concentrations of active ingredients necessary to inhibit the cell growth by 50%) were calculated as described previously [41]. In addition, the cytotoxicity of similar liposomal delivery systems containing DOX, siRNA targeted to MDR1 and BCL2 mRNA and their combinations was studied in human MDR A2780/AD ovarian and MCF-7/AD breast cancer cells.
Gene expression
Quantitative reverse transcription (RT)-PCR was used for the analysis in H69AR cell line expression of genes encoding MRP1 and BCL2 protein, as described previously [1,2]. Cells were incubated separately for 48 h with the following formulations: control (fresh media); cationic liposomes; free DOX; cationic liposomes containing DOX; cationic liposomes-mock non-targeting control siRNA; cationic liposomes containing MRP1 or BCL2 siRNA; and cationic liposomes containing DOX and MRP1 or BCL2 siRNA. Concentrations of DOTAP, DOX and siRNA were 0.07 mM, 34.5 µM and 123 µM, respectively. RNA was isolated using a RNeasy kit (Qiagen, Valencia, CA, USA). The following pairs of primers were used (5′ to 3′): MRP1 – ATG TCA CGT GGA ATA CCA GC (sense), GAA GAC TGA ACT CCC TTC CT (antisense); BCL2 – GGA TTG TGG CCT TCT TTG AG (sense), CCA AAC TGA GCA GAG TCT TC (antisense); β2-microglobulin (β2-m, internal standard) – ACC CCC ACT GAA AAA GAT GA (sense), ATC TTC AAA CCT CCA TGA TG (antisense). PCR products were separated in 4% NuSieve 3:1 Reliant® agarose gels (BMA, Rockland, ME, USA) in 1 × TBE (Tris/Borate/EDTA) buffer (0.089 M Tris/Borate, 0.002 M EDTA, pH 8.3; Research Organics Inc., Cleveland, OH, USA) by submarine electrophoresis. The gels were stained with ethidium bromide, digitally photographed and scanned using Gel Documentation System 920 (NucleoTech, San Mateo, CA, USA). Gene expression was calculated as the ratio of mean-band density of analyzed RT-PCR product to that of the internal standard (β2-m).
Protein expression
To confirm the RT-PCR data, the expression of MRP1 and BCL2 proteins was analyzed. The identification of these proteins was made by immunocytochemical staining of the human MDR H69AR SCLC cells. Cells were treated for 48 h with the following compositions: control (fresh media); empty cationic liposomes; DOX; cationic liposomes containing DOX; cationic liposomes containing siRNA targeted to MRP1 mRNA; cationic liposomes containing siRNA targeted to BCL2 mRNA; cationic liposomes containing DOX and siRNA targeted to MRP1 mRNA; and cationic liposomes containing DOX and siRNA targeted to BCL2 mRNA. Concentrations of DOTAP and DOX were 0.07 mM and 34.5 µM, respectively. Concentrations of siRNA were 123 µM when MRP1 and BCL2 siRNA were used separately and 61.6 µM of each siRNA when they were used in combination. Before staining, cells were washed three times in ice cold phosphate-buffered saline (PBS), then fixed with ice cold acetone at −20°C for 7 min. The slide chambers were then washed again three times with PBS and stained using RTU VECTASTAIN® Universal Elite ABC-Peroxidase Kit (Catalog no. PK7200, Vector Laboratories, Inc., Burlingame, CA, USA). Mouse monoclonal antibodies to MRP-1 (ab24102, 1:20 dilution, Abcam, Cambridge, MA, USA) and BCL2 protein (CP-B201, 1:80 dilution, Vector Laboratories) were used as primary antibodies for the detection of MRP1 and BCL2 proteins, respectively. Biotinylated anti-mouse IgG reagent (Vector Laboratories) and Horseradish Peroxidase (HRP)–Streptavidin Detection System ready-to-use stabilized ABC reagent (Vector Laboratories, Inc.) in combination with ImmPACT 3,3′-dichlorobenzidine (DAB) substrate kit for peroxidase (cat. no. SK-4105, Vector Laboratories, Inc.) were used for visualization. Nuclear staining was done with VECTOR Hematoxylin QS (cat. no. H-3404, Vector Laboratories, Inc.). The slides were cleared by washing in 95% ethanol, 100% ethanol and then xylene. Mounting was done with VectaMount permanent-mounting medium (cat. no. H-5000, Vector Laboratories, Inc.). After staining, the slides were analyzed by a light microscope (Olympus) and photographed.
The expression of p-glycoprotein, MRP1 and BCL2 proteins was also analyzed by western immunoblotting analysis. To this end, harvested cells were lysed in a RIPA buffer (Santa Cruz Biotechnologies) using a needle and syringe. Following incubation on ice for 45 min, the cells were centrifuged at 10,000 g for 10 min. Protein content in the supernatant was determined using the BCA Protein Assay Kit (Pierce, Rockford, IL, USA) and 50 µg of protein was run on a 15% sodium dodecyl sulphate (SDS) polyacrylamide gel immersed in Tris/Glycine/ SDS buffer (BioRad, Hercules, CA, USA) for 90 min at 70 V. Proteins were transferred to an Immobilon-P nitrocellulose membrane (Millipore, Bedford, MA, USA) in a Tris/ Glycine buffer (cat. no. 161–0771, BioRad) for 90 min at 100 V. The membrane was blocked in non-fat milk for 30 min at room temperature on a rotating shaker to prevent nonspecific binding, washed and incubated overnight with the rabbit primary anti-p-glycoprotein (code no. Z5116, 1:50 dilution, DAKO, Carpinteria, CA, USA), rabbit anti-Bcl-2 (cat. no. AAP070, 1:280 dilution, Stress Gen Biotechnologies, Victoria State, BC, Canada) and mouse anti-MRP1 (1:250 dilution, Chemicon International, Temecula, CA, USA) primary antibodies at 4°C. Following further washing, the membrane was immersed in a goat anti-rabbit or goat anti-mouse IgG biotinylated antibody (1:3000 dilution and 1:1000 dilution, respectively, BioRad) at room temperature for 1 h on a rotating shaker. Bands were visualized using an alkaline phosphatase color-development reagent (cat. no. 1706412, BioRad).
Apoptosis
The analysis of apoptosis was based on the detection of ssDNA and dsDNA breaks (nicks) by an in situ cell death-detection kit (Roche, Nutley, NJ, USA) using terminal deoxynucleotidyl transferase-mediated dUTP–fluorescein nick-end labeling (TUNEL) method, as described previously [7,42]. Briefly, cells were fixed, permeabilized and incubated with the TUNEL-reaction mixture. The label incorporated at the damaged sites of the DNA was visualized by a fluorescence microscope. Digital images of TUNEL-stained cells were scanned and intensity of fluorescence (apoptosis induction) was presented in relative units (the fluorescence intensity in control cells was set to 1 unit).
Statistical analysis
Data obtained were analyzed using descriptive statistics, single factor analysis of variance (ANOVA) and presented as mean value ± standard deviation (SD) from four to eight independent measurements in separate experiments.
Results
Characteristics of multifunctional NDS
The size of positively charged DOTAP liposomes after extrusion was approximately 100–140 nm and zeta potential was approximately +20 mV. Inclusion of DOX did not change the size and charge of the liposomes. Mixing of cationic liposomes with negatively charged siRNA in the ratio of 6:1 (lipid:siRNA v/v) led to the formation of larger DOTAP:siRNA complexes with the size of approximately 500 nm and decreased the resulting surface charge of the entire complex to +4 mV owing to the electrostatic interactions between lipids and siRNA.
Intracellular localization of liposomes, siRNA & DOX
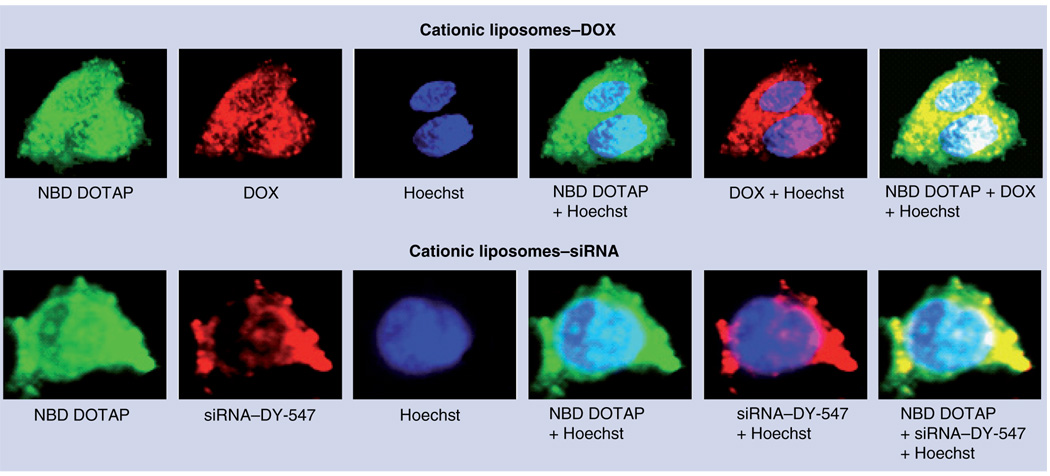
To evaluate the penetration of cationic liposomes into cancer cells and the intracellular localization of delivered siRNA and DOX, we used NBD DOTAP (green fluorescence) and DY-547 dye (red fluorescence) to label cationic liposomes and siRNA, respectively. DOX by itself possesses an intrinsic red fluorescence. MDR human H69AR lung cancer cells were incubated separately with free siRNA, cationic liposomes containing siRNA and similar cationic liposomes containing DOX. In addition, cell nuclei were stained with nuclear-specific dye Hoechst 33258 (blue fluorescence). Fluorescence was registered with a fluorescent microscope and images were overlaid digitally to analyze intracellular co-localization of liposomes and their active components (FIGURE 3). Superimposition of green and red fluorescence gives yellow color and enables the detection of cytoplasmic co-localization of cationic liposomes and siRNA or DOX. Superimposition of green and red with blue fluorescence enables us to detect nuclear localization of liposomes (cyan), DOX or siRNA (pink) or nuclear co-localization of liposomes with DOX and siRNA (white color). An absence of red fluorescence in cancer cells after incubation with free-labeled siRNA (data not shown) supports our previous finding and shows that free siRNA is unable to penetrate the plasma membrane of cancer cells [43,44]. Cationic liposomes were able to penetrate into cancer cells. They delivered their contents effectively into the cytoplasm (siRNA and DOX) and nuclei (DOX). These data clearly show that the proposed NDS can be used for co-delivery of anticancer drugs and siRNA into cancer cells.
Figure 3. Intracellular localization of cationic liposomes, siRNA and the anticancer drug doxorubicin.
Typical images of multidrug-resistant H69AR human lung cancer cells incubated with cationic liposomes containing DOX or siRNA. Liposomes were prepared using NBD DOTAP (1-oleoyl-2-[6-[(7-nitro-2-1,3-benzoxadiazol-4 yl)amino]hexanoyl]-3 trimethylammonium propane chloride salt, green fluorescence), siRNA was labeled with DY-547 (red fluorescence), DOX possesses an intrinsic red fluorescence. In addition, cell nuclei were stained with nuclear-specific dye Hoechst 33258 (blue fluorescence). Superimposition of images allows for detecting of cytoplasmic co-localization of liposomes with DOX or siRNA (yellow) and nuclear localization of liposomes (cyan) or DOX (pink). Nuclear co-localization of liposomes and DOX or siRNA gives a white color.
DOX: Doxorubicin.
Suppression of targeted mRNA & proteins
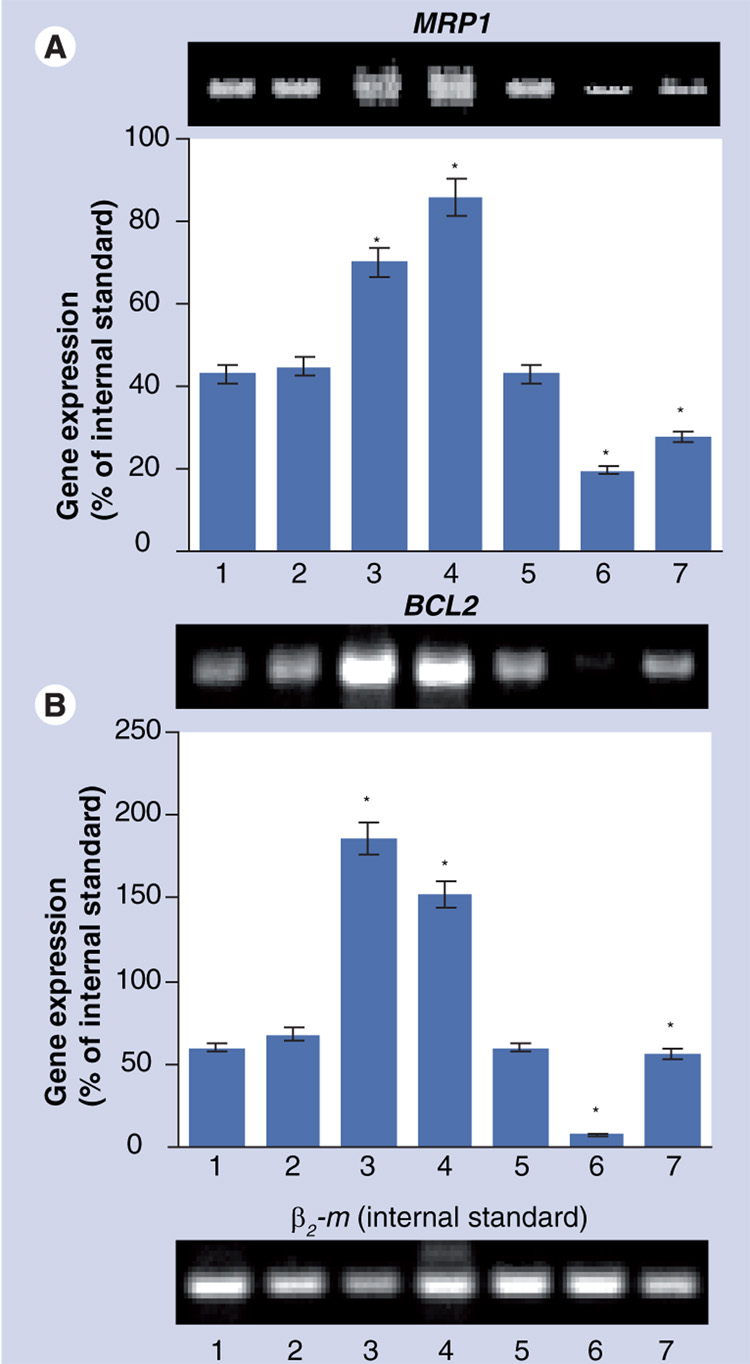
Two methods have been used to estimate the effectiveness of the suppression of targeted MRP1 and BCL2 proteins. First, the expression of targeted mRNA in MDR human lung cancer cells was measured by RT-PCR. The results of these measurements are shown in FIGURE 4. As expected, both MRP1 and BCL2 mRNA were expressed in these MDR cancer cells (FIGURE 4A & B, BAR 1). Exposure with empty cationic liposomes did not significantly change the expression of either type of mRNA (FIGURE 4A & B, Bar 2). Treatment of the cells with free DOX (FIGURE 4A & B, Bar 3) and liposomal DOX (FIGURE 4A & B, Bar 4) led to the significant overexpression of both MRP1 and BCL2 mRNA. These data support our previous findings that chemotherapy with an anticancer drug leads to the activation of both types of resistance: pump and nonpump resistance (FIGURE 2) [1–3,29,30]. After the delivery of DOX with cationic liposomes, both types of mRNA still remained overexpressed, however, the dynamic of changes in the expression was opposite for MRP1 and BCL2 mRNA (COMPARE BARS 3 & 4, FIGURE 4A & B). The expression of MRP1 mRNA after treatment with liposomal DOX was further elevated, whereas the expression of BCL2 was decreased when compared with treatment by free DOX. Incubation of cells with cationic liposomes complexed with mock nontargeting control siRNA does not change the expression of targeted MRP1 and BCL2 mRNA (FIGURE 4A & B, BAR 5). The delivery of siRNA by cationic liposomes led to the substantial suppression of targeted mRNA:MRP1 (FIGURE 4A, BAR 6) and BCL2 (FIGURE 4B, BAR 6). The combination of one type of siRNA targeted to MRP1 or BCL2 in one delivery system with DOX significantly decreased the overexpression of corresponding mRNA when compared with free and liposomal DOX without siRNA (COMPARE BAR 7 WITH BARS 3 & 4, FIGURE 4A & B). In fact, inclusion of siRNA targeted to MRP1 mRNA in the delivery system containing DOX led to a 2.5- and 3.1-times decrease in the expression of MRP1 when compared with free and liposomal DOX, respectively (p <0.05 in both cases). Similarly, BCL2 siRNA decreased the expression of targeted mRNA 3.3 and 2.7 times when compared with free and liposomal DOX, respectively (p < 0.05 in both cases).
Figure 4. Typical images of gel electrophoresis of the reverse transcription-PCR products and mRNA levels for MRP1 and BCL2 in H69AR human multidrug-resistant lung cancer cells.
Cells were incubated for 48 h with the indicated formulations. β2 microglobulin (β2 m) was used as an internal standard. Means ± standard deviation are shown. (A) 1: Control (fresh media); 2: Cationic liposomes; 3: Free DOX; 4: Cationic liposomes–DOX; 5: Cationic liposomes–mock nontargeting control siRNA; 6: Cationic liposomes–MRP1 siRNA; 7: Cationic liposomes–DOX–MRP1 siRNA. (B) 1: Control (fresh media); 2: Cationic liposomes; 3: Free DOX; 4: Cationic liposomes-DOX; 5: Cationic liposomes–mock nontargeting control siRNA; 6: Cationic liposomes–BCL2 siRNA; 7: Cationic liposomes–DOX–BCL2 siRNA.
*p <0.05 when compared with control.
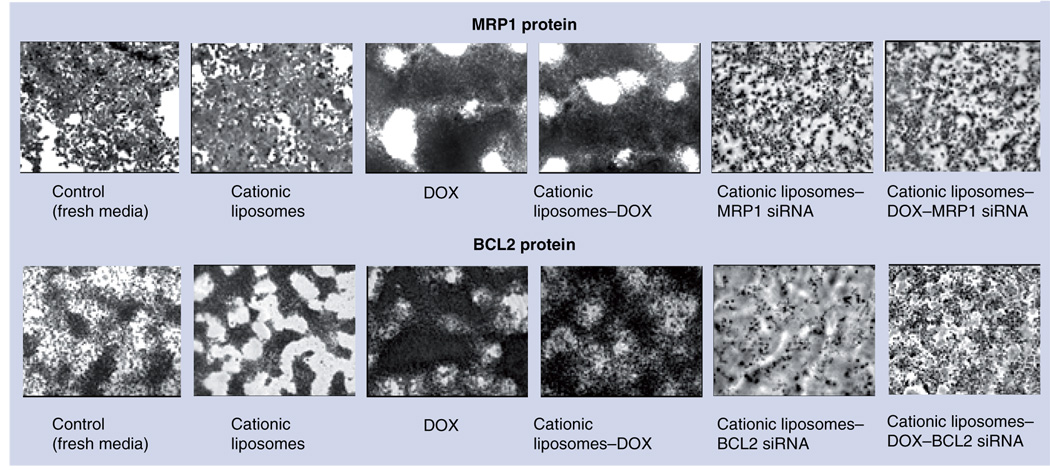
Analysis of protein expression (FIGURE 5) supports data obtained using the RT-PCR method (FIGURE 4). Again, treatment with the anticancer drug (DOX) induced overexpression of proteins responsible for active drug efflux (MRP1) and cellular anti-apoptotic defense (BCL2). Incubation of MDR SCLC cells with the NDS containing the same concentration of DOX but in the presence of siRNA targeted to MRP1 mRNA (suppressor of pump resistance) and BCL2 mRNA (suppressor of nonpump resistance) led to the suppression of MRP1 and BCL2 proteins, respectively.
Figure 5. Typical images of in multidrug-resistant H69AR human lung cancer cells stained with antibody against MRP1 (top panel) and BCL2 (bottom panel) proteins.
Dark color indicates high protein concentration. Cells were incubated for 48 h with the indicated formulations. DOX: Doxorubicin.
Apoptosis induction
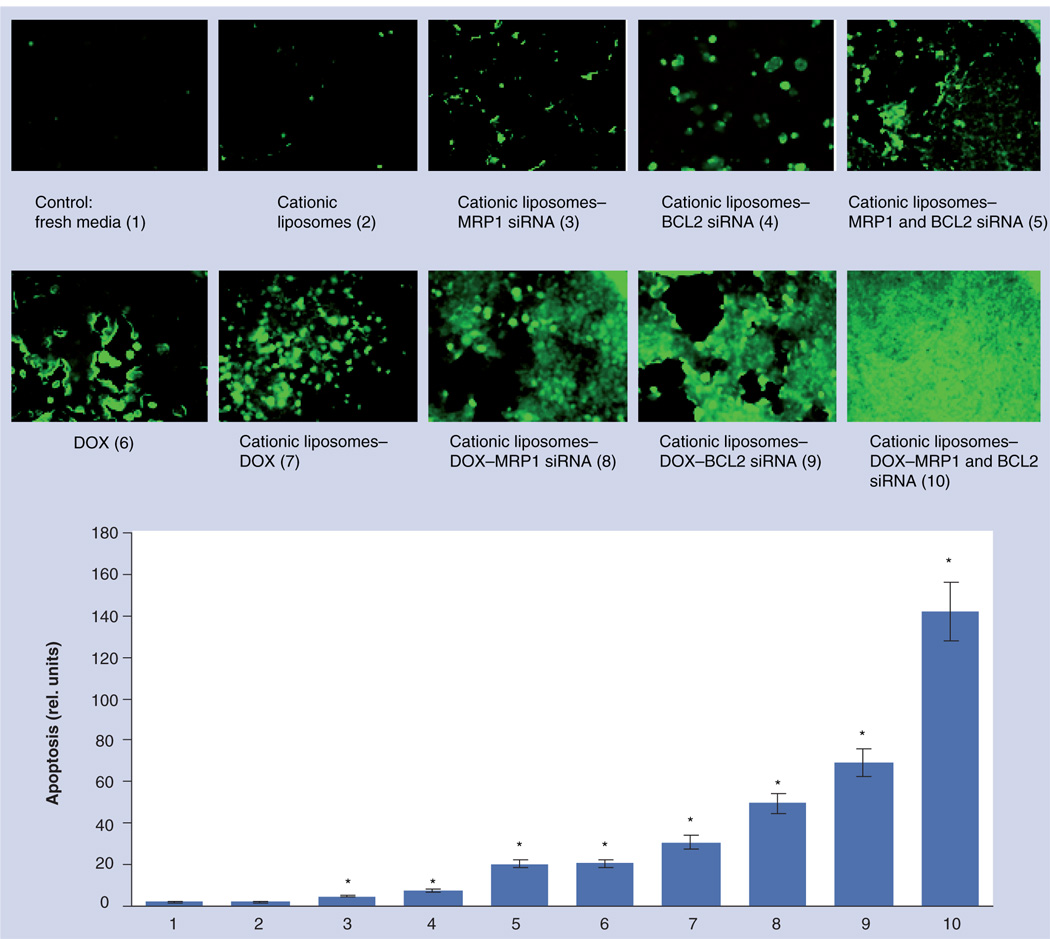
Apoptosis was studied in cells incubated for 48 h with different formulations and stained by TUNEL (FIGURE 6). Cationic liposomes containing only MRP1 or BCL2 siRNA or their combination were able to slightly induce cell death by apoptosis in MDR human lung cancer cells (FIGURE 6, UPPER PANEL, BARS 3 & 4). It is interesting that the degree of apoptosis provoked by the combination of both types of siRNA in one liposomal delivery system was comparable with that induced by free DOX (COMPARE BARS 5 & 6, FIGURE 6). Delivery of DOX by liposomes enhanced its cell death-inducing ability. The delivery of DOX by liposomes in combination with siRNA targeted to MRP1 or BCL2 enhanced the degree of apoptosis. Simultaneous cell-death induction by DOX in combination with the suppression of pump and nonpump cellular resistance led to the induction of apoptosis to a level that cannot be achieved by each component of the complex delivery system applied separately (FIGURE 6, BAR 10; p <0.05 when compared with all other formulations containing DOX and control).
Figure 6. Apoptosis induction in multidrug-resistant H69AR human lung cancer cells.
Typical fluorescent microscopy images of cancer cells incubated for 48 h with the indicated formulations and stained by TUNEL. Quantitative analysis of the intensity of fluorescence (apoptosis induction) is presented on the bottom panel. Means ± standard deviation are shown.
*p < 0.05 when compared with control.
Cytotoxicity
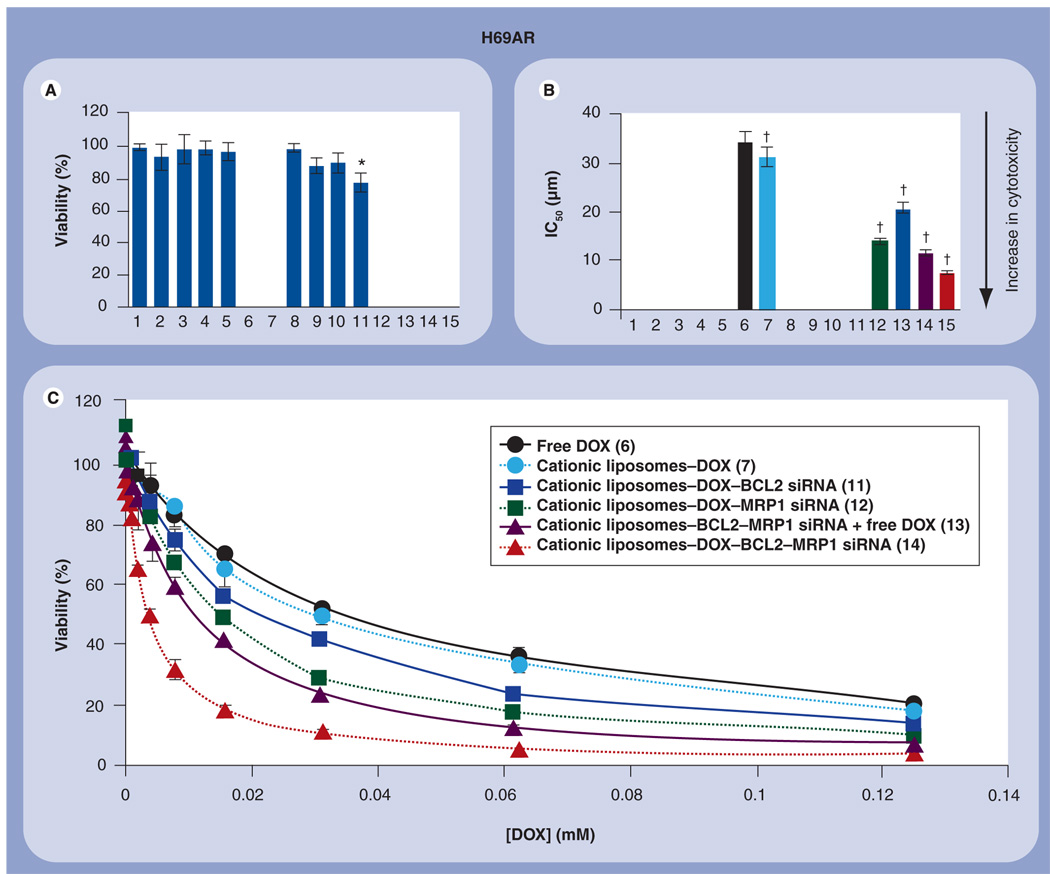
Cytotoxicity of different formulations was analyzed by a modified MTT assay (FIGURE 7). Empty liposomes and free siRNA were nontoxic (FIGURE 7A, BARS 1–5). Cationic liposomes-mock nontargeting control siRNA complexes did not influence viability of cancer cells (FIGURE 7, BAR 8). Although liposomal siRNA targeted to MRP1 and BCL2 mRNA slightly (but statistically significantly; p <0.05) induced apoptosis in its initial stages, as detected by the TUNEL staining (FIGURE 6, BARS 3 & 4), these liposomal siRNA only slightly, yet statistically insignificantly (p > 0.05), decreased the cell viability. It seems that moderate DNA strand breaks detected by the TUNEL reaction on the initial states of apoptosis were later compensated and did not result in substantial cell death. A moderate (~22%) but statistically significant decrease in the viability of MDR human lung cancer cells was found after their incubation for 48 h with cationic liposomes containing a combination of MRP1 and BCL2 siRNA (FIGURE 4A, BAR 11). As expected, delivery of DOX by liposomes slightly (~10%) but statistically significantly increased its toxicity (FIGURE 7B, BAR 7). A further improvement in toxicity of liposomal DOX was achieved by incorporating MRP1 or BCL2 siRNA in the same delivery system. The IC50 of liposomal DOX combined with MRP1 or BCL2 siRNA was approximately 60 and 40% less than free DOX, respectively (p < 0.05 in both cases). The combination of liposomal MRP1 and BCL2 siRNA with free DOX was more effective when compared with liposomal DOX combined with only one type of siRNA. The maximal enhancement in cytotoxicity was achieved under a concurrent cell-death induction by DOX and simultaneous suppression of pump (by MRP1 siRNA) and nonpump (by BCL2 siRNA) resistance (FIGURE 7B & C). The IC50 dose of this combination was only approximately 20% of that in free DOX (COMPARE BAR 15 WITH BAR 6 IN FIGURE 7B & CORRESPONDING CURVES IN FIGURE 7C). In other words, cytotoxicity of liposomes containing DOX and two types of siRNA targeted against MRP1 and BCL2 mRNA was almost 4.5-times higher than that of free DOX, 4.1-times higher than liposomal DOX, 1.8–2.7-times higher than liposomal DOX formulation containing only one type of siRNA and more than 1.5-times higher that the mixture of both liposomal siRNA with free DOX.
Figure 7. Viability of multidrug-resistant H69AR human lung cancer cells incubated 48 h with the indicated formulations.
(A) Cytotoxicity of formulations that do not contain DOX; (B) cytotoxicity of formulations that contain DOX; (C) actual dose–response curves of formulations that contain DOX. The concentration of siRNA and composition of cationic liposomes in all formulations were the same. Means ± standard deviation are shown. 1: Control (fresh media); 2: Empty cationic liposomes; 3: Free BCL2 siRNA; 4: Free MRP1 siRNA; 5: Free BCL2 siRNA–MRP1 siRNA; 6: Free DOX; 7: Cationic liposomes–DOX; 8: Cationic liposomes–mock nontargeting control siRNA; 9: Cationic liposomes–BCL2 siRNA; 10: Cationic liposomes–MRP1 siRNA; 11: Cationic liposomes-MRP1 siRNA–BCL2 siRNA; 12: Cationic liposomes-DOX-BCL2 siRNA; 13: Cationic liposomes–DOX–MRP1 siRNA; 14: Cationic liposomes–BCL2 siRNA–MRP1 siRNA + Free DOX; 15: Cationic liposomes–DOX–BCL2 siRNA–MRP1 siRNA.
*p <0.05 when compared with control; ‡p <0.05 when compared with free DOX.
DOX: Doxorubicin.
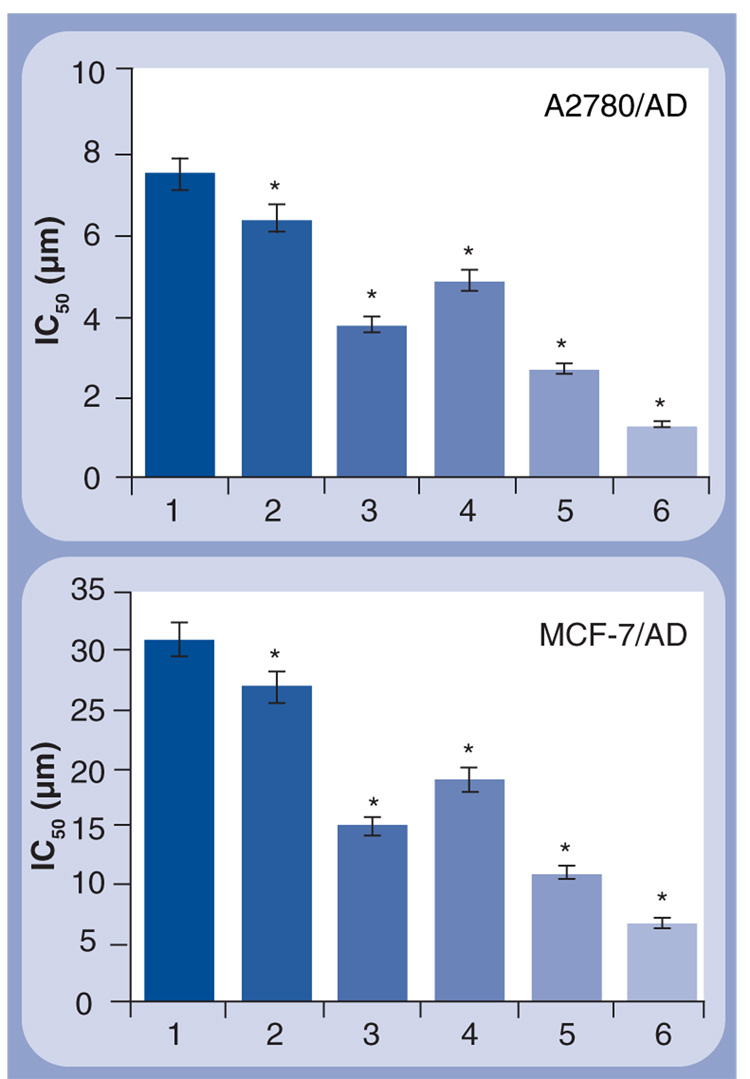
To show that the proposed concept of simultaneous apoptosis induction by the anticancer drug and suppression of pump and nonpump resistance is not limited to a single cell type, we measured the cytotoxicity of similar cationic liposomes–DOX–siRNA complexes in human MDR A2780/AD ovarian and MCF-7/AD breast cancer cells. In this series of experiments, we used siRNA targeted to BCL2 mRNA to suppress nonpump resistance and siRNA targeted to MDR1 mRNA encoding p-glycoprotein efflux pump. The predominant role of p-glycoprotein encoded by the MDR1 gene in the development of pump resistance under the action of DOX in these cells (FIGURE 2) served as a reason for the use of MDR1 siRNA instead of MRP1-targeted siRNA. The results of these series (FIGURE 8) support our finding on MDR lung cancer cells. The combination in one liposomal drug-delivery system of the anticancer drug (DOX) with suppressors of pump (MDR1 siRNA) and nonpump (BCL2 siRNA) resistance led to the enhancement of the cytotoxicity of DOX to a level that cannot be achieved by each component applied separately or by a combination of liposomal siRNA and free DOX.
Figure 8. Cytotoxicity of different formulations in human multidrug-resistant ovarian (A2780/AD) and breast (MCF-7/AD) cancer cells.
The cells were incubated for 48 h with the indicated formulations. The concentration of siRNA and composition of cationic liposomes in all formulations were the same. Means ± standard deviation are shown. 1: Free DOX; 2: Cationic liposomes–DOX; 3: Cationic liposomes–BCL2 siRNA; 4: Cationic liposomes–DOX–MDR1 siRNA; 5: Cationic liposomes-MDR1 siRNA–BCL2 siRNA + Free DOX; 6: Cationic liposomes–DOX–MDR1 siRNA–BCL2 siRNA.
*p <0.05 when compared with free DOX.
Discussion
The effectiveness of chemotherapy is often constrained by the limited accumulation of active ingredients in MDR cancer cells. The accumulation of low-molecular-weight anticancer drugs is restricted by the activation of drug-efflux pumps in MDR cancer cells. By contrast, cellular uptake of relatively high molecular-weight components, including antisense oligonucleotides, siRNA and other nucleotide-based therapeutics, is limited by their intrinsic characteristics (i.e., size, charge and so on). To simultaneously solve both problems and enhance the efficacy of chemotherapy of drug-resistant cancers, we are proposing co-delivery of an anticancer drug with siRNA-based suppressors of pump and nonpump cellular resistance. We constructed a multifunctional NDS that consisted of cationic liposomes as a carrier, DOX as a cell death induces/anticancer agent, siRNA targeted to MRP1 mRNA as a suppressor of pump resistance and siRNA targeted to BCL2 mRNA as a suppressor of nonpump resistance. This NDS was evaluated in MDR lung cancer cells. The results of experimental testing of this multifunctional system showed that cationic liposomes were able to deliver siRNA and DOX efficiently inside MDR lung cancer cells. Simultaneous delivery of suppressors of pump and nonpump resistance in combination with the anticancer drug led to the effective apoptosis induction and killing of drug-resistant lung cancer cells. The data obtained also support our hypothesis that simultaneous suppression of both pump and nonpump cellular resistance in combination with cell-death induction by an anticancer drug is able to effectively kill MDR lung cancers.
MRP proteins (including MRP1) are members of the superfamily of ATP-binding cassette (ABC) transporters that transport various molecules across extra- and intracellular membranes. MRP proteins are mainly responsible for the pump resistance in SCLC cells [2,4,5]. The main role of the MRP1 protein in the development of multidrug resistance in SCLC is active efflux of anticancer drugs out from the cells, decreasing their intracellular concentration, thus limiting their cell death-inducing activity. However, MRP proteins can have other roles related to drug metabolism and drug detoxification inside cancer cells. In particular, MRP proteins can transfer drugs (conjugated after enzymatic inactivation and degradation) outside the cells or into cellular organelles for further degradation [45–49]. Similar mechanisms also protect cells from endogenously generated cytotoxic substances. One can hypothesize that the suppression of MRP1 protein by disturbing such cellular-protective mechanisms can potentially induce apoptosis in cancer cells even in the absence of an anticancer drug. The results of the present study support such an assumption and show that siRNA targeted to MRP1 mRNA delivered into lung cancer cells by a cationic nanocarrier was able to induce apoptosis in SCLC cells. However, the level of apoptosis induced by the suppression of MRP1 protein alone was substantially lower when compared with the degree of apoptosis induced by liposomal DOX or the complex delivery system that includes DOX in combination with one or two types of siRNA (i.e., liposomal DOX combined with siRNA targeted to BCL2 mRNA or/and siRNA targeted to MRP1 siRNA).
A major function of BCL2 protein in the cellular anti-apoptotic defense includes the prevention of cytochrome c release from the mitochondrion [8,11,50,51]. Consequently, the suppression of BCL2 protein and associated leakage of cytochrome c into the cytosol leads to the formation of an apoptosome – the combination of cytochrome c, procaspase 9, dATP and apoptotic protease-activating factor-1. This converts inactive procaspase 9 into its active form. Active caspase 9 initiates a cascade of downstream caspases and these caspases, in turn, activate apoptosis [45]. Therefore, the suppression of BCL2 protein alone, even without an anticancer drug, is able to induce apoptosis, probably by triggering the aforementioned caspase-dependent mechanism initiated by the release of mitochondrial cytochrome c into the cytosol. However, as can be seen from the present experimental results, the level of apoptosis achieved by this mechanism is significantly lower when compared with cell death induced by the combination of siRNA targeted to BCL2 mRNA with the anticancer drug.
As expected, free and liposomal DOX were able to induce cell death in MDR lung cancer cells. However, this induction was accompanied by the simultaneous activation of pump and non-pump resistance associated with overexpression of MRP1 and BCL2 proteins. The activation of both types of cellular resistance decreased the efficiency of DOX as an anticancer drug. By contrast, the suppression of pump and nonpump resistance enhanced its anticancer activity and led to the effective induction of cell death in MDR cancer cells.
Many different nanoscale delivery systems, including polymer- and lipid-based nano-particles, liposomes and organic–inorganic hybrid carriers, have been used successfully for the delivery of anticancer drugs and siRNA [3,29,33,52–59]. However, despite the high efficiency of certain carriers in terms of delivery of either siRNA or a drug, the combination in one delivery system of siRNA as suppressors of cellular resistance and an anticancer drug as a cell-death inducer remains unexploited. In the present paper, we used cationic liposomes for co-delivery of two types of siRNA and an anticancer drug into cancer cells. A liposomal delivery system was chosen because of its simplicity and high efficiency for the in vitro delivery of both the water-soluble drug and siRNA. This work was designed as a proof-of-concept study, in which we examined the effectiveness of a novel approach to treatment of MDR lung and other cancer cells. The selection of the most efficient delivery system for the delivery of two different types of siRNA and an anticancer drug in vivo requires a separate extensive study. It is clear, however, that a simple liposomal system used in the present research cannot be used effectively in vivo. We have already started investigations for the comparison of different known and novel nanoscale-based systems as well as various routes of their administration to select a most effective carrier and method of its injection for the delivery of anticancer drugs and other active ingredients [3,44,60]. We have also studied previously the effectiveness of antisense oligonucleotides targeted to MDR1, MRP1 and BCL2 as suppressors of pump and nonpump resistance [1,2]. A synthetic analog of BH3 peptide has been used previously for the suppression of anti-apoptotic cellular defense [7,10,15–19]. Other agents have also been used for the suppression of pump cellular resistance [12,14,24–27]. The final selection of such components designed for the simultaneous suppression of pump and nonpump cellular resistance and cell-death induction is the aim of the following extensive investigations. However, the results of the present proof-of-concept study clearly show that this direction in anticancer research is worth studying and can lead to novel effective approaches in cancer treatment and anticancer delivery systems that can be used effectively in clinical practice.
The results of the present study show that simultaneous suppression of pump and non-pump resistance enhanced toxicity of DOX substantially. Consequently, such an increase in the DOX efficacy may also enhance its side effects in vivo. The possibility of severe adverse effects of the proposed siRNA–DOX combination requires local or targeted systemic delivery of active ingredients specifically to tumor cells. Such tumor-targeting delivery systems are being developed currently in our laboratory [13,15,29,40,42]. In the future, for in vivo experiments for the treatment of an orthotopic model of lung tumor, we are planning to use local targeted delivery of siRNA and DOX specifically to lung tumor cells.
Therefore, we were able to verify our hypothesis and show that cell-death induction by an anticancer drug in combination with the suppression of both pump and nonpump resistance is required for effective killing of MDR cancer cells. Although each active ingredient (DOX, MRP1 and BCL2 siRNA) alone delivered by liposomes was capable of inducing cell death in MDR cancer cells, only the co-delivery of all active components in one multifunctional NDS improved cellular drug uptake and led to the simultaneous cell-death induction and suppression of drug cellular resistance, as well as enhanced the efficacy of chemotherapy of MDR cancer cells substantially. The experiments on other MDR human cancer cells (breast and ovarian) support the data obtained on lung cancer cells and show that our hypothesis is not limited to lung cancer but has a more general application and can probably be applied to other, if not all, cancer cells. The comparison of the present results obtained with a liposomal delivery system with our previous studies with different polymeric delivery systems [3,15,33,40,44,61] and different suppressors of cellular resistance [7,13,15,29,42,45,62] show that the enhancement of cytotoxicity of anticancer drugs depends mostly on the degree of the suppression of pump and nonpump resistance but not on the method that is used for such a suppression.
Conclusion
A simultaneous co-delivery of DOX as a cell-death inducer/anticancer agent with siRNA targeted to MRP1 mRNA as a suppressor of drug-efflux pumps (pump resistance) and siRNA targeted to BCL2 mRNA as a suppressor of cellular anti-apoptotic defense (nonpump resistance) by cationic nanocarriers-enhanced efficacy of chemotherapy to a level that cannot be achieved by separate treatment with an anticancer drug or siRNA alone.
Future perspective
A strategy that includes the simultaneous suppression of drug-efflux pumps and anti-apoptotic cellular defense combined with cell-death induction can probably be applied to other, if not all, MDR cancers. However, an exceptionally high cytotoxicity of an anticancer drug combined with suppressors of pump and nonpump resistance requires a special targeted-delivery system that will deliver all these components specifically to tumor cells, preventing their accumulation in healthy organs. The further enhancement of the treatment of MDR lung cancer may also require local inhalatory co-delivery of anticancer drug(s) and suppressors of pump and nonpump resistance directly to lung tumor cells and prevent their penetration into the systemic circulation. The development and testing of such advanced complex anticancer delivery systems should be an immediate task for research in cancer chemotherapy.
Executive summary
A novel nanomedicine approach for the treatment of multidrug-resistant cancer by combining an anticancer drug and suppressors of cellular resistance within one multifunctional nanocarrier-based delivery system was developed.
The system consists of cationic liposomes (carrier), doxorubicin (anticancer drug), siRNA targeted to mRNA-encoding proteins responsible for the cellular drug efflux and antiapoptotic cellular defense (suppressors of pump and nonpump cellular resistance, respectively).
For the treatment of multidrug-resistant lung cancer, the system includes siRNA targeted to MRP1 and BCL2 mRNA, whereas breast and ovarian cancers require siRNA targeted to MDR1 and BCL2 mRNA.
The developed system enhanced efficiency of chemotherapy to a level that cannot be achieved by applying any active component separately.
Footnotes
Financial & competing interests disclosure
This research was supported by Rutgers, The State University of New Jersey, NIH grants CA100098 and CA111766 from the National Cancer Institute and by LCD-23812-N grant from the American Lung Association. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Bibliography
- 1.Pakunlu RI, Cook TJ, Minko T. Simultaneous modulation of multidrug resistance and antiapoptotic cellular defense by MDR1 and BCL-2 targeted antisense oligonucleotides enhances the anticancer efficacy of doxorubicin. Pharm. Res. 2003;20(3):351–359. doi: 10.1023/a:1022687617318. [DOI] [PubMed] [Google Scholar]
- 2.Pakunlu RI, Wang Y, Tsao W, Pozharov V, Cook TJ, Minko T. Enhancement of the efficacy of chemotherapy for lung cancer by simultaneous suppression of multidrug resistance and antiapoptotic cellular defense: novel multicomponent delivery system. Cancer Res. 2004;64(17):6214–6224. doi: 10.1158/0008-5472.CAN-04-0001. [DOI] [PubMed] [Google Scholar]
- 3.Minko T, Khandare JJ, Vetcher AA, et al. Multifunctional nanotherapeutics for cancer (Chapter 10) In: Torchilin VP, editor. Multifunctional Pharmaceutical Nanocarriers. Series Fundamental Biomedical Technologies. New York, NY, USA: Springer; 2008. [Google Scholar]
- 4.Hsia TC, Lin CC, Wang JJ, Ho ST, Kao A. Relationship between chemotherapy response of small cell lung cancer and P-glycoprotein or multidrug resistance-related protein expression. Lung. 2002;180(3):173–179. doi: 10.1007/s004080000091. [DOI] [PubMed] [Google Scholar]
- 5.Scagliotti GV, Novello S, Selvaggi G. Multidrug resistance in non-small-cell lung cancer. Ann. Oncol. 1999;10 Suppl. 5:S83–S86. doi: 10.1093/annonc/10.suppl_5.s83. [DOI] [PubMed] [Google Scholar]
- 6.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281(5381):1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 7.Dharap SS, Chandna P, Wang Y, et al. Molecular targeting of BCL2 and BCLXL proteins by synthetic BCL2 homology 3 domain peptide enhances the efficacy of chemotherapy. J. Pharmacol. Exp. Ther. 2006;316(3):992–998. doi: 10.1124/jpet.105.094243. [DOI] [PubMed] [Google Scholar]
- 8.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13(15):1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 9.Kassim SK, Ali HS, Sallam MM, et al. Increased bcl-2 expression is associated with primary resistance to chemotherapy in human epithelial ovarian cancer. Clin. Biochem. 1999;32(5):333–338. doi: 10.1016/s0009-9120(99)00026-0. [DOI] [PubMed] [Google Scholar]
- 10.Minko T, Dharap SS, Fabbricatore AT. Enhancing the efficacy of chemotherapeutic drugs by the suppression of antiapoptotic cellular defense. Cancer Detect. Prev. 2003;27(3):193–202. doi: 10.1016/s0361-090x(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 11.Reed JC. Dysregulation of apoptosis in cancer. J. Clin. Oncol. 1999;17(9):2941–2953. doi: 10.1200/JCO.1999.17.9.2941. [DOI] [PubMed] [Google Scholar]
- 12.Aszalos A, Ross DD. Biochemical and clinical aspects of efflux pump related resistance to anti-cancer drugs. Anticancer Res. 1998;18(4C):2937–2944. [PubMed] [Google Scholar]
- 13.Minko T, Dharap SS, Pakunlu RI, Wang Y. Molecular targeting of drug delivery systems to cancer. Curr. Drug Targets. 2004;5(4):389–406. doi: 10.2174/1389450043345443. [DOI] [PubMed] [Google Scholar]
- 14.Takara K, Sakaeda T, Okumura K. An update on overcoming MDR1-mediated multidrug resistance in cancer chemotherapy. Curr. Pharm. Des. 2006;12(3):273–286. doi: 10.2174/138161206775201965. [DOI] [PubMed] [Google Scholar]
- 15.Chandna P, Saad M, Wang Y, et al. Targeted proapoptotic anticancer drug delivery system. Mol. Pharm. 2007;4(5):668–678. doi: 10.1021/mp070053o. [DOI] [PubMed] [Google Scholar]
- 16.Cosulich SC, Worrall V, Hedge PJ, Green S, Clarke PR. Regulation of apoptosis by BH3 domains in a cell-free system. Curr. Biol. 1997;7(12):913–920. doi: 10.1016/s0960-9822(06)00410-6. [DOI] [PubMed] [Google Scholar]
- 17.Dharap SS, Minko T. Targeted proapoptotic LHRH-BH3 peptide. Pharm. Res. 2003;20(6):889–896. doi: 10.1023/a:1023839319950. [DOI] [PubMed] [Google Scholar]
- 18.Dharap SS, Qiu B, Williams GC, Sinko P, Stein S, Minko T. Molecular targeting of drug delivery systems to ovarian cancer by BH3 and LHRH peptides. J. Control. Release. 2003;91(1–2):61–73. doi: 10.1016/s0168-3659(03)00209-8. [DOI] [PubMed] [Google Scholar]
- 19.Holinger EP, Chittenden T, Lutz RJ. Bak BH3 peptides antagonize Bcl-xL function and induce apoptosis through cytochrome c-independent activation of caspases. J. Biol. Chem. 1999;274(19):13298–13304. doi: 10.1074/jbc.274.19.13298. [DOI] [PubMed] [Google Scholar]
- 20.Lutz RJ. Role of the BH3 (Bcl-2 homology 3) domain in the regulation of apoptosis and Bcl-2-related proteins. Biochem. Soc. Trans. 2000;28(2):51–56. doi: 10.1042/bst0280051. [DOI] [PubMed] [Google Scholar]
- 21.Jekerle V, Kassack MU, Reilly RM, Wiese M, Piquette-Miller M. Functional comparison of single- and double-stranded mdr1 antisense oligodeoxynucleotides in human ovarian cancer cell lines. J. Pharm. Pharm. Sci. 2005;8(3):516–527. [PubMed] [Google Scholar]
- 22.Lopes de Menezes DE, Hu Y, Mayer LD. Combined treatment of Bcl-2 antisense oligodeoxynucleotides (G3139), p-glycoprotein inhibitor (PSC833), and sterically stabilized liposomal doxorubicin suppresses growth of drug-resistant growth of drug-resistant breast cancer in severely combined immunodeficient mice. J. Exp. Ther. Oncol. 2003;3(2):72–82. doi: 10.1046/j.1359-4117.2003.01075.x. [DOI] [PubMed] [Google Scholar]
- 23.Wu H, Hait WN, Yang JM. Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res. 2003;63(7):1515–1519. [PubMed] [Google Scholar]
- 24.Zhou J, Liu M, Aneja R, Chandra R, Lage H, Joshi HC. Reversal of P-glycoprotein-mediated multidrug resistance in cancer cells by the c-Jun NH2-terminal kinase. Cancer Res. 2006;66(1):445–452. doi: 10.1158/0008-5472.CAN-05-1779. [DOI] [PubMed] [Google Scholar]
- 25.Hatanaka H, Abe Y, Naruke M, et al. Modulation of multidrug resistance in a cancer cell line by anti-multidrug resistance-associated protein (MRP) ribozyme. Anticancer Res. 2001;21(2A):879–885. [PubMed] [Google Scholar]
- 26.Ihnat MA, Nervi AM, Anthony SP, et al. Effects of mitomycin C and carboplatin pretreatment on multidrug resistance-associated P-glycoprotein expression and on subsequent suppression of tumor growth by doxorubicin and paclitaxel in human metastatic breast cancer xenografted nude mice. Oncol. Res. 1999;11(7):303–310. [PubMed] [Google Scholar]
- 27.Flescher E. Jasmonates in cancer therapy. Cancer Lett. 2007;245(1–2):1–10. doi: 10.1016/j.canlet.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Minko T, Kopeckova P, Kopecek J. Comparison of the anticancer effect of free and HPMA copolymer-bound adriamycin in human ovarian carcinoma cells. Pharm. Res. 1999;16(7):986–996. doi: 10.1023/a:1018959029186. [DOI] [PubMed] [Google Scholar]
- 29.Minko T, Pakunlu RI, Wang Y, Khandare JJ, Saad M. New generation of liposomal drugs for cancer. Anticancer Agents Med. Chem. 2006;6(6):537–552. doi: 10.2174/187152006778699095. [DOI] [PubMed] [Google Scholar]
- 30.Pakunlu RI, Wang Y, Saad M, Khandare JJ, Starovoytov V, Minko T. In vitro and in vivo intracellular liposomal delivery of antisense oligonucleotides and anticancer drug. J. Control. Release. 2006;114:153–162. doi: 10.1016/j.jconrel.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Lopes de Menezes DE, Hudon N, McIntosh N, Mayer LD. Molecular and pharmacokinetic properties associated with the therapeutics of bcl-2 antisense oligonucleotide G3139 combined with free and liposomal doxorubicin. Clin. Cancer Res. 2000;6(7):2891–2902. [PubMed] [Google Scholar]
- 32.Lopez-Barcons LA, Polo D, Llorens A, Reig F, Fabra A. Targeted adriamycin delivery to MXT-B2 metastatic mammary carcinoma cells by transferrin liposomes: effect of adriamycin ADR-to-lipid ratio. Oncol. Rep. 2005;2005(5):1337–1343. [PubMed] [Google Scholar]
- 33.Minko T, Khandare J, Jayant S. Polymeric drugs. In: Matyjaszewski K, Gnanou Y, Leibler L, editors. Macromolecular Engineering: From Precise Macromolecular Synthesis to Macroscopic Material Properties and Application. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co.; 2007. [Google Scholar]
- 34.Schroeder A, Avnir Y, Weisman S, et al. Controlling liposomal drug release with low frequency ultrasound: mechanism and feasibility. Langmuir. 2007;23(7):4019–4025. doi: 10.1021/la0631668. [DOI] [PubMed] [Google Scholar]
- 35.Palizban AA, Salehi R, Nori N, Galehdari H. In vivo transfection rat small intestine K-cell with pGIP/Ins plasmid by DOTAP liposome. J. Drug Target. 2007;15(5):351–357. doi: 10.1080/10611860701349364. [DOI] [PubMed] [Google Scholar]
- 36.Ruozi B, Battini R, Montanari M, et al. DOTAP/UDCA vesicles: novel approach in oligonucleotide delivery. Nanomed. 2007;3(1):1–13. doi: 10.1016/j.nano.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Palmerini CA, Cametti C, Sennato S, et al. Role of cholesterol, DOTAP, and DPPC in prostasome/spermatozoa interaction and fusion. J. Membr. Biol. 2006;211(3):185–190. doi: 10.1007/s00232-006-0009-2. [DOI] [PubMed] [Google Scholar]
- 38.Buyens K, Lucas B, Raemdonck K, et al. A fast and sensitive method for measuring the integrity of siRNA–carrier complexes in full human serum. J. Control. Release. 2008;126(1):67–76. doi: 10.1016/j.jconrel.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 39.Ma Z, Li J, He F, Wilson A, Pitt B, Li S. Cationic lipids enhance siRNA-mediated interferon response in mice. Biochem. Biophys. Res. Commun. 2005;330(3):755–759. doi: 10.1016/j.bbrc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 40.Khandare JJ, Chandna P, Wang Y, Pozharov VP, Minko T. Novel polymeric prodrug with multivalent components for cancer therapy. J. Pharmacol. Exp. Ther. 2006;317(3):929–937. doi: 10.1124/jpet.105.098855. [DOI] [PubMed] [Google Scholar]
- 41.Minko T, Kopeckova P, Kopecek J. Chronic exposure to HPMA copolymer-bound adriamycin does not induce multidrug resistance in a human ovarian carcinoma cell line. J. Control. Release. 1999;59(2):133–148. doi: 10.1016/s0168-3659(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 42.Dharap SS, Wang Y, Chandna P, et al. Tumor-specific targeting of an anticancer drug delivery system by LHRH peptide. Proc. Natl Acad. Sci. USA. 2005;102(36):12962–12967. doi: 10.1073/pnas.0504274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betigeri S, Pakunlu RI, Wang Y, Khandare JJ, Minko T. JNK1 as a molecular target to limit cellular mortality under hypoxia. Mol. Pharm. 2006;3(4):424–430. doi: 10.1021/mp060014x. [DOI] [PubMed] [Google Scholar]
- 44.Patil ML, Zhang M, Betigeri S, Taratula O, He H, Minko T. Surface-modified and internally cationic polyamidoamine dendrimers for efficient siRNA delivery. Bioconjug. Chem. 2008;19(7):1396–1403. doi: 10.1021/bc8000722. [DOI] [PubMed] [Google Scholar]
- 45.Minko T. Mechanisms of cellular drug resistance and strategies to overcome it. In: Mahato RI, editor. Biomaterials for Delivery and Targeting of Proteins and Nucleic Acids. New York, Washington, DC, USA: CRC Press; 2005. [Google Scholar]
- 46.Hammond CL, Marchan R, Krance SM, Ballatori N. Glutathione export during apoptosis requires functional multidrug resistance-associated proteins. J. Biol. Chem. 2007;282(19):14337–14347. doi: 10.1074/jbc.M611019200. [DOI] [PubMed] [Google Scholar]
- 47.Homolya L, Varadi A, Sarkadi B. Multidrug resistance-associated proteins: Export pumps for conjugates with glutathione, glucuronate or sulfate. Biofactors. 2003;17(1–4):103–114. doi: 10.1002/biof.5520170111. [DOI] [PubMed] [Google Scholar]
- 48.Kigawa J, Minagawa Y, Cheng X, Terakawa N. γ-glutamyl cysteine synthetase up-regulates glutathione and multidrug resistance-associated protein in patients with chemoresistant epithelial ovarian cancer. Clin. Cancer Res. 1998;4(7):1737–1741. [PubMed] [Google Scholar]
- 49.Watts RN, Hawkins C, Ponka P, Richardson DR. Nitrogen monoxide (NO)-mediated iron release from cells is linked to NO-induced glutathione efflux via multidrug resistance-associated protein 1. Proc. Natl Acad. Sci. USA. 2006;103(20):7670–7675. doi: 10.1073/pnas.0602515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 51.Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21(3):485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 52.Akita H, Harashima H. Nonviral gene delivery. Contrib. Nephrol. 2008;159:13–29. doi: 10.1159/000125560. [DOI] [PubMed] [Google Scholar]
- 53.Liang MT, Davies NM, Blanchfield JT, Toth I. Particulate systems as adjuvants and carriers for peptide and protein antigens. Curr. Drug Deliv. 2006;3(4):379–388. doi: 10.2174/156720106778559029. [DOI] [PubMed] [Google Scholar]
- 54.Parveen S, Sahoo SK. Polymeric nanoparticles for cancer therapy. J. Drug Target. 2008;16(2):108–123. doi: 10.1080/10611860701794353. [DOI] [PubMed] [Google Scholar]
- 55.Pirollo KF, Chang EH. Targeted delivery of small interfering RNA: approaching effective cancer therapies. Cancer Res. 2008;68(5):1247–1250. doi: 10.1158/0008-5472.CAN-07-5810. [DOI] [PubMed] [Google Scholar]
- 56.Putnam D, Doody A. RNA-interference effectors and their delivery. Crit. Rev. Ther. Drug Carrier Syst. 2006;23(2):137–164. doi: 10.1615/critrevtherdrugcarriersyst.v23.i2.30. [DOI] [PubMed] [Google Scholar]
- 57.Ramon AL, Bertrand JR, Malvy C. Delivery of small interfering RNA. A review and an example of application to a junction oncogene. Tumori. 2008;94(2):254–263. doi: 10.1177/030089160809400218. [DOI] [PubMed] [Google Scholar]
- 58.Sanguino A, Lopez-Berestein G, Sood AK. Strategies for in vivo siRNA delivery in cancer. Mini Rev. Med. Chem. 2008;8(3):248–255. doi: 10.2174/138955708783744074. [DOI] [PubMed] [Google Scholar]
- 59.Wagner E. Advances in cancer gene therapy: tumor-targeted delivery of therapeutic pDNA, siRNA, and dsRNA nucleic acids. J. BUON. 2007;12 Suppl. 10:S77–S82. [PubMed] [Google Scholar]
- 60.Saad M, Garbuzenko OB, Ber E, et al. Receptor targeted polymers, dendrimers, liposomes: which nanocarrier is the most efficient for tumor-specific treatment and imaging? J. Control. Release. 2008;130(2):107–114. doi: 10.1016/j.jconrel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khandare J, Minko T. Polymer–drug conjugates: progress in polymeric prodrugs. Progr. Polym. Sci. 2006;31:359–397. [Google Scholar]
- 62.Wang Y, Saad M, Pakunlu RI, et al. Nonviral nanoscale-based delivery of antisense oligonucleotides targeted to hypoxia-inducible factor 1α enhances the efficacy of chemotherapy in drug-resistant tumor. Clin. Cancer Res. 2008;14(11):3607–3616. doi: 10.1158/1078-0432.CCR-07-2020. [DOI] [PubMed] [Google Scholar]