Abstract
Distal nephron renin may provide a possible pathway for angiotensin (Ang) I generation from proximally delivered angiotensinogen. To examine the effects of Ang II on distal nephron renin, we compared renin protein and mRNA expression in control and Ang II—infused rats. Kidneys from sham (n=9) and Ang II—infused (80 ng/kg per minute, 13 days, n=10) Sprague-Dawley rats were processed by immunohistochemistry, Western blot, reverse transcriptase—polymerase chain reaction (RT-PCR), and quantitative real-time RT-PCR. Ang II infusion increased systolic blood pressure (181±4 versus 115±5 mm Hg) and suppressed plasma and kidney cortex renin activity. Renin immunoreactivity was suppressed in juxtaglomerular apparatus (JGA) cells in Ang II—infused rats compared with sham (0.1±0.1 versus 1.0±0.1 relative ratio) but increased in distal nephron segments (6.4±1.4 versus 1.0±0.1 cortex; 2.5±0.3 versus 1.0±0.2 medulla). Tubular renin immunostaining was apically distributed in principal cells colocalizing with aquaporin-2 in connecting tubules and cortical and medullary collecting ducts. Renin protein levels were decreased in the kidney cortex of Ang II—infused rats compared with that of sham (0.4±0.2 versus 1.0±0.4) rats but higher in the kidney medulla (1.2±0.4 versus 1.0±0.1). In kidney medulla, RT-PCR and quantitative real-time PCR showed similar levels of renin transcript in both groups. In summary, the detection of renin mRNA in the renal medulla, which is devoid of JGA, indicates local synthesis rather than an uptake of JGA renin. In contrast to the inhibitory effect of Ang II on JGA renin, Ang II infusion stimulates renin protein expression in collecting ducts and maintains renin transcriptional levels in the medulla, which may contribute to the increased intrarenal Ang II levels in Ang II—dependent hypertension.
Keywords: renin, angiotensin II, angiotensinogen, immunohistochemistry, Western blot
Renin is synthesized primarily by the juxtaglomerular apparatus (JGA).1 However, renin mRNA and protein have been detected in proximal, connecting tubule and collecting duct cells of human and mouse kidneys as well as in extrarenal tissues.2–7 Although regulation of renin synthesis and secretion from JGA cells has been extensively studied,1,8–13 very little is known about the regulation of tubular renin.7,14–16
Renin formation in tubular segments may have greater importance than previously thought in view of evidence of high concentrations of angiotensinogen (AGT), as well as angiotensin (Ang) I and Ang II in proximal tubule fluid,17,18 and in view of the enhancement of renal AGT mRNA and protein levels in Ang II—dependent hypertension.19,20 Recent studies in Ang II—infused hypertensive rats have shown that there is an increased urinary excretion of AGT,21 which is closely correlated with intrarenal Ang II content.21 Enhancement of urinary AGT excretion suggests increased distal nephron AGT delivery and subsequent Ang I and Ang II formation as long as there is availability of an adequate source of renin and angiotensin-converting enzyme (ACE). Therefore, activation of distal nephron renin could be a contributing factor to the development and maintenance of hypertension by causing continued intrarenal and intratubular formation of Ang II, despite suppressed JGA renin.22–24 In this study, we compared renal medullary renin mRNA and protein levels in Sprague-Dawley Ang II—infused and normotensive sham-operated rats. We conclude that (1) distal nephron cells, specifically principal cells of connecting tubules and collecting ducts, have the capability to synthesize renin and (2) renin protein in connecting tubules and cortical-and medullary-collecting ducts are increased in response to chronic Ang II infusion, thus suggesting differential regulation from JGA renin.
Methods
Animal and Tissue Preparation
All experimental protocols were approved by the Tulane Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, Mass) were cage-housed and maintained in a temperature-controlled room with 12-hour light/dark cycle, with free access to tap water and standard rat chow (Ralston Purina) for 2 weeks. For minipump implantation (Alzet model 2002, Alza Corp), rats were selected at random and divided into 2 groups: control (sham-operated, n=9) and Ang II—infused rats (n=10) that had an osmotic minipump containing Ang II (Human Ang II, Sigma, St Louis, Mo) implanted subcutaneously at the dorsum of the neck. Ang II was infused at a rate of 80 ng/min for 13 days. Systolic blood pressure was monitored by tail-cuff plethysmography (Visitech, BP-2000) 1 day before and 3, 7, and 11 days following sham operation or minipump implantation as previously described.19,21 A separate group of sham (n=6) and Ang II—infused (n=6) rats was used to determine renal renin activity.
Sample Collection and Evaluation
Blood and kidney samples were harvested on day 13. Trunk blood was collected in 2 chilled tubes containing 5.0 mmol/L EDTA, which were centrifuged at 4000 rpm for 30 minutes at 4°C. Plasma fractions were removed and assayed as previously described.23 Plasma renin activity (PRA) was expressed as ng/mL per hour of generated Ang I.
After decapsulation, the left kidney was sagitally sectioned and fixed in zinc-saturated formalin for immunohistochemical studies as previously described.25 The results are expressed in arbitrary units of the relative intensity normalized to the renin immunostaining average of the sham group.
To avoid the contribution of JGA renin to distal nephron renin expression, protein and total RNA were extracted from renal medulla dissected from cortex of the right kidney. In addition, renin activity was determined separately in renal cortex and papillary tips as previously described.9
Renin Western blot analysis using 10 μg of protein against rat renin (1:4000 and β-actin) was performed with a standard protocol as described previously.19–21,23
For reverse transcriptase—polymerase chain reaction (RT-PCR) purposes, first strand cDNA synthesis was performed using 5 μg of total RNA and SuperScript II RNase H-reverse transcriptase system (Invitrogen Life Technologies Co, Carlsbad, Calif). Synthetic specific primers located in exons 1 and 5 of renin 1c gene (sense 5′-ATGCCTCTCTGGGCACTCTT-3′ and antisense 5′-GTCAAACTTGGCCAGCATGA-3′) were used with standard experimental conditions as previously described.26,27
A detailed description of these procedures is available in the online supplement at http://www.hypertensionaha.org.
For quantitative real-time PCR (qRT-PCR), a specific probe (5′-TTCAAAGTCATCTTTGACACGGGTTCAG-3′) labeled with 5′-6FAM and 3′-black hole quencher-1 and a set of primers (sense 5′-AGTACTATGGTGAGATCGGCATT-3′; antisense 5′-AGATTCACAACCTCTATGACTCCTC-3′) were designed from the published cDNA sequences of the rat renin 1c gene28 for a given amplified PCR product of 123 bp. Values were extrapolated from separate standard curves. Rat renin 1c gene expression was compared with 3-fold dilutions of control rat kidney total RNA (Ambion Inc., Austin, Tex) and intensity was normalized to ROX fluorescent dye used as internal reference. Total RNA (10 ng/well) from each sample in triplicate was applied to Mx3000P System using the Brilliant Single-Step QRT-PCR Master Mix kit (Stratagene) following the RT-PCR conditions according to manufacturer’s instructions and amplifications during 60 cycles of 95°C for 15 seconds and an annealing/extension 60°C for 60 seconds. Values were normalized by the average of the values of renin expressed in kidney cortex samples from sham-operated rats, and fold induction was determined.
Statistical Analysis
Results are expressed as mean±SEM. The data were analyzed using unpaired t test between groups. Statistical significance is defined at a value of P≤0.05.
Results
Body Weight, Blood Pressure, Plasma, and Renal Renin Activity
Body weights were similar at the initiation of the study (Ang II, 221±4 g; sham 220±2 g), but on day 11 of infusion, body weights were significantly lower in the Ang II—treated animals than the sham-operated rats (297±7 versus 325±9 g; P<0.05). Systolic blood pressure values for the 2 groups were similar before implantation of the osmotic minipumps (Ang II, 116±2 mm|Hg; sham, 115±4 mm|Hg). On day 7 of infusion, systolic blood pressure was significantly elevated in Ang II—infused rats (154±4 mm|Hg versus 113±5 mm|Hg) and was increased further by day 11 (181±3 mm|Hg versus 115±5 mm|Hg; Figure 1A; P<0.001). PRA (Figure 1B) and renin content in the kidney cortex rats were suppressed in Ang II—infused rats (1.5±0.5 ng Ang I/mL per hour and 41.2±19.2 μg Ang I/g of tissue per hour) compared with the sham-operated rats (8.3±0.9 ng Ang I/mL per hour and 177.8±20 μg Ang I/g of tissue per hour; P<0.001). The measured values of renin activity in the papillary tips were much lower and ranged between 0.3 and 3.2 μg Ang I/g of tissue per hour.
Figure 1.
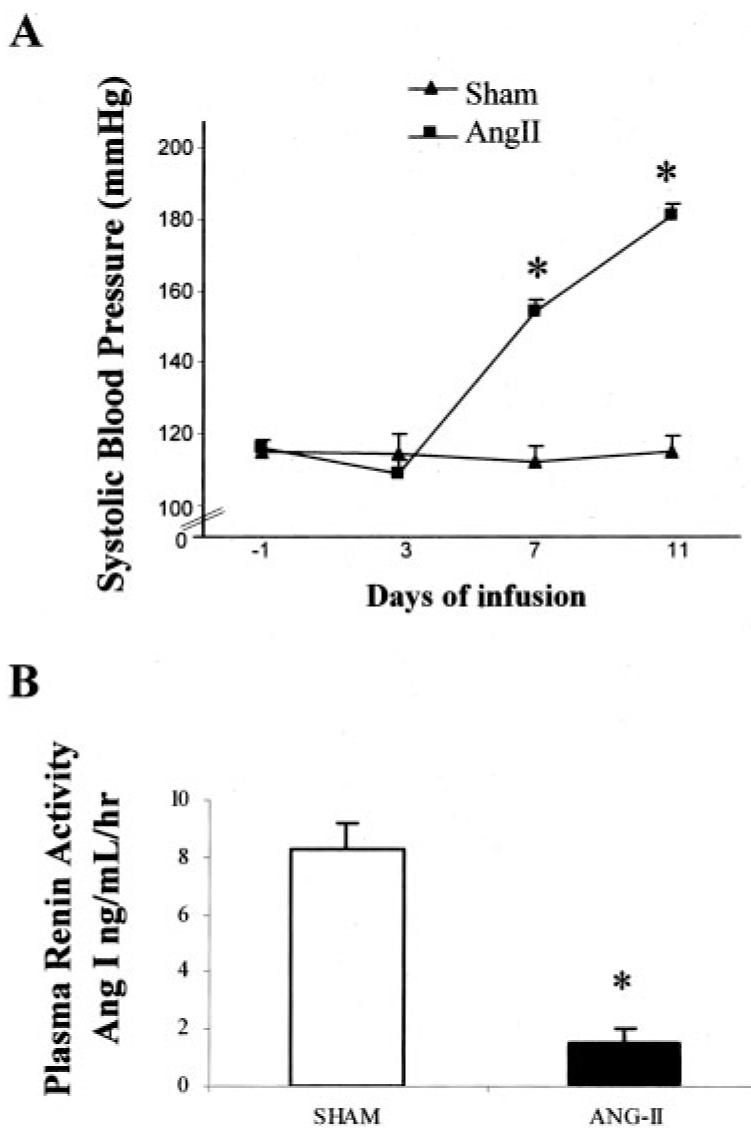
Comparison of systolic blood pressure (A) and plasma renin activity (B) in sham (n=9) and Ang II—infused (n=10) rats. Values are mean±SE *P<0.001 vs sham rats.
Immunolocalization of Renin in Cortical and Medullary Distal Nephron Segments
Figure 2A (red, arrows) shows a dual-color immunostaining for renin in JGA cells, connecting tubules and collecting duct cells (Figure 2A and 2B, AEC substrate, red, arrow heads), which can be identified as principal cells based on the colocalization with aquaporin 2 (AQP-2; Figure 2A and 2B, DAB substrate, brown, asterisks). Renin and AQP-2 negative cells correspond to intercalated cells.
Figure 2.
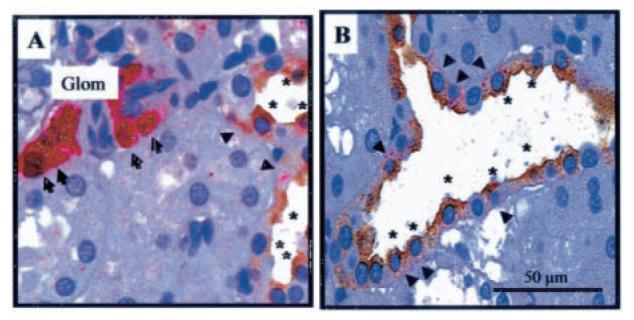
Immunocolocalization of renin and AQP-2 in a paraffin-embedded rat kidney section. Renin immunostaining is observed in juxtaglomerular cells (A, arrows, fast red chromogen) and distal tubular cells (B, arrows, fast red chromogen). AQP-2 immunostaining is present in distal tubular cells (B, asterisks, DAB chromogen) and connecting tubule cells (A, asterisks, DAB chromogen). Observe immunocolocalization of both, renin and AQP2, in principal cells of a connecting tubule (A). Renin antibody concentration 1:4000. AQP-2 antibody concentration 1:500.
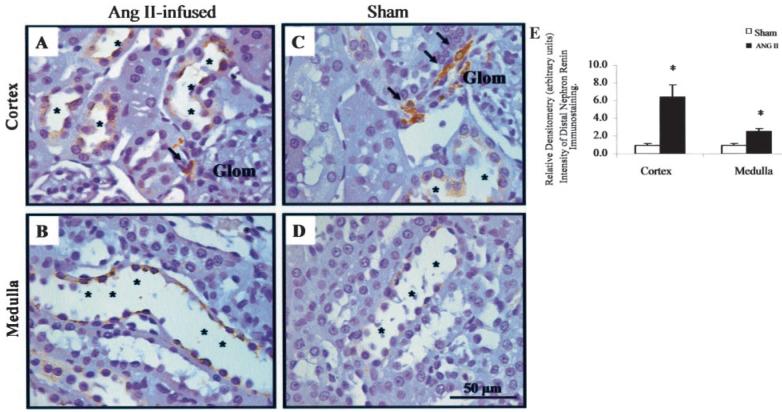
Figure 3 shows renin immunostaining in cortical (asterisks, Figure 3A and 3C) and medullary collecting duct cells (asterisks, Figure 3B and 3D) from kidney sections immunostained in the same run using an automatic immunostainer system and a polyclonal rat anti-renin antibody raised in rabbit at 1:8000 dilution. Ang II—infused rats exhibited higher renin immunoreactivity in cortical and medullary collecting duct cells (asterisks, Figure 3A and 3B). In contrast, sham-operated rats showed high renin immunoreactivity in JGA cells (arrows, Figure 3C) with low intensity signals in distal nephron cells (cortex, asterisks, Figure 3C) and even less in medullary collecting duct cells (asterisks, Figure 3D). Densitometry of the intensity of renin immunoreactivity using Image ProPlus software showed a marked suppression in JGA cells of Ang II—infused rats compared with sham (0.14±0.05 versus 1.0±0.11 DU, P<0.001). However, spatial density of distal nephron segment renin immunoreactivity was higher in Ang II—infused than in sham rats (6.40±1.4 versus 1.0±0.1 cortex; 2.5±0.3 versus 1.0±0.2 DU medulla; P<0.001); (Figure 3E).
Figure 3.
Quantification of intensity of distal nephron renin immunoreactivity in rat kidney cortex and medulla of Ang II—infused (A and B) and control rats (C and D). A shows increased renin immunoreactivity in cortical collecting duct cells with juxtaglomerular renin suppression in Ang II—infused in comparison to sham-operated rat kidney (C). Renin immunoreactivity in medullary collecting duct cells is shown in B and D. Observe higher renin immunoreactivity in Ang II—infused medullary collecting duct cells (B) than sham rat (D). E, a densitometric analysis of the renin intensity immunoreactivity in cortex and medulla of Ang II—infused rats (n=6; 4 kidney sections/animal; 10 analyzed microscopic fields/kidney section) relative to sham (n=5; 4 kidney sections/animal; 10 analyzed microscopic fields/kidney section). Glom indicates glomerulus. Values are mean±SE. *P<0.0001 vs sham rats.
Immunoadsorption of the renin antibody by its peptide (1×10-4 mol/L, 5× in excess) for 72 hours eliminated distal tubular renin and highly attenuated juxtaglomerular renin immunoreactivity.
Protein Expression of Renin in Kidney Cortex and Medulla
Renin Western blot analysis is shown in Figure 4. A prominent band with an estimated molecular weight of 57 kDa was observed in all protein samples extracted from kidney cortex and medulla. In the kidney cortex, Ang II infusion significantly decreased renin levels (Figure 4A, 0.43±0.2 versus 1.0±0.4 densitometric ratio compared with the average of sham-operated rats; P<0.05). In contrast, in the kidney medulla an enhancement of renin protein expression was observed in the Ang II—infused rats in comparison to the sham-operated rats (Figure 4B, 1.22±0.4 versus 1.0±0.1, densitometric ratio to the average of sham rats; P≤0.05).
Figure 4.
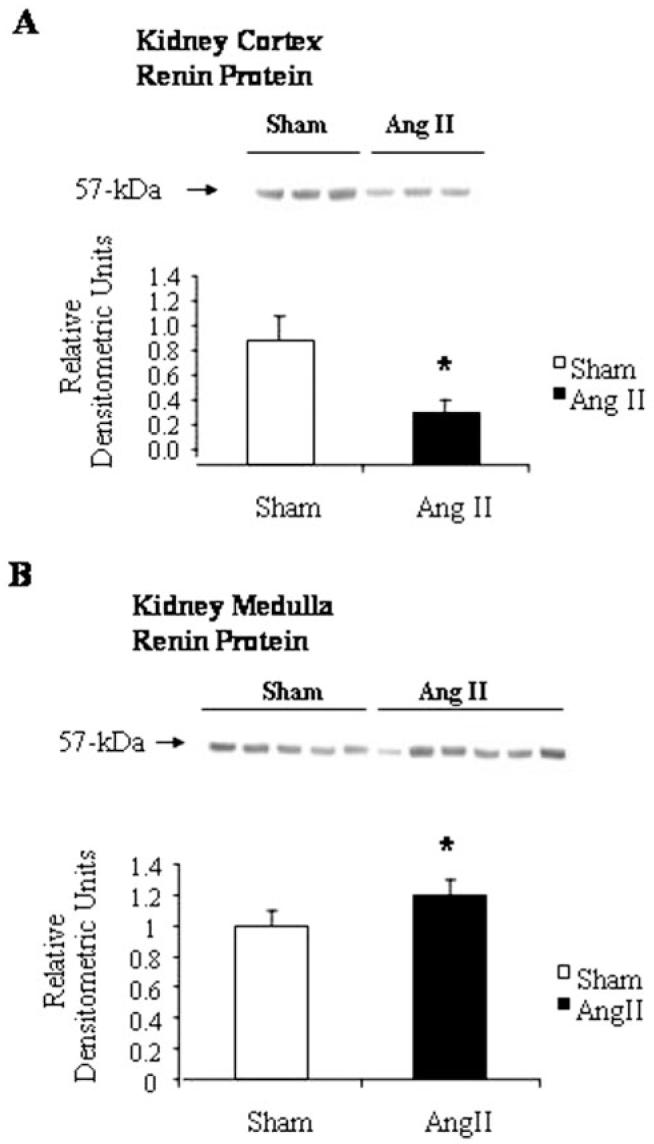
Renin Western blot analyses in kidney cortex and medulla from Ang II—infused and sham-operated rats. A, a representative autoradiography of renin kidney cortex from sham-operated and Ang II—infused rats. Densitometric analysis of the immunoreactive band (57 kDa) showed that Ang II infusion significantly decreased renin levels in the cortex (A), but in contrast it increased renin levels in the medulla (B). Renin antibody concentration 1:4000. *P<0.05 vs sham rats.
RT-PCR for Renin in Rat Kidney Medulla
Figure 5 shows that renin mRNA was present in the kidney cortex (560 bp, positive control) as well as in the renal medulla; however, no significant differences were observed between Ang II—infused and sham-operated rats (0.99±0.50 versus 0.67±0.11; Figure 5B). Omission of RT did not lead to product amplification, ruling out genomic DNA contamination. DNA sequencing of the renin PCR products from kidney cortex and medulla reported 99% identity to preprorenin/prorenin. Relative to sham cortex, quantitation of mRNA showed no significant difference in kidney medulla renin transcript levels between Ang II—infused and sham-operated rats (0.31±0.05 versus 0.28±0.04; Figure 5C); however, in kidney cortex renin, expression was suppressed in Ang II—infused rats as compared with sham-operated rats (0.26±0.01 versus 1.00±0.31). These results indicate that rat kidney medulla expresses the renin 1c gene and that chronic Ang II infusion does not exert an inhibitory effect on medullary renin mRNA as it does on JGA renin message.
Figure 5.
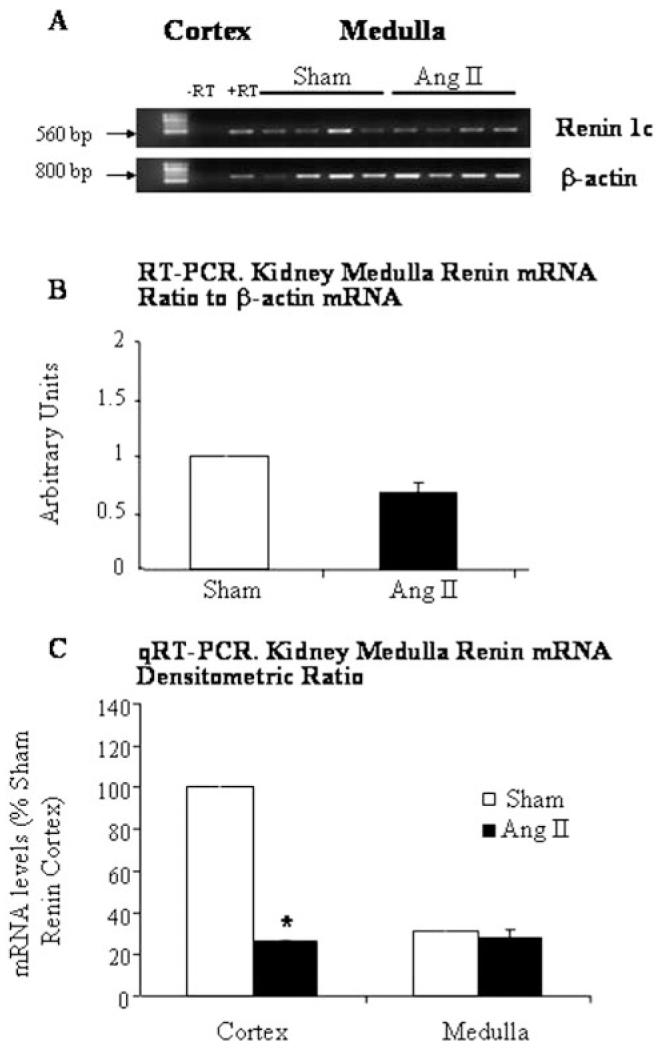
Renin mRNA in rat kidney cortex and medulla. Representative ethidium bromide-stained gel (A) showing renin (560 bp) and corresponding β-actin (800 bp) mRNA levels in the medulla from Ang II—infused and sham-operated rats. Renin mRNA in rat kidney medulla did not show significant differences either by RT-PCR (B) or qRT-PCR (C); however, cortex renin mRNA levels were markedly suppressed in Ang II—infused rats compared with sham-operated rats (C). Normal rat kidney cortex was used as positive control (+RT). Absence of a PCR product with omission of reverse transcriptase enzyme (-RT) ruled out amplification of genomic DNA.
Discussion
Tubular renin immunoreactivity has been previously reported on the apical side of proximal tubule cells1,3,5,6 and in connecting and cortical collecting tubule cells from rat, mouse, and human.4,7 Our results demonstrating positive renin immunoreactivity in cortical connecting tubules and collecting ducts are consistent with these previous reports. In the present study, however, we also showed that positive renin immunoreactivity is present not only in the cortex but also in the medullary collecting duct cells and, furthermore, that distal nephron renin is augmented by chronic Ang II infusions.
Rohrwasser et al7 demonstrated positive renin immunoreactivity in principal cells based on the negative staining for H+-ATPase and peanut lectin (markers for intercalated cells). In this study, using AQP-2 as a positive marker for principal cells, we found that renin colocalizes in principal cells of cortical connecting tubules and cortical and medullary collecting ducts. Although uptake of renin from circulation has been reported for cardiac cells,29 this has not been reported in renal tubular cells. It is unlikely that the increase in tubular renin immunoreactivity and medullary renin protein levels could be due to a contribution from circulating or JGA renin because PRA and cortical renin activity were markedly suppressed by the chronic Ang II infusions. Furthermore, the discrete renin localization specifically to principal cells argues against nonspecific uptake being responsible for the presence of renin in cortical and medullary collecting duct cells. However, this was not reflected by renin activity in the papillary tissue, which was markedly lower than in the cortex. These findings suggest that the distal nephron renin regulation by Ang II differs from that in JGA cells. Although it is known that Ang II directly suppresses JGA renin formation and release, our data indicate a differential regulation on distal nephron renin formation. The enhanced renin protein levels suggest augmented distal nephron Ang II formation under these conditions. The findings of high Ang II type-1 receptor density in distal tubules,30 coupled with the recent evidence that Ang II directly stimulates epithelial sodium channel activity in cortical collecting duct cells,31 provide further support to the potential role of renin formation in principal cells. Under high Ang II states, the augmented distal nephron renin may contribute to an increase in intratubular Ang II formation leading to augmented Ang II actions on distal nephron sodium reabsorption, thus contributing to the sustained elevated blood pressure in Ang II—dependent hypertension.
Despite the growing interest in the existence of a local RAS in the kidney along the nephron, there are few studies characterizing the response of tubular renin to agonists or antagonists and even less with regard to distal nephron renin levels.14,32,33 Rohrwasser et al7 showed in mice that the combination of high sodium diet and amiloride treatment leads to an increase of renin immunoreactivity in connecting tubule cells in association with decreases in JGA renin.7 Moreover, it has been demonstrated that, in marked contrast to the changes in plasma renin content, connecting tubule cell renin immunoreactivity in mice did not exhibit significant variation in response to changes in diet from high to low dietary sodium.15 Because it is well known that Ang II exerts a direct feedback inhibitory effect on JGA renin,34 we sought to determine whether distal tubular renin expression is also inhibited in Ang II—infused rats. As expected, following chronic Ang II infusions, renin immunoreactivity was suppressed in the JGA cells. In contrast, we observed increased renin immunoreactivity in cortical and medullary collecting duct cells and protein levels in renal medulla as demonstrated by immunohistochemistry and Western blot analyses, respectively. Collectively, these findings provide further evidence that the regulation of tubular renin differs from that of JGA renin. From these results, it is not possible to determine whether the enhancement of renin protein in connecting tubules and collecting duct cells is due to a direct effect of the increased intrarenal Ang II levels, a secondary factor stimulated by Ang II, or a consequence of the high blood pressure produced by Ang II. Because Ang II directly inhibits renin synthesis in JGA cells, it would be paradoxical if it stimulates renin in tubular cells. It is also unlikely that the elevated arterial pressure would be reflected to a significant extent at the level of the collecting ducts, but this cannot be ruled out from the present results. Further studies on distal nephron renin in other Ang II—dependent hypertensive models, including the clipped and nonclipped kidneys in Goldblatt hypertensive rats, will be required to address this issue.
Although the apparent molecular weight of renin by gel filtration or sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) has been reported to be in the 38 to 42 kDa range,35–37 it has also been reported that renin in kidney tissue has variable degrees of glycosylation, which may account for the reported variations in molecular weight.36,37 Inagami et al identified in rat and hog kidneys a renin form responsible for all renin activity with a molecular weight between 55 to 60 kDa when they avoided the acidification step during the purification procedure.38,39 These investigators reasoned that the native form of renin in the kidney was a 60 kDa molecule, which was converted during extraction to 40 kDa renin by a sulfhydryl-dependent protease.38 In the present study, using a specific polyclonal rat anti-renin antibody, we detected a prominent band with an estimated molecular weight of 57 kDa.
The presence of renin mRNA in renal distal nephron segments has been reported.7,40 In subtotally nephrectomized rats, distal nephron renin immunoreactivity and renin transcript disappear after chronic administration of perindropil, an ACE inhibitor.40 In contrast, during blockade of RAS by converting enzyme inhibition, renin-expressing smooth muscle cells can be detected beyond the afferent arteriole including the interlobular arteriole, suggesting a recruitment of these cells to the renin-expressing population.41–43 Although we did not demonstrate in the present study that Ang II directly exerts a positive transcriptional effect on distal tubular renin, the presence of the renin transcript detected in kidney medulla with no quantitative changes occurring after chronic treatment with Ang II suggests that renin mRNA in the kidney medulla is not inhibited by Ang II and is not due to contamination from plasma or JGA renin.
Previous studies have demonstrated that there is substantial ACE activity in collecting duct fluid and urine44 associated with an increment of intrarenal ACE binding and activity by chronic Ang II infusions.45 In addition, intraluminal conversion of Ang I to Ang II in the cortical collecting duct and stimulation of distal apical sodium reabsorption have also been reported.46 Taken together, the Ang II stimulatory effects on distal nephron renin could help to explain the marked impairment of sodium excretion and suppression of the pressure—natriuresis relationship observed in this model.47 Because renal AGT mRNA and protein levels are upregulated by increases in circulating Ang II levels,19,20 we postulate that renin in connecting tubule and collecting duct cells may be secreted into the tubular fluid and acts on proximally-delivered AGT to form Ang I in the luminal fluid. In turn, the presence of ACE in the distal nephron would lead to maintained renal Ang II—generating capacity that occurs in Ang II—dependent hypertension leading to high intrarenal Ang II and the maintenance of high blood pressure.
Perspectives
On the basis of these results, we conclude that increased distal nephron renin expression may contribute to the maintenance of intrarenal Ang II levels observed in Ang II—infused hypertensive rats. Although PRA and JGA renin are markedly suppressed in Ang II—induced hypertension, increased distal nephron renin associated with an increased proximal tubular AGT production and spillover into the distal nephron segments may collectively contribute to elevated and sustained intratubular Ang I and Ang II formation in this hypertensive model. In perspective, the Ang II—mediated actions on the distal nephron could be acting synergistically and in concert with the actions of aldosterone to stimulate distal nephron sodium reabsortion and contribute to overall enhanced sodium reabsorption.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL26731 and P20RR017659), American Heart Association (SDG 9930120N and 0325269B), and the Louisiana Board Regents Millennium Health Excellence Fund (2001–06 to 07). We thank Dr Tadashi Inagami (Vanderbilt University) for providing the rat anti-renin antibody. The authors express their gratitude to Faith Shiro, Dale Seth, My-Linh Rauv, and Helena Pappas Le-Bau for their excellent technical assistance. Digital images of histological specimens were obtained at the Imaging Core Facility of Hypertension and Renal Center of Excellence, Department of Physiology at Tulane University Health Sciences Center.
References
- 1.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
- 2.Yanagawa N, Capparelli AW, Jo OD, Friedal A, Barrett JD, Eggena P. Production of angiotensinogen and renin-like activity by rabbit proximal tubular cells in culture. Kidney Int. 1991;39:938–941. doi: 10.1038/ki.1991.117. [DOI] [PubMed] [Google Scholar]
- 3.Taugner R, Hackenthal E, Inagami T, Nobiling R, Poulsen K. Vascular and tubular renin in the kidneys of mice. Histochemistry. 1982;75:473–484. doi: 10.1007/BF00640599. [DOI] [PubMed] [Google Scholar]
- 4.Deschepper CF, Mellon SH, Cumin F, Baxter JD, Ganong WF. Analysis by immunocytochemistry and in situ hybridization of renin and its mRNA in kidney, testis, adrenal, and pituitary of the rat. Proc Natl Acad Sci USA. 1986;83:7552–7556. doi: 10.1073/pnas.83.19.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M, Harris MP, Rose D, Smart A, He XR, Kretzler M, Briggs JP, Schnermann J. Renin and renin mRNA in proximal tubules of the rat kidney. J Clin Invest. 1994;94:237–243. doi: 10.1172/JCI117312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J Clin Invest. 1993;91:774–779. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 8.DiBona GF. Neural control of the kidney: functionally specific renal sympathetic nerve fibers. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1517–R1524. doi: 10.1152/ajpregu.2000.279.5.R1517. [DOI] [PubMed] [Google Scholar]
- 9.Kobori H, Ichihara A, Suzuki H, Miyashita Y, Hayashi M, Saruta T. Thyroid hormone stimulates renin synthesis in rats without involving the sympathetic nervous system. Am J Physiol. 1997;272:E227–E232. doi: 10.1152/ajpendo.1997.272.2.E227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang W, Sjoquist M, Skott O, Stricker EM, Sved AF. Oxytocin-induced renin secretion in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2000;278:R226–R230. doi: 10.1152/ajpregu.2000.278.1.R226. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi N, Smithies O. Gene targeting approaches to analyzing hypertension. J Am Soc Nephrol. 1999;10:1598–1605. doi: 10.1681/ASN.V1071598. [DOI] [PubMed] [Google Scholar]
- 12.Leyssac PP, Holstein-Rathlou NH, Skott O. Renal blood flow, early distal sodium, and plasma renin concentrations during osmotic diuresis. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1268–R1276. doi: 10.1152/ajpregu.2000.279.4.R1268. [DOI] [PubMed] [Google Scholar]
- 13.Thrasher TN, Chen HG, Keil LC. Arterial baroreceptors control plasma vasopressin responses to graded hypotension in conscious dogs. Am J Physiol Regul Integr Comp Physiol. 2000;278:R469–R475. doi: 10.1152/ajpregu.2000.278.2.R469. [DOI] [PubMed] [Google Scholar]
- 14.Henrich WL, McAllister EA, Eskue A, Miller T, Moe OW. Renin regulation in cultured proximal tubular cells. Hypertension. 1996;27:1337–1340. doi: 10.1161/01.hyp.27.6.1337. [DOI] [PubMed] [Google Scholar]
- 15.Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, Thomas J, Xiao S, Ishigami T, Herrmann T, Terreros DA, Ward K, Lalouel JM. Effects of dietary sodium and genetic background on angiotensinogen and renin in mouse. Hypertension. 2002;39:1007–1014. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]
- 16.Brooks HL, Ageloff S, Kwon TH, Brandt W, Terris JM, Seth A, Michea L, Nielsen S, Fenton R, Knepper MA. cDNA array identification of genes regulated in rat renal medulla in response to vasopressin infusion. Am J Physiol Renal Physiol. 2003;284:F218–F228. doi: 10.1152/ajprenal.00054.2002. [DOI] [PubMed] [Google Scholar]
- 17.Braam B, Mitchell KD, Fox J, Navar LG. Proximal tubular secretion of angiotensin II in rats. Am J Physiol Renal Physiol. 1993;264:F891–F898. doi: 10.1152/ajprenal.1993.264.5.F891. [DOI] [PubMed] [Google Scholar]
- 18.Navar LG, Imig JD, Zou L, Wang C-T. Intrarenal production of angiotensin II. Sem Nephrol. 1997;17:412–422. [PubMed] [Google Scholar]
- 19.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol Endocrinol Metab. 1992;263:E863–E869. doi: 10.1152/ajpendo.1992.263.5.E863. [DOI] [PubMed] [Google Scholar]
- 23.Von Thun AM, Vari RC, El-Dahr SS, Navar LG. Augmentation of intrarenal angiotensin II levels by chronic angiotensin II infusion. Am J Physio Renal Physiol. 1994;266:F120–F128. doi: 10.1152/ajprenal.1994.266.1.F120. [DOI] [PubMed] [Google Scholar]
- 24.Zou L, Imig JD, Von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal Ang II augmentation in Ang II-infused rats. Hypertension. 1996;28:669–677. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]
- 25.Prieto M, Dipp S, Meleg-Smith S, El Dahr SS. Ureteric bud derivatives express angiotensinogen and AT1 receptors. Physiol Genomics. 2001;6:29–37. doi: 10.1152/physiolgenomics.2001.6.1.29. [DOI] [PubMed] [Google Scholar]
- 26.Burnham CE, Hawelu-Johnson CL, Frank BM, Lynch KR. Molecular cloning of rat renin cDNA and its gene. Proc Natl Acad Sci USA. 1987;84:5605–5609. doi: 10.1073/pnas.84.16.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norwood VF, Garmey M, Wolford J, Carey RM, Gomez RA. Novel expression and regulation of the renin-angiotensin system in metanephric organ culture. Am J Physiol Regul Integr Comp Physiol. 2000;279:R522–R530. doi: 10.1152/ajpregu.2000.279.2.R522. [DOI] [PubMed] [Google Scholar]
- 28.Stubbe J, Jensen BL, Bachmann S, Morsing P, Skott O. Cyclooxygenase-2 contributes to elevated renin in the early postnatal period in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1179–R1189. doi: 10.1152/ajpregu.00340.2002. [DOI] [PubMed] [Google Scholar]
- 29.Saris JJ, Derkx FH, Lamers JM, Saxena PR, Schalekamp MA, Danser AH. Cardiomyocytes bind and activate native human prorenin: role of soluble mannose 6-phosphate receptors. Hypertension. 2001;37:710–715. doi: 10.1161/01.hyp.37.2.710. [DOI] [PubMed] [Google Scholar]
- 30.Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, El-Dahr SS. Immunohistochemical localization of Ang II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol Renal Physiol. 1997;273:F170–F177. doi: 10.1152/ajprenal.1997.273.1.F170. [DOI] [PubMed] [Google Scholar]
- 31.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 32.Churchill PC. Second messengers in renin secretion. Am J Physiol. 1985;249:F175–F184. doi: 10.1152/ajprenal.1985.249.2.F175. [DOI] [PubMed] [Google Scholar]
- 33.Henrich WL, Campbell WB. Importance of calcium in renal renin release. Am J Physiol. 1970;56:1056. Abstract.
- 34.Kammerl MC, Richthammer W, Kurtz A, Kramer BK. Angiotensin II feedback is a regulator of renocortical renin, COX-2, and nNOS expression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1613–R1617. doi: 10.1152/ajpregu.00464.2001. [DOI] [PubMed] [Google Scholar]
- 35.Matoba T, Murakami K, Inagami T. Rat renin: purification and characterization. Biochim Biophys Acta. 1978;526:560–571. doi: 10.1016/0005-2744(78)90146-8. [DOI] [PubMed] [Google Scholar]
- 36.Dzau VJ, Slater EE, Haber E. Complete purification of dog renal renin. Biochemistry. 1979;18:5224–5228. doi: 10.1021/bi00590a029. [DOI] [PubMed] [Google Scholar]
- 37.Corvol P, Devaux C, Ito T, Sicard P, Ducloux J, Menard J. Large scale purification of hog renin. Physicochemical characterization. Circ Res. 1977;41:616–622. doi: 10.1161/01.res.41.5.616. [DOI] [PubMed] [Google Scholar]
- 38.Inagami T, Hirose S, Murakami K, Matoba T. Native form of renin in the kidney. J Biol Chem. 1977;252:7733–7737. [PubMed] [Google Scholar]
- 39.Inagami T, Murakami K. Purification of high molecular weight forms of renin from hog kidney. Circ Res. 1977;41:11–16. doi: 10.1161/01.res.41.4.11. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert RE, Wu LL, Kelly DJ, Cox A, Wilkinson-Berka JL, Johnston CI, Cooper ME. Pathological expression of renin and angiotensin II in the renal tubule after subtotal nephrectomy. Implications for the pathogenesis of tubulointerstitial fibrosis. Am J Pathol. 1999;155:429–440. doi: 10.1016/S0002-9440(10)65139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindop GB, Lever AF. Anatomy of the renin-angiotensin system in the normal and pathological kidney. Histopathology. 1986;10:335–362. doi: 10.1111/j.1365-2559.1986.tb02489.x. [DOI] [PubMed] [Google Scholar]
- 42.Gomez RA, Lynch KR, Chevalier RL, Everett AD, Johns DW, Wilfong N, Peach MJ, Carey RM. Renin and angiotensinogen gene expression and intrarenal renin distribution during ACE inhibition. Am J Physiol Renal Physiol. 1988;254:F900–F906. doi: 10.1152/ajprenal.1988.254.6.F900. [DOI] [PubMed] [Google Scholar]
- 43.Gomez RA, Chevalier RL, Everett AD, Elwood JP, Peach MJ, Lynch KR, Carey RM. Recruitment of renin gene-expressing cells in adult rat kidney. Am J Physiol Renal Physiol. 1990;259:F660–F665. doi: 10.1152/ajprenal.1990.259.4.F660. [DOI] [PubMed] [Google Scholar]
- 44.Casarini DE, Boim MA, Stella RCR, Krieger-Azzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol Renal Physiol. 1997;272:F405–F409. doi: 10.1152/ajprenal.1997.272.3.F405. [DOI] [PubMed] [Google Scholar]
- 45.Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT1 receptor and ACE binding in angiotensin II-induced hypertensive rats. Am J Physiol Renal Physiol. 2001;281:F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 47.Wang C-T, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in Ang II—infused hypertensive rats. Am J Physiol Renal Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.