Abstract
Background
Recent data suggest that Clostridium difficile-associated diarrhea is becoming more severe and difficult to treat. Antibody responses to C. difficile toxin A are protective against symptomatic disease and recurrence. We examined the safety and pharmacokinetics (pk) of a novel neutralizing human monoclonal antibody against C. difficile toxin A (CDA1) in healthy adults.
Methods
Five cohorts with 6 subjects each received a single intravenous infusion of CDA1 at escalating doses of 0.3, 1, 5, 10, and 20 mg/kg. Safety evaluations took place on days 1, 2, 3, 7, 14, 28, and 56 post-infusion. Samples for pk analysis were obtained before and after infusion, and at each safety evaluation. Serum CDA1 antibody concentrations and human anti-human antibody (HAHA) titers were measured with enzyme-linked immunosorbent assays. A noncompartmental model was used for pk analysis.
Results
Thirty subjects were enrolled. The median age was 27.5 yrs. There were no serious adverse events related to CDA1. Twenty-one of the 48 reported non-serious adverse events were possibly related to CDA1, and included transient blood pressure changes requiring no treatment, nasal congestion, headache, abdominal cramps, nausea, and self-limited diarrhea. Serum CDA1 concentrations increased with escalating doses: mean Cmax ranged from 6.82 mcg/ml for the 0.3 mg/kg cohort to 511 mcg/ml for the 20mg/kg cohort. The geometric mean values of the half-life of CDA1 ranged between 25.3 and 31.8 days, and the volume of distribution approximated serum. No subject formed detectable HAHA titers.
Conclusion
Administration of CDA1 as a single intravenous infusion was safe and well tolerated. Cmax increased proportionally with increasing doses. A randomized study of CDA1 in patients with C. difficile associated diarrhea is underway.
Introduction
Clostridium difficile is an important cause of antibiotic-associated diarrhea, and results in a wide spectrum of disease, ranging from asymptomatic colonization to fulminant pseudomembranous colitis.1 Pathogenic strains produce two protein exotoxins: toxin A and toxin B.1 Experiments in rabbits, hamsters, mice, and rats have shown that toxin A causes a severe inflammatory response, epithelial damage, and hemorrhagic fluid accumulation in intestinal loops.2–4 Toxin B induces cytotoxic effects on cultured intestinal epithelial cells, but lacks enterotoxicity in rodents in vivo.5–7 Active immunization of hamsters with toxin A was sufficient to prevent fatal clindamycin-induced C. difficile-associated ileocecitis.8 However, immunization with toxin B alone was not protective in this model.8 Passive immunization with monoclonal antibodies against toxin A protected axenic mice from pseudomembranous cecitis.9 Therefore, toxin A has been considered to be the principal mediator of C. difficile-associated disease in animals.
Conventional management of C. difficile-associated diarrhea consists of discontinuing the precipitating antimicrobial agent(s) if possible, and providing supportive care.1 If improvement does not occur, therapy with oral metronidazole or vancomycin is recommended.1, 10–13 Although the majority of cases respond to such therapy, 15–30% of patients experience recurrent diarrhea after treatment is discontinued.12, 13 Treating recurrent disease is often problematic, and an optimal therapeutic approach remains to be elucidated.12, 13
Humoral immune responses to C. difficile toxins play an important role in determining the severity of disease induced by the organism.14–17 Approximately 60% of healthy individuals produce serum immunoglobulin (Ig) G antitoxin A antibodies and intestinal secretory antitoxin A antibodies.14 Patients with low antibody levels against toxin A are at risk of experiencing severe, prolonged, or recurrent C. difficile-associated disease.15,–17 A prospective study of hospitalized patients who received antibiotics and were colonized by toxigenic C. difficile showed that those with low serum IgG antitoxin A antibody levels were 48 times more likely to develop C. difficile-associated diarrhea compared to individuals with high antibody levels.15 Small case series have demonstrated that administering pooled human immunoglobulin to children and adults with severe or protracted C. difficile-associated disease can lead to clinical improvement.18–21 Therefore, passive immunization may be a promising adjunct to standard therapies for severe or recurrent disease.
Given the above observations, a novel human monoclonal antibody against C. difficile toxin A designated CDA1 was developed by the Massachusetts Biologic Laboratories in partnership with Medarex, Inc. CDA1 is an IgG1κ molecule that was generated against C. difficile toxin A using genetically altered mice (Hco7 HuMAB mice) which produce human antibodies. CDA1 binds to the receptor binding domain of toxin A with high affinity and has demonstrated efficacy in reducing mortality in the established hamster model for C. difficile associated disease.19 An open-label, dose escalation study was therefore undertaken to assess the safety and pharmacokinetics of single infusions of CDA1 in healthy adult volunteers.
Subjects and Methods
Subject Population
Thirty subjects were enrolled at 2 centers (Beth Israel Deaconess Medical Center, Boston, MA and Tufts-New England Medical Center, Boston, MA). Inclusion criteria were as follows: age between 18 and 55 years, inclusive; good general health without a history of any of the conditions listed in the exclusion criteria; and screening laboratory values that met the following criteria: 1) WBC >= 4500 and <=13,000 cells/μl;2)Platelet count >= 150,000 cells/μl; 3) hemoglobin level >= 12 gm/dl; 4)creatinine level and bilirubin level < 1.1 times the upper limit of normal (ULN); 5) blood urea nitrogen, aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase level < 1.25 times the ULN; and 6) nonfasting glucose level <= 115 mg/dl. Exclusion criteria included previous receipt of a humanized monoclonal antibody (licensed or investigational); receipt of a licensed vaccine or investigational agent within 30 days of enrollment; personal history of cancer, heart disease, diabetes mellitus, respiratory conditions (e.g., asthma requiring daily medication), autoimmune disorders, or blood dyscrasias; any chronic condition requiring daily prescription or over-the-counter medications, except for birth control products; personal history of severe allergic reactions with generalized urticaria, angioedema, or anaphylaxis; clinically significant physical examination findings (e.g., heart murmurs, hepatosplenomegaly, lymphadenopathy, or focal neurologic deficits); blood pressure > 160/100 or < 90/70 on two separate readings; urinalysis positive for protein, >5 red cells/hpf, or >5 white cells/hpf; positive serology for HIV-1 antibody or HCV antibody; positive hepatitis B surface antigen; positive urine pregnancy test during an initial screening visit or within 24 hours of the administration of CDA1; unwillingness to undergo pregnancy testing; breast-feeding; and any other condition that in the opinion of the investigators would jeopardize the safety or rights of the volunteer, or make study completion unlikely. Female subjects of childbearing potential and male subjects were required to use effective contraception during the study. Women were asked to avoid becoming pregnant during the study, and for at least three months after the infusion of CDA1.
This study followed human experimentation guidelines of the US Department of Health and Human Services and those of the authors’ institutions. It was approved by the institutional review boards of both participating centers. Written informed consent was obtained from each subject prior to study participation.
Study Product Administration
Subjects were sequentially enrolled into 5 dosing cohorts of 6 subjects each. Subjects were admitted to the General Clinical Research Center at each study site for study drug administration and monitoring during the first 8 hours after infusion. CDA1, a humanized IgG1κ monoclonal antibody, was manufactured under GMP (Good Manufacturing Practice)-compliant conditions with appropriate chemistry, manufacturing and control information provided to the Food and Drug Administration at the time of filing and Investigational New Drug application. CDA1 was manufactured by Massachusetts Biologic Laboratories (MBL, 305 South Street, Jamaica Plain, MA 02130) and supplied at a concentration of 10 mg/ml as a sterile product in 10 mM PBS buffer, pH 6.0, containing 0.01% polysorbate 80 and 0.15 M NaCl. Each calculated dose of CDA1 was diluted to a total volume of 200 ml in 0.9% NaCl. For administration, CDA1 was injected through a sterile 0.22 micron filter with the infusion controlled by a volumetric pump. The first cohort received a single intravenous infusion of CDA1 at 0.3 mg/kg over 2 hours. Subsequent cohorts were administered increasing doses of CDA1 at 1 mg/kg, 5 mg/kg, 10 mg/kg, and 20 mg/kg. CDA1 was infused over 2 hours for the 1 mg/kg and 5 mg/kg cohorts. For the 10 and 20 mg/kg doses, a test dose was first given at 180 mg/hr for 30 minutes. If this rate was tolerated, the infusion rate was increased to a maximum rate of 550 mg/hr for a total infusion time of not less than 2 hours.
Safety Monitoring
Before escalation to the next dosing cohort was permitted, the day 7 safety data of all subjects in the prior cohort were reviewed by the principal investigator and sponsor. An independent safety monitoring committee reviewed the safety data collected through day 14 for the first 18 subjects, then after the day 14 evaluation of the next 12 subjects, and at study completion of all subjects. Adverse events (AE) were assessed and reported using the Division of Microbiology and Infectious Diseases (DMID) Adult Toxicity Table (May 2001, National Institute of Allergy and Infectious Diseases, National Institutes of Health)
Each study subject was monitored during the infusion of CDA1 and in the immediate 8 hour post-infusion periodfor vital signs and emergence of adverse events. Each subject was re-assessed at study visits on days 1, 2, 3, 7, 14(+/−1), 28(+/−3), and 56(+/−7). At each study visit, interim medical histories, concomitant medication use, and clinical adverse events were assessed, and physical examinations were performed. Safety labs (complete blood cell counts, serum electrolytes, blood urea nitrogen, creatinine, glucose, liver function tests, and urinalysis with microscopic examination) were measured on days 0 (baseline), 1, 3, 7, and 28+/−3. Day 56+/−7 safety labs were measured only if day 28 results were abnormal. All safety laboratory measurements were performed by the local clinical laboratories of the study centers.
Pharmacokinetics
The immunogenicity of CDA1 was determined by measuring serum concentrations of human anti-human antibody (HAHA) with a bridging enzyme-linked immunosorbent-assay (ELISA) on days 0 (prior to the infusion of CDA1), 14+/−1, and 56+/−7. Serum CDA1 antibody titers were measured using an ELISA for measurement of IgG antibody concentrations to C. difficile Toxin A. The assays for HAHA titers and CDA1 antibody concentrations were performed at the Massachusetts Biologic Laboratories.
Blood samples for pk analysis were obtained pre-infusion, and 0.5, 1, 2, 4, 6, and 8 hours post-infusion on day 0; and on days 1, 2, 3, 7, 14+/1, 28+/−3, and 56+/−7. It should be noted that the Tmax and time to Cmax were calculated with reference to the start, not the mid-point or end, of the infusions and that for all of the cohorts except the 20 mg/kg group, the infusion time was 2 hours.
Statistical Methods
Summary descriptive statistics were used to describe the study population and safety data. Pk parameters were calculated using noncompartmental methods with WinNonlin Professional software (version 4.1; Pharsight).
Results
Study Population Demographics
Thirty subjects were enrolled at 2 centers between September 2004 and April 2005. Overall, 33% of the subjects were men, and the median age was 27.5 years (range, 20–53 years). Seventy-three percent of subjects were white, 3% were African American, 13% were Asian, 3% were American Indian/Alaskan Native, and 7% were of other ethnicities. All subjects completed the study.
The majority of AE were mild to moderate in severity and assessed as unlikely related or unrelated to receipt of CDA1 (Table 1). There were no serious AE assessed as possibly, probably, or definitely related to the administration of CDA1. One serious AE occurred that was assessed to be unrelated to receipt of the study product: a subject who was enrolled in the 10 mg/kg dosing cohort was hospitalized on day 44 post-infusion following a prescription drug and alcohol overdose, and subsequently recovered. A total of 21 AE were assessed as possibly related to infusion of CDA1. Three of these events were of moderate severity, and included a transient drop in systolic blood pressure by more than 20 mmHg during the 8-hour post-infusion monitoring period that required no treatment (2 subjects), and diarrhea lasting 24 hours requiring treatment with loperamide (1 subject). The remaining 18 possibly related AE were mild in severity, and included headache (2 subjects), nasal congestion (1 subject), abdominal discomfort (1 subject), nausea and loose stools (1 subject), transient changes in blood pressure (13 subjects). Twenty-seven AE were deemed to be unlikely to be related or unrelated to the study drug, and were mild to moderate in severity (Table 1) There were no laboratory abnormalities that were associated with clinical signs or symptoms or required therapy.
Table 1.
Adverse events
| Number | Severity | Relationship to CDA1 | Description (number of subjects) |
|---|---|---|---|
| 1 | Serious | Unrelated | Alcohol and drug overdose requiring hospitalization, recovered fully (1) |
| 3 | Moderate | Possibly |
|
| 18 | Mild | Possibly |
|
| 27 | Mild to Moderate | Unlikely or Uunrelated |
|
Pharmacokinetics
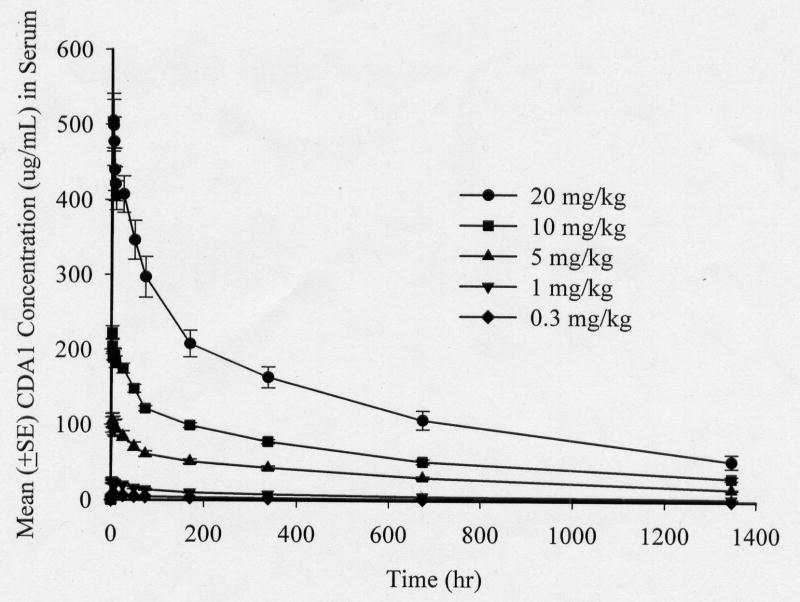
CDA1 exhibited dose-dependent pharmacokinetics at the doses administered (Table 2 and Figure). Cmax (maximum serum concentration) increased proportionally with increasing doses as did the AUC (area under the serum concentration time curve to the last collection time). Mean Cmax were 6.82 μg/ml, 26.3μg/m, 109μg/m, 223μg/m and 511μg/m for the 0.3, 1.0, 5.0, 10 and 20 mg/kg cohorts respectively. The volume of distribution approximated that of serum, suggesting that drug distribution was mostly confined to the intravascular space. The median Tmax (time to Cmax) was 3 hours. The mean residence time was long (28.5 days) consistent with a long terminal disposition phase of CDA1. Similarly, the median terminal elimination half-life ranged between 22.9 and 30.3 days. CDA1 clearance was low (median 0.0014 ml/min/kg) and was independent of the administered dose. In general, the variability of the pk parameters exhibited a coefficient of variance in the range of 10% to 30%, which is within the normal variability that is observed in human populations.
Table 2.
Summary of pharmacokinetic parameters of healthy subjects given a single intravenous infusion of CDA1 by dose level
| Dose, mg/kg | Tmax, h | Cmax μg/ml | AUC, h*μg/ml | Vd, L/kg | MRT, h | T1/2, h | CL, ml/min/kg |
|---|---|---|---|---|---|---|---|
| 0.3 | 5.0 (2.4, 25.9) | 7.0 (5.9, 7.4) | 3042 (2516, 4022) | 0.070 (0.064, 0.081) | 926 (843, 1873) | 684 (628, 1350) | 0.0012 (0.0006, 0.0015) |
| 1.0 | 2.6 (2.3, 3.0) | 24.7 (18.8, 35.4) | 8902 (6457, 10804) | 0.081 (0.062, 0.097) | 917 (724, 1258) | 684 (548, 953) | 0.0014 (0.0010, 0.0019) |
| 5.0 | 3.0 (2.5, 4.7) | 112.7 (77.9, 134.4) | 47530 (41162, 52660) | 0.071 (0.067, 0.093) | 981 (812, 1155) | 728 (537, 824) | 0.0013 (0.011, 0.0015) |
| 10.0 | 2.6 (2.5, 10.0) | 227.7 (194.5, 245.0) | 84357 (72193, 86444) | 0.079 (0.076, 0.097) | 913 (663, 1365) | 660 (481, 1015) | 0.0014 (0.0012, 0.0019) |
| 20.0 | 3.9 (3.0, 5.0) | 479.1 (430.1, 638.0) | 174632 (144521, 242411) | 0.076 (0.048, 0.085) | 704 (615, 1136) | 550 (472, 846) | 0.0014 (0.0011, 0.0020) |
NOTE. Data are median (range). Tmax, time to maximum serum drug concentration after start of infusion; Cmax, maximum serum concentration; AUC, area under the serum concentration time curve to the last collection time; Vd, volume of distribution; MRT, mean residence time; T1/2, terminal elimination phase half-life; CL, drug clearance.
Figure.
Mean CDA1 concentration(μg/ml) after the start of a single intravenous infusion of 5 different dose levels to healthy adults
Discussion
C. difficile-associated diarrhea usually responds to conventional treatment with metronidazole or vancomycin.1, 10–13 A significant proportion of patients, however, develop recurrent disease that is associated with significant morbidity.12, 13 Humoral immune responses are thought to play an important role in determining the severity of C. difficile-associated disease,14–17 which can range from asymptomatic carriage to severe colitis with life-threatening complications.1 Inadequate antibody levels to C. difficile toxins predispose individuals to severe or protracted disease,15–17 while passive immunotherapy with pooled human immunoglobulin can lead to resolution of diarrhea among patients who have not responded to conventional therapies.18–21 However, IgG titers against C. difficile toxins in commercial immunoglobulin preparations have been shown to vary by as much as fourfold.18 In addition, the pharmacokinetics of these preparations for treating C. difficile-associated infection has not been characterized.
CDA1 is a novel human monoclonal antibody against C. difficile toxin A that has been developed as a potential adjunctive therapy for C. difficile-associated disease. In this study we examined its safety and pharmacokinetics in healthy adults. We found that CDA1 was safe and well tolerated at single doses between 0.3 mg/kg and 20 mg/kg. The majority of observed AE were mild to moderate in severity and assessed as unrelated to receipt of CDA1. There were no reports of serious AE associated with study product.
A therapeutic protein such as a monoclonal antibody may be immunogenic and lead to the development of human anti-monoclonal antibodies (HAMA). Immunogencity and HAMA production is most frequent with murine monoclonals, there is less immuogenicity with chimeric monoclonals and least with humanized monoclonals such as CDA1. Higher monoclonal antibody doses and frequent administration are also important factors that contribute to immunogenicity. In this study where subjects received a single dose of a human monoclonal the development of HAHA was not detected in any subject as might be expected.
Passive antibody-based therapy dates back to the late 19th century, when Behring first discovered that a component of serum with anti-toxin activity could protect rabbits from lethal doses of tetanus toxin.22 Subsequently, passive immunotherapy was developed to treat many infectious diseases, but was largely abandoned in the 1940s, when effective antimicrobial agents were discovered and became widely available.23 Renewed interest in immunoglobulins to treat infections has occurred in recent years, however, due to the emergence of drug-resistant microorganisms and new pathogenic strains with potentially increased virulence.23 Although only one monoclonal antibody for an infectious agent has been approved in the United States (i.e., palivizumab for respiratory syncytial virus),24 a number of antibody molecules are currently being evaluated for various infections. For example, tefibazumab (a humanized monoclonal antibody against a surface protein of Staphylococcus aureus) has undergone preclinical and phase I testing.25
Recent data indicate that C. difficile-associated disease has become more prevalent, more aggressive and more difficult to treat. Geographically distributed medical centers and the National Nosocomial Infections Surveillance (NNIS) system in the United States have reported increases in the incidence and severity of C. difficile-associated disease.27–29 NNIS data from 1987–2001 showed that intensive care units in large hospitals experienced significant increases in the frequency of C. difficile-associated disease.29 Outbreaks of severe cases have also occurred in Canada, the United Kingdom, and the Netherlands.30, 31 Typing techniques have revealed dominant epidemic genotypes in North America and the United Kingdom with strains that exhibit resistance to certain antibiotics (e.g. clindamycin and fluoroquinolones), and may produce greater amounts of toxin A and toxin B.31–33 Collectively, these observations highlight the need for new treatment strategies, including those that target C. difficile toxins.
CDA1 was developed to neutralize C. difficile toxin A. Given its demonstrated safety in healthy adults in this study, a randomized double blind, placebo-controlled study has been undertaken to determine its safety and effectiveness as an adjunct to standard of care treatment in hospitalized patients with C. difficile associated diarrhea. We acknowledge, however, that neutralizing C. difficile toxin B may also be a necessary component of treating patients who do not respond to conventional therapies. Because of its lack of enterotoxic effects in animal intestine in vivo, toxin B was not thought to be an important mediator of C. difficile-associated disease in humans. However, recent evidence suggests otherwise.34, 35 Toxin B is a potent cytotoxin on cultured mammalian cells.5, 6 It induced cell rounding, and disrupted actin-containing myofilament bundles in human lung fibroblasts6 and human intestinal epithelial cell monolayers that were derived from a colonic adenocarcinoma.7 Toxin B was also found to be 10 times more potent than toxin A at causing electrophysiologic changes in human colonic strips in vitro.34 It had potent inflammatory enterotoxic effects on human intestinal xenografts that were subcutaneously transplanted into immunodeficient mice.35 In addition, an increasing number of reports have detected toxin-A negative, toxin-B positive strains of C. difficile among symptomatic individuals,36–40 and outbreaks associated with such strains have occurred in Canada and the Netherlands.41, 42 Patients have exhibited a wide spectrum of disease, including recurrent diarrhea and fatal pseudomembranous colitis.36–42 Studies attempting to characterize such strains have detected deletions in the repetitive regions of the toxin A gene, and altered restriction sites in the toxin B gene.39, 41 Certain strains have also been noted to produce functionally inactive forms of toxin A.39, 41, 43 Therefore, future treatments for C. difficile-associated disease may require targeted therapies against both toxin A and toxin B. Our preclinical studies support this approach since the use of a monoclonal antibody against toxin B in addition to CDA1 resulted in substantially increased efficacy in a hamster CDAD model.19
The mechanisms by which systemic antibody responses help to minimize C. difficile-mediated enteric infections are not well understood. Toxin A is synthesized in the colonic lumen, where it elicits severe damage to the intestinal mucosa.44 Investigators have proposed that IgG antitoxin may leak from the circulation into the colonic lamina propria or intestinal lumen.44, 45 It may then bind to and neutralize C. difficile toxins directly, or prevent their binding to intestinal epithelial cell receptors.44, 45 Data supporting the exudation of IgG across the colonic mucosa can be found in animal models8 and in observational studies in which investigators measured fecal IgG levels of infected patients.17 Hamsters which were immunized against C. difficile toxin A or B and subsequently challenged with the toxins were found to have antibodies against the immunizing toxin in their sera and cecal homogenates.8 ELISAs of the cecal homogenates detected the presence of only the toxin against which the animals were not immunized.8 In a human study patients with C. difficile-associated diarrhea who had high serum IgG antitoxin A titers were also found to have high levels of fecal IgG to toxin A.17 Accordingly, an examination of the colonic contents of treated subjects for the presence of CDA1 may be instructive in determining whether or not the monoclonal antibody is also secreted or exuded into the colonic lumen during CDAD.
The clinical indications for passive immune-based therapies for C. difficile-associated disease are not fully defined. Pooled human immunoglobulin preparations have been administered to patients with recurrent C. difficile associated diarrhea.16,20–21 Patients with severe, refractory and fulminant CDAD are another patient population that may benefit substantially from passive immunotherapy.18 The clinical outcomes in this situation are often grim including multi-organ failure, colectomy and death. There is no available treatment with proven efficacy beyond antibacterial therapy with vancomycin and metronidazole. Normal pooled immunoglobulin infusion has been used for this indication but its efficacy is unclear since no prospective, controlled clinical trials have been reported.18 Aside from treating refractory and recurrent disease, passive immunotherapy could also be administered prophylactically to hospitalized patients at high risk of developing nosocomial C. difficile diarrhea. Future studies will also need to address how best to apply what may prove to be a useful new form of therapy for this increasingly difficult to treat nosocomial infectious disease.
Acknowledgments
This study was sponsored by Massachusetts Biologic Laboratories, University of Massachusetts medical school, Jamaica Plain, MA and Medarex, INC, Bloomsbury, NJ.
This work was supported in part by grants from the National Institutes of Health (RO-1 AI053069 to CPK, K30-HL04095 to the Scholars in Clinical Science Program at Harvard Medical School, in which CPT was enrolled, T32- DK0776 to the Division of Gastroenterology, Beth Israel Deaconess Medical Center, RR 01032 to BIDMC-General Clinical Research Center, and M01RR 000054 to Tufts-New England Medical Center-General Clinical Research Center). We thank the staff of the General Clinical Research Centers at Beth Israel Deaconess Medical Center and Tufts-New England Medical Center for their excellent nursing care. We also acknowledge the generous contributions of the individuals who volunteered for this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med. 1994;330:257–262. doi: 10.1056/NEJM199401273300406. [DOI] [PubMed] [Google Scholar]
- 2.Lyerly DM, Lockwood DE, Richardson SH, Wilkins TD. Biological activities of toxin A and B of Clostridium difficile. Infect Immun. 1982;35:1147–1150. doi: 10.1128/iai.35.3.1147-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell TJ, Ketley JM, Haslam SC, Stephen J, Burdon DW, Candy DCA, Daniel R. Effect of toxin A and B of Clostridium difficile on rabbit ileum and colon. Gut. 1986;27:78–85. doi: 10.1136/gut.27.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triadafilopoulos G, Pothoulakis C, O’Brien MJ, LaMont JT. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology. 1987;93:273–279. doi: 10.1016/0016-5085(87)91014-6. [DOI] [PubMed] [Google Scholar]
- 5.Wedel N, Toselli P, Pothoulakis C, Faris B, Oliver P, Franzblau C, LaMont T. Ultrastructural effects of Clostridium difficile toxin B on smooth muscle cells and fibroblasts. Exp Cell Res. 1983;148:413–422. doi: 10.1016/0014-4827(83)90163-5. [DOI] [PubMed] [Google Scholar]
- 6.Pothoulakis C, Barone LM, Ely R, Faris B, Clark ME, Franzblau C, LaMont JT. Purification and properties of Clostridium difficile cytotoxin B. J Biol Chem. 1986;261:1316–1321. [PubMed] [Google Scholar]
- 7.Hecht G, Koutsouris A, Pothoulakis C, Lamont JT, Madara JL. Clostridium difficile toxin B disrupts the barrier function of T84 monolayers. Gastroenterology. 1992;102:416–423. doi: 10.1016/0016-5085(92)90085-d. [DOI] [PubMed] [Google Scholar]
- 8.Kim P-H, Iaconis JP, Rolfe RD. Immunization of adult hamsters against Clostridium difficile-associated ileocecitis and transfer of protection to infant hamsters. Infect Immun. 1987;55:2984–2992. doi: 10.1128/iai.55.12.2984-2992.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corthier G, Muller MC, Wilkins TD, Lyerly D, L’Haridon RL. Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A. Infect Immun. 1991;59:1192–1195. doi: 10.1128/iai.59.3.1192-1195.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teasley DG, Gerding DN, Olson MM, Peterson LR, Gebhard RL, Schwartz MJ, Lee JT. Prospective randomised trial of metronidazole versus vancomycin for Clostridium difficile-associated diarrhea and colitis. Lancet. 1983;2:1043–1046. doi: 10.1016/s0140-6736(83)91036-x. [DOI] [PubMed] [Google Scholar]
- 11.Wenisch C, Parschalk B, Hasenhundl M, Hirschl AM, Graninger W. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile-associated diarrhea. Clin Infect Dis. 1996;22:813–818. doi: 10.1093/clinids/22.5.813. [DOI] [PubMed] [Google Scholar]
- 12.Fekety R. Guidelines for the diagnosis and management of Clostridium difficile-associated diarrhea and colitis. Am J Gastroenterol. 1997;92:739–750. [PubMed] [Google Scholar]
- 13.Maroo S, LaMont JT. Recurrent Clostridium difficile. Gastroenterology. 2006;130:1311–1316. doi: 10.1053/j.gastro.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 14.Kelly CP, Pothoulakis C, Orellana J, LaMont JT. Human colonic aspirates containing immunoglobulin A antibody to Clostridium difficile toxin A inhibit toxin A-receptor binding. Gastroenterology. 1992;102:35–40. doi: 10.1016/0016-5085(92)91781-x. [DOI] [PubMed] [Google Scholar]
- 15.Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390–397. doi: 10.1056/NEJM200002103420604. [DOI] [PubMed] [Google Scholar]
- 16.Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357:189–193. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 17.Warny M, Vaerman J-P, Avesani V, Delmee M. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect Immun. 1994;62:384–389. doi: 10.1128/iai.62.2.384-389.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salcedo J, Keates S, Pothoulakis C, Warny M, Castagliuolo I, LaMont JT, Kelly CP. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut. 1997;41:366–370. doi: 10.1136/gut.41.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babcock GJ, Broering TJ, Hernandez HJ, Mandell RB, Donahue K, Boatright N, Stack AM, Lowy I, Graziano R, Molrine D, Ambrosino DM, Thomas WD. Human mmoclonal antibodies directed against Toxins A and B Prevent Clostridium difficile-induced mortality in hamsters. Infect Immun. 2006;74:6339–6347. doi: 10.1128/IAI.00982-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilcox MH. Descriptive study of intravenous immunoglobulin for the treatment of recurrent Clostridium difficile diarrhoea. J Antimicrob Chemo. 2004;53:882–884. doi: 10.1093/jac/dkh176. [DOI] [PubMed] [Google Scholar]
- 21.Leung DY, Kelly CP, Boguniewicz M, Pothoulakis C, LaMont JT, Flores A. Treatment with intravenously administered gamma globulin of chronic relapsing colitis induced by Clostridium difficile toxin. J Pediatr. 1991;118:633–637. doi: 10.1016/s0022-3476(05)83393-1. [DOI] [PubMed] [Google Scholar]
- 22.Behring E. Uber das Zustandekommen der Diphterie-Immunitat und der Tetanus-Immunitat bei Thieren. Deutsche Medicinische Wochenschrift. 1890;14:1113–1114. [PubMed] [Google Scholar]
- 23.Casadevall A, Dadachova E, Pirofski LA. Passive antibody therapy for infectious diseases. Nature Rev Microbiol. 2004;2:695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- 24.The Impact RSV study group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus in high-risk infants. Pediatrics. 1998;102:531–537. [PubMed] [Google Scholar]
- 25.Reilley S, Wenzel E, Reynolds L, Bennett B, Patti JM, Hetherington S. Open-label, dose escalation study of the safety and pharmacokinetic profile of tefibazumab in healthy volunteers. Antimicrob Agents Chemother. 2005;49:959–962. doi: 10.1128/AAC.49.3.959-962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 27.Dallal RM, Harbrecht BG, Boujoukas AJ, Sirio CA, Farkas LM, Lee KK, Simmons RL. Fulminant Clostridium difficile: An underappreciated and increasing cause of death and complications. Ann Surg. 2002;3:363–372. doi: 10.1097/00000658-200203000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muto CA, Pokrywka M, Shutt K, Mendelsohn AB, Nouri K, Posey K, Roberts T, Croyle K, Krystofiak S, Patel-Brown S, Pasculle W, Paterson DL, Saul M, Harrison LH. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005;26:273–280. doi: 10.1086/502539. [DOI] [PubMed] [Google Scholar]
- 29.Archibald LK, Banerjee SN, Jarvis WR. Secular trends in hospital-acquired Clostridium difficile disease in the United States, 1987–2001. J Infect Dis. 2004;189:1585–1589. doi: 10.1086/383045. [DOI] [PubMed] [Google Scholar]
- 30.Pepin J, Valiquette L, Alary M-E, Villemure P, Pelletier A, Forget K, Pepin K, Chouinard D. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171:466–472. doi: 10.1503/cmaj.1041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, Vibien A, Brassard P, Fenn S, Dewar K, Hudson TJ, Horn R, Rene P, Monczak Y, Dascal A. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353:2442–2449. doi: 10.1056/NEJMoa051639. [DOI] [PubMed] [Google Scholar]
- 32.McDonald LC, Killgore GE, Thompson A, Owens RC, Kazakova SV, Sambol SP, Johnson S, Gerding DN. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353:2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 33.Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, Frost E, McDonald LC. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 34.Riegler M, Sedivy R, Pothoulakis C, Hamilton G, Zacherl J, Bischof G, Cosentini E, Feil W, Schiessel R, LaMont JT, Wenzl E. Clostridium difficile toxin B is more potent than toxin A in damaging human colonic epithelium in vitro. J Clin Invest. 1995;95:2004–2011. doi: 10.1172/JCI117885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savidge TC, Pan W-H, Newman P, O’Brien M, Anton PM, Pothoulakis C. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology. 2003;125:413–420. doi: 10.1016/s0016-5085(03)00902-8. [DOI] [PubMed] [Google Scholar]
- 36.Boriello SP, Wren BW, Hyde S, Seddon SV, Sibbons P, Krishna MM, Tabaqhali S, Manek S, Price AB. Molecular, immunological, and biological characterization of a toxin A-negative toxin B-positive strain of Clostridium difficile. Infect Immun. 1992;60:4192–4199. doi: 10.1128/iai.60.10.4192-4199.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson S, Kent SA, O’Leary KJ, Merrigan MM, Sambol SP, Peterson LR, Gerding DN. Fatal pseudomembranous colitis associated with a variant Clostridium difficile strain not detected by toxin A immunoassay. Ann Intern Med. 2001;135:434–438. doi: 10.7326/0003-4819-135-6-200109180-00012. [DOI] [PubMed] [Google Scholar]
- 38.Limaye AP, Turgeon DK, Cookson BT, Fritsche TR. Pseudomembranous colitis caused by a toxin A (-) B (+) strain of Clostridum difficile. J Clin Microbiol. 2000;38:1696–1697. doi: 10.1128/jcm.38.4.1696-1697.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyerly DM, Barroso LA, Wilkins TD, Depitre C, Corthier G. Characterization of a toxin A-negative, toxin B-positive strain of Clostridium difficile. Infect Immun. 1992;60:4633–4639. doi: 10.1128/iai.60.11.4633-4639.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komatsu M, Kato H, Aihara M, Shimakawa K, Iwasaki M, Nagasaka Y, Fukuda S, Matsuo S, Arakawa Y, Watanabe M, Iwatani Y. High frequency of antibiotic-associated diarrhea due to toxin A-negative, toxin B-positive Clostridium difficile in a hospital in Japan and risk factors for infection. Eur J Clin Microbiol Infect Dis. 2003;22:525–529. doi: 10.1007/s10096-003-0992-5. [DOI] [PubMed] [Google Scholar]
- 41.Alfa MJ, Kabani A, Lyerly D, Moncrief S, Neville LM, Al-Barrak A, Harding GKH, Dyck B, Olekson K, Embil JM. Characterization of a toxin A-negative toxin B-positive strain of Clostridium difficile responsible for a nosocomial outbreak of Clostridium difficile-associated diarrhea. J Clin Microbiol. 2000;38:2706–2714. doi: 10.1128/jcm.38.7.2706-2714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kuijper EJ, de Weerdt J, Kato H, Kato N, van Dam AP, van der Vorm ER, Weel J, van Rheenen C, Dankert J. Nosocomial outbreak of Clostridium difficile-associated diarrhoea due to a clindamycin-resistant enterotoxin A-negative strain. Eur J Clin Microbiol Infect Dis. 2001;20:528–534. doi: 10.1007/s100960100550. [DOI] [PubMed] [Google Scholar]
- 43.von Eichel-Streiber C, Zec-Pirnat I, Grabnar M, Rupnik M. A nonsense mutation abrogates production of a functional enterotoxin A in Clostridium difficile toxinotype VIII strains of serogroups F and X. FEMS Microbiol Lett. 1999;178:163–168. doi: 10.1111/j.1574-6968.1999.tb13773.x. [DOI] [PubMed] [Google Scholar]
- 44.Kelly CP. Immune response to Clostridium difficile infection. Eur J Gastroenterol Hepatol. 1996;8:1048–1053. doi: 10.1097/00042737-199611000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Kelly CP, Becker S, Linevsky JK, Joshi MA, O’Keane JC, Dickey BF, LaMont JT, Pothoulakis C. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J Clin Invest. 1994;93:1257–1265. doi: 10.1172/JCI117080. [DOI] [PMC free article] [PubMed] [Google Scholar]