Abstract
Background
The study was conducted to determine the impact of switching from oral to transdermal patch or vaginal ring contraception on biomarkers of thrombosis.
Study Design
Current healthy oral contraceptive (OC) users were randomized to switch to either a contraceptive ring (CR) or patch (CP) and underwent phlebotomy to measure surrogate biomarkers of thrombosis (sex hormone binding globulin (SHBG), free protein S, and activated protein C-resistance (APC-r)) before switching, and during the 4th cycle of use of the new method.
Results
Of 142 reproductive age women enrolled, 120 sample pairs were available for analysis. SHBG increased significantly from baseline in CP users (mean change (95% CI) +29.9 nM (9.6, 50) but not in CR users −1.6 (−16.6, 13.5). Protein S decreased significantly from baseline in CP users (mean change −7.1% (−12.1, −2.1), but increased significantly in CR users +5.3% (1.1, 9.6). The APC-r ratio did not undergo a significant change from baseline in either group [CP +0.06 (−0.06, 0.18), CR +0.02 (−0.10, 0.14)] Compared to CR users, subjects using the CP had significantly higher SHBG (187.5 (167.0, 208), 146 (132.6,159.4), p = 0.012), significantly lower protein S (81.8 (76.8, 86.8), 93.6 (89.1, 98.1), p = 0.001), and similar APC-r ratios (2.99 (2.85,3.14), 3.09 (2.96, 3.22), p = 0.3) at the cycle 4 visit.
Conclusion
OC users who switch to the ring exhibit beneficial changes in biomarkers of thrombosis while those switching to the patch display a shift favoring clot formation.
Keywords: Transdermal, Transvaginal, Hormonal Contraception, Thrombosis, Randomized
1. Introduction
Since receiving regulatory approval for marketing, both vaginal ring (NuvaRing™) and transdermal patch (Ortho Evra™) contraceptive delivery systems have become popular contraceptive options. Both of these products release ethinyl estradiol (EE), the same synthetic estrogen used in combination oral contraceptives (OCs). Since both routes of administration avoid hepatic first-pass metabolism and demonstrate lower peak levels of hormones than seen following oral dosing, the impact on hepatic induction of coagulation factors might be different from OC users [1].
Epidemiological studies have shown that both the estrogen dose and type of progestogen in a combination OC contribute to the increased risk of venous thrombosis seen in OC users compared to non-users [2]. A dose-dependent effect with estrogen is noted; OCs containing 50 mcg or more of EE are associated with a higher risk of thrombosis than formulations containing 20 to 30 mcg EE [3]. More recently, epidemiologic studies investigating the impact of the progestogen component of combined OCs have suggested that the so-called “third-generation” progestogens gestodene and desogestrel or the progestogen cyproterone acetate are more thrombogenic than OCs containing the “second-generation” progestogen levonorgestrel [4, 5].
Steady-state levels of EE are higher in patch users than in ring users [1]. Highly publicized reports of fatal venous thromboembolism (VTE) in patch users in 2004 led to the addition of a specific warning regarding an increase in estrogen exposure (e.g., average steady-state concentrations of EE are 60% higher than in users of a 35 mcg EE OC) in the prescribing information for the product [6, 7]. The results of epidemiologic studies have been inconsistent. A nested case-control study design using an insurance claims database found that the odds ratio for venous thromboembolism was 2.4 (95% CI 1.1–5.5) in patch users compared to users of OCs containing norgestimate, the parent compound of the progestogen norelgestromin used in the patch [8]. However, Jicks et al. [9, 10], used a similar study design and found no increase in risk (OR 1.1 (95% CI 0.6–2.1).
Women using contraceptive pills may consider switching to either the patch or the ring to enjoy the benefits of long-acting contraceptive delivery systems. Although the absolute risk of thrombosis is low in healthy reproductive age women, not all hormonal contraceptive users are at low risk [11]. Compared to use of the pill, patch use results in a shift in hemostasis variables favoring clot formation [12–14]. In order to obtain more information on how transdermal and vaginal contraceptive delivery systems may affect thrombosis risk, we measured three surrogate markers of thrombosis risk in healthy OC users who agreed to be randomized to receive either the patch or ring: sex hormone binding globulin (SHBG), protein S, and activated protein C-resistance (APC-r). Our hypothesis was that, compared to ring users and former pill users, patch users will exhibit a net change in these markers favoring clot formation.
2. Materials and methods
2.1. Study population
All women enrolled in the Patch-Ring Acceptability Study (PARIS)[15], an open-label, prospective, multicenter, randomized comparative trial at three [Oregon Health & Science University (OHSU), the Johns Hopkins University, and the University of Pennsylvania] of the 10 study sites were asked to participate in a substudy investigating the effects of the contraceptive products on surrogate serum markers of thrombosis. All three participating sites obtained approval from their Institutional Review Board for the main study and substudy prior to initiation of the protocol. The details and findings of the PARIS study have been published [15]. Eligible subjects were satisfied current or recent users of a combined OC with no past experience using either the contraceptive ring or patch. Subjects agreed to discontinue their current oral contraceptive method and be randomized to either the patch or ring and continue use of the new method for four cycles. Group assignment was determined in the main PARIS study by a randomization sequence created using STATA 9.0 (Stata Corp., College Station, TX) statistical software, stratified by center with a 1:1 allocation using random block sizes of 2, 4 and 6. An individual unassociated with the clinical portion of the study prepared sequentially numbered opaque envelopes containing the product assignment, and research staff at the study site opened the next envelope for each enrolled subject.
Age, height, and weight were recorded for the substudy. BMI was calculated as weight (in pounds)/(height in inches)2 × 703, and classified as underweight (BMI < 18.5), normal weight (BMI 18.5 – 24.9), overweight (BMI 25– 29.9), and obese (BMI >30) [16]. Subjects were asked about their current use of OCs, as well as the use of any OC in the last three months. For the purposes of data analysis, women who had used OCs within eight weeks of enrollment, but were not currently using the pill, were classified as recent users. All women enrolled in the study were either current or recent users of OCs.
All subjects underwent phlebotomy to obtain a blood sample for measurement of SHBG, protein S, and APC-r at the enrollment visit (prior to initiation of the patch or ring), and at the final scheduled follow-up visit during the first week of the fourth cycle of patch or ring use (or sooner if early discontinuation). Following separation of serum and plasma, samples were shipped frozen on dry ice to the Oregon Clinical & Translational Research Institute (OCTRI) Laboratory at Oregon Health & Science University where they were stored between −70 to −80°C until analysis. Samples from Johns Hopkins and Pennsylvania were stored at −70 to −80°C and shipped on dry ice to OHSU in batches. A unique identifying number was used to link the initial and follow-up samples to each other and to demographic data. Only subjects providing an initial and follow-up blood sample contributed data to the primary outcome. Women who discontinued the protocol prior to completing four months of use underwent the second blood draw within one week of stopping the ring or patch system.
2.2. Laboratory methods
The assays were performed in batches using the same standards following collection of all the samples. Protein S antigen determination was performed in the OCTRI Laboratory using a commercially available ELISA-based kit (Corgenix Inc, Denver, CO) that measures free protein S as a percent of normal, with a reference range of 73–149%. Only free protein S has biologic activity. The kit has an inter-assay coefficient of variance (CV) of 6.7%. SHBG levels were also measured in the OHSU OCTRI Laboratory using a chemiluminescent based immunoassay (Immulite, Siemens Medical Solutions Diagnostics, Tarrytown, NY) with an inter-assay CV of 6.2%, and a normal range for women of 20–175 ng/mL. APC-r was determined using a highly sensitive and specific plasma based functional clotting assay, (Pefakit APC-R Factor V Leiden assay, Pentapharm LTD, Basel, Switzerland) performed in the OHSU Special Immunology and Coagulation lab (Hemostasis and Thrombosis Section). Results are expressed as ratios between activated partial thromboplastin time with and without activated protein C, with an inter-assay CV of 4.26%. The normal range is a value greater than 2.19, and a drop in APC-r ratio with this assay indicates a shift toward greater thrombosis risk.
2.3. Primary objective
The primary objective of this study was to compare the change from baseline to cycle four in levels of SHBG, protein S, and APC-r between women in the contraceptive ring (CR) and contraceptive patch (CP) groups.
2.4. Secondary objectives
The secondary objectives were to assess the influence of prior OC use, OC type, and BMI on SHBG, protein S, and APC-r following use of the contraceptive ring or patch system. For purposes of data analysis, oral contraceptives were classified according to progestin type and estrogen dose. Dose of ethinyl estradiol was considered very low (20 mcg) or low (> 20 mcg). No subject reported use of a 50 mcg or higher pill. Progestins were classified as either 3rd generation (norgestimate, desogestrel), 1st and 2nd generation (levonorgestrel, norgestrel, ethynodiol diacetate, norethindrone), or other (drosperinone) [17].
2.5. Sample size
Sample sizes were based on the PARIS study, which expected to enroll 50 subjects at each of the three sites. We planned to enroll all of these women in the substudy (n=150). We estimated that enrolling 64 subjects in each group (assuming 15% dropout) would detect a difference in SHBG of 21.2 nmol/L (assuming a common standard deviation (SD) of 30 nmol/L) and conservatively assuming zero correlation between baseline and follow-up measurements) with 80% power (at significance level 0.05) using a paired t-test.
2.6. Statistical analysis
Simple comparisons for differences between baseline and follow-up measurements of APC-r, SHBG, and protein S were analyzed by two-tailed paired t-tests; differences in overall mean changes between contraceptive ring and contraceptive patch participants were analyzed by independent samples t-tests. Demographic differences between the contraceptive ring and contraceptive patch groups were assessed via t-tests and chi-square tests. p-values of less than 0.05 were considered statistically significant. We did not adjust the p-value for significance for multiple comparisons.
To adjust for confounders, linear mixed modeling was used to compare APC-r, SHBG, and protein S scores between the contraceptive ring and contraceptive patch groups. Primary predictors of interest included time (baseline vs. follow-up measurement), group (contraceptive ring (CR) vs. contraceptive patch (CP)), and OC use (current OC use vs. recent OC use). Potential confounders included in the analyses were study site (OHSU, John Hopkins, Penn); age (continuous); race (white vs. non-white); number of past pregnancies (0 vs.≥1); and BMI (underweight/normal vs. overweight/obese). All possible two-way and three-way interactions were assessed between group, time, and OC use. Only interactions with a p-value of <0.05 were retained in the models.
Secondary analyses were conducted using linear mixed models to assess differences between groups among only the women who were current OC users at baseline (n = 102). Two separate models were analyzed: 1) with primary predictors group, time, and estrogen dose, and 2) with primary predictors group, time, and progestogen type. The same potential confounders and interactions were assessed as for the primary analysis described above.
All analyses were conducted using SAS version 9.1 (SAS Institute Inc., Cary, NC).
3. Results
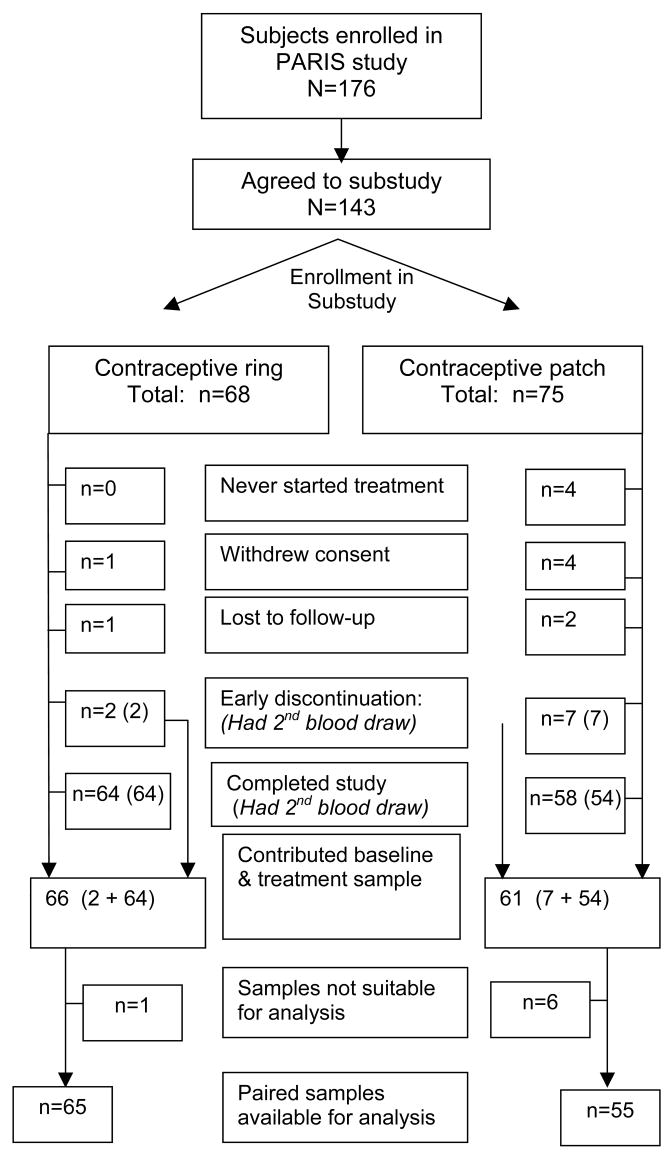
Between June 2005 and September 2006, 176 subjects were enrolled in the PARIS study at the three sites. Of these, 143 enrolled in the substudy and 131 contributed two blood samples. Labeling errors led to the exclusion of several samples (CR =1, CP = 6) leaving 120 subjects that contributed two samples suitable for analysis for paired analysis of APC-r and SHBG, and 119 for protein S (Fig. 1).
Fig. 1.
Enrollment in substudy, and samples available for evaluation.
Of the 120 subjects in the analysis group, 65 received a contraceptive ring and 55 a contraceptive patch at randomization. Baseline characteristics were similar between the two treatment groups after randomization. The mean age of all 120 subjects was 27 years with the majority current OC users (85%), nulligravid (73%), white (81%) and non-Hispanic (97%) (Table 1). The demographics of the substudy sample were similar to those in the PARIS study [15].
Table 1.
Baseline characteristics of the study participants.
| Variable | Contraceptive ring n= 65 | Contraceptive patch n= 55 | p-valueb | |
|---|---|---|---|---|
| Age at enrollment (years) | 27.5 (6.5) | 26.7 (5.5) | 0.520 | |
| BMI | <18.5 Underweight 18.5 – | 2 (3.1%) | 2 (3.6%) | 0.854 |
| 24.9 Normal | 49 (75.4%) | 38 (69.1%) | ||
| 25–29.9 Overweight | 10 (15.4%) | 10 (18.2%) | ||
| >30 Obese | 4 (6.1%) | 5 (9.1%) | ||
|
| ||||
| Pregnancies | 0 | 50 (76.9%) | 38 (69.1%) | 0.334 |
| ≥1 | 15 (23.1%) | 17 (30.9%) | ||
|
| ||||
| Race | White | 52 (80%) | 45 (81.8%) | 0.977 |
| Black | 4 (6.2%) | 3 (5.5%) | ||
| Asian | 4 (6.2%) | 4 (7.3%) | ||
| Other | 5 (7.7%) | 3 (5.4%) | ||
|
| ||||
| Ethnicity | Hispanic | 1 (1.5%) | 3 (5.4%) | 0.332 |
| Not Hispanic | 64 (98.5%) | 52 (94.6%) | ||
|
| ||||
| OC use | Recent | 8 (12.3%) | 10 (18.2%) | 0.369 |
| Current | 57 (87.7%) | 45 (81.8%) | ||
|
| ||||
| Current OC preparation (n %) | ||||
| EE >20 mcg | 48 (84.2%) | 38 (84.4%) | 0.974 | |
| 3rd generation progestogen | 39 (68.4%) | 27 (60.0%) | 0.732 | |
Progestins were classified as either 3rd generation (norgestimate, desogestrel), 1st and 2nd generation (levonorgestrel, norgestrel, ethynodiol diacetate, norethindrone, or other (drosperinone)
For both the CR and the CP groups, the baseline values for APC-r ratio, SHBG, and protein S fell within the normal ranges for healthy premenopausal women, with no significant differences between treatment groups (Table 2).
Table 2.
Mean change in APC-r, SHBG, and protein S before (pre), and after (post) treatment with a contraceptive ring or contraceptive patch, all subjects
| APC-r ratio (95% CI)1 | SHBG nmol/l (95% CI)2 | Protein S% (95% CI)3 | ||
|---|---|---|---|---|
| Contraceptive ring | Pre Post | 3.07 2.93, 3.20) | 147.6 (128.5, 166.7) | 88.3 (83.3, 93.3) |
| Mean change | 3.09 (2.96, 3.22) | 146.0 (132.6, 159.4) | 93.6 (89.1, 98.1) | |
| p value | 0.02 (−0.10, 0.14) 0.698 |
−1.6 (−16.6, 13.5) 0.834 |
5.3 (1.1, 9.6) 0.014 |
|
| Contraceptive patch | Pre Post | 2.93 (2.79, 3.08) | 157.6 (136.5, 178.8) | 88.9 (82.9, 94.9) |
| Mean change | 2.99 (2.85, 3.14) | 187.5 (167.0, 208.0) | 81.8 (76.8, 86.8) | |
| p value | 0.06 (−0.06, 0.18) 0.296 |
29.9 (9.6, 50.1) 0.005 |
−7.1 (−12.1, −2.1) 0.006 |
|
| p-value between groups | Mean change | 0.647 | 0.012 | <0.001 |
APC-r ratio normal range > 2.19
SHBG normal range 20–175 ng/mL
Protein S % of normal activity, normal range 73–149%
Non-significant increases in APC-r ratio occurred during treatment in both CR (mean change 0.02 (95% CI −0.10, 0.14)) and CP (0.06 (−0.06, 0.18)) users, with no significant difference in the mean change between groups. In contrast, while SHBG declined slightly in ring users (mean change −1.6 nmol/L (−16.6, 13.5)), it increased significantly in patch users (mean increase 29.9 nmol/p (9.6, 50.1)). This difference in the mean change in SHBG was significant between the ring and patch treatments (p=0.012). While protein S increased significantly in CR users (mean change 5.3 (1.1, 9.6)), CP users demonstrated a significant drop in activity (mean decrease −7.1 (−12.1, −2.1), and these mean changes in protein S activity between treatment groups were significantly different (p <0.001). Adjusting for BMI or other demographic characteristics did not alter the direction of the general conclusions.
The effects of OC use and formulation (e.g. progestogen type, estrogen dose) at baseline, are summarized in Table 3. In general, the direction of the effect for all of the assays mirrored the overall comparison. Current OC users showed smaller mean changes in SHBG [CR −9.6 (−26.4, 7.2), CP 18.4 (−0.5, 37.3), p = 0.03] and protein S [6.1 (1.5, 10.6), −4.6 (−9.8, 0.6), p =0.003] than recent users [SHBG: 55.8 (11.0, 100.7), 81.3 (41.2, 121.4), p = 0.4; protein S: 0.3 (−11.9, 12.4), −18.3 (−29.2, −7.4), p = 0.026]. Few subjects (n=16) reported use of very low 20 mcg EE pills, and the confidence intervals for all comparisons were very broad. Results from subjects using estrogen doses > 20 mcg at baseline were the same as the overall group, with an increase in SHBG [CP 23.6 (0.6, 46.6), CR −12.0 (−28.0, 3.9), p = 0.013) and decrease in protein S [CP −6.5 (−12.2, −0.7), CR 7.5 (2.5, 12.6), p = 0.001) observed in patch users. Users of third generation progestins (e.g. desogestrel and norgestimate) did not experience changes in SHBG or protein S while using the patch, but showed a significant reduction in SHBG (mean change −17.4 (−34.5, −0.2)) and a non-significant increase in protein S (5.0 (−0.7, 10.6)). Former users of 1st/2nd generation progestins experienced non- significant increases in SHBG when switching to either new method, but protein S increased in CR (5.5 (−4.2, 15.3)) and decreased in CP users (−13.9 (−24.1, −3.8), p = 0.007. Since only 11 women (6 CR, 5 CP) reported use of drosperinone, these results were not separately analyzed. Similar to the overall comparison, OC use and type did not influence activated protein C resistance.
Table 3.
Effect of OC use status, and OC type on APC-r ratio, SHBG (nmol/L), and protein S (% of normal activity); Adjusted means with 95% confidence intervals (CI). The mean change is statistically significant within groups if the 95% CI does not include zero, and between groups if the p-value is <0.05.
| Effect | Group | Time | APC-r ratio (95% CI)1 | SHBG nmol/L (95% CI)2 | Protein S % (95% CI)3 |
|---|---|---|---|---|---|
|
OC use status | |||||
| Current OC use (n = 102) | Contraceptive ring (n = 57) | Pre | 2.93 (2.75, 3.11) | 145.0 (121.1, 169.0) | 85.1 (78.2, 92.0) |
| Post | 2.93 (2.75, 3.11) | 135.4 (111.5, 159.3) | 91.2 (84.3, 98.1) | ||
| Mean change | −0.003 (−0.12, 0.12) | −9.6 (−26.4, 7.2) | 6.1 (1.5, 10.6) | ||
| Contraceptive patch (n = 45) | Pre | 2.80 (2.62, 2.98) | 159.2 (135.0, 183.4) | 83.1 (76.1, 90.1) | |
| Post | 2.81 (2.63, 2.99) | 177.6 (153.4, 201.8) | 78.5 (71.5, 85.5) | ||
| Mean change | 0.01 (−0.12, 0.14) | 18.4 (−0.5, 37.3) | −4.6 (−9.8, 0.6) | ||
| Between groups p-value4 | 0.737 | 0.030 | 0.003 | ||
|
| |||||
| Recent OC use (n = 18) | Contraceptive ring (n = 8) | Pre | 2.65 (2.29, 3.02) | 51.1 (2.8, 99.5) | 91.7 (77.9, 105.6) |
| Post | 2.86 (2.50, 3.22) | 106.9 (58.6, 155.3) | 92.0 (78.2, 105.8) | ||
| Mean change | 0.21 (−0.11, 0.52) | 55.8 (11.0, 100.7) | 0.3 (−11.9, 12.4) | ||
| Contraceptive patch (n = 10) | Pre | 2.82 (2.50, 3.14) | 99.2 (56.2, 142.1) | 101.7 (89.4, 113.9) | |
| Post | 3.10 (2.78, 3.42) | 180.5 (137.6, 223.4) | 83.4 (71.1, 95.7) | ||
| Mean change | 0.28 (−0.003, 0.56) | 81.3 (41.2, 121.4) | −18.3 (−29.2, −7.4) | ||
| Between groups p-value4 | 0.861 | 0.403 | 0.026 | ||
|
| |||||
|
Effect of estrogen dose | |||||
| EE = 20 mcg (n = 16) | Contraceptive ring (n = 9) | Pre | 3.03 (2.65, 3.42) | 144.6 (89.9, 199.3) | 97.7 (83.9, 111.4) |
| Post | 2.92 (2.53, 3.31) | 147.9 (108.3, 187.5) | 95.8 (82.0, 109.6) | ||
| Mean change | −0.11 (−0.41, 0.18) | 3.3 (−33.5, 40.0) | −1.9 (−13.5, 9.9) | ||
| Contraceptive patch (n = 7) | Pre | 2.68 (2.28, 3.09) | 176.2 (121.0, 231.3) | 79.4 (64.9, 93.2) | |
| Post | 2.57 (2.17, 2.98) | 166.5 (113.7, 219.3) | 84.7 (70.2, 99.2) | ||
| Mean change | −0.11 (−0.45, 0.23) | −9.7 (−63.2, 43.8) | 5.3 (−8.0, 18.5) | ||
| Between groups p-value4 | 0.993 | 0.692 | 0.427 | ||
|
| |||||
| EE >20 mcg (n = 86) | Contraceptive ring (n = 48) | Pre | 2.94 (2.74, 3.15) | 142.0 (114.8, 169.3) | 82.8 (75.5, 90.0) |
| Post | 2.96 (2.76, 3.17) | 130.0 (108.1, 151.8) | 90.3 (83.1, 97.6) | ||
| Mean change | 0.02 (−0.11, 0.15) | −12.0 (−28.0, 3.9) | 7.5 (2.5, 12.6) | ||
| Contraceptive patch (n = 38) | Pre | 2.84 (2.64, 3.05) | 152.0 (124.8, 179.1) | 83.7 (76.3, 91.1) | |
| Post | 2.88 (2.67, 3.09) | 175.6 (149.3, 201.9) | 77.2 (69.7, 84.7) | ||
| Mean change | 0.04 (−0.11, 0.18) | 23.6 (0.6, 46.6) | −6.5 (−12.2, −0.7) | ||
| Between groups p-value4 | 0.854 | 0.013 | 0.001 | ||
|
| |||||
|
Effect of progestogen type | |||||
| 1st/2nd Generation (n = 25) | Contraceptive ring (n = 13) | Pre | 2.94 (2.62, 3.28) | 90.9 (47.2, 134.6) | 86.1 (74.2, 98.0) |
| Post | 2.89 (2.56, 3.23) | 109.6 (76.6, 142.6) | 91.6 (79.7, 103.6) | ||
| Mean change | −0.05 (−0.30, 0.19) | 18.7 (−11.1, 48.4) | 5.5 (−4.2, 15.3) | ||
| Contraceptive patch (n = 12) | Pre | 2.83 (2.50, 3.15) | 96.0 (58.2, 133.8) | 89.8 (78.2, 101.5) | |
| Post | 2.98 (2.66, 3.30) | 151.0 (110.3, 191.7) | 75.9 (64.3, 87.5) | ||
| Mean change | 0.15 (−0.11, 0.41) | 55.0 (15.4, 94.6) | −13.9 (−24.1, −3.8) | ||
| Between groups p-value4 | 0.259 | 0.149 | 0.007 | ||
|
| |||||
| 3rd generation (N = 66) | Contraceptive ring (n = 39) | Pre | 2.98 (2.76, 3.20) | 153.8 (126.1, 181.5) | 85.0 (77.2, 92.9) |
| Post | 3.00 (2.78, 3.21) | 136.4 (114.2, 158.7) | 90.0 (82.2, 97.9) | ||
| Mean change | 0.01 (−0.13, 0.16) | −17.4 (−34.5, −0.2) | 5.0 (−0.7, 10.6) | ||
| Contraceptive patch (n = 27) | Pre | 2.80 (2.56, 3.04) | 184.1 (156.8, 211.5) | 80.8 (72.3, 89.3) | |
| Post | 2.74 (2.51, 2.98) | 185.1 (156.0, 214.3) | 80.4 (71.8, 89.1) | ||
| Mean change | −0.06 (−0.23, 0.12) | 1.0 (−25.4, 27.4) | −0.4 (−7.2, 6.5) | ||
| Between groups p-value4 | 0.542 | 0.249 | 0.235 | ||
APC-r ratio, normal range > 2.19
SHBG, normal range 20–175 ng/mL
Protein S % of normal activity, normal range 73–149%
For comparison of pre-post mean change between ring and patch users within each category
Progestins were classified as either 3rd generation (norgestimate, desogestrel), 1st and 2nd generation (levonorgestrel, norgestrel, ethynodiol diacetate, norethindrone, or other (drosperinone).
4. Discussion
Thrombosis represents one of the greatest risks of combined hormonal contraception, and the impact of a combined hormonal contraceptive method on coagulation pathways has important implications for safety. Our results demonstrate that OC users who switch to the contraceptive patch exhibit unfavorable changes in prothrombotic markers, while women switching to the contraceptive ring tend to show values more similar to non-users.
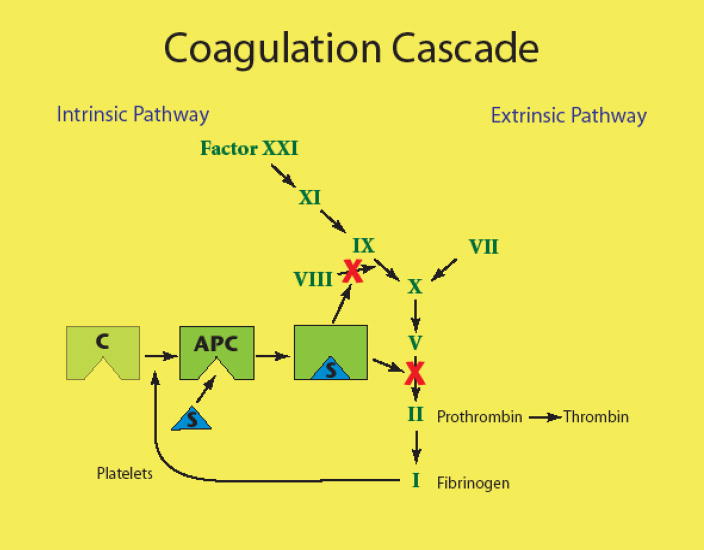
Oral contraceptive use produces changes in procoagulant, anticoagulant, and fibrinolytic parameters resulting in a net prothrombotic effect [2]. One suggested method of measuring the total effect is the thrombin generation-based activated protein C sensitivity ratio (APC-r) test [18]. The APC-r test has been shown to predict the risk of venous thrombosis in users of OCs as well as in non-users and men [19]. The direction of the effect of a hormone combination on APC-r supports observations noted in epidemiological studies of OC use and thrombosis [18]. However, APC acts as an anticoagulant through interaction with Factor V (see Figure 2)[20]. At least 90% of resistance to activated protein C is explained by point mutation in the gene for coagulation factor V, with the Leiden mutation most common [20]. Acquired resistance to APC then occurs primarily due to interactions with other plasma factors, principally protein S. Since the APC-r is sensitive to changes in multiple coagulation factors beyond Factor V Leiden it represents a milieu effect, rather than specific activity of Factor V. In other words, the APC-r assay is sensitive to a number of plasma factors affected by hormonal contraception that might influence blood clotting.
Fig. 2.
A simplified illustration of the coagulation cascade. Activated protein C (APC) exerts an anticoagulant effect primarily through inhibition of factor V. Protein S is required for this interaction.
It is important to note that the APC-r test used in our study differs from the APC-r test, and is not expected to change with hormonal contraception[21] Therefore, the similarity of our groups with respect to APC-r pre-treatment demonstrates the absence of a congenital defect in Factor V, and the absence of a change following switching to the CR or CP confirms that the impact of these hormonal therapies on the coagulation cascade is independent of factor V and related to other plasma factors.
Two studies have also suggested that an OC’s effect on SHBG levels could possibly be an indicator of the thrombotic risk of that particular formulation [22, 23]. The extent of the estrogen-related increase in SHBG depends not only on the dose of EE, but also on both the dose and type of progestogen [24–27]. This has led to the advancement of the use of SHBG levels as a marker of the total estrogenicity (the sum of estrogenic effect of EE and the anti-estrogenic effect of the progestogen) in an OC [22, 28]. Odlind et al. [22] found that SHBG levels were highest in users of pills shown in epidemiologic studies to have the highest risk of venous thrombosis. Consistent with these results, a randomized controlled trial comparing SHBG levels in women using a desogestrel-containing OC to levels in women using levonorgestrel-containing OCs confirmed higher levels of SHBG and APC-r in desogestrel-containing pill users [23].
Protein S is a vitamin K–dependent anticoagulant protein. Its major function is as a cofactor to facilitate the action of APC on its substrates (see Figure 2). Protein S deficiency usually manifests clinically as venous thromboembolism and may be hereditary or acquired. Protein S levels decrease in pregnancy and can fall into the abnormal-low laboratory range. However, low levels of protein S in pregnancy do not normally cause thrombosis by themselves [29].
Our results are consistent with studies documenting similar changes in these markers between users of 2nd and 3rd generation OCs [4, 5], and between users of OCs and the contraceptive patch [29]. Johnson et al. [29] randomized non-users of hormonal contraception to either the patch or a 35 mcg EE/250 mcg norgestimate-containing OC in a crossover design. Consistent with our findings, protein S decreased with both treatments in the Johnson study, with a larger decline seen in patch users. Resistance to APC also increased with both treatments, and was significantly greater among patch users in one of the two assays used. In contrast, all of our APC-r ratios were within the normal range, with only small non-significant differences seen between or within treatment groups. This difference reflects the underlying sensitivity of the APC-r assay used in the two studies. Johnson measured the normalized APC sensitivity ratio (nAPCsr) using two thrombin generation assay methods that are sensitive to changes in multiple coagulation factors beyond Factor V Leiden. Our study used a functional APC-r ratio assay kit that shows no interference from changes in levels of protein C, protein S, lupus anticoagulants, or elevated Factor VIII levels.
Although we enrolled fewer subjects than planned, we were still able to find significant changes in both SHBG and protein S between treatment groups. We tested the main effect of use of the contraceptive patch or ring with possible interactions of BMI, recent or past OC use, and type of OC used, but did not enroll sufficient numbers to fully evaluate interactions. Small sample size in subgroups and the hazards of applying statistical tests over multiple outcomes limit our ability to make inferences about the effect of OC type used at baseline. Given the small observed differences seen in APC-r ratio using this assay, a much larger study would be needed to demonstrate a difference in this outcome.
Strengths of the study include the randomized design, and excellent participation. Of the 142 subjects who consented to participate, 120 (85%) contributed two samples suitable for analysis. All of the assays were performed at a central laboratory and run against the same standard curves.
It is important to recognize the limitations of using surrogate markers to assess risk of a hormonal product. Clarkston et al. [30] found that OC use protected cynomologous monkeys from the negative impact of an atherogenic diet, even with unfavorable lipid changes. While the surrogate markers measured in our study support a shift favoring clot formation in patch users, epidemiologic studies of thrombosis have not consistently demonstrated an increased risk in women using the patch [9, 10]. The largest case-control study of thrombosis in patch users to date by Jicks et al. [9, 10] found no increase in risk (OR 1.1 (95% CI 0.6–2.1). Furthermore, the overall risk of a thrombotic event in a healthy reproductive age woman is low, and the risk of thrombosis associated with pregnancy exceeds that of combined hormonal contraception [31]. However, subgroups that are at slightly higher risk of thrombosis (e.g., obese women) have not been well studied. It is unknown if small differences in prothrombotic markers produced using different combined hormonal contraceptives may influence the likelihood of a thrombotic event in women at increased baseline risk for thrombosis.
Our data support that users of OCs who switch to the contraceptive ring tend to exhibit favorable changes in biomarkers of clotting, while patch users undergo a shift toward a more pro-thrombotic profile. Given that OC users who switch to the ring are also more satisfied, and more likely to continue with the method than those that switch to the patch [15], clinicians may feel more comfortable recommending ring use to OC switchers and new patients.
Acknowledgments
The authors wish to thank the staff of the Women’s Health Research Unit at OHSU for their assistance with the protocol, Dr. Clive Woffendin for assistance with the assays, Dr. Thomas Deloughery for review of the manuscript, and Dr. Mitch Crenin for the concept and execution of the PAIR study.
Funding
This publication was made possible in part with biostatistics support from the Biostatistics Shared Resource of Oregon Health and Science University and laboratory support from the Oregon Clinical and Translational Research Institute (OCTRI), grant numbers UL1 RR024140 01 & M01 RR000334 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Funding for the main study was directly from the Magee-Women’s Research Institute Foundation using funds from Organon USA. Organon USA, Inc., played no role in the design of either study; in the acquisition, analysis, and interpretation of the data; in the drafting or revision of the manuscript; or technical support or supervision of the study.
Footnotes
Conflict of interest
Dr. Jensen: research funding: Berlex (Bayer), Organon, Galen, Symbollon, Wyeth, Pfizer, Novartes; speakers bureau: Berlex (Bayer), Wyeth; consulting: Berlex (Bayer), Wyeth, Novartes
Dr. Burke: research funding: Organon, Duramed, Berlex
Dr. Barnhart: research funding: Organon, Wyeth-Ayerst, Johnson & Johnson, Duramed, Xanodyne, Boehringer Ingelheim, Third Wave, Pfeizer, MGI Pharma; speakers bureau: Organon; consulting: Novo Nordisk
Dr. Peters, Ms. Messerle-Forbes, Ms. Tillotson: No conflicts
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168–74. doi: 10.1016/j.contraception.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 2.Vandenbroucke JP, Rosing J, Bloemenkamp KW, et al. Oral contraceptives and the risk of venous thrombosis. N Engl J Med. 2001;344:1527–35. doi: 10.1056/NEJM200105173442007. [DOI] [PubMed] [Google Scholar]
- 3.Rosendaal FR, Van Hylckama Vlieg A, Tanis BC, et al. Estrogens, progestogens and thrombosis. J Thromb Haemost. 2003;1:1371–80. doi: 10.1046/j.1538-7836.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 4.Vasilakis-Scaramozza C, Jick H. Risk of venous thromboembolism with cyproterone or levonorgestrel contraceptives. Lancet. 2001;358:1427–9. doi: 10.1016/S0140-6736(01)06522-9. [DOI] [PubMed] [Google Scholar]
- 5.Kemmeren JM, Algra A, Grobbee DE. Third generation oral contraceptives and risk of venous thrombosis: meta-analysis. BMJ. 2001;323:131–134. doi: 10.1136/bmj.323.7305.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Srivastava A, Weiss M. First Patch Death; Coroner Confirms It. New York Post. 2004 April 28;2004:13. [Google Scholar]
- 7.Edelman S, Kranes M. ‘Patch’ Shock Grows: Kin ID new ‘victims’. New York Post. 2004 May 16;2004:1. [Google Scholar]
- 8.Cole JA, Norman H, Doherty M, et al. Venous thromboembolism, myocardial infarction, and stroke among transdermal contraceptive system users. Obstet Gynecol. 2007;109:339–46. doi: 10.1097/01.AOG.0000250968.82370.04. [DOI] [PubMed] [Google Scholar]
- 9.Jick S, Kaye JA, Li L, et al. Further results on the risk of nonfatal venous thromboembolism in users of the contraceptive transdermal patch compared to users of oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception. 2007;76:4–7. doi: 10.1016/j.contraception.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Jick SS, Kaye JA, Russmann S, et al. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception. 2006;73:223–8. doi: 10.1016/j.contraception.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Teal SB, Ginosar DM. Contraception for women with chronic medical conditions. Obstet Gynecol Clin North Am. 2007;34:113–26. ix. doi: 10.1016/j.ogc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Cornelis K, Piet M, Katherine DL, et al. Comparison of a transdermal contraceptive patch vs. oral contraceptives on hemostasis variables. Contraception. 2008;77:77–83. doi: 10.1016/j.contraception.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JV, Lowell J, Badger GJ, et al. Effects of oral and transdermal hormonal contraception on vascular risk markers: a randomized controlled trial. Obstet Gynecol. 2008;111:278–84. doi: 10.1097/AOG.0b013e3181626d1b. [DOI] [PubMed] [Google Scholar]
- 14.White T, Ozel B, Jain JK, et al. Effects of transdermal and oral contraceptives on estrogen-sensitive hepatic proteins. Contraception. 2006;74:293–6. doi: 10.1016/j.contraception.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Creinin MD, Meyn LA, Borgatta L, et al. Multicenter comparison of the contraceptive ring and patch: a randomized controlled trial. Obstet Gynecol. 2008;111:267–77. doi: 10.1097/01.AOG.0000298338.58511.d1. [DOI] [PubMed] [Google Scholar]
- 16.Thomas AE, McKay DA, Cutlip MB. A nomograph method for assessing body weight. Am J Clin Nutr. 1976;29:302–4. doi: 10.1093/ajcn/29.3.302. [DOI] [PubMed] [Google Scholar]
- 17.Sitruk-Ware R. Pharmacological profile of progestins. Maturitas. 2004;47:277–83. doi: 10.1016/j.maturitas.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Rosing J, Tans G. Effects of oral contraceptives on hemostasis and thrombosis. Am J Obstet Gynecol. 1999;180:S375–S382. doi: 10.1016/s0002-9378(99)70699-x. [DOI] [PubMed] [Google Scholar]
- 19.Tans G, van Hylckama Vlieg A, Thomassen MC, et al. Activated protein C resistance determined with a thrombin generation-based test predicts for venous thrombosis in men and women. Br J Haematol. 2003;122:465–70. doi: 10.1046/j.1365-2141.2003.04443.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosen SB, Sturk A. Activated protein C resistance — a major risk factor for thrombosis. Eur J Clin Chem Clin Biochem. 1997;35:501–16. [PubMed] [Google Scholar]
- 21.Curvers J, Thomassen MC, Nicolaes GA, et al. Acquired APC resistance and oral contraceptives: differences between two functional tests. Br J Haematol. 1999;105:88–94. [PubMed] [Google Scholar]
- 22.Odlind V, Milsom I, Persson I, et al. Can changes in sex hormone binding globulin predict the risk of venous thromboembolism with combined oral contraceptive pills? Acta Obstet Gynecol Scand. 2002;81:482–90. [PubMed] [Google Scholar]
- 23.van Rooijen M, Silveira A, Hamsten A, et al. Sex hormone-binding globulin--a surrogate marker for the prothrombotic effects of combined oral contraceptives. Am J Obstet Gynecol. 2004;190:332–7. doi: 10.1016/s0002-9378(03)00950-5. [DOI] [PubMed] [Google Scholar]
- 24.Anderson DC. Sex-hormone-binding globulin. Clin Endocrinol (Oxf) 1974;3:69–96. doi: 10.1111/j.1365-2265.1974.tb03298.x. [DOI] [PubMed] [Google Scholar]
- 25.El Makhzangy MN, Wynn V, Lawrence DM. Sex hormone binding globulin capacity as an index of oestrogenicity or androgenicity in women on oral contraceptive steroids. Clin Endocrinol (Oxf) 1979;10:39–45. doi: 10.1111/j.1365-2265.1979.tb03031.x. [DOI] [PubMed] [Google Scholar]
- 26.Knopp RH, Broyles FE, Cheung M, et al. Comparison of the lipoprotein, carbohydrate, and hemostatic effects of phasic oral contraceptives containing desogestrel or levonorgestrel. Contraception. 2001;63:1–11. doi: 10.1016/s0010-7824(00)00196-7. [DOI] [PubMed] [Google Scholar]
- 27.van der Vange N, Blankenstein MA, Kloosterboer HJ, et al. Effects of seven low-dose combined oral contraceptives on sex hormone binding globulin, corticosteroid binding globulin, total and free testosterone. Contraception. 1990;41:345–52. doi: 10.1016/0010-7824(90)90034-s. [DOI] [PubMed] [Google Scholar]
- 28.van Kammen E, Thijssen JH, Rademaker B, et al. The influence of hormonal contraceptives on sex hormone binding globulin (SHBG) capacity. Contraception. 1975;11:53–9. doi: 10.1016/0010-7824(75)90050-5. [DOI] [PubMed] [Google Scholar]
- 29.Kupferminc MJ. Thrombophilia and pregnancy. Reprod Biol Endocrinol. 2003;1:111. doi: 10.1186/1477-7827-1-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarkson TB, Shively CA, Morgan TM, et al. Oral contraceptives and coronary artery atherosclerosis of cynomolgus monkeys. Obstet Gynecol. 1990;75:217–22. [PubMed] [Google Scholar]
- 31.Blickstein I. Thrombophilia and women’s health: An overview. Obstet Gynecol Clin North Am. 2006;33:347–56. doi: 10.1016/j.ogc.2006.05.003. [DOI] [PubMed] [Google Scholar]