Abstract
Pseudomonas aeruginosa (PA) from acute and chronic (e.g. cystic fibrosis [CF]) infections differ in several respects though they can worsen prognosis in each context. Factors that facilitate conversion from an acute to chronic phenotype are poorly understood. Type III (T3) secretion proteins are virulence factors associated with poorer outcomes in acute infections, but little is known about their role in CF. We wished to characterize T3 secretion in CF PA isolates and examine its role in clinical outcomes. One-hundred fourteen CF subjects were divided into 3 cohorts: 1st infected individuals, chronically infected (CI) children, and adults. Serial respiratory cultures were analyzed for T3 secretion. Serial spirometry and exacerbation data were prospectively collected. In 1st infection, 45.2% +/− 9.1% of PA isolates secreted T3 proteins compared to 29.1% +/− 4.2% and 11.5% +/− 3.0% in CI children and CI adults, respectively (p<0.001). There was an inverse correlation between duration of PA infection and percent T3 positive isolates (r=−0.32, p<0.001). Overall there was no association between T3 secretion and pulmonary outcomes, but in the subgroup of subjects who had at least one T3 positive organism, T3 secretion was inversely correlated with FEV1 decline (r=−0.35, p=0.02). In 1st infection, 82% of cultures grew either all or no T3 positive organisms. In these patients, T3 secretion was associated with greater risk of subsequent PA isolation (p<0.001). In CF, PA T3 secretion decreases with residence time in lung, may predict FEV1 decline in patients who have detectable T3 organisms, and may facilitate persistence following 1st infection.
Keywords: cystic fibrosis, Pseudomonas aeruginosa, type III secretion, virulence factors, outcome, FEV1
Introduction
Acute infections with Pseudomonas aeruginosa (PA) cause up to 20% of hospital-acquired pneumonias1 and a small percentage of severe community-acquired pneumonias 2. In addition PA is one of the few organisms that has been independently associated with increased mortality in the intensive care unit 3. It has a large genome and the ability to adapt rapidly, which makes it a particularly difficult organism to treat. A number of PA virulence factors have been associated with worse outcomes in models of acute PA infection, including flagella4, pili5, quorum-sensing signaling6, pyocyanin7, pyoverdin8, and exotoxin A9. An additional important PA virulence factor is the type III secretion system10 which uses secretion/translocation machinery to inject effector proteins directly into eukaryotic cells. All PA strains harbor the type III secretion apparatus genes although they differ in the effector proteins they secrete 11–13. Three effector proteins implicated in disease are ExoS, ExoT and ExoU. ExoS and ExoT function as activating proteins for Rho GTP-ases and ribosylate host proteins 14 while ExoU is a phospholipase that can rapidly kill macrophages, epithelial cells and fibroblasts15. We and others have reported that 77–90% of PA isolates from patients with acute respiratory infections secreted type III proteins and that the presence of type III secreting isolates is associated with worse outcomes in this setting 11,12.
PA is also adept at causing chronic infections. This bacterium infects the bronchiectatic airways of nearly 80% of adults with cystic fibrosis 16. In contrast to acute pneumonia, PA infection of CF patients may last for years or decades. There are specific benchmarks of PA infection in CF. One is the onset of 1st PA infection and a second is the transition of intermittent to chronic PA infection. Factors that facilitate these transitions are poorly understood but likely important to CF pathophysiology. PA isolates from 1st time infected CF patients resemble those from acutely infected non-CF patients, in that they tend to be non-mucoid and antibiotic susceptible17 18. However, over time PA evolves and adapts to the CF lung environment, perhaps to facilitate evasion of local host defense mechanisms and/or immune surveillance19. Interestingly, many of the virulence factors upon which PA relies during acute infections are selected against during chronic infection. For example, production of functional flagella, pili, quorum-sensing signaling, pyocin, pyoverdin, and exotoxin A are attenuated in many isolates from chronically infected CF patients19–24. Adaptation in the form of decreased expression of such factors may be associated with changes in patient respiratory status in CF 17,20,25.
Recent studies suggest that the type III secretion system of PA also undergoes adaptation in CF. In a small pilot study, we previously showed that the proportion of PA strains secreting type III effector proteins decreased from 49% to 4% with increasing age of patient26. Thus type III secretion may be a potent and toxic virulence determinant that helps PA cause acute cellular injury in the lung but is less compatible with the chronic infections such as in CF. Here we examine this phenomenon in a larger cohort of patients and determine whether increased prevalence of type III secretion positive PA isolates is associated with worse pulmonary outcomes. Finally, we examine the association between type III secretion in 1st PA infection and persistence of PA.
Materials and Methods
Study design, Subjects and definitions
This longitudinal study included 3 cohorts of CF patients who were followed between the years 2003–2006. The first two cohorts consisted of adults and children with a confirmed CF diagnosis (positive sweat chloride or 2 CFTR mutations) and history of a respiratory culture positive for PA in the 2 years preceding study entry. This group was considered chronically infected with PA. Patients were considered adults if they reached 18 years of age during the study. The third cohort consisted of patients without a previous history or PA or who grew PA for the first time following a period of at least 2 years before study entry. This group was considered 1st time infected. Patients’ time accrual in the study began with their 1st positive PA culture after study enrollment. Subjects with a respiratory culture growing Burkholderia cepacia were excluded. Written informed consent was obtained from all participants. For CI patients, respiratory specimens were collected prospectively every 6 months for approximately 2 years. For patients without prior PA isolates, surveillance cultures were obtained prospectively every 3–6 months and PA isolates were studied at time of first PA positive culture. Spirometry was obtained at each visit, and clinical characteristics, pulmonary exacerbation data and year of first PA infection was ascertained from the medical chart or the Cystic Fibrosis Foundation (CFF) registry if otherwise unavailable. Duration of PA infection was calculated as the difference in time between 1st infection and enrollment in the study. A severe pulmonary exacerbation (PE) was defined as a worsening of pulmonary status deemed to require the use of intravenous antibiotics by the treating physician. Use of oral or inhaled antibiotics was not included in the definition in order to facilitate treatment decision homogeneity. This study was approved by the Northwestern University and Children’s Memorial Hospital Institutional Review Boards.
Bacterial Isolates
Respiratory samples (sputum, throat swabs, or bronchoalveolar lavage) were processed by microbiology laboratories at Northwestern Memorial Hospital and Children’s Memorial Hospital, and PA was identified using criteria approved by the Clinical and Laboratory Standards Institute. Five PA colonies from each sample were randomly selected for type III secretion analysis.
Immunoblot analysis
Bacteria were grown in MINS medium 27 at 37°C for approximately 17 hours with vigorous shaking to induce type III secretion28. Auxotrophic isolates that did not grow in this minimal medium were cultured in Luria Bertani broth supplemented with 125 mM EGTA, a rich medium that also induces type III secretion 10. Supernatants were collected by centrifugation at 6,000 × g at 4°C for 20 minutes, concentrated and partially purified as previously described 12. Immunoblot analysis using antisera against ExoS, ExoT, ExoU, PopB and PopD was performed as previously described 12. Isolates that secreted at least one type III protein were scored as secretion positive.
Statistics
Data were summarized using means and standard errors for continuous variables and frequency distributions for categorical variables. Deterioration in pulmonary function was measured as the slope of FEV1 and FVC decline. PE frequency was determined by dividing the number of PEs by years in the study. A Pearson’s correlation coefficient was determined between various clinical parameters. Baseline spirometry, spirometric decline and type III secretion status were compared between adults and children using t-tests. Differences in percent type III isolates in 1st infected patients who grew PA subsequently and those who did not were compared by Chi-square. Analyses were conducted using SAS statistical software, and significance was drawn at α=0.05.
Results
Subject population
One-hundred and fourteen patients grew PA at least once and were included in the study. Selected demographics are shown in table 1. Thirty-eight (69%) of 55 children and all 49 adults were chronically infected (CI) with PA at study entry. Twenty-seven children had first detection of PA during the study and were characterized as 1st infected. Seventeen (63%) of 27 1st infected children also had subsequent respiratory cultures positive for PA. These subsequent PA cultures were included in the CI children cohort for type III secretion prevalence analysis (Figure 1).
Table 1.
Selected demographics of subject population
| Demographics of Patient Population (n=114) | |
|---|---|
| Race/Ethnicity | Caucasian-84% |
| Hispanic-11% | |
| Afro-american-3% | |
| Asian-Indian-2% | |
| Sex | Female-53% |
| Male-43% | |
| Age | ≥ 18y- 27% |
| <18y - 73% | |
| Pancreas status | Insufficient- 92% |
| Sufficient-8% | |
| Inhaled Tobramycin | 55% |
| Azithromycin | 63% |
Figure 1. Enrollment and subcohorts in this study.
There were a total of 114 subjects included in the study, 83 children and 31 adults. At study entry, children were split into a 1st infection cohort and chronically infected cohort. If the 1st infection cohort grew PA at the next culture (3–6 months later), they were then rolled into the chronically infected children’s cohort. All adults were chronically infected with PA.
Mean age of CF diagnosis was 3.0 yr (+/− 4.8 yr) for CI patients and 1.2 yr (+/− 1.7 yr) for 1st infected patients. The mean age for first PA isolation was 10.2 yr (+/− 7.5 yr) for CI patients and 5.3 yr (+/− 4.3 yr) for 1st infected patients. This may reflect more aggressive PA detection strategies over time. Comparison of various variables between the chronically infected children and adult cohorts are shown in table 2.
Table 2.
Comparison of Adult and Children chronically-infected cohort
| Comparison of Chronically Infected Cohorts | |||
|---|---|---|---|
| >= 18 years (n=49) | < 18 years (n=55) | ||
| Age of CF Diagnosis | 4.1 +/− 0.9 | 1.9+/− 0.4 | p<0.02 |
| Age of PA Colonization | 14.9 +/−1.1 | 6.1 +/− 0.6 | p<0.001 |
| Age at study end | 27.4 +/−0.9 | 11.9 +/− 0.6 | p<0.001 |
| Total PA isolates analyzed | 24.8 +/− 2.4 | 23.6 +/− 2.4 | p=ns |
| Spirometries Obtained | 6.1 +/− 0.5 | 7.1 +/− 0.7 | p=ns |
| Years in Study | 2.3 +/− 0.2 | 1.9 +/− 0.2 | p=ns |
| Duration of PA colonization (years) | 13.4 +/− 0.7 | 6.3+/− 0.5 | p<0.001 |
The mean length of follow-up for the full CI cohort (both children and adults) was 2.1 yr +/− 1.1 yr. Sixty-eight patients (35 children and 33 adults) had 1 year of serial spirometry data and 54 patients had 2 years.
Spirometry and pulmonary exacerbation data
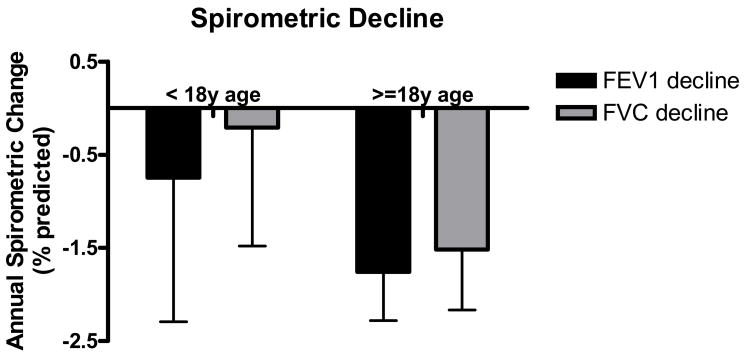
The mean FEV1 and FVC were 62.1% +/− 3.4% and 77.0% +/− 3.1%, and 75.1% +/− 3.8% and 81.4% +/− 3.4%, for CI adults and children respectively. For the seven 1st infected patients who could perform spirometry, the mean FEV1 and FVC were 81.7% +/− 8.3% and 87.5% +/− 5.9%. The subgroup of patients with at least 1 year of data was used to calculate spirometric trends. For CI adults, the mean annual change in FEV1 and FVC was −1.75% +/− 0.52% and −1.52% +/− 0.64%, respectively, and for CI children −0.75% +/− 1.54% and −0.21% +/− 1.27% (Fig. 2). The trends were similar for the complete adult and children’s CI cohorts. The difference in spirometric decline was not statistically different between CI adults and children in the overall cohort or 1- or 2-year subgroups. There were not enough patients from the 1st infected cohort to determine meaningful spirometric trends.
Figure 2. Spirometric decline for chronically infected cohorts.
FEV1 and FVC were obtained at the time of the first respiratory sample collection at study entry. Subsequent spirometry measurements were used to determine the average annual decrease in FEV1 and FVC. Shown is the annual decline in FEV1 and FVC for patients who had at least 1 year of spirometry data after baseline.
For the combined CI cohort there were a cumulative 193 person-years of follow-up during which there were 195 severe PEs. The mean annual severe PE rate for the combined CI cohort was 1.0 +/− 0.2. In adults the rate was 0.9 +/− 0.2, and in the children’s subgroup the rate was 1.3 +/− 0.2, (p=ns). Approximately 7% of the patients averaged between 4–7 PE per year.
Pseudomonas aeruginosa type III secretion decreases with duration of infection
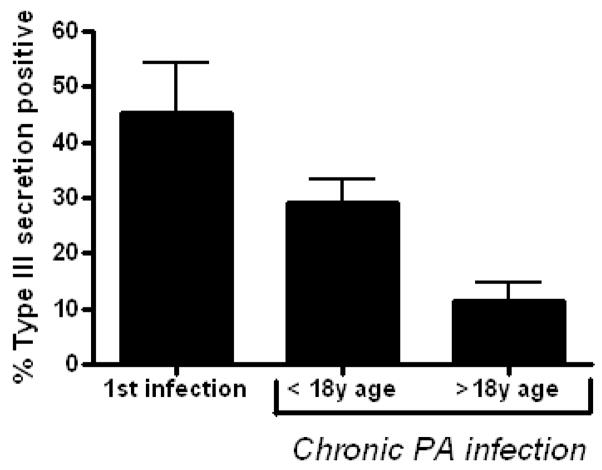
We collected 2516 PA isolates from 104 CI patients: 1217 from adults and 1299 from children. We also collected 135 PA isolates from 1st infected patients, all children. The proportion of type III secretion positive isolates from each group is shown in figure 3: CI adults, 11.5% +/− 3.0%; CI children, 29.1% +/− 4.2; and 1st infected patients, 45.2% +/− 9.1. In the combined CI cohort, there was a significant inverse correlation between percentage of type III secreting isolates and duration of PA infection (Fig. 4). In the subpopulation of CI patients who had at least one type III secretion positive isolate, the inverse correlation was stronger (data not shown). Thus increased residence in CF airways was associated with fewer type III secreting isolates.
Figure 3. Proportion of PA isolates that secrete type III proteins decreases with age of patients with CF.
All PA isolates from each cohort were analyzed in vitro for type III secretion phenotype, and the percent that secreted at least one type III protein is shown. The differences between the 3 groups were statistically significant (p< 0.001).
Figure 4. Type III secretion correlates inversely with duration of PA infection.
The percent of PA isolates that secreted type III proteins in vitro was compared to the number of years that a subject was known to grow PA in respiratory cultures. The correlation coefficient between the two variables was r = −0.32 (p<0.001).
The relationship between type III secretion and clinical variables
We then compared the proportion of type III secreting isolates from each CI patient to baseline spirometric function and decline in FEV1. There was no significant correlation between type III secretion percentage and baseline FEV1 or annual change in FEV1 (Fig. 5a). However, since 40% of CI patients failed to grow a type III secretion positive isolate during the study, which limited discriminatory power, we analyzed the subgroup that had at least one type III secreting organism detected during the study. In this subgroup with detectable type III secreting organisms, there was a significant inverse correlation between percentage of type III secreting organisms and FEV1 decline (Fig. 5b).
Figure 5. The relationship between type III secretion and FEV1 decline.
The percentage of type III isolates grown during the course of the study was compared to change in FEV1 for each patient in the whole cohort of chronically infected patients (panel a) and in the subcohort of patients who grew at least one type III secreting isolate (panel b).
No statistically significant association between percentage of type III secreting isolates and PE rate was noted in the CI cohort (data not shown). Results did not differ significantly when the analysis was restricted to patients for who at least 2 years of PE data were available.
In a subgroup of patients treated for a severe PE, we analyzed percent of type III secreting isolates before and after treatment with 2 antibiotics with anti-PA activity. The percentage of type III secreting isolates prior to a pulmonary exacerbation was 9.23%, and the percentage after a course of intravenous antibiotics was 13.3% which were not significantly different, (p=ns).
Type III secretion and 1st isolation
Prior to chronic PA infection, many CF patients are transiently infected with different strains of this bacterium 18. Eventually a single strain becomes established and progresses to chronic infection, although the strain attributes that enable this persistence are unclear. We investigated whether type III secretion was associated with persistence during early infection. As shown in figure 2, the percentage of type III secretion positive isolates was significantly higher in the twenty-seven 1st infected patients. When this group was examined more closely, a bimodal distribution for type III secreting isolates became apparent (Fig. 6a). Thirteen (48%) of these 27 patients had no type III secretion positive isolates identified from their cultures, whereas 12 (44%) had virtually all type III secretion positive isolates. We assessed whether differential use of antibiotics in an attempt to eradicate PA could explain this difference. Overall 22 out of 27 1st infected patients received oral and/or inhaled antibiotics and 2 others received intravenous antibiotics. There was no significant difference in the frequency or route of administration between patients with all or no type 3 secreting isolates. We then examined whether presence of type III secreting isolates in the 1st culture predicted growth of PA from subsequent cultures. Seventeen (63%) of 27 patients in the 1st infection cohort grew PA in their next culture. We found that 42/85 (49%) isolates from the initial cultures of these 17 patients secreted type III proteins whereas only 14/50 (28%) of isolates from the remaining 10 patients secreted type III proteins, a statistically significant difference (p< 0.002). The presence of type III organisms at time of 1st PA culture was associated with an odds ratio of 2.52 (1.19–5.32) for a subsequent positive PA culture. Thus type III secretion may be a marker for strains more likely to persist following initial infection.
Figure 6. A bimodal distribution of type III secretion phenotypes in PA isolates from 1st infected patients.
From each subject who grew PA for the first time, 5 colonies were selected and tested for type III secretion. Shown is the percent distribution of type III secreting isolates from the cohort (panel A). In nearly 75% of CI patients, all PA isolates were type III secretion negative, and the remaining patients had an even distribution of type III secretion positive and negative isolates. From the CI cohort, only subjects who had at least 8 separate cultures and 40 individual PA isolates were included. Shown is the percent distribution of type III secreting isolates from individual respiratory culture from the CI cohort (panel B).
Finally we analyzed the distribution of type III secretion isolates in individual cultures from CI patients to compare with 1st infected patients. Approximately 75% of individual respiratory cultures from CI patients failed to grow single type III secreting organism. In the remaining 25% there was nearly an even distribution in the proportion of type III secreting organisms from 0% up to 100% (Fig. 6b). These data suggest that with respect to type III secretion, significant diversity of CF PA subpopulations develops over time, though eventually type III secretion falls to a low level.
Discussion
In this report, we highlight four findings. First, we confirm in a sample of more than 2500 PA isolates that prevalence of type III secreting organisms wanes with residence time in the CF lung. Second there may be an association between the proportion of type III protein secreting isolates and the rate of FEV1 decline in patients who still harbor at least some type III positive isolates. Third, in 1st infected patients either nearly all or no PA isolates secrete type III proteins. Finally, the presence of type III secreting isolates at initial infection may be associated with subsequent PA positive cultures.
This study included twice as many patients who were followed for a significantly longer period of time than the cohort in our pilot study. In addition, we ascertained the number of years that each patient had grown PA. This allowed discernment of a significant inverse relationship between duration of PA airways infection and proportion of type III secreting isolates. We were also able to include a larger cohort of 1st infected patients and confirm that prevalence of type III secreting isolates in this group was between that of acute PA infections and CI PA patients. Together, these results indicate that PA adapts to chronic residence within the CF airways by gradually stopping the secretion of type III proteins.
A recent report examined genomic changes that had occurred in two PA clonal isolates collected 8 years apart from an individual with CF19. Full sequence analysis revealed 68 total mutations, the vast majority of which predicted a change in protein production. Mutations in genes affecting virulence factors, including the type III secretion system, were the most commonly observed. These authors found that mutations in the gene exsA, a transcriptional regulator of type III secretion, were relatively common. Such mutations are one mechanism by which type III secretion negative isolates may emerge in the CF lung over time. It is unclear why the absence of virulence factors may provide an adaptive advantage to PA in the CF lung. Such factors may be recognized by the host immune response, and loss of expression may help evade host defenses. Alternatively, the marked tissue damage and inflammation induced by many of these factors may not be compatible with long-term persistence. In any case, decreased expression of type III effector proteins over time may be an adaptation by PA to ensure survival in the CF host 29.
Interestingly, we found that the distribution of type III secreting isolates of 1st infected PA patients was bimodal, with most patients being infected with all type III secreting isolates or no type III secreting isolates. There are two possible explanations for this finding. One is that CF patients acquire PA strains from two different reservoirs, one with a high prevalence of type III secreting isolates (e.g. the environment) and the other with a low prevalence of type III secreting isolates (e.g. CI CF patients). A second possibility is that all patients become infected with type III secreting PA strains but that some strains adapt quickly and rapidly lose the ability to secrete type III proteins. Thus they already have a secretion negative phenotype at the time of 1st culture detection.
Our study was potentially limited because approximately 40% of our CI cohort grew no type III secreting organisms during the study, and patient follow up was for a relatively short time in comparison to duration of PA infection. Both factors contribute to decreasing discriminatory power. Despite these limitations, our subgroup analysis of CI patients who had at least one type III secreting isolate suggests that there may be a significant inverse relationship between percentage of type III secreting isolates and FEV1 decline. We did not see a similar relationship with PE. For the purposes of our study, a decision to treat with intravenous antibiotics for severe PE was made by the treating physician and was not part of the study protocol. This may have confounded the pulmonary exacerbation analysis. We acknowledge, however, that subgroup analyses have to interpreted with caution and that whether the presence of type III secreting isolates, like mucoidy, are truly associated with more rapid pulmonary deterioration in CF needs to be validated in large prospective study. Also, we used in vitro secretion of type III proteins to categorize isolates as type III secretion positive or negative, but it is conceivable that secretion properties of these isolates differed under conditions present in the CF airways. Methods are currently not available to determine the in vivo secretion characteristics of large numbers of PA isolates. However several studies have validated the use of in vitro type III secretion status to predict clinical outcomes11,12, and the in vitro measurement of effector protein secretion correlates with cell injury in cell culture models30. Thus our approach has a high likelihood of accurately reflecting type III secretion status of PA isolates and its clinical impact in CF patients.
Over the last several years, detecting 1st PA infection has rapidly gained increased practical importance with adoption of aggressive PA eradication strategies aimed at preserving lung function. Some PA strains are capable of only transient infection whereas others persist and eventually result in chronic infection 18. Little is known about PA attributes that determine this dichotomy, but our data suggest that functional type III secretion is a risk factor for subsequent positive PA cultures. Validation of this finding would provide a rationale for testing therapies directed against the PA type III secretion system in newly infected CF patients 31.
Speculations
Chronic bacterial infection differs in many respects from acute infection as highlighted in our study. Loss of type III secretion may be one mechanism by which PA evades the immune system and causes chronic infection. Mutations in the bacterial genome are but one mechanism by which this change in phenotype might occur, but there are likely to be others such as local airway conditions, epigenetic and host factors. This may lead to the evolution of related, but diverse communities of PA organisms in the CF lung. One clinical implication of this possibility is that there are subpopulations of bacteria with differing antibiotic susceptibilities in the CF lung. This may explain why routine antibiotic susceptibility testing has never been associated with improved clinical response to pulmonary exacerbations.
Acknowledgments
This work was supported by the Cystic Fibrosis Foundation Therapeutics, Inc. [A.R.H.] and NIH (K02 AI065615 [A.R.H.], NHLBI/NCRR 1K12RR017707-02 [MJ] and M01 RR-00048 [MJ]).
There is no personal or financial support with any entity that may have financial interest in the subject matter of this manuscript.
We wish to thank Xiaotian Zheng and the personnel at the microbiology laboratories at Children’s Memorial Hospital and Northwestern Memorial Hospital for their assistance in completing this work.
Abbreviations
- CF
cystic fibrosis
- CFF
cystic fibrosis foundation
- CFTR
cystic fibrosis transmembrane regulator
- CI
chronically infected
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- PA
Pseudomonas Aeruginosa
- PE
pulmonary exacerbation
- T3
type III
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Rello J, Ausina V, Ricart M, Castella J, Prats G. Impact of previous antimicrobial therapy on the etiology and outcome of ventilator-associated pneumonia. Chest. 1993;104(4):1230–1235. doi: 10.1378/chest.104.4.1230. [DOI] [PubMed] [Google Scholar]
- 2.Micek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Health care-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother. 2007;51(10):3568–3573. doi: 10.1128/AAC.00851-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rello J, Jubert P, Valles J, Artigas A, Rue M, Niederman MS. Evaluation of outcome for intubated patients with pneumonia due to Pseudomonas aeruginosa. Clin Infect Dis. 1996;23(5):973–978. doi: 10.1093/clinids/23.5.973. [DOI] [PubMed] [Google Scholar]
- 4.Feldman M, Bryan R, Rajan S, et al. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun. 1998;66(1):43–51. doi: 10.1128/iai.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohama M, Hiramatsu K, Miyajima Y, Kishi K, Nasu M, Kadota J. Intratracheal immunization with pili protein protects against mortality associated with Pseudomonas aeruginosa pneumonia in mice. FEMS Immunol Med Microbiol. 2006;47(1):107–115. doi: 10.1111/j.1574-695X.2006.00069.x. [DOI] [PubMed] [Google Scholar]
- 6.Tateda K, Ishii Y, Horikawa M, et al. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect Immun. 2003;71(10):5785–5793. doi: 10.1128/IAI.71.10.5785-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Malley YQ, Abdalla MY, McCormick ML, Reszka KJ, Denning GM, Britigan BE. Subcellular localization of Pseudomonas pyocyanin cytotoxicity in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;284(2):L420–430. doi: 10.1152/ajplung.00316.2002. [DOI] [PubMed] [Google Scholar]
- 8.Meyer JM, Neely A, Stintzi A, Georges C, Holder IA. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect Immun. 1996;64(2):518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurahashi K, Kajikawa O, Sawa T, et al. Pathogenesis of septic shock in Pseudomonas aeruginosa pneumonia. J Clin Invest. 1999;104(6):743–750. doi: 10.1172/JCI7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank DW. The exoenzyme S regulon of Pseudomonas aeruginosa. Mol Microbiol. 1997;26(4):621–629. doi: 10.1046/j.1365-2958.1997.6251991.x. [DOI] [PubMed] [Google Scholar]
- 11.Roy-Burman A, Savel RH, Racine S, et al. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. Journal of Infectious Diseases. 2001;183(12):1767–1774. doi: 10.1086/320737. [DOI] [PubMed] [Google Scholar]
- 12.Hauser AR, Cobb E, Bodi M, et al. Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med. 2002;30(3):521–528. doi: 10.1097/00003246-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology. 2001;147(Pt 10):2659–2669. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- 14.Barbieri JT, Sun J. Pseudomonas aeruginosa ExoS and ExoT. Rev Physiol Biochem Pharmacol. 2004;152:79–92. doi: 10.1007/s10254-004-0031-7. [DOI] [PubMed] [Google Scholar]
- 15.Sato H, Frank DW. ExoU is a potent intracellular phospholipase. Mol Microbiol. 2004;53(5):1279–1290. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- 16.FitzSimmons SC. The changing epidemiology of cystic fibrosis. J Pediatr. 1993;122(1):1–9. doi: 10.1016/s0022-3476(05)83478-x. [DOI] [PubMed] [Google Scholar]
- 17.Fegan M, Francis P, Hayward AC, Davis GH, Fuerst JA. Phenotypic conversion of Pseudomonas aeruginosa in cystic fibrosis. J Clin Microbiol. 1990;28(6):1143–1146. doi: 10.1128/jcm.28.6.1143-1146.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns JL, Gibson RL, McNamara S, et al. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. Journal of Infectious Diseases. 2001;183(3):444–452. doi: 10.1086/318075. [DOI] [PubMed] [Google Scholar]
- 19.Smith EE, Buckley DG, Wu Z, et al. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc Natl Acad Sci U S A. 2006;103(22):8487–8492. doi: 10.1073/pnas.0602138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallant CV, Raivio TL, Olson JC, Woods DE, Storey DG. Pseudomonas aeruginosa cystic fibrosis clinical isolates produce exotoxin A with altered ADP-ribosyltransferase activity and cytotoxicity. Microbiology. 2000;146(Pt 8):1891–1899. doi: 10.1099/00221287-146-8-1891. [DOI] [PubMed] [Google Scholar]
- 21.De Vos D, De Chial M, Cochez C, et al. Study of pyoverdine type and production by Pseudomonas aeruginosa isolated from cystic fibrosis patients: prevalence of type II pyoverdine isolates and accumulation of pyoverdine-negative mutations. Arch Microbiol. 2001;175(5):384–388. doi: 10.1007/s002030100278. [DOI] [PubMed] [Google Scholar]
- 22.Lee B, Haagensen JA, Ciofu O, Andersen JB, Hoiby N, Molin S. Heterogeneity of biofilms formed by nonmucoid Pseudomonas aeruginosa isolates from patients with cystic fibrosis. J Clin Microbiol. 2005;43(10):5247–5255. doi: 10.1128/JCM.43.10.5247-5255.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luzar MA, Thomassen MJ, Montie TC. Flagella and motility alterations in Pseudomonas aeruginosa strains from patients with cystic fibrosis: relationship to patient clinical condition. Infect Immun. 1985;50(2):577–582. doi: 10.1128/iai.50.2.577-582.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romling U, Fiedler B, Bosshammer J, et al. Epidemiology of chronic Pseudomonas aeruginosa infections in cystic fibrosis. J Infect Dis. 1994;170(6):1616–1621. doi: 10.1093/infdis/170.6.1616. [DOI] [PubMed] [Google Scholar]
- 25.Lechtzin N, John M, Irizarry R, Merlo C, Diette GB, Boyle MP. Outcomes of adults with cystic fibrosis infected with antibiotic-resistant Pseudomonas aeruginosa. Respiration. 2006;73(1):27–33. doi: 10.1159/000087686. [DOI] [PubMed] [Google Scholar]
- 26.Jain M, Ramirez D, Seshadri R, et al. Type III secretion phenotypes of Pseudomonas aeruginosa strains change during infection of individuals with cystic fibrosis. J Clin Microbiol. 2004;42(11):5229–5237. doi: 10.1128/JCM.42.11.5229-5237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang PJ, Hauser AR, Apodaca G, et al. Identification of Pseudomonas aeruginosa genes required for epithelial cell injury. Mol Microbiol. 1997;24(6):1249–1262. doi: 10.1046/j.1365-2958.1997.4311793.x. [DOI] [PubMed] [Google Scholar]
- 28.Thompson MRBM, Sokol PA, Lile JD, Iglewski BH. Exoenzyme S: an ADP-ribosyltransferase produced by Pseudomonas Aeruginosa. In: Smulson MST, editor. Novel ADP-ribosylation of regulatory enzymes and proteins. Amsterdam: Elsevier/North Holland Inc.; 1980. pp. 425–433. [Google Scholar]
- 29.Nguyen D, Singh PK. Evolving stealth: genetic adaptation of Pseudomonas aeruginosa during cystic fibrosis infections. Proc Natl Acad Sci U S A. 2006;103(22):8305–8306. doi: 10.1073/pnas.0602526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulert GS, Feltman H, Rabin SD, et al. Secretion of the toxin ExoU is a marker for highly virulent Pseudomonas aeruginosa isolates obtained from patients with hospital-acquired pneumonia. J Infect Dis. 2003;188(11):1695–1706. doi: 10.1086/379372. [DOI] [PubMed] [Google Scholar]
- 31.Imamura Y, Yanagihara K, Fukuda Y, et al. Effect of anti-PcrV antibody in a murine chronic airway Pseudomonas aeruginosa infection model. Eur Respir J. 2007;29(5):965–968. doi: 10.1183/09031936.00147406. [DOI] [PubMed] [Google Scholar]