Abstract
Background
Catecholamines govern stress blood pressure responses. Catecholaminergic responses may be partially genetic and contribute to the complex heritability of hypertension.
Methods and Results
To evaluate catecholaminergic responses without systemic counterregulation, we infused graded concentrations of tyramine, an indirect presynaptic norepinephrine releaser, into dorsal hand veins of 49 normotensive men and women of 5 ethnicities. Vascular responses were coupled to common (minor allele frequency >10%) single-nucleotide polymorphisms at adrenergic target loci within presynaptic pathways. Significance was set at P<0.003 after Bonferroni correction. Generalized analysis of molecular variance (GAMOVA) was performed to determine whether genetic admixture contributed to results. Venoconstriction progressed to 47% with increasing concentrations of tyramine (0.129 to 25.8 mmol/L; P<0.001). Family history of hypertension (P<0.001) and female sex (P=0.02) predicted blunted tyramine responses. Two genetic loci significantly predicted vascular response: chromogranin B, which encodes a protein that catalyzes catecholamine vesicle formation (CHGB, exon 4, Glu348Glu; P=0.002), and cytochrome b-561 (CYB561, intron 1, C719G; P<0.001), an electron shuttle for catecholamine synthesis. Stepwise regression suggested important effects for the CHGB locus, with polymorphisms for the vacuolar-ATPase β-subunit (ATP6V1B1, exon 1, Ile30Thr) and flavin-containing monooxygenase-3 (FMO3, exon 3, Lys158Glu, P=0.002). GAMOVA did not show a significant relationship between overall genetic profile and hand-vein constriction (P=0.29), which indicates that population stratification did not contribute to this phenotype.
Conclusions
Locally infused tyramine produced dose-dependent pressor responses, predicted by family history of hypertension, sex, and genetic variants at loci, particularly CHGB, that encode the biosynthesis, storage, and metabolism of catecholamines. Such variants may influence the complex heritability of adrenergic responses and perhaps hypertension.
Keywords: genetics, genes, hypertension, veins, catecholamines
Hypertension, which affects >25% of US adults, is associated with significant comorbidities, such as stroke, cardiac dysfunction, and renal failure.1 It is a complex trait, influenced by multiple environmental and genetic factors (estimated ≈30% heritability).2 The sympathetic nervous system (SNS), with catecholamines as its neurotransmitters, has been implicated in the development of essential hypertension given its role in minute-to-minute regulation of blood pressure, especially in stress responses. Hypersensitivity and overactivity of the SNS have been noted in the hypertensive state,3 and previous reviews of catecholamines suggest elevated levels in hypertensive individuals.4
A physical map has been created for sympathetic candidate genetic loci for hypertension in humans.5 Previous emphasis has been on the postsynaptic β1- and β2-adrenergic receptors, although efforts to link hypertension to such single-nucleotide polymorphisms (SNPs) have yielded mixed results.6,7 Thus, we currently focus on targeted polymorphisms in relation to local activity rather than disease state. For example, Dishy et al8 found 2 SNPs within the postsynaptic β2-adrenergic receptor that correlated with local vascular response to agonists.
The present study aimed to evaluate genetic variations within the presynaptic neuroeffector SNS pathway for their influence on local vascular responses to adrenergic stimulation. Tyramine, an indirect sympathomimetic amine, was infused locally, and vascular responses were correlated with SNPs at loci that encode proteins involved in the synthesis, storage, release, and metabolism of catecholamines.
Methods
Subjects
We studied 49 unrelated ambulatory, nonsmoking, normotensive men and women. Demographic information (Table I, Data Supplement) and subject instructions are noted in the online Data Supplement. Family history of hypertension (history of high blood pressure before the age of 60 years in a first-degree relative) was determined by self-report in 84% of subjects. Biogeographic ancestry was self-classified as white (including Hispanic), black, East Asian, South Asian, or mixed. Subjects did not take any medications regularly, except for oral contraceptives in 16% (3/19) of women and subcutaneous insulin in 1 subject with stable type 1 diabetes mellitus.
Our hand-vein study protocol has been published previously9 and was approved by the Human Subjects Committee of the University of California at San Diego. Written informed consent was obtained from each subject.
Measurement of Vascular Responses
Local venous responses were measured in a cannulated dorsal hand vein with a linear variable differential transformer, as previously described and noted in the Data Supplement.9-11 Adrenergic vasoconstriction was achieved in the dorsal hand vein by infusion of increasing concentrations of tyramine (tyramine HCl, molecular weight 173.7 g/mol; Sigma, St Louis, Mo; purity >99% by thin-layer chromatography) in 0.5% albumin in normal saline solution. Tyramine (at doses of 4.49, 22.4, 89.2, and 449 pmol · kg-1 · min-1) was freshly mixed on the morning of each study and then filter sterilized with a 0.2-μm filter. The rate of infusion was 6 to 12 mL/h, paralleled by infusion of the albumin in saline solution at a rate of 0 to 6 mL/h, so that the total infusion rate was consistently 12 mL/h. Each dose was infused for 8 minutes, with responses recorded during the last 2.5 minutes of each dose infusion. Details of evaluation for desensitization, reproducibility, and half-life are noted in the online Data Supplement.
SNP Assays/Genotyping
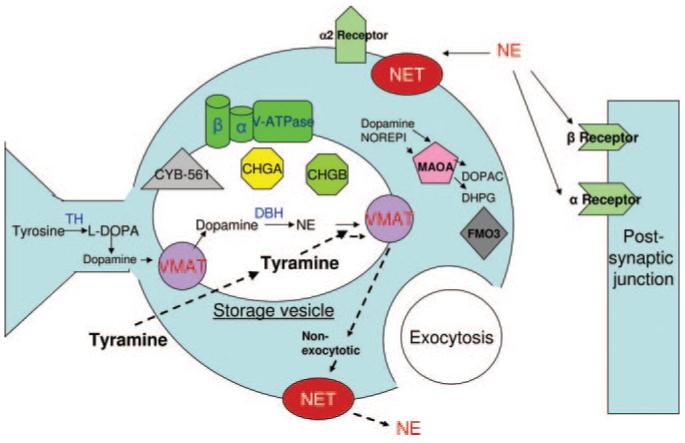
Seventeen SNPs at loci that encode components of the presynaptic adrenergic pathway, with common minor alleles (frequencies >10%), were evaluated, as listed in Table 1 and illustrated in Figure 1. These particular polymorphisms were studied because of their high minor allele frequencies (>10%), locations in coding or promoter regions, or previous indications of biological activity or functionality. Genomic DNA was prepared from leukocytes with PureGene extraction columns (Gentra Biosystems, Minneapolis, Minn). SNPs were scored by use of 1 of 2 extension-based techniques, as described in the Data Supplement.12-14
Table 1.
SNPs Evaluated for Dose-Dependent Response With Tyramine Infusion
| Gene Function/Gene Name | Reference SNP* | Locus Name | Domain | Position† | Biological Effect |
|---|---|---|---|---|---|
| Catecholamine biosynthesis | |||||
| Tyrosine hydroxylase | rs10770141 | TH | Promoter | C-824T | Converts tyrosine to L-Dopa, which can be converted to DA |
| Dopamine β-hydroxylase | rs1611115 | DBH | Promoter | C-1021T | Converts DA to NE, also released with NE |
| Cytochrome b-561 | rs2058203 | CYB561 | Intron 1 | C719G | Shuttles electrons for catecholamine synthesis |
| Catecholamine storage | |||||
| Chromogranin A | rs7610 | CHGA | 3′-UTR | C11825T | Aids in formation of secretory vesicle |
| Chromogranin A | rs9658655 | CHGA | Exon 6 | Glu246Asp | |
| Chromogranin A | rs9658634 | CHGA | Promoter | G-462A | |
| Chromogranin B | rs2821 | CHGB | 3′-UTR | C13612A | |
| Chromogranin B | rs236153 | CHGB | Exon 4 | Glu348Glu | |
| Catecholamine transport | |||||
| Vesicular monoamine transporter 1 |
rs1497020 | VMAT1=SLC18A1 | 3′-UTR | T38292C | Transports catecholamines in and out of secretory vesicles |
| Vesicular monoamine transporter 2 |
rs363227 | VMAT2=SLC18A2 | Intron 12 | C25851T | |
| NE transporter | rs5569 | NET1=SLC6A2 | Exon 9 | Thr429Thr | Reuptakes and releases NE from axon |
| Storage vesicle acidification | |||||
| Vacuolar ATP synthase catalytic α-subunit |
rs12636577 | ATP6V1A | Intron 1 | A31599G | Acidification of the secretory vesicle |
| Vacuolar ATP synthase catalytic β-subunit |
rs17720303 | ATP6V1B1 | Exon 1 | Ile30thr | |
| Catecholamine metabolism | |||||
| Monoamine oxidase A | rs6323 | MAOA | Exon 8 | Arg297Arg | Catalyzes oxidative deamination of catecholamines, to inactivate |
| Monoamine oxidase B | rs1799836 | MAOB | Intron 13 | A113683G | |
| Flavin-containing monooxygenase 3 |
rs2266782 | FMO3 | Exon 4 | Glu158Lys | Degrades catecholamines |
| Catechol-O- methyltransferase |
rs4680 | COMT | Exon 4 | Met158Val | Transfers methyl group to catecholamines, to inactivate |
DA indicates dopamine; NE, norepinephrine; and UTR, untranslated region.
Reference SNP refers to National Center for Biotechnology Information classification (http://www.ncbi.nlm.nih.gov/projects/SNP).
Position of the SNPs within the promoters indicates base pairs upstream from the gene: for introns and 3′ UTRs, the base pairs from the CAP (transcription initiation) site; for exons, the location of the amino acid (Glu indicates glutamate; Asp, aspartate; Thr, threonine; Ile, isoleucine; Arg, arginine; Lys, lysine; Met, methionine; and Val, valine).
Figure 1.
The postganglionic sympathetic neuroeffector junction, indicating the locations and functions of the proteins targeted in the present study. Catecholamine synthesis requires tyrosine hydroxylase (TH) and dopamine β-hydroxylase (DBH), as well as cytochrome b-561 (CYB561) as an electron shuttle. Transport and vesicle formation is aided by vesicular monoamine transporters (VMAT), norepinephrine transporter (NET), chromogranins A and B (CHGA, CHGB), and the vacuolar-ATPase (V-ATPase). Catecholamine degradation and metabolism may utilize flavin-containing monooxygensase-3 (FMO3) or monoamine oxidase A (MAOA). Dotted lines illustrate the mechanisms of NE release in a nonexocytotic mechanism by tyramine. L-DOPA indicates L-3,4-dihydroxyphenylalanine; DHPG, dihydroxyphenylglycol; and DOPAC, dihydroxyphenylacetic acid.
Analysis and Statistics of Hand-Vein Responses
Vasoconstriction was expressed as the percent reduction in vein diameter from baseline. The baseline was defined as the mean of 3 stable (±5% coefficient of variation) baseline measurements during the infusion of 0.5% albumin in saline solution. When 100% constriction was reached in a subject, we ceased the infusion and tabulated further values as 100%.
Results are presented as the mean value ±1 SEM. Additive general linear models were used as association tests. Dose-response curves in different groups were compared by 2-way repeated-measures ANOVA (SPSS 11.5 for Windows, SPSS Inc, Chicago, Ill), with 9 repetitions considered, including a baseline value with 0% constriction. When a Bonferroni correction was applied to probability values for drug-by-gene interactions (assuming independence of genetic effects by loci on different autosomes), significance was set at P<0.003 (0.05/17). False-discovery rates were also estimated to minimize the impact of the multiple comparisons performed.15,16 Repeated-measures ANOVA was also performed for each of the significant SNPs in the 2 dominant ethnic groups, whites (n=23) and East Asians (n=14), alone. Where appropriate, such as for differences between ethnic groups, diploid genotype frequencies were compared between 2 ethnicities by χ2 on 3-by-2 contingency tables (diploid genotype by ethnic group, df=2). Multiple stepwise linear regression models were performed for the highest tyramine infusion rate with P≤0.05 for inclusion and P≥0.1 for exclusion.
The power calculator G*Power317 (http://www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3) was used to estimate statistical power of the F tests for within/between interactions of repeated-measures ANOVA analyses for a range of sample and effect sizes, detailed in the Data Supplement. With the use of diploid genotype frequencies in the present study group, Hardy-Weinberg equilibrium (HWE) was determined for each SNP by χ2 analysis with probability values, also noted in the Data Supplement.
Population Stratification
We studied subjects with different biogeographic origins (Table I, Data Supplement). To confirm that the observed associations were not simply an artifact of population stratification, GAMOVA18 (generalized analysis of molecular variance) was used to test and quantify the relationship between the overall genetic background of the subjects and the percent constriction from the highest tyramine dose, with an identity-by-state distance matrix based on genotypes at 17 biallelic markers distributed across the autosomes. The analysis was performed in 43 individuals who had all polymorphisms scored.
Evaluation of Dopamine in Tyramine Samples
To evaluate the tyramine samples for the possibility of trace amounts of dopamine contamination, freshly diluted samples of 2 distinct concentrations of tyramine used in the present study were assayed in duplicate: 0.000547 mg/mL (3.14 μmol/L) and 0.0547 mg/mL (0.314 mmol/L). Dopamine was assayed by a modification of the sensitive catechol-O-methyltransferase (COMT)-based radioenzymatic assay, as described by Kennedy and Ziegler19 and in the Data Supplement.
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Subject Characteristics
The demographic characteristics of the subjects are shown in Table I in the Data Supplement. Forty-nine normotensive and nonsmoking subjects were studied (30 males and 19 females) between the ages of 18 and 48 years (mean 23.7 years), with 47% white (including 8% Hispanic), 29% East Asian, 12% South Asian, and 6% black. Thirty-three percent reported a positive and 51% a negative family history of hypertension, whereas family history was unknown in 16%.
Dorsal Hand-Vein Vasoconstriction and Tyramine Infusion
Baseline dorsal hand-vein measurements were obtained during saline and albumin infusion. All subjects had reproducibility with their first 3 readings within the requirement of coefficient of variation <5%, which permitted the experiment to continue. Tyramine was then infused in each subject for up to 8 graded doses or until 100% venoconstriction occurred. Eighty-two percent of subjects received all 8 doses of tyramine, thus not reaching 100% constriction. Systolic and diastolic blood pressure values were normal and did not differ significantly before and after the study, whereas heart rate decreased slightly (P=0.02) at the end of the study (Table I, Data Supplement).
Progressive vasoconstriction with increasing doses of tyramine (F=29, P<0.001) was noted. In assuming dorsal hand-vein blood flow of ≈2.5 mL/min,20 Figure 2 illustrates the percent constriction of the dorsal hand vein as a function of both tyramine infusion rate and estimated mean local concentration. Noting the degree of constriction at maximum concentration, the effective concentration for 50% of maximal constriction (EC50) was ≈9 mmol/L. No associations of drug response were observed by age, ethnicity, baseline hand-vein diameter, or body mass index, but a significantly blunted venoconstrictive response was seen in those with a family history of hypertension (F=2.53, P=0.011), depicted in Figure I in the Data Supplement. Males were also noted to exhibit more venoconstriction than females (maximally 55% versus 35%, F=2.31, P=0.02). Figure II in the Data Supplement illustrates the interindividual diversity of tyramine response by dividing subjects into approximate quartiles of response.
Figure 2.
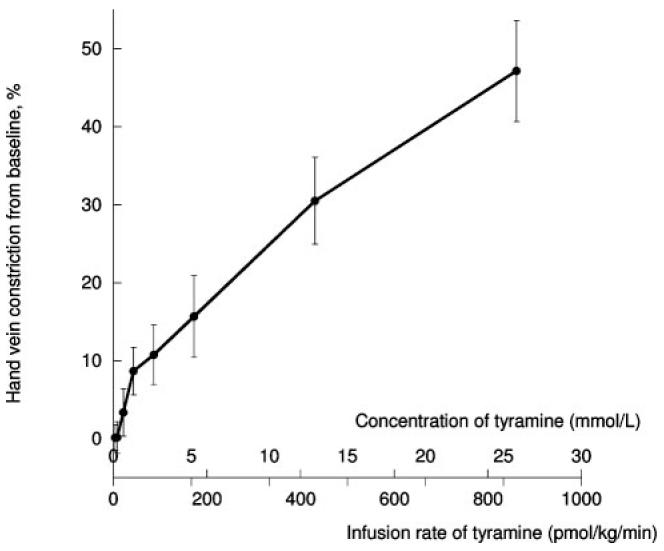
Hand-vein constriction by concentration and infusion of tyramine. Error bars on y-axis illustrate the variation of percent constriction among subjects. Concentration assumes a dorsal hand-vein blood flow of 2.5 mL/min. Analysis by 2-way repeated-measures ANOVA P<0.001, with df=8 within subjects and df=1 between subjects.
Vascular Responses to Tyramine by Genotype
Seventeen genetic variants that spanned components of the catecholamine synthesis, storage, transport, and metabolism pathway were evaluated for tyramine response (Table 1), each with common minor alleles (>10% frequency; Table II, Data Supplement). Figure 1 illustrates their presynaptic locations.
Because of racial variation in SNP frequencies (noted by the National Center for Biotechnology Information, at www. NCBI.nlm.nih.gov), ethnicity was factored as a covariate in all analyses. Table 2 indicates the results of the effect of genotypes of each SNP on tyramine response, in which significant associations persisted at 3 loci after both Bonferroni correction (P<0.003 threshold) and false-discovery-rate estimates. The first was chromogranin B, a protein that aids in catecholamine secretory vesicle formation (CHGB, exon 4, Glu348Glu; F=2.44, P=0.002), in which the minor allele homozygotes (G/G) achieved more vasoconstriction with increasing doses of tyramine (Figure 3). When the 2 largest ethnicity subgroups were evaluated, associations continued to be significant for East Asian subjects alone (n=14; F=5.269, P<0.001) or whites alone (n=23; F=2.824, P=0.001). These 2 groups also had indistinguishable diploid genotype frequencies at Glu348Glu (χ2=2.07, P=0.36).
Table 2.
Dose-Dependent Tyramine Response: Prediction by SNPs in Presynaptic Pathway
| Repeated-Measures ANOVA |
||||
|---|---|---|---|---|
| Gene Function/Locus Name | Domain | Position* | F Statistic | P |
| Catecholamine biosynthesis | ||||
| TH | Promoter | C-824T | 0.59 | 0.88 |
| DBH | Promoter | C-1021T | 0.31 | 0.99 |
| CYB561 | Intron 1 | C719G | 2.80 | <0.001 |
| Catecholamine storage | ||||
| CHGA | 3′-UTR | C11825T | 1.62 | 0.06 |
| CHGA | Exon 6 | Glu246Asp | 0.88 | 0.60 |
| CHGA | Promoter | G-462A | 1.28 | 0.21 |
| CHGB | 3′-UTR | C13612A | 1.45 | 0.12 |
| CHGB | Exon 4 | Glu348Glu | 2.44 | 0.002 |
| Catecholamine transport | ||||
| VMAT1=SLC18A1 | 3′-UTR | T38292C | 1.30 | 0.20 |
| VMAT2=SLC18A2 | Intron 12 | C25851T | 1.32 | 0.23 |
| NET1=SLC6A2 | Exon 9 | Thr429Thr | 1.25 | 0.23 |
| Storage vesicle acidification | ||||
| ATP6V1A | Intron 1 | A31599G | 1.00 | 0.46 |
| ATP6V1B1 | Exon 1 | Ile30thr | 1.74 | 0.038 |
| Catecholamine metabolism and degradation |
||||
| MAOA | Exon 8 | Arg297Arg | 3.32† (1.26‡) | <0.001 (0.27) |
| MAOB | Intron 13 | A113683G | 0.75† (0.81‡) | 0.73 (0.60) |
| FMO3 | Exon 4 | Glu158Lys | 1.33 | 0.18 |
| COMT | Exon 4 | Met158Val | 0.56 | 0.91 |
All analyses performed with ethnicity as a covariate. Sexes were evaluated separately for SNPs on the X chromosome (MAOA, MAOB).
For position notations, see legend for Table 1.
From female subjects only
from male subjects only.
Data in parentheses are for male subjects only. Degrees of freedom for repeated-measures ANOVA: df=8 within subjects, df=2 between subjects, and df=16 for genotype-by-dose interaction.
Figure 3.
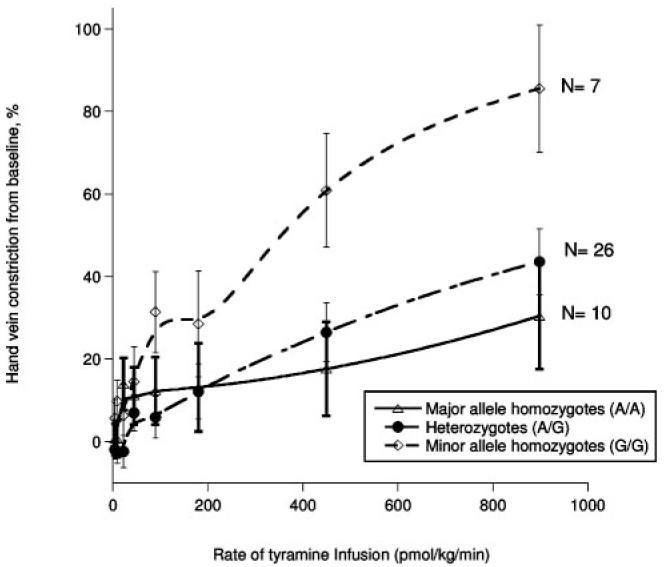
An example of genotype effects on tyramine-induced vasoconstriction: dose response by genotype of chromogranin B, exon 4, Glu348Glu. Here, the minor allele homozygotes (G/G) had greater vasoconstriction than the heterozygotes (G/A) and the minor allele homozygotes (A/A); by 2-way repeated-measures ANOVA, P=0.003, with df=8 within subjects, df=16 for genotype-by-dose interaction, and df=2 between subjects.
With regard to the second significant loci, cytochrome b-561, a storage vesicle membrane electron shuttle for catecholamine synthesis, the variant (CYB561, intron 1, C719G; F=2.80, P<0.001) predicted tyramine response such that those with homozygous minor and major alleles (G/G and C/C) had significantly more vasoconstriction than those who were heterozygous (C/G). This association was not maintained when we evaluated the white (n=23) and East Asian (n=14) subjects separately, which likely reflects a loss of statistical power attendant on subdivision of groups. The alternative explanation of a false conclusion secondary to population stratification is unlikely, because the diploid genotype frequencies at CYB561 were indistinguishable in these 2 subgroups (χ2=0.69, P=0.71).
The SNP for MAOA (exon 8, Arg297Arg) also demonstrated significance (F=3.31, P<0.001) when the 16 female subjects were analyzed separately, with heterozygotes (T/G) having significant vasodilation, whereas those with homozygous major or minor alleles exhibited vasoconstriction. However, diploid genotype frequencies for the MAOA variants deviated somewhat from HWE (P=0.019; Table II, Data Supplement), even when we evaluated only women in the dominant (white) ethnic group. Inclusion of sex as a covariate did not alter the marker-on-trait associations.
The allele frequencies from the subjects in the present study are shown in Table II of the Data Supplement, with χ2 analysis for HWE. Two autosomal SNPs were out of HWE when all of the subjects were considered together regardless of ethnicity (n=49): tyrosine hydroxylase (TH, promoter C-824T) and chromogranin A (CHGA 3′-UTR C11825T). However, within the dominant ethnic group (white), HWE was maintained for each (χ2=0.196, P>0.05 and χ2=2.38, P>0.05, respectively).
Regression models were used to explore the hierarchy of genetic determination of maximal hand-vein constriction with the highest infusion rate of tyramine. When all of the autosomal SNPs were included in a stepwise entry/exit fashion, the first significant model contained only the SNP for CHGB (exon 4, Glu348Glu; F=6.51, R2=0.118, R=0.374, P=0.015). A second model with CHGB in addition to a polymorphism for flavin-containing monooxygenase-3 (FMO3, exon 3, Lys158Glu), an enzyme that degrades nucleophilic heteroatom-containing compounds, perhaps including catecholamines and tyramine, was also significant (F=6.08, R2=0.198, R=0.487, P=0.005). A third model included the previous 2 predictor loci (CHGB and FMO3) with the addition of the β-subunit of vacuolar-ATPase (V-ATPase), which acidifies the core of the secretory vesicle (ATP6V1B1, exon 1, Ile30Thr; F=6.04, R2=0.323, R=0.568, P=0.002) and explained the greatest percentage of trait variance (32.3% versus 19.8% and 11.8%).
Population Stratification
To further evaluate whether hand-vein response to tyramine was influenced by the global degree of biogeographic ancestral admixture (ie, the genetic background) of the subjects, GAMOVA was performed. The genotypes of the subjects with all genotypes scored (n=43) were assessed at 17 widely dispersed biallelic markers. The GAMOVA indicated that people with similar predictor variables (venoconstriction at the maximal tyramine dose) were not genetically more related to each other than expected by chance alone (P=0.29). Despite the use of only 17 biallelic markers, GAMOVA was successful in discerning the groups of self-identified ethnicity (P=0.0001 for East Asian subjects and P=0.0002 for white subjects). Similarly, univariate analysis showed that percent constriction from the maximal tyramine dose was not predicted by self-identified ethnicity (P=0.81).
Dopamine
Because tyramine may undergo slow, nonenzymatic transformation to dopamine on prolonged storage,21 we assayed the freshly prepared tyramine samples at 2 concentrations for the presence of dopamine. The low-concentration tyramine sample, 3.14 μmol/L, did not generate counts above blank. The higher concentration of tyramine, 0.314 mmol/L, had a mean concentration of 17.0 nmol/L dopamine. Thus, the mean ratio within this sample was 0.542 μmol/L dopamine to 1 mol/L tyramine, or 1:1845 (ie, 0.000054%).
Discussion
Overview
Alterations in activity of the SNS, with its minute-to-minute regulation of the stress blood pressure response, appear to contribute to the development of human hypertension.3,4 There have been recent discoveries of blood pressure-associated single-nucleotide variants within the genes that encode for the synthesis, storage, transport, actions, and metabolism of catecholamines.5,22 We hypothesized that such variants and their resultant genotypes may contribute to adrenergic responses and therefore to hypertension. Using exogenous tyramine infusion to induce a sympathetic response (by norepinephrine [NE] release)23,24 in a local vascular bed, we found that tyramine induced dose-dependent vasoconstriction (Figure 2), and the response could be predicted by common (>10% minor allele frequency) genetic variation at multiple steps within the presynaptic adrenergic pathway (Table 2). These variants included SNPs within genes that encode proteins for catecholamine synthesis, such as cytochrome b-561, and proteins for the storage of catecholamines in secretory vesicles, enabled by chromogranin B.25
Unusual Features of the Study
The present study exemplifies many unique aspects of the catecholaminergic response to the sympathomimetic agent tyramine not explored in previous studies. The dorsal hand-vein technique was first described in 1981 by Aellig,26 who used NE and showed that only minute amounts reached the circulation.10,11 The present study is the first report to use this local vascular bed for exogenous tyramine infusion to elicit an adrenergic response, evidenced by measurement of changes in vein diameter; this design was intended to avoid confounding by activation of systemic baroreceptor responses, although a previous group had noted tyramine effects on hydrostatic pressure in the dorsal hand vein.27 No change in systemic blood pressure was noted before or after infusion (Table I, Data Supplement); thus, the modest reduction in pulse rate was likely due to relaxation and immobilization of the subjects throughout the study.
High concentrations of tyramine, well above endogenous plasma levels of ≈7.5 nmol/L in healthy subjects,28 were required to produce a vasoconstrictive response in subjects in the present study (Figure 2). Their responses were seen over local 0.129- to 25.8-mmol/L concentrations in a dorsal hand vein, and the EC50 (threshold concentration) was estimated at ≈9 mmol/L tyramine, consistent with the viewpoint that tyramine acts as a sympathomimetic amine, which functions only indirectly by displacing NE from storage vesicles, allowing the nonexocytotic release of the transmitter into the synaptic cleft.23,24 Such “amphetamine-like” effects require concentrations that are multiple orders of magnitude above normal physiological levels, well above the typical agonist concentrations required for activation of metabotropic or ionotropic receptors.29,30 Given the lack of saturability even at the very high concentrations of tyramine within the dorsal hand vein (Figure 2), its effects are unlikely to be directly receptor-mediated. Trace amine G-protein—coupled receptors have recently been identified in the central nervous system but with much lower EC50 values of ≈200 nmol/L for tyramine.31
Role of Genetic Variation
We observed large interindividual differences in response to tyramine infusion (Figure II, Data Supplement), similar to those previously reported with NE constriction on the dorsal hand vein.32 Large interindividual differences have also been observed in resting sympathetic nerve activity by muscle measurements,33,34 and this contributes to physiological variation in blood pressure regulation.
We hypothesized that such trait variability may result in part from genetic variation, focusing on the presynaptic catecholaminergic pathway (Figure 1; Table 1). A genetic locus that encodes a component for catecholamine biosynthesis (CYB561) was associated with vascular response due to tyramine sympathetic stimulation (Table 2); CYB561 encodes a chromaffin granule membrane cytochrome that shuttles electrons, which accumulated from the catalysis of the formation of NE by mixed function oxidation, out of the vesicle.35 Without this cytochrome, catecholamine biosynthesis comes to a halt due to unfavorable redox forces within the secretory vesicle. A polymorphism for chromogranin B (CHGB) was associated with the response (Table 2; Figure 3). These proteins are necessary to form catecholamine secretory vesicles,25 and their deficiency leads to dysregulation of catecholamine release.36
In testing the hierarchy of independence of the adrenergic genetic variants on the response trait by simultaneously considering all of the variants in a stepwise regression, 3 significant, nested genetic predictors arose. The first predictor was CHGB alone (exon 4, Glu348Glu, P=0.015), which accounted for ≈11.8% of variation in the hand-vein response. The second predictor, with 2 independent variables (CHGB and FMO3), was more significant (P=0.005, accounting for 19.8% of trait variance), but a third model with 3 independent variables (CHGB, FMO3, and ATP6V1B1) accounted for the largest fraction of trait variance (≈32.3%), with a highly significant probability value (P=0.002). FMO3 is an enzyme that oxygenates nucleophilic heteroatom-containing compounds, including tyramine itself and perhaps catecholamines.37 Vesicular acidification by the V-ATPase complex (including ATP6V1B1) to an interior pH of ≈5.5, achieving an ≈100-fold H+ gradient across the membrane, is necessary for accumulation of catecholamines. Disruption of this pH gradient leads to acute catecholamine release, followed by chronic vesicular transmitter depletion.38
Tyramine and Dopamine
A prior study found that tyramine that underwent prolonged storage developed up to 0.7% dopamine contamination over the course of several weeks by a nonenzymatic transformation in vitro, which was enough to induce forearm vasodilation.21,39 Because ≈20% of subjects in the present study experienced dilation after tyramine (Figure II, Data Supplement), we investigated our samples, as detailed in the Data Supplement. In radioenzymatic assay, our freshly prepared tyramine solution had a ratio of 0.541 μmol/L dopamine to 1 mol/L tyramine, or contamination of <0.000054%. Owing to our weight-based infusions, the maximal local dopamine concentration would be no more than 20.5 nmol/L. Given 50 nmol/L as the threshold concentration for effects of dopamine on the human vasculature in vivo,21 the 0.000054% dopamine in the infusate used here should not trigger dopamine receptor—mediated vasodilation in the present subjects. Thus, the apparent vasodilation after tyramine in a subset of study subjects (Figure II, Data Supplement) must have another explanation, perhaps more prominent β2-adrenergic receptor—mediated vasodilation8 in response to tyramine-released NE or even intravesicular conversion of tyramine to octopamine, catalyzed by dopamine β-hydroxylase (DBH).40
Tyramine and Hypertension
Response to tyramine infusion in normotensive subjects in the present study was not associated with age, in agreement with previous studies,32,41 nor were significant associations by ethnicity or baseline hand-vein diameter observed. Rather, significance was noted for sex (F=2.312, P=0.02) and for self-reported family history of hypertension (F=2.53, P=0.011; Figure I, Data Supplement). Subjects who self-reported a family history of hypertension had significantly less vasoconstriction than those with a negative family history. This phenomenon may be illuminated by the findings of Charkoudian et al,42 in which healthy subjects with lower resting muscle sympathetic nerve activity had greater vasoconstrictive responsiveness to adrenergic agonists than those with higher activity. Thus, the vasoconstrictive effects of further increased sympathetic activity may be blunted in subjects with high levels of basal activity, perhaps because of prior agonist-induced desensitization of the constrictor response. Such adaptations may aid in the maintenance of stable blood pressure despite large differences in sympathetic nerve activity. Previous studies have indicated higher levels of plasma NE in normotensive subjects with a family history of hypertension43 but a greater systolic blood pressure increment after epinephrine infusion in those without a family history of hypertension.44
Study Limitations
Use of Tyramine
We used tyramine as an indirect sympathomimetic agent that displaces NE from sympathetic nerve termini in a nonexocytotic manner, using NE (NET) and vesicular monoamine (VMAT) transporters (Figure 1). We chose tyramine because it evaluates endogenous intraneuronal NE stores. Because its release mechanism involves transport by NET and VMAT, subjects with defects in these transporters may experience alterations in vasoconstriction, a process proposed as a potential cause of hypertension.45 We therefore probed polymorphisms in the VMAT and NET genes, but the vascular response was not affected by such genotypes (Table 2). Infusion of exogenous NE might have bypassed this limitation, yet studies that have compared in vivo administration of exogenous NE and endogenous NE released by infusion of tyramine have indicated that the responses may have different cardiovascular effects, with the former mediated by α-adrenoceptors (both α1 and α2) and the latter by α1-46 or β-adrenoceptors.47 Given different receptor-mediated actions,48 results of exogenous NE action and that of endogenous NE may not be directly comparable in the dorsal hand vein. The variation in exogenous and endogenous transmitter responses may reflect the type and location of the blood vessels studied.
We did not measure local or systemic levels of NE after tyramine infusion, in part because the critical site for NE increments, the vascular neuroeffector junction, cannot be readily sampled for assay of NE. Previous studies have clarified a nonexocytotic “spillover” mechanism for NE release in forearm venous tyramine infusion.23,24 Even during systemic infusion of tyramine, changes in blood pressure and NE levels may be difficult to correlate, perhaps because of the rapidity of change in blood pressure and NE release.23,39 Scriven et al48 found dose-related increases in systolic blood pressure and systemic NE levels after intravenous tyramine infusion but with large interindividual variability that could be reduced by correlating the change in systolic blood pressure with plasma NE levels. In the present study design, we aimed to minimize any change in systemic blood pressure or NE levels (Table I, Data Supplement).
Tyramine responses involve not only presynaptic but also postsynaptic effectors (Figure 1), such as adrenergic receptors and postreceptor signaling. Here, we focused only on presynaptic events, perhaps overlooking other important determinants of the responses we measured. Additional genetic studies might probe the role of such pathways.
The Hand-Vein System
The present study used a superficial dorsal hand-vein protocol to evaluate vascular responses to endogenous NE release. Veins do not experience the elevated hydrostatic pressure of the arterial tree, and hence, interindividual differences in venous responses cannot be ascribed to the consequences of systemic hypertension. However, arterial and venous β-adrenergic receptor sensitivity and innervation may differ, and thus, the present results may not be readily extrapolated to all vascular beds in vivo.49 Similar responses in the venous and arterial systems of hypertensive individuals to isoproterenol infusion suggest that arterial and venous responses to exogenous catecholamines are directionally similar,50 although arterial beds, with their direct role in total peripheral resistance, likely have a closer relationship with systemic hypertension.
Ethnic Diversity
The present study included subjects of multiple ethnicities, which presents both strengths and limitations. This enrollment reflects the current US urban population and thus represents a strength in that the study’s inferences arose from a broad base. However, simultaneous analysis of such groups with different biogeographic ancestries (or even population admixture within a self-identified group) might confound our conclusions; indeed, several of the loci we studied may differ in allele frequencies across ethnic groups (www.ncbi.nlm.nih.gov/SNP). We used ethnicity as a covariate in each analysis; in no case did ethnicity exert a significant effect on a genotypic association. Furthermore, ethnicity did not influence the overall phenotypic response to infusion (P=0.9). In stratifying subjects of the dominant ethnic groups (white and East Asian) in the present study, the association of the SNP from CHGB maintained significance (P=0.001 in whites, P<0.001 in East Asians). Such subdivisions for the CYB561 SNP yielded too few subjects per group for adequate statistical power.
GAMOVA was performed to further evaluate whether hand-vein response to tyramine was influenced by the global degree of biogeographic ancestral admixture (ie, the genetic background) of the subjects.18 Seventeen widely dispersed biallelic markers were genotyped in 86% of the cohort (43 subjects) and analyzed with GAMOVA, which indicated that people with similar predictor variables (maximal venoconstriction trait) were not genetically more related to each other than expected by chance alone (P=0.29). Therefore, the specific adrenergic genotype—frequency differences in the maximal venoconstriction trait means that we observed cannot be readily attributed to differential population stratification. With only 17 biallelic markers, GAMOVA was still able to discern the groups of self-identified ethnicity (P=0.0001 for East Asian subjects and P=0.0002 for white subjects), which further supports the conclusion that the different ethnicities did not artifactually induce the marker-on-trait associations we found (Table 2; Figure 3). An alternative approach to population stratification would have been to perform family-based association tests, but we genotyped only pheno-typed individuals and did not obtain parental DNA.
Hardy-Weinberg Equilibrium
HWE was generally obtained for diploid genotypes at candidate loci in the present subject group (Table II, Data Supplement), especially if computed in a single/dominant ethnic group (in the present study, white). One locus deviated systematically from HWE, MAOA (exon 8, Arg297Arg), even after stratification by gender and ethnicity, which reflects a relatively low frequency of heterozygous genotypes in the dominant ethnic group (0 of 8 females). At this locus, we cannot exclude assay artifact, wherein the heterozygous genotype was not detected properly . Thus, the otherwise computationally significant (P<0.0001) MAOA results are of uncertain significance in the present study.
Conclusions
In conclusion, this study shows evidence of significant heritable influences on pressor responses to adrenergic presynaptic stimulation. To gain a comprehensive picture of the adrenergic stimulus, we used a large spectrum (Figure 2) of infusion rates of tyramine as an indirect sympathomimetic agent in a local vascular bed. The vascular response was significantly coupled to genetic differences at candidate loci that encode components of the catecholamine biosynthesis, storage, and metabolic pathway. The large interindividual differences may reflect variable responses to sympathetic activity, may be accounted for by genetic inheritance, and may play a role in the development of hypertension.
Supplementary Material
Acknowledgment
We appreciate the assistance of the National Institutes of Health (NIH)—sponsored General Clinical Research Center (NIH RR00827).
Sources of Funding
This work was funded by grants from the NIH and the Department of Veterans Affairs. Studies were performed in the General Clinical Research Center (NIH RR00827), with support from the Comprehensive Research Center of Excellence in Minority Health and Health Disparities (CRCOE, NIH MD00020).
Footnotes
Disclosures
None.
References
- 1.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 2.Ward R. Familial aggregation and genetic epidemiology of blood pressure. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis and Management. Raven Press; New York, NY: 1990. pp. 81–100. [Google Scholar]
- 3.Grassi G. Role of the sympathetic nervous system in human hypertension. J Hypertens. 1998;16:1979–1987. doi: 10.1097/00004872-199816121-00019. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein DS. Plasma catecholamines and essential hypertension: an analytical review. Hypertension. 1983;5:86–99. doi: 10.1161/01.hyp.5.1.86. [DOI] [PubMed] [Google Scholar]
- 5.Chitbangonsyn SW, Mahboubi P, Walker D, Rana BK, Diggle KL, Timberlake DS, Parmer RJ, O’Connor DT. Physical mapping of autonomic/sympathetic candidate genetic loci for hypertension in the human genome: a somatic radiation hybrid library approach. J Hum Hypertens. 2003;17:319–324. doi: 10.1038/sj.jhh.1001550. [DOI] [PubMed] [Google Scholar]
- 6.Shioji K, Kokubo Y, Mannami T, Inamoto N, Morisaki H, Mino Y, Tago N, Yasui N, Iwa N. Association between hypertension and the alpha adducing, beta1-adrenoreceptor, and G protein beta3 subunit genes in the Japanese population: the Suita study. Hypertens Res. 2004;27:31–37. doi: 10.1291/hypres.27.31. [DOI] [PubMed] [Google Scholar]
- 7.Galletti F, Iacone R, Ragone E, Russo O, Valle ED, Siani A, Barba G, Farinaro E, Strazzullo V, Strazzullo P. Lack of association between polymorphisms in the beta2-adrenergic receptor gene, hypertension, and obesity in the Olivetti heart study. Am J Hypertens. 2004;17:718–720. doi: 10.1016/j.amjhyper.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Dishy V, Sofowora GG, Xie HG, Kim RB, Byrne DW, Stein CM, Wood AJJ. The effect of common polymorphisms of the beta2 adrenergic receptor on the agonist mediated vascular desensitization. N Engl J Med. 2001;345:1030–1035. doi: 10.1056/NEJMoa010819. [DOI] [PubMed] [Google Scholar]
- 9.King D, Etzel JP, Chopra S, Smith J, Cadman PE, Rao F, Funk SD, Rana BK, Schork NJ, Insel PA, O’Connor DT. Human response to alpha2-adrenergic agonist stimulation studied in an isolated vascular bed in vivo: biphasic influence of dose, age, gender, and receptor genotype. Clin Pharm Ther. 2005;77:388–403. doi: 10.1016/j.clpt.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Aellig WH. Clinical pharmacology, physiology, and pathophysiology of superficial veins, 1. Br J Clin Pharmacol. 1994;38:181–196. doi: 10.1111/j.1365-2125.1994.tb04341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aellig WH. Clinical pharmacology, physiology, and pathophysiology of superficial veins, 2. Br J Clin Pharmacol. 1994;38:289–305. doi: 10.1111/j.1365-2125.1994.tb04357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buetow KH, Edmondson M, MacDonald R, Clifford R, Yip P, Kelley J, Little DP, Strausberg R, Koester H, Cantor CR, Braun A. Highthroughput development and characterization of a genome-wide collection of gene-based single nucleotide polymorphism markers by chip-based matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Proc Natl Acad Sci U S A. 2001;98:581–584. doi: 10.1073/pnas.021506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferraris A, Rappaport E, Santacroce R, Pollak E, Krantz I, Toth S, Lysholm F, Margaglione M, Restagno G, Dallapiccola B, Surrey S, Fortina P. Pyrosequencing for detection of mutations in the connexin 26 (GJB2) and mitochondrial 12S RNA (MTRNR1) genes associated with hereditary hearing loss. Hum Mutat. 2002;20:312–320. doi: 10.1002/humu.10127. [DOI] [PubMed] [Google Scholar]
- 14.Ronaghi M. Pyrosequencing sheds light on DNA sequencing. Genome Res. 2001;11:3–11. doi: 10.1101/gr.11.1.3. [DOI] [PubMed] [Google Scholar]
- 15.Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- 16.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300. [Google Scholar]
- 17.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2003;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 18.Nievergelt CM, Libiger O, Schork NJ. Generalized analysis of molecular variance. PLoS Genet. 2007;3:e51. doi: 10.1371/journal.pgen.0030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy B, Ziegler MG. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–2153. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- 20.Chalon S, Moreno H, Jr, Benowitz NL, Hoffman BB, Blaschke TF. Nicotine impairs endothelium-dependent dilatation in human vein in vivo. Clin Pharmacol Ther. 2000;67:391–397. doi: 10.1067/mcp.2000.105153. [DOI] [PubMed] [Google Scholar]
- 21.Holmes C, Moak J, Eldadah B, Zimmerly E, Sharabi Y, Goldstein DS. Dopamine contamination of infused tyramine. Clin Chem. 2005;51:1733–1735. doi: 10.1373/clinchem.2005.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rana BK, Insel PA, Payne SH, Abel K, Beutler E, Ziegler MG, Schork NJ, O’Connor DT. Population-based sample reveals gene-gender interactions in blood pressure in white Americans. Hypertension. 2007;49:96–106. doi: 10.1161/01.HYP.0000252029.35106.67. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein DS, Nurnberger J, Simmons S, Gershon ES, Polinsky R, Keiser HK. Effects of injected sympathomimetic amines on plasma catecholamine and circulatory variables. Life Sci. 1982;32:1057–1063. doi: 10.1016/0024-3205(83)90110-8. [DOI] [PubMed] [Google Scholar]
- 24.Frewin DB, Whelan RF. The mechanism of action of tyramine on the blood vessels of the forearm in man. Br J Pharmacol Chemother. 1968;33:105–116. doi: 10.1111/j.1476-5381.1968.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taupenot L, Harper KL, O’Connor DT. Mechanism of disease: the chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 26.Aellig WH. A new technique for recording compliance of human hand veins. Br J Clin Pharmacol. 1981;11:237–243. doi: 10.1111/j.1365-2125.1981.tb00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sicuteri F, Del Bianco PL, Anselmi B. Morphine abstinence and serotonin supersensitivity in man: analogies with the mechanism of migraine? Psychopharmacology (Berl) 1979;65:205–209. doi: 10.1007/BF00433050. [DOI] [PubMed] [Google Scholar]
- 28.Faraj BA, Bowen PA, Isaacs JW, Rudman D. Hypertyraminemia in cirrhotic patients. N Engl J Med. 1976;294:1360–1364. doi: 10.1056/NEJM197606172942502. [DOI] [PubMed] [Google Scholar]
- 29.Berry MD. Mammalian central nervous system trace amines: pharmacologic amphetamines, physiologic neuromodulators. J Neurochem. 2004;90:257–271. doi: 10.1111/j.1471-4159.2004.02501.x. [DOI] [PubMed] [Google Scholar]
- 30.Brandao F, Rodrigues-Pereira E, Monteiro JG, Davidson R. A kinetic study of the release of noradrenaline by tyramine. Arch Pharmacol. 1981;318:83–87. doi: 10.1007/BF00508830. [DOI] [PubMed] [Google Scholar]
- 31.Borowsky B, Adham N, Jones KA, Raddatz R, Artymyshyn R, Ogozalek KL, Durkin MM, Laklani PP, Bonini JA, Pathirana S, Boyle N, Pu X, Kouranova E, Lichtblau H, Ochoa FY, Branchek TA, Gerald C. Trace amines: identification of a family of mammalian G protein coupled receptors. Proc Natl Acad Sci U S A. 2001;98:8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin SA, Alexieva S, Carruthers SG. The influence of age on dorsal hand vein responsiveness to norepinephrine. Clin Pharmacol Ther. 1986;40:257–260. doi: 10.1038/clpt.1986.172. [DOI] [PubMed] [Google Scholar]
- 33.Sundlof G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol (London) 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fagius J, Wallin BG. Long term variability and reproducibility of resting human muscle nerve sympathetic nerve activity at rest, as reassessed after a decade. Clin Auton Res. 1993;3:201–205. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- 35.Okuyama E, Yamamoto R, Ichikawa Y, Tsubaki M. Structural basis for the electron transfer across the chromaffin vesicle membranes catalyzed by cytochrome b561: analyses of cDNA nucleotide sequences and visible absorption spectra. Biochim Biophys Acta. 1998;1383:269–278. doi: 10.1016/s0167-4838(97)00216-1. [DOI] [PubMed] [Google Scholar]
- 36.Huh YH, Jeon SH, Yoo SH. Chromogranin B-induced secretory granule biogenesis: comparison with the similar role of chromogranin A. J Biol Chem. 2003;278:40581–40589. doi: 10.1074/jbc.M304942200. [DOI] [PubMed] [Google Scholar]
- 37.Cashman JR, Zhang J. Human flavin-containing monooxygenases. Annu Rev Pharmacol Toxicol. 2006;46:65–100. doi: 10.1146/annurev.pharmtox.46.120604.141043. [DOI] [PubMed] [Google Scholar]
- 38.Camacho M, Machado JD, Montesinos MS, Criado M, Borges R. Intragranular pH rapidly modulates exocytosis in adrenal chromaffin cells. J Neurochem. 2006;96:324–334. doi: 10.1111/j.1471-4159.2005.03526.x. [DOI] [PubMed] [Google Scholar]
- 39.Jacob G, Gamboa A, Diedrich A, Shibao C, Robertson D, Biaggioni I. Tyramine induced vasodilation mediated by dopamine contamination. Hypertension. 2005;46:355–359. doi: 10.1161/01.HYP.0000172353.62657.8b. [DOI] [PubMed] [Google Scholar]
- 40.Poch GR, Kopin IJ. The role of octopamine in tachyphylaxis to tyramine. Biochem Pharmacol. 1966;15:210–212. doi: 10.1016/0006-2952(66)90065-7. [DOI] [PubMed] [Google Scholar]
- 41.Harada K, Ohmori M, Kito Y, Fujimura A. Effects of dopamine on veins in humans: comparison with noradrenaline and influence of age. Eur J Clin Pharmacol. 1998;54:227–230. doi: 10.1007/s002280050450. [DOI] [PubMed] [Google Scholar]
- 42.Charkoudian N, Joyner MJ, Johnson CP, Eisenach JH, Dietz NM, Curry TB, Wallin BG. Vascular adrenergic responsiveness is inversely related to tonic activity of sympathetic vasoconstrictor nerves in humans. J Physiol. 2006;572(pt 3):821–827. doi: 10.1113/jphysiol.2005.104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrier C, Cox H, Esler M. Elevated total body noradrenaline spillover in normotensive members of hypertensive families. Clin Sci. 1993;84:225–230. doi: 10.1042/cs0840225. [DOI] [PubMed] [Google Scholar]
- 44.Bachmann AW, Ballantine DM, Gordon RD. Effect of positive family history of hypertension on the blood pressure and catecholamine responses to a 6 hour adrenaline infusion. Clin Exp Pharmacol Physiol. 1993;20:395–398. doi: 10.1111/j.1440-1681.1993.tb01715.x. [DOI] [PubMed] [Google Scholar]
- 45.Rumantir MS, Kaye DM, Jennings GL, Vaz M, Hastings JA, Esler MD. Phenotypic evidence of faulty neuronal norepinephrine reuptake in essential hypertension. Hypertension. 2000;36:824–829. doi: 10.1161/01.hyp.36.5.824. [DOI] [PubMed] [Google Scholar]
- 46.Jie K, Van Brummelen P, Vermey P, Timmermans PBMWM, Van Zwieten PA. Post-synaptic α1 and α2 adrenoreceptors in human blood vessels: interactions with exogenous and endogenous catecholamines. Eur J Clin Invest. 1987;17:174–181. doi: 10.1111/j.1365-2362.1987.tb02397.x. [DOI] [PubMed] [Google Scholar]
- 47.Schafers RF, Poller U, Ponicke K, Geissler M, Daul AE, Michel MC, Brode O-E. Influence of adrenoreceptor and muscarinic receptor blockade on the cardiovascular effects of exogenous noradrenaline and of endogenous noradrenaline released by infused tyramine. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:239–249. doi: 10.1007/pl00004938. [DOI] [PubMed] [Google Scholar]
- 48.Scriven AJ, Dollery CT, Murphy MB, Macquin I, Brown MJ. Blood pressure and plasma norepinephrine concentrations after endogenous norepinephrine release by tyramine. Clin Pharmacol Ther. 1983;33:710–716. doi: 10.1038/clpt.1983.97. [DOI] [PubMed] [Google Scholar]
- 49.Stein CM, Deegan R, Wood AJJ. Lack of correlation between arterial and venous β-adrenergic receptor sensitivity. Hypertension. 1997;29:1273–1277. doi: 10.1161/01.hyp.29.6.1273. [DOI] [PubMed] [Google Scholar]
- 50.Stein CM, Nelson R, Brown M, He H, Deegan R, Wood M, Wood AJJ. Forearm beta-adrenergic receptor-mediated vasodilation is impaired without alteration of norepinephrine spillover in borderline hypertension. J Clin Invest. 1995;96:579–585. doi: 10.1172/JCI118070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.