SUMMARY
During cardiogenesis, perturbation of a key transition at midgestation from cardiac patterning to cardiac growth and chamber maturation often leads to diverse types of congenital heart disease, such as ventricular septal defect (VSD), myocardium noncompaction, and ventricular hyper-trabeculation. This transition, which occurs at embryonic day (E) 9.0-9.5 in murine and E24-28 in human embryos, is crucial for the developing heart to maintain normal cardiac growth and function in response to an increasing haemodynamic load. Although, ventricular trabeculation and compaction are key morphogenetic events associated with this transition, its molecular and cellular mechanism is currently unclear. Initially, cardiac restricted cytokine Bone Morphogenetic Protein 10 (BMP-10) was identified as being up-regulated in hypertrabeculated hearts from mutant embryos deficient in FK506 Binding Protein 12 (FKBP12). To determine the biological function of BMP-10 during cardiac development, we generated BMP-10-deficient mice. Here we describe an essential role of BMP-10 in regulating cardiac growth and chamber maturation. BMP-10 null mice display ectopic and elevated expression of p57kip2 and a dramatic reduction in proliferative activity in cardiomyocytes at E9.0-E9.5. BMP-10 is also required for maintaining normal expression levels of several key cardiogenic factors (e.g., Nkx2.5 and MEF2C) in the developing myocardium at midgestation. Furthermore, BMP-10 conditioned medium is able to rescue BMP-10-deficient hearts in culture. Our data suggest an important pathway that involves a genetic interaction between BMP-10, cell cycle regulatory proteins, and several major cardiac transcription factors in orchestrating this transition in cardiogenesis at midgestation. This may provide an underlying mechanism for understanding the pathogenesis of both structural and functional congenital heart defects.
Keywords: BMP-10, p57kip2, Nkx2.5 and MEF2C, cardiac growth and development, ventricular trabeculation and compaction
INTRODUCTION
Cardiac development is a complex and sequential process that is composed of a series of morphogenetic events, such as cardiogenic induction and patterning, cardiomyocyte growth and differentiation, multiple cell lineage specification, and terminal differentiation and chamber maturation (review see Srivastava and Olson, 2000). These events are regulated by cardiogenic transcriptional factors and growth/differentiation factors. Genetic mutations of these genes lead to either early embryonic lethality or a vast majority of congenital heart defects (CHDs) that affect 1/200 live births. One of many forms of CHDs is ventricular non-compaction or spongy myocardium, which is based on its characteristic feature of abnormal ventricular myocardium with hyper-trabeculation and non-compaction (Dusek et al., 1975; Ichida et al., 2001). It is a unique type of cardiomyopathy generally found in infant patients. These patients may proceed to have dilated hearts and congestive heart failure. Commonly associated defects are ventricular septal defect (VSD) and pulmonic stenosis (PS). This non-compaction of ventricular myocardium has been defined as a morphogenetic abnormality and is clearly due to defects in the ventricular trabeculation and compaction.
Ventricular trabeculation and compaction are important morphogenetic processes and are closely associated with cardiac growth regulation at midgestation (Rumyantsev, 1991; Icardo, 1984). At the final stage of cardiac looping (E9.0-E9.5), initially, the endocardium penetrates through the cardiac jelly and evaginates at discrete points of myocardium to form “out pockets” directed toward the myocardium. These endocardium out pockets initiate the cardiac trabeculation. Further expansion of primitive trabecular myocardium between E9.5 to E13.5, via either myocyte recruitment or proliferation, is an important step to generate matured trabeculae. Later in development (E14.5-E15.5), trabecular myocytes in the developing myocardium undergo “compaction” and gradually become part of compact wall, papillary muscles, interventricular septum, and conductive system cells (Moorman and Lamers, 1999), respectively. One of the scientific challenges is to determine the molecular mechanism by which cardiac trabeculation and subsequent compaction are regulated. Several endocardial growth factors have been identified that are required for the development of trabeculae, such as neuregulin (Meyer and Birchmeier, 1995) and its receptors ErbB (Gassmann et al., 1995, Lee et al., 1995), vascular endothelial growth factor (VEGF) (Ferrara et al., 1996), and angiopoietin-1 (Suri et al., 1996). Mutant mice deficient in these genes have severe defects in ventricular trabeculation. However, more detailed analysis is still required to determine their precise role in the process of ventricular trabeculation and compaction.
Bone morphogenetic proteins (BMPs), named for their initial biological activity of inducing ectopic bone formation, belong to the transforming growth factor β (TGFβ) superfamily. They mediate a diverse spectrum of developmental events from insects to mammals (review see Ducy and Karsenty, 2000; Nakayama et al., 2000). BMP signals have been shown to link to multiple steps of cardiac development, including cardiogenic induction and endocardial cushion formation (review see Schneider et al., 2003). Unlike other BMPs, BMP-10 expression is restricted to the developing and postnatal heart (Neuhaus et al., 1999). The most interesting feature of BMP-10 is its transient presence in the developing trabecular myocardium. In this study, we found that BMP-10 is up-regulated in trabecular myocardium of a genetically manipulated mutant mice deficient in FK506 binding protein 12 (FKBP12) (Shou et al, 1998). FKBP12 is able to bind type I receptors for BMP/Activin/TGFβ and possibly plays a role in preventing premature activation of type I receptors (Wang et al, 1994; Wang et al, 1996, review see Massague and Chen, 2000). FKBP12-defcient mice are embryonic lethal due to enormous overproduction of ventricular trabeculae that severely impairs cardiac development and function. In this study, we generated BMP-10-deficient mice and analyzed the biological function of BMP-10 during cardiac trabeculation and uncovered a BMP-10 mediated molecular pathway that is essential for regulating cardiac growth and function at midgestation.
MATERIALS and METHODS
RNA Differential display and molecular cloning of BMP-10
The PCR based RNA differential display was based on the previously described protocol (Bao, 1998). Briefly, cellular mRNA samples were isolated from pooled FKBP12-deficient and littermate control E14.5 embryonic cardiac tissues, respectively. S35-labeled cDNA fragments were amplified by PCR using a set of palindromic primers and rTh DNA polymerase and Mn2+ and Mg2+ reaction buffer system (Perkin Elmer Cetus). The differentially expressed gene products revealed by the mRNA display were confirmed by Northern blot before being processed for sequencing. The mouse BMP-10 full-length cDNA was obtained by RT-PCR and was confirmed by sequencing.
RT-PCR reactions
MicroPoly(A)Pure kit (Ambion) was used to isolate mRNA from mouse embryos or pooled embryonic hearts. The first-strand synthesis and PCR reaction were performed using RETROscript kit (Ambion) according the manufacture’s instruction. The sets of primers are as following, 5′ BMP-10 primer: ACCAGACGTTGGCAAAAGTCAGGC; 3′ BMP-10 primer: GATGATCCAGGAGTCCCACCCAAT; 5′ p57kip2 primer: AGTCTGTGCCCGCCTTCTAC; 3′ p57kip2 primer:CTCAGTTCCCAGCTCATCACCC; 5′ FKBP12 primer: CACGTGGATCTGCCATGGAGGAA; 3′ FKBP12 primer: GTGGAAGGACTGACAGAAGCCAA; 5′ GAPDH primer: GGGTGGAGCCAAACGGGTC; 3′ GAPDH primer: GGAGTTGCTGTTGAAGTCGCA.
Targeted deletion and generation of BMP-10-deficient mice
A BMP-10 genomic clone was isolated from a mouse 129SvEv genomic BAC library (RPCI-22 129 mouse library from BAC/PAC Resources, Children’s Hospital Oakland). The mouse BMP-10 gene contains two exons (Figure 3A). Linearized targeting vector (25μg) was electroporated into ES cells (CCE916 ES cell line), clones were selected in G418 and gancyclovir, DNA from the clones was analyzed by Southern blot, and targeted ES cell lines BMP10-B12 and BMP10-F8 were expanded and injected into blastocysts. Male chimeras were bred to C57BL/6J or 129SvEv females to generate F1 offspring. Mutant mice generated from both targeted ES cell lines had identical phenotype.
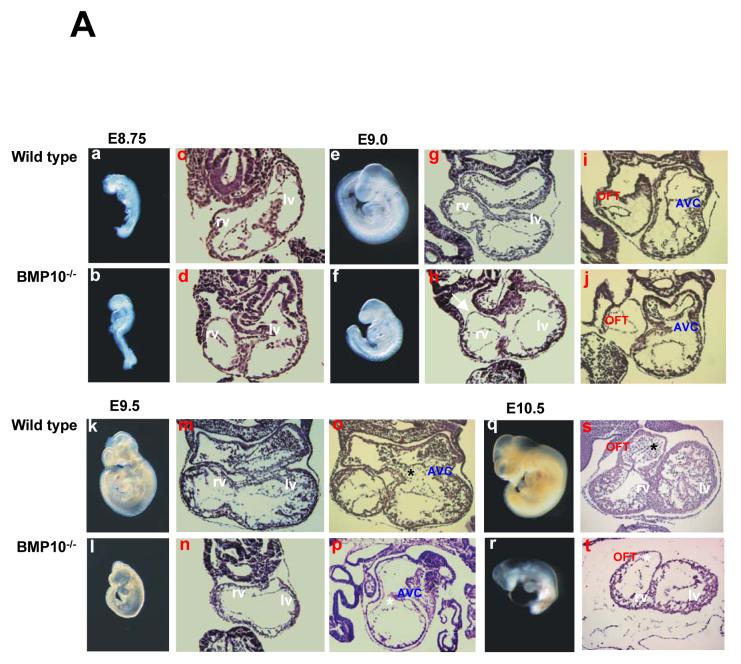
Figure 3.
Morphological and histological analysis of BMP-10-deficient embryos and hearts.
(A) In a and b, e and f, k and l, q and r, comparison of gross morphology of normal littermate control and BMP-10-deficient embryos from E8.75 to E10.5. (a and b) No apparent abnormality was detected at E8.75. (e and f) Some BMP10-deficient embryos were slightly growth retarded at E9.0. (k and l) Severe growth retardation was seen in BMP-10-deficient embryo at E9.5, however, mutants had an identical number of somite pairs and normal allantoic connection when compared to littermate controls. Over 50% of BMP-10-deficient embryos have severe edema and expanded pericardiac sacs, suggesting poor cardiac function in these mutants. (q and r) BMP-10-deficient embryos were dead by E10.5. In c and d, g and h, i and j, m and n, o and p, and s and t, comparison of histological sections of normal control and BMP-10-deficient hearts from E8.75 to E10.5 embryos stained with haematoxylin and eosin. (d, h and j) At E8.75-E9.0, BMP-10-deficient embryos had normal rightward looped heart and primitive ventricular chambers, suggesting that BMP-10 is not required for the early phases of cardiogenesis. Also, the size of the heart in BMP-10-deficient embryos was grossly normal compared to littermate control, but exhibited some thinned myocardium (white arrow). Acellular endocardial cushions were formed in both the outflow track (OFT) and atrial-ventricular canal (AVC). (n, p and t) Compared with wild-type normal heart at E9.5-E10.5, BMP-10-deficient hearts were growth retarded, had hypoplastic walls, and failed to develop normal ventricular trabeculae and endocardial cushions. While endocardial cushions in OFT and AVC of wild type control hearts have begun to be seeded after epithelialmesenchymal transformation of adjacent endocardium (black asterisks), acellular endocardial cushions were remained in BMP-10-deficient hearts (white asterisks).
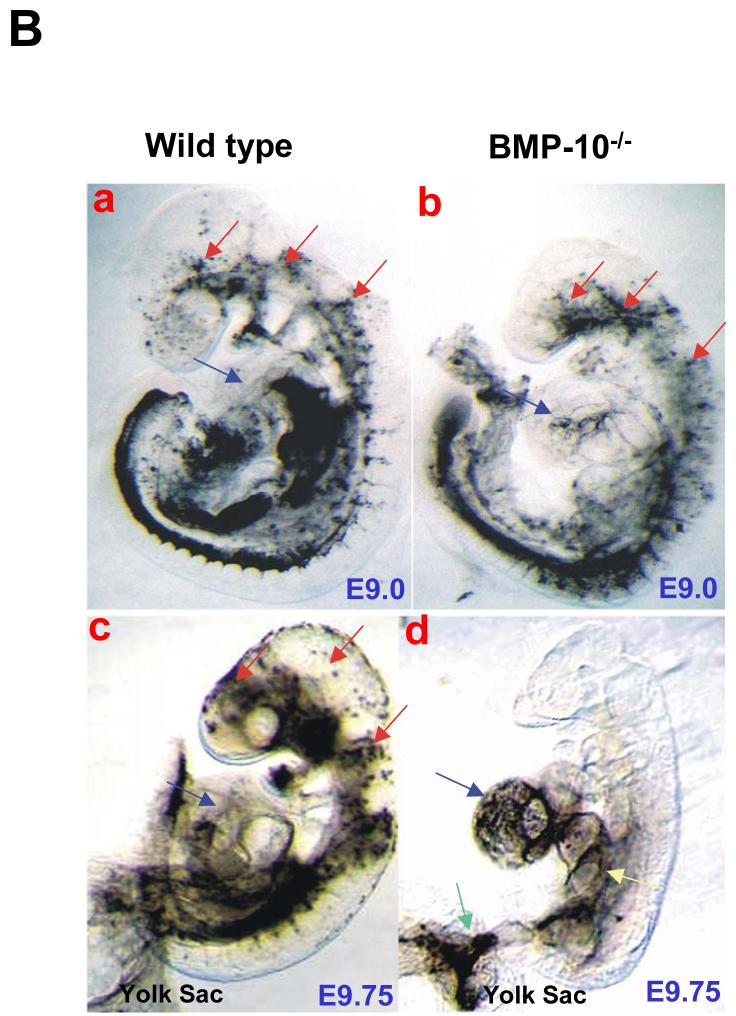
(B) Ink-injection was used to visualize the cardiac contractile function and blood flow in E9.0 and E9.5-E9.75 embryos. (a and c) Ink injected in the primitive left ventricle was efficiently pumped throughout entire cardiovascular system in control embryos. (b) At E9.0, the circulation was established in BMP-10-deficient embryos, however, not as efficiently as littermate controls, which might reflect the weaker/slower heart rate in BMP-10 mutants. (d) At E9.5-9.75, ink remained in the BMP-10-deficient ventricles, suggesting a poor cardiac function. Note that the ink within the BMP-10-deficient heart has diffused in a retrograde direction into the sinus venous (yellow arrow) and yolk sac (green arrow) due to lack of adequate cardiac contraction and circulation. Red arrows indicate circulated ink around embryonic head region in a control embryo. Blue arrows indicate hearts. The posterior portions of the embryos were removed to help visualize the hearts (c and d).
Histological, morphological, ink injection, whole-mount and section in situ hybridization, and immunohistochemistry analyses
Embryos were harvested by caesarean section. Embryos and isolated hearts were fixed in 10% neutral buffered formalin, paraffin embedded, and sectioned (6μm), and stained with hematoxylin and eosin. To analyze the proliferative activity of the developing heart, timed-mated females were given a single injection of tritiated thymidine (200 μCi ip at 28 Ci/mM; Amersham Biosciences Corp.). Embryos were harvested after a 3-hour labeling period, and followed by fixation (10% neutral buffered Formalin) and paraffin sectioning. Deparaffined sections were stained with Hoechst in PBS to identify the cell nucleus. The Hoechst-stained slides were coated with photographic emulsion (Polysciences, Inc., Warrington, PA, USA) and further processed for autoradiography. 3H-thymidine labeling index was the percent of labeled nuclei versus the total number of nuclei. Ink-injection analysis was performed as previously described (Winnier et al, 1999). Both whole mount and section in situ hybridization were performed as previously described (Franco, 2001). Complementary RNA probes of various cardiac markers were labeled with digoxingenin-UTP using Roche DIG RNA Labeling system according to the manufacturer’s guidelines. HOP probe was a generous gift of Dr. Epstein (Chen et al., 2002). These probes were hybridized with paraformaldehyde fixed embryos or hearts for whole-mount in situ staining or with either frozen or deparaffined sectioned samples for in situ staining. To analyze p57kip2 expression in the heart, we used a monoclonal antibody against mouse p57kip2 (Labvision, ms-897-P1) and a Vector staining system (Vector, PK-2200) according to the manufacturer’s instructions.
Whole-mount immunostaining and confocal microscopic imaging
Embryos were washed 3 times in PBS, fixed for 10 minutes in pre-chilled acetone before treated with blocking solution containing 3% non-fat dry milk (Bio-Rad Laboratories, Hercules, CA) and 0.025% Triton X-100 for 1 hour. Directly conjugated primary antibodies were then added to a final concentration of 1 μg/ml for 12 to 18 hours at 4°C. Anti-Flk-1 (PharMingen, San Diego, CA) and MF-20 monoclonal antibodies (Hybridoma bank, University of Iowa) were labeled with Alexa Fluor 488 and Alexa Fluor 647, respectively, using a monoclonal antibody labeling kit (Molecular Probes, Eugene, OR). Samples were analyzed using a Bio-Rad MRC 1024 Laser Scanning Confocal Microscope (Bio-Rad Microscopy Division, Cambridge, MA) equipped with a Krypton-Argon laser (488, 647 nm). 3D series (Z series) were obtained by imaging serial confocal planes at 512 × 512 pixel resolution with a Nikon 20X oil-immersion objective (2 μm intervals).
Generation of BMP-10 expressing NIH3T3 cells
The coding region of the mouse BMP-10 cDNA was subcloned 5′ of an IRES-EGFP cassette in the retroviral vector MIEG3. Ecotropic packaging cell lines were established for MIEG3-BMP-10 and MIEG3 vector control as described (Haneline, 2003). NIH3T3 cells were transduced with retroviral supernatants (containing about 1×105 viral particles/ml) similar to previously published methods (Haneline, 2003). Transduced cells were kept in DMEM containing 10% FBS for 3 days before sorting for EGFP positive cells using FACSVantage SE (Becton Dickinson). Northern blot analysis confirmed that the BMP-10 transcript was only detected in NIH3T3 cells transduced with MIEG3-BMP-10 (NIH3T3/BMP-10) and not in cells transduced with vector control (NIH3T3/EGFP).
Cardiomyocyte-NIH3T3 cell co-culture to assay the proliferative activity of cardiomyocytes
NIH3T3/BMP-10 and NIH3T3/EGFP cells were used as feeders in our cardiomyocyte co-culture assay. We first treated feeder cells with mitomycin C (10 μg/ml) for 2 hours to prevent proliferation. After washing twice in PBS, mitomycin C-treated cells were trypsinized and resuspended in DMEM containing 2% FBS and were mixed with freshly isolated mouse embryonic cardiomyocytes (E12.5). Cells were plated on one-well Lab-Tek chamber slides. The final plating density was 2-3 ×105 total cells for each well. Cells were labeled with 3H-thymidine for 2 hours and fixed in methanol and processed for autoradiography and Periodic Acid-Schiff (PAS) staining. The DNA-3H-thymidine labeling index of PAS positive cells (cardiomyocytes) were recorded and compared between experimental and control groups.
Culture of isolated embryonic hearts
Based on the method previously described (Conway et al., 1997, Rentschler et al., 2002), embryonic hearts were dissected from E9.25 or E9.5 embryos in DMEM containing 10% FBS, and washed twice in PBS and cultured for 8, 12, 24 hours in BMP-10 conditioned or control media. BMP-10 conditioned media were collected from overnight culture of BMP-10 expressing cells (NIH3T3/BMP-10). Control media were collected from overnight culture of control cells (NIH3T3/EGFP). Both BMP-10 conditioned and control media were derived from DMEM containing 1% FBS. Conditioned media for BMP-2, -4, -5, -6 and TGFβ-1 (final concentration: 50ng/ml) and neuregulin (NRG-1, final concentration: 2.5×10-9 M) were prepared by adding each activated growth factors to DMEM containing 1% serum right before the culture to reach the final concentration (Barron et al, 2000, Rentschler et al., 2002). BMP-2, -4, -5, -6 and TGFβ-1 were from Sigma. NRG-1 was from R & D Systems.
RESULTS
BMP-10 is up-regulated in hyper-trabeculated hearts deficient in FKBP12
FKBP12-deficient mice develop severe cardiac defects with the unique phenotype of hyper-trabeculation and non-compaction (Shou et al., 1998), suggesting that FKBP12-deficient mouse is a valuable mouse model to study the molecular mechanism of cardiac trabeculation. To screen for candidate genes that were abnormally expressed in FKBP12-deficient hearts, specifically in over-produced trabecular myocardium, we used RNA differential display. RNAs were isolated from pooled E14.5 hearts of FKBP12-deficient and control embryos. RNA differential displays (data not shown) were performed as previously described (Bao et al., 1998). BMP-10 was identified in this gene profiling study. BMP-10 expression was significantly elevated in FKBP12-deficient hearts as confirmed by Northern blot analysis (Figure 1A-k).
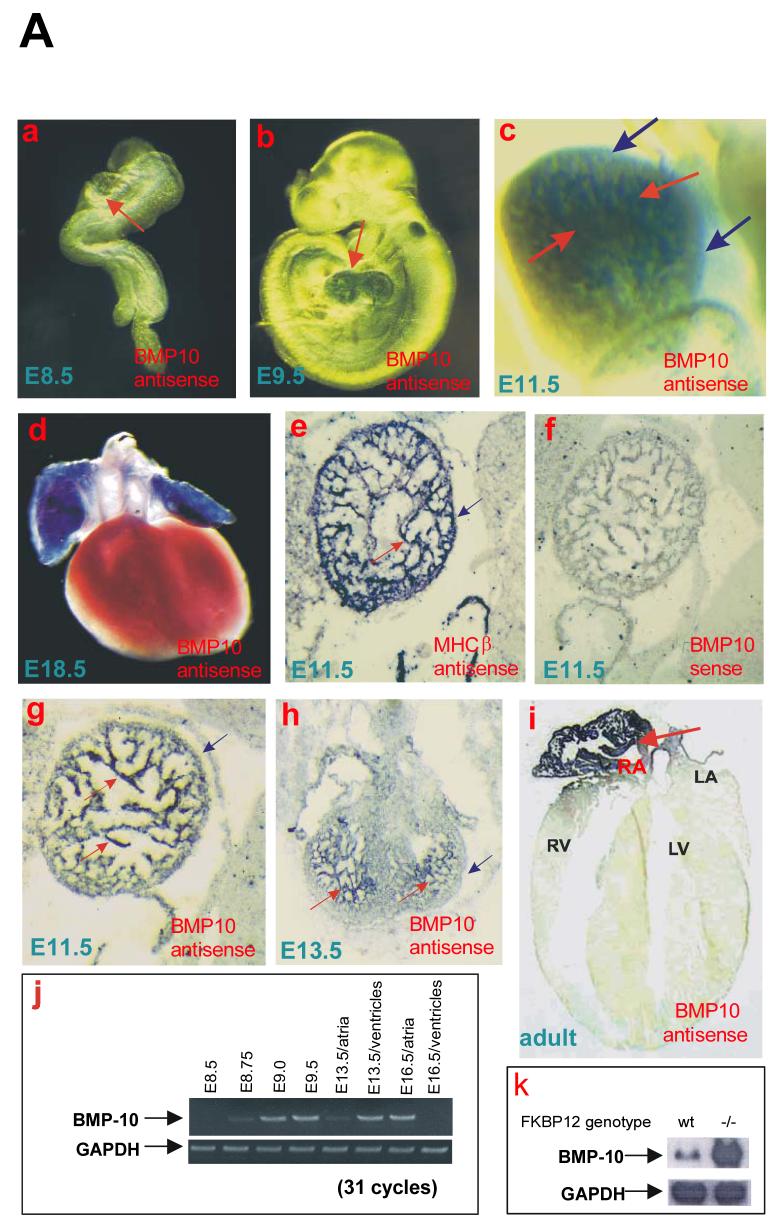
Figure 1.
Expression pattern of BMP-10 during cardiogenesis.
(A) In (a-d), using whole-mount in situ hybridization, BMP-10 transcripts were not detected in E8.5 embryonic hearts (a), but were detected in developing ventricles and atria at E9.5 (b), and at E11.5 (c). BMP-10 expression was restricted to trabecular myocardium. Red arrows indicate the heart in (a and b) and trabecular myocardium in (c), and blue arrows indicate the ventricular compact wall. (d) BMP-10 expression was only detected in atria at E18.5. In (e-i), in situ hybridizations were performed on sagittal sections of E11.5, transverse sections of E13.5 embryos, and transverse sections of an adult mouse heart. (e) Myosin heavy chain β (MHCβ) transcripts were detected throughout the myocardium. (f) BMP-10 sense probe was used as a negative control. (g and h) BMP-10 expression in ventricle was restricted to trabecular myocardium, and (i) became restricted to the right atrium in adult. (j) BMP-10 transcripts were detected in the heart as early as E8.75 using RT-PCR. By E16.5, BMP-10 is hardly detectable in ventricles. (k) Using Northern blot to confirm BMP-10 expression in FKBP12-deficient heart (E14.5). BMP-10 transcripts were significantly up-regulated in FKBP12-deficient heart.
(B) Multiple-tissue quantitative mRNA level analysis. (a) Quantitative dot blot analysis of evenly loaded 84 mRNA samples from varying adult human tissues (Clontech). Only mRNA samples isolated from whole heart (A4) and right atrium (D4) showed significant BMP-10 mRNA level when compared to negative control samples. (b) Phosphorimage analysis on the relative level of expression of BMP-10 in different regions of the adult heart. The phosphorimaging values were corrected for the mean background consisting of negative controls (yeast total RNA, yeast tRNA, E.coli rRNA, E. Coli DNA) and empty fields.
BMP-10 expression in developing and adult heart
We carefully analyzed the expression pattern of BMP-10 in developing and adult mouse hearts using whole-mount and section in situ hybridization analyses (Figure 1A-a to -i). During cardiac development, BMP-10 is expressed transiently in the ventricular trabecular myocardium from E9.0 to E13.5, a critical time span when cardiac development shifts from patterning to growth and chamber maturation (Figure 1A-b, -c, and -e to -h). Using RT-PCR, we could detect BMP-10 expression as early as E8.75, but not in E16.5 ventricles (Figure 1A-j). By E16.5-E18.5, BMP-10 was only detectable in atria (Figure 1A-d and -j). This finding is consistent with previously published work (Neuhaus et al., 1999). Interestingly, in adult heart BMP-10 was present in right atrium, but not in left atrium (Figure 1A-i), which was further confirmed by a quantitative RNA analysis using Clontech adult tissue mRNA array (Figure 1B-a and -b).
Generation of BMP-10-deficient mice
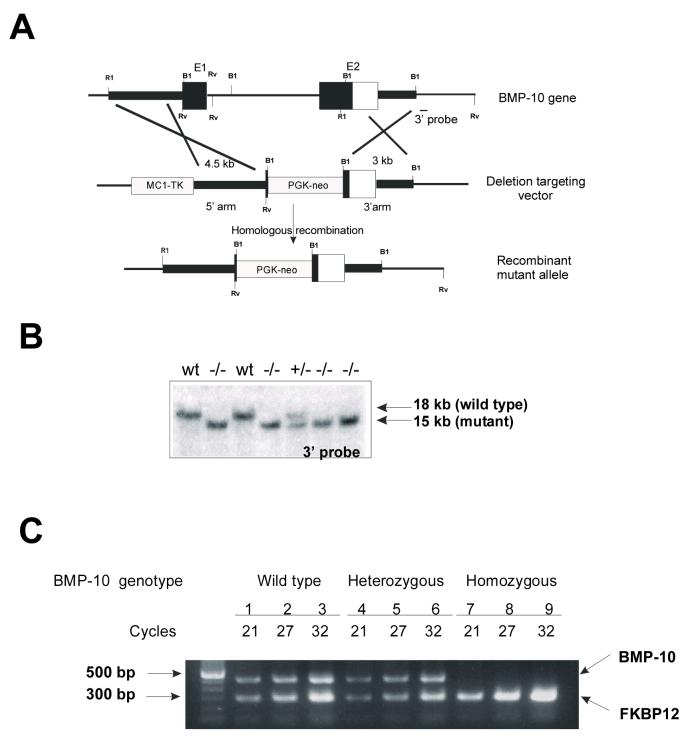
The mouse BMP-10 gene contains only two exons. The second exon encodes a proteolytic processing site and the mature peptide. To generate a null mutation in the mouse BMP-10 gene, we deleted most of the coding region (bmp10m1) using mouse embryonic stem cell technology (Figure 2A). Genomic Southern blot using 3′-probe (Figure 2B) and PCR analyses were used to genotype targeted ES cells and mutant mice. RT-PCR was used to confirm that BMP-10 expression was absent in BMP-10-deficient embryos (Figure 2C).
Figure 2.
Generation of BMP-10-deficient mice.
Targeting vector to mutate the mouse BMP-10 gene in ES cells (a), Southern blot analysis of genomic DNA (digested with EcoRV and probed with 3′-probe) derived from a single litter of E9.5 embryos after mating of bmp10m1/+ mice (b), and RT-PCR analysis to confirm the inactivation of BMP-10 expression in bmp10m1/bmp10m1 embryos (E9.0) (c). Expression of FKBP12 was used as loading control.
Heterozygous (bmp10m1/+) mice were viable and fertile and were intercrossed to obtain homozygous (bmp10m1/ bmp10m1) mutants. Genotyping analysis of 191 F2 129SvEv inbred and 230 F2 C57BL6/129SvEv hybrid offspring at weaning demonstrated no viable BMP-10-deficient mice. The distribution of genotypes in E9.5 embryos (105 wild-type [24%], 244 heterozygotes [56%], and 87 homozygotes [20%]) and in E10.5 embryos (95 wild-type [30%], 190 heterozygotes [61%], and 28 homozygotes [9%]) suggested that BMP-10-deficient mice were dying in utero between E9.5 to E10.5.
Embryonic lethality of BMP-10-deficient mice is due to severely impaired cardiac development and function
We carried out morphological and histological analyses on embryos from E8.5 to E10.5 (Figure 3A). At E8.5-8.75 (8-12 somite pair stage), BMP-10-deficient embryos appeared normal compared to wild type and heterozygous littermate controls, suggesting that BMP-10 was not required for cardiac patterning (Figure 3A-a, -b, -c and -d). At E9.0-E9.5 (15-20 somite pair stage), while having normal pairs of somite in mutant embryos, normal allantoic/umbilical connection, and normal vasculature development in mutant yolk sacs and embryos, cardiogenesis appeared arrested in BMP-10-deficient embryos (Figure 3A-e, -f, -g, -h, -i, -j, -k, -l, -m, n, -o and -p). These mutant embryos displayed cardiac dysgenesis with profound hypoplastic ventricular walls and absence of ventricular trabeculae. The development of endocardial cushions was abnormal in both outflow track (OFT) and atrial-ventricular canal (AVC) and was halted at the acellular stage. At this developmental stage, BMP-10-deficient hearts exhibit rhythmic contraction, but at a significantly slower rate compared to littermate controls (43 ± 6 beats/minute in mutant hearts [n=12] versus 96 ± 7 beats/minute in wild type and heterozygous littermate controls [n=15], p<0.0001). To visualize and compare the cardiac function and circulation in mutants and controls, ink was injected into primitive left ventricle of E9.0 and E9.5-9.75 embryos. As shown in Figure 3B, while circulation was established in BMP-10 mutant embryos at E9.0, severely impaired cardiac function and circulation was observed in mutant embryos at E9.5-9.75 (Figure 3B). By E10.0-E10.5, mutant embryos appeared to be dying (Figure 3A-q, -r, -s and -t).
The outgrowth of ventricular trabecular myocardium is defective in BMP-10-deficient heart
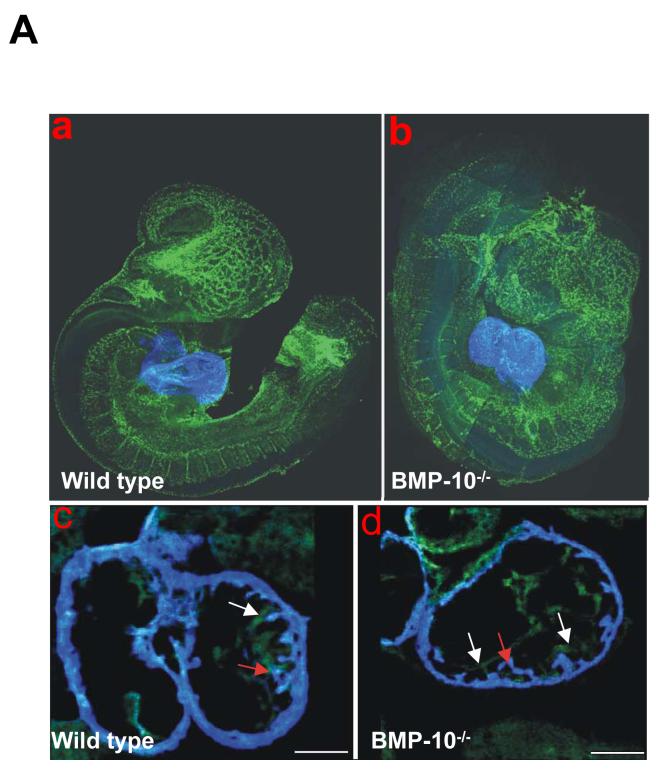
It has been suggested that cardiac trabeculation is the consequence of interactions between the developing myocardium and the “out pocketing” endocardium at E9.0-E9.5 (Icardo, 1984). To better understand if the lack of ventricular trabeculae in the BMP-10-deficient heart was due to the failure of endocardial out pocketing (i.e., initiation of trabeculation) or the failure of a myocardial response (i.e., further expansion of trabecular myocardium through either myocyte recruitment or growth), we used confocal microscopy to analyze the structural relationship between endocardium and myocardium in BMP-10-deficient hearts at E9.5 (Figure 4). Endothelial receptors for vascular endothelial growth factor (Flk1) (Millauer et al., 1993) and angiopoietin (Tek/Tie2) (Dumont et al., 1994) (data not shown), were normal in BMP-10-deficient endothelial cells throughout the developing cardiovasculature compared to wild type and heterozygous littermate controls at E9.5 (Figure 4-a and -b). MF20 staining (anti-myosin heavy chain) was also maintained in the BMP-10-deficient myocardium despite overall hypoplasia (Figure 4-c and -d). These observations confirmed that BMP-10 was not required for differentiation of either endocardium or myocardium. Furthermore, the developing endocardium was in normal proximity to the ventricular wall and the primitive ventricular trabeculae were indeed formed in the BMP-10 mutants (Figure 4-c and -d). These findings strongly suggested that BMP-10 was not critical to the initiation of cardiac trabeculation nor myocyte recruitment, but was essential to the further growth of both ventricular wall and trabeculae.
Figure 4.
Whole mount immunostaining and confocal microscopic analysis of control (a and c) and BMP-10-deficient embryos (b and d) at E9.25. Fluorescence conjugated anti-Flk-1 monoclonal antibody stains the endothelial cells (green) in the developing vasculature and endocardium of the developing heart, while anti-myosin heavy chain monoclonal antibody MF-20 stains the myocardium (blue). In a and b, comparison of endothelial development in both control and BMP-10-deficient embryos at E9.25. Endothelial development was not affected in BMP-10-deficient embryos. In c and d, comparison of endocardium and myocardium development in control and BMP-10-deficient ventricles. The BMP-10-deficient heart displayed a much thinner ventricular wall compared to control heart, however, the endocardium was in normal proximity to the myocardium. Some primitive trabeculae were formed in the BMP-10-deficient ventricles at this age. Red arrows point to the primitive trabecular myocardium, while white arrows indicate the endocardium.
Growth deficiency and ectopic expression of P57kip2 in BMP-10-deficient myocardium
Surprisingly, TUNEL assays showed no significant cellular apoptosis in E9.5 BMP-10 mutant hearts despite severely thinned ventricular wall (data not shown). This finding suggested that a defect in myocyte proliferation was the main reason for the hypoplastic wall seen in BMP-10-deficient hearts. We used 3H-thymidine labeling to determine the proliferative activity in BMP-10-deficient heart. 3H-thymidine labeling index in the mutant hearts was significantly lower than that in littermate controls (10.1%±2, n=5, in mutants versus 29.0%±3, n=4, in controls, p<0.001). These results further suggested that a marked reduction in proliferation of BMP-10-deficient cardiomyocytes was responsible for the hypoplastic ventricular wall and failure of normal ventricular trabeculation.
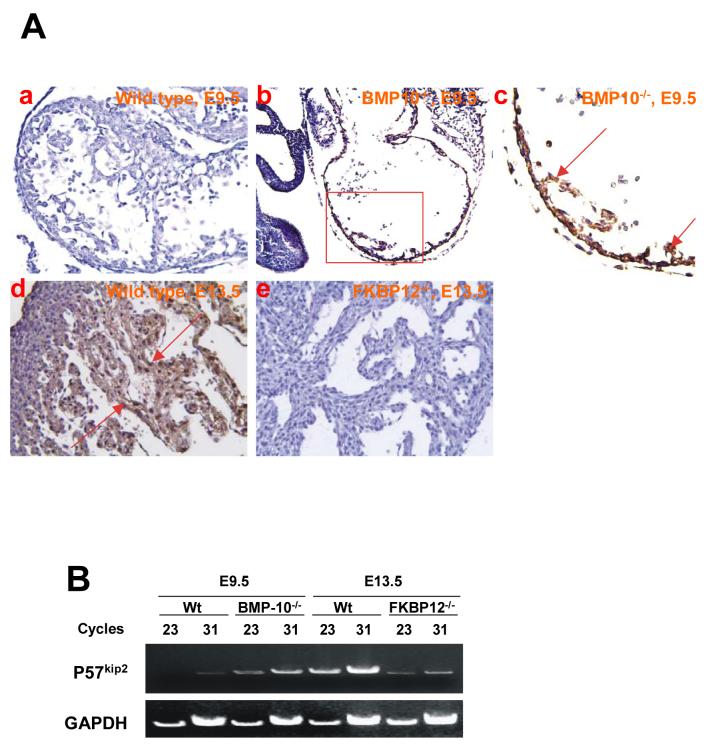
Among multiple cell cycle regulators, p57kip2 expression is first detectable in the developing heart at E10.5 by in situ hybridization and RT-PCR, and is restricted to ventricular trabeculae (Kochilas, 1999). Therefore, p57kip2 is considered a key negative regulator involved in cardiac cell cycle exit within the developing ventricular trabeculae during chamber maturation (Kochilas, 1999). Immunohistochemistry staining revealed that p57kip2 was up-regulated and ectopically expressed throughout the ventricular wall in BMP-10-deficient hearts at E9.5 compared to littermate controls (Figure 5A-a, -b and -c). FKBP12-deficient hearts (E13.5), which have elevated BMP-10 levels and an overproduction of trabeculae, exhibited significantly lower p57kip2 expression in trabecular myocardia compared to littermate controls (Figure 5A-d and -e), suggesting a mechanistic relationship between p57kip2 and BMP-10. RT-PCR analysis confirmed this observation (Figure 5B) and further suggested that BMP-10 regulates p57kip2 at the transcriptional level. Together these data show that elimination or elevation of BMP-10 expression jeopardizes the regulation of ventricular growth and chamber maturation.
Figure 5.
Distribution and expression of p57kip2 in BMP-10-deficient and FKBP12-deficient hearts using immunohistochemistry staining. (A) At E9.5, p57kip2 expression was undetectable in wild type heart (a), but was ectopically present in the BMP-10-deficient heart (b and c) under identical staining conditions. The expression of p57kip2 was abundant in E13.5 ventricular trabecular myocardium of wild type heart (d), but was significantly down-regulated in the E13.5 FKBP12-deficient trabecular myocardium (e). Arrows indicate areas of positive staining.
(B) Using RT-PCR to confirm the mRNA level of p57kip2 in BMP-10-deficient hearts (E9.5) and FKBP12-deficient hearts (E13.5). The expression of p57kip2 was up-regulated in BMP-10-deficient hearts and down-regulated in FKBP12-deficient hearts.
Defects in cardiogenic pathway in BMP-10-deficient heart
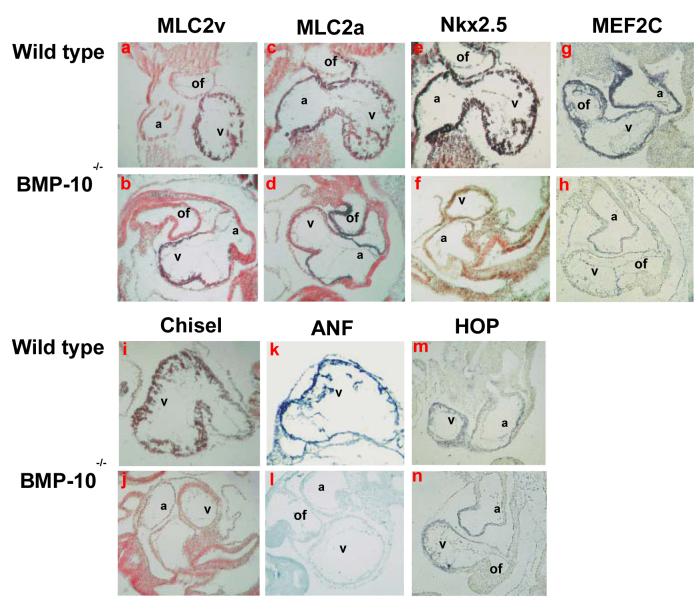
To determine if cardiogenic pathways were affected by the loss of BMP-10, we evaluated the expression patterns of key cardiac transcriptional factors and chamber-restricted markers. The expression of cardiac chamber-specific markers MLC2v and MLC2a (Franco and Icardo, 2001) were normal in BMP-10-deficient hearts at E8.5 (data not shown) and E9.5 (Figure 6A-a, -b, -c, and -d), which further confirmed our observation that BMP-10 was not required for cardiac patterning and cardiogenic differentiation. While the expression of cardiogenic transcriptional factors Nkx2.5 (Schott, 1998; review see Harvey, 1999) and MEF2C (review see Black and Olson, 1999) was normal in BMP-10-deficient embryos at E8.5-E8.75 (data not shown), these molecules were dramatically down-regulated in the BMP-10-deficient hearts at E9.25-E9.5 (Figure 6A-e, -f, -g, and -h). Whole mount in situ hybridization revealed that the down-regulation of Nkx2.5 was only restricted to the heart, as Nkx2.5 expression in the branchial arches and developing gut remained normal at E9.5 (data not shown). ANF and Chisel are known downstream targets of Nkx2.5 (Durocher et al., 1996; Palmer et al., 2001). Both Chisel and ANF were significantly down-regulated (Figure 6A-i, -j, -k and -l). Another Nkx2.5 downstream target HOP (Chen et al., 2002; Shin et al., 2002), which is a novel homeodomain protein that serves a negative feedback for Nkx2.5 function, remained normal in the mutant hearts at E9.5 (Figure 6A-m and -n). In addition, cardiogenic transcriptional factor eHand also remained normal in BMP-10-deficient hearts, while dHand was slightly down-regulated (data not shown). These finds suggested that Nkx2.5 and MEF2C were regulated by BMP-10.
Figure 6.
Analysis of cardiac markers in BMP-10-deficient hearts.
(A) Using in situ hybridization to analyze the expression of cardiac markers at E9.5. The cardiac chamber markers MLC2v (a and b) and MLC2a (c and d) were not altered in the BMP-10-deficient hearts, suggesting that cardiac patterning and chamber specification are normal in the BMP-10-deficient heart. Cardiogenic transcription factors Nkx2.5 (e and f) and MEF2C (g and h) were significantly down-regulated at E9.5 in the BMP-10-deficient hearts. The expression of Chisel (i and j) and ANF (k and l) was also reduced in the BMP-10-deficient heart, while the expression of HOP (m and n) remained at a similar level in the BMP-10-deficient heart when compared to the control heart. a: atrium; v: ventricle; of: outflow tract.
BMP-10 is able to promote embryonic cardiomyocyte growth and rescue BMP-10-deficient hearts in culture
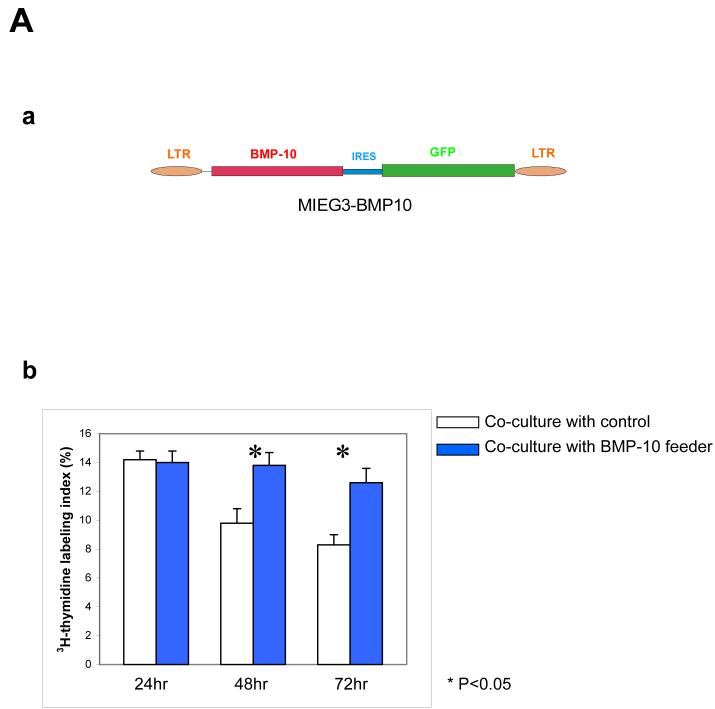
To further analyze the biological activity of BMP-10, we generated BMP-10 expressing NIH3T3 cells using a retroviral gene delivery system (Figure 7A-a). The expression of BMP-10 in these cells was confirmed by Northern analysis (data not shown). Using BMP-10 expressing cells as feeders, we established a co-culture system in which freshly isolated wild type embryonic cardiomyocytes were laid on top of mitomycin C pre-treated (non-mitotic) BMP-10 expressing cells or control cells. BMP-10 expressing feeder cells maintained a higher 3H-thymidine labeling index in embryonic cardiomyocytes after 48 hours of co-culture compared to control co-cultures (Figure 7A-b), indicating that BMP-10 has growth promoting activity for embryonic cardiomyocytes. This growth promoting activity of BMP-10 was also confirmed using BMP-10 conditioned media in both cell culture and organ culture (Figure 7B).
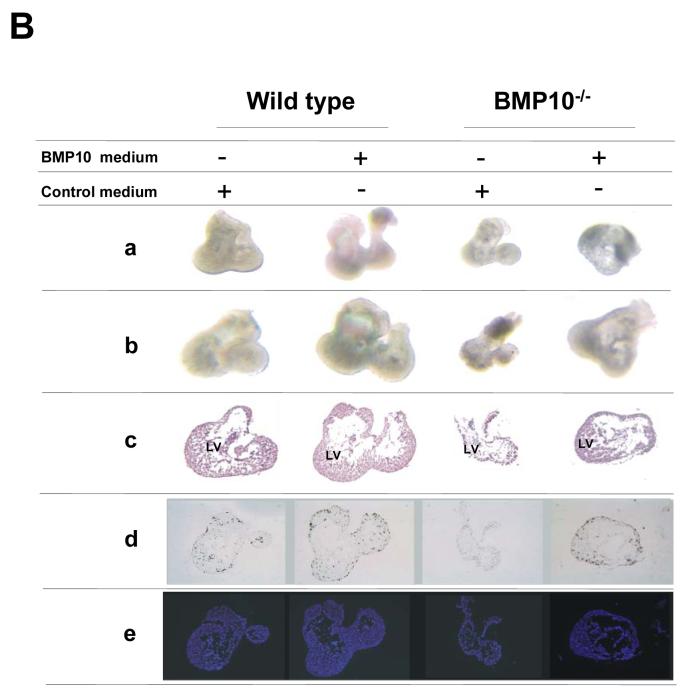
Figure 7.
The effect of BMP-10 to cultured cardiomyocytes and hearts.
(A) Generation of BMP-10 expressing NIH 3T3 cell lines and using cardiomyocyte co-culture assay to determine the biological activity of BMP-10. (a) Schematic diagram of BMP-10 retroviral vector used to express BMP-10. (b) Co-culture of embryonic cardiomyocytes with BMP-10 feeder cells (NIH3T3/BMP-10) and control feeder cells (NIH3T3/EGFP). The proliferative activity of cardiomyocytes in culture after 24, 48 and 72 hours were determined by using 3H-thymidine labeling index of PAS positive cells as described in Methods. BMP-10 feeders were able to maintain higher proliferative activity of cardiomyocytes compared to control feeder cells.
(B) In vitro culture of embryonic hearts in BMP-10 conditioned and control media. Embryonic hearts were isolated from E9.0-E9.25 embryos harvested from bmp10m1/+ matings. Each heart was photographed before (a) and after (b) 24 hours of culture. (c), haematoxylin and eosin stained histological sections of cultured hearts. (d) 3H-thymidine labeling was used to determine the proliferative activity of cultured hearts. Autoradiographs of 3H-thymidine labeled hearts. (e) Hoechst staining to show nuclei. The images of each column were from the same heart.
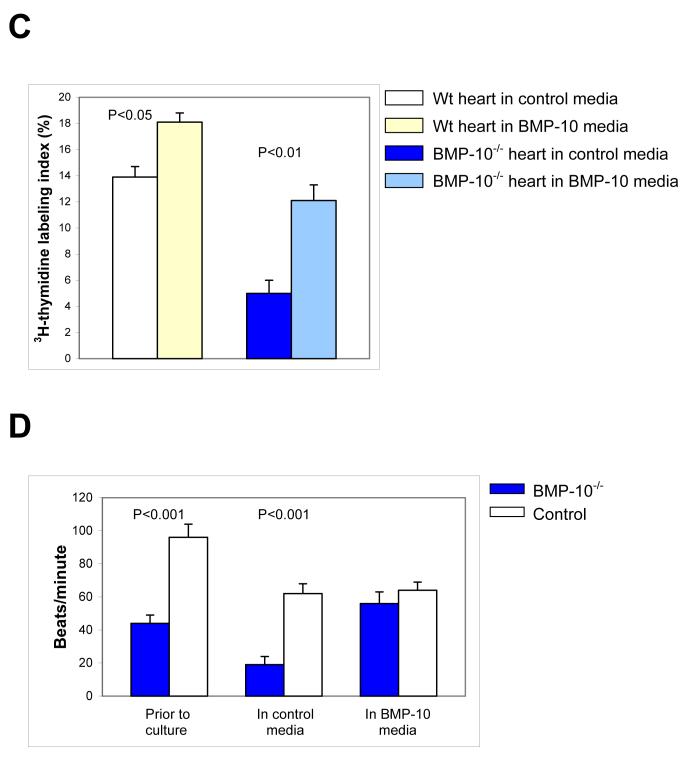
(C) 3H-thymidine labeling index of cultured embryonic hearts.
(D) Heart rates of BMP-10-deficient and control hearts prior to culture, and following culture in control and BMP-10 conditioned media. BMP-10 conditioned media is able to rescue the heart rates of BMP-10-deficient embryos.
When we applied BMP-10 conditioned media to isolated BMP-10-deficient hearts in culture, we found BMP-10 conditioned media rescued BMP-10-deficient hearts (Figure 7B-b). The rescued BMP-10-deficient hearts displayed significant improvements in cardiac size, 3H-thymidine labeling (Figure 7B-d and 7C), and cardiac performance as measured by the improved heart rate (Figure 7D). This experiment further delineated the essential role for BMP-10 in cardiac development.
Mutant mice deficient in neuregulin or its receptor ErbB developed severe defects in ventricular trabeculation (Meyer and Birchmeier, 1995, Gassmann et al., 1995, Lee et al., 1995). To determine if the strong effect of BMP-10 to myocardium was via neuregulin-ErbB mediated pathway, we cultured BMP-10-deficient hearts in the neuregulin (NRG-1) containing media (Rentschler et al., 2002). Unlike BMP-10 conditioned medium, NRG-1 medium was not able to rescue the growth deficiency phenotype of BMP-10-deficient heart (data not shown). This observation suggests that BMP-10 is either downstream of or irrelevant to the neuregulin-ErbB mediated pathway.
Importantly, when E9.5 mutant hearts were cultured in BMP-10 conditioned media, both Nkx2.5 (Figure 8A) and MEF2C (data not shown) expression was restored, and p57kip2 expression was significantly down-regulated in these rescued hearts (Figure 8B). This observation further indicated the close relationship between BMP-10 and cardiogenic transcriptional factors Nkx2.5 and MEF2C and cell cycle regulator p57kip2.
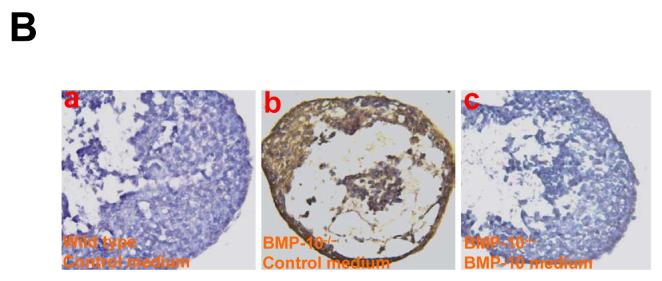
Figure 8.
BMP-10 conditioned medium restores normal genetic program in BMP-10-deficient hearts.
(A) Whole mount in situ hybridization of Nkx2.5 expression in cultured embryonic hearts. Embryonic hearts were isolated at E9.0 and cultured in BMP-10 conditioned media over night. Prior to the culture, the BMP-10-deficient heart (n=3) (a) had a significantly lower Nkx2.5 expression than the littermate control heart (3) (b). After culture, the BMP-10-deficient heart (n=4) (c) had restored Nkx2.5 expression. (d) Littermate control heart (n=3) cultured in BMP-10 conditioned medium.
(B) Immunohistological staining of p57kip2 expression in cultured embryonic hearts. Embryonic hearts were isolated at E9.0 and cultured in BMP-10 conditioned media or control medium over night. (a) wild type heart cultured in control medium (n=6). (b) BMP-10-deficient heart cultured in control medium (n=4). (c) BMP-10-deficient heart cultured in BMP-10 conditioned medium (n=4). The expression of p57kip2 in mutant hearts cultured in BMP-10 conditioned medium was significantly down-regulated when compared to the mutant hearts cultured in control medium.
In summary, our studies demonstrated that the presence of BMP-10 in the developing ventricle at E8.75-E9.0 is critical to maintain the proliferative activity of the embryonic cardiomyocytes by preventing premature activation of the negative cell cycle regulator p57kip2 and to maintain the required expression level of two very important cardiogenic factors, Nkx2.5and MEF2C, in the developing heart at midgestation (Figure 9).
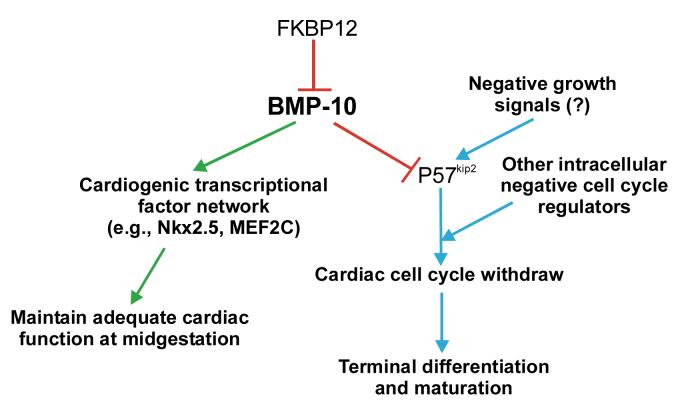
Figure 9.
A model for the modulation of cardiac growth and function by BMP-10 during midgestation. FKBP12 negatively regulates BMP-10 possibly via its interaction to type I receptor for BMP-10. BMP-10 has double biological activities: 1) prevents the premature activation and/or antagonizes the activity of negative cell cycle regulators such as p57kip2; 2) maintains cardiac function by regulating level of expression of several key cardiogenic transcriptional factors during midgestation.
DISCUSSION
The importance of BMP-10 to cardiac development is reflected by its temporal and spatial pattern of expression. It is known that cardiac cell cycle withdraw is associated with ventricular chamber maturation, myocyte terminal differentiation and lineage specification. The fact that trabecular myocytes have lower proliferative activity than that of compact wall suggests that trabecular myocytes initiate the process of terminal differentiation earlier (Rumyantsev, 1991; Icardo, 1984). In this regard, cardiac growth and chamber maturation has to be regulated by a temporally and spatially controlled process and a balance between proliferation and terminal differentiation. During trabeculation (E9.0 to E13.5 in mouse), trabecular myocardium is required to expand in order to generate sufficient number of myocytes that contribute to future ventricular septum, papillary muscles, and conductive system cells, while at same time, these cells are also required to reduce proliferative activity for terminal differentiation and lineage specification. Although it is still not clear about the identities of negative growth signals, p57kip2, a negative cell cycle regulator, is one of the intracellular candidates and is initially found in trabecular myocardium at E10.5 (Kochilas et al, 1999). Interestingly, cardiac defects have not been reported in p57kip2-deficient mice (Zhang et al, 1997; Yan et al., 1997), suggesting that there are other negative regulators, in a cooperative way with p57kip2, involved in the cardiac cell cycle withdraw and terminal differentiation. In fact, there is evidence that indicates that p57kip2 and other negative cell cycle regulators (e.g., p21cip1 and p27kip1) cooperate to regulate cell cycle exit and cell differentiation of skeletal muscle cells, lens fiber cells and placenta trophoblasts (Zhang et al., 1998; Zhang et al., 1999). Our data support the suggestion that BMP-10 provides a positive growth signal for cardiomyocytes that antagonizes negative regulators such as p57kip2. Perturbation of this balance would lead to either cardiac hyperplasia and hyper-trabeculation and noncompaction (e.g., elevation of BMP-10 expression in FKBP12-deficient heart) or the cardiac hypoplasia seen in BMP-10-deficient heart.
Another important finding in our studies is that BMP-10 is specifically required to maintain the expression level of cardiogenic transcription factors Nkx2.5 and MEF2C during midgestation. Both Nkx2.5 and MEF2C have been shown to be critical for cardiac patterning in the early phase of cardiogenesis (review see Harvey et al., 1999; Black and Olson, 1999). BMP-10-deficient hearts have normal cardiac patterning, which is consistent with the normal expression of Nkx2.5 and MEF2C before E9.0. However, the rapid down-regulation of Nkx2.5 and MEF2C in E9.5 mutant heart may help to explain the severely impaired cardiac function seen in BMP-10-deficient hearts, since many of the Nkx2.5 and MEF2C downstream gene products are crucial for cardiac function.
A related observation is that the down-regulation of Nkx2.5 and MEF2C is not restricted to ventricular chamber where BMP-10 is expressed. In fact, similar to other BMPs, BMP-10 is a secreted peptide that can function as an autocrine and paracrine growth and differentiation signals, which would lead to a broader effect during cardiac development. The lack of seeded mesenchymal cells in endocardial cushions in BMP-10-deficient hearts and the relative normal cushion structure in rescued hearts suggest that BMP-10 is able to contribute to the epithelial-mesenchymal transformation, a key step in endocardial cushion development. However, we cannot yet exclude the likelihood that the cushion defect in BMP-10 mutant heart is secondary to severely impaired cardiac growth in vivo.
In addition to the cardiac-specific BMP-10, other BMPs (e.g., BMP-2, -4, -5, -6, -7) and TGFβ family members and receptors (review see Schneider, 2003), which have much wider expression patterns during embryonic development, are also found in the developing heart. These BMPs appear to form an important network that controls several crucial morphogenetic events during cardiac development. Obviously, these BMPs are not able to compensate for the loss of BMP-10 in vivo. Likely, it is partially due to their differences in temporal and spatial pattern of expression and partially due to their differences in ligand-receptor specificity. During cardiac development, BMP2/4 is more restricted to the myocardium adjacent to the endocardial cushion region (Nakajima, 2000). BMPs 5, 6 and 7 have overlapping expression patterns and redundant functions in cardiac development (Solloway and Robertson, 1999; Kim, 2001). Mice deficient in both BMP-6 and BMP-7 develop outflow tract and valvo-septation anomalies (Kim, 2001), while mice deficient in both BMP-5 and BMP-7 have defects in cardiac looping and ventricular chamber formation (Solloway and Robertson, 1999). In our on-going rescue experiments using different BMPs (e.g., BMP-2, -4, -5 and —6, and TGFβ-1), we clearly observed the differences in their ability to rescue the BMP-10-deficient hearts in culture (data not shown). This finding suggests a complicated BMP-receptor network in the developing heart. It is consistent with recent data in cardiac conditional knockout of type I receptor for BMP (Alk3) (Gaussin, 2002). Cardiac myocyte-specific deletion of Alk3 leads to major defects in endocardial cushion and ventricular wall, but very little effect to cardiac trabeculation. BMP-10 expression is also normal in these mutant mice. Interestingly, Nkx2.5 and other cardiogenic transcription factors are not altered in Alk3 mutant hearts, suggesting that Alk3 is not the receptor mediating BMP-10 signaling.
Recently, analysis of Nkx2.5 promoter showed that Nkx2.5 expression in both early and late cardiac development is mediated by multiple conserved Smad binding sites (Liberatore et al., 2002; Lien et al., 2002). This finding further suggests that BMP signaling is required beyond the initial step of cardiogenic induction. Previously, BMP2/4 has been shown to mediate cardiac induction via activation of Nkx2.5 in chick embryos at early stage (Schultheiss et al., 1997; review see Srivastava and Olson, 2000). Our data support the observation from Nkx2.5 promoter analysis. BMP-10 is a strong candidate that is responsible for maintaining the expression of Nkx2.5 during midgestation possibly via a Smad-Nkx2.5 pathway.
Our studies have demonstrated an important genetic pathway that regulates ventricular trabeculation and chamber maturation. The regulation of BMP-10 expression may be an important mechanism for normal cardiac trabecular growth and compaction in mice. Genetic investigation of human patients with non-compaction myocardial defects may demonstrate a role for BMP-10 in this pathological condition as well.
Acknowledgement
We wish to thank Dr. Shaolian Jing and Mr. William Carter of Indiana University Mouse Core for their superb assistance in generating BMP-10 knockout mice, Dr. Loren Field for his generous support and valuable discussion, Dr. Michael Rubart-Von Der Lohe, Dr. Kishore Pasumarthi and Dr. Hisako Nakajima for their generous help in this study. This study was supported in part by National Institute of Health HL70259 (WS), HL63196 (MY), HLOK04071 (LH), HL60714 (SJC), Showalter Research Foundation (WS and LH), Muscular Dystrophy Association (WS), the Riley Children’s Foundation (WS, MY, LH).
References
- Andree B, Duprez D, Vorbusch B, Arnold HH, Brand T. BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech Dev. 1998;70:119–131. doi: 10.1016/s0925-4773(97)00186-x. [DOI] [PubMed] [Google Scholar]
- Bao S, Shen X, Shen K, Liu Y, Wang XF. The mammalian Rad24 homologous to yeast Saccharomyces cerevisiae Rad24 and Schizosaccharomyces pombe Rad17 is involved in DNA damage checkpoint. Cell Growth Differ. 1998;9:961–967. [PubMed] [Google Scholar]
- Barron M, Gao Min., Lough J. Requirement for BMP and FGF signaling during cardiogenic induction in non-precardiac mesoderm is specific, transient, and cooperative. Dev. Mech. 2000;218:383–393. doi: 10.1002/(SICI)1097-0177(200006)218:2<383::AID-DVDY11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Black BL, Olson EN. Control of cardiac development by the MEF2 family of transcription factors. In: Harvey RP, Rosenthal N, editors. Heart Development. Academia press; 1999. pp. 131–142. [Google Scholar]
- Chen F, Kook H, Milewski Rita., Gitler AD, Lu MM, Nazarian R, Schnepp R, Jen K, Biben C, Runke G, Mackay JP, Novotny J, Schwatz RJ, Harvey RP, Mullins MC, Epstein JA. Hop is an unusual homeobox gene that modulates cardiac development. Cell. 2002;110:713–723. doi: 10.1016/s0092-8674(02)00932-7. [DOI] [PubMed] [Google Scholar]
- Conway SJ, Henderson DJ, Copp AJ. Pax3 is required for cardiac neural crest migration in the mouse: evidence from the splotch (Sp2h) mutant. Development. 1997;124:505–514. doi: 10.1242/dev.124.2.505. [DOI] [PubMed] [Google Scholar]
- Ducy P, Karsenty G. The family of bone morphogenetic proteins. Kidney International. 2000;57:2207–2214. doi: 10.1046/j.1523-1755.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- Durocher D, Chen C-Y, Ardati A, Schwartz RJ, Nemer M. The atrial natriuretic factor promoter is a downstream target for Nkx-2.5 in the myocardium. Mol. Cell. Biol. 1996;16:4648–4655. doi: 10.1128/mcb.16.9.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek J, Ostadal B, Duskova M. Postnatal persistence of spongy myocardium with embryonic blood supply. Arch Pathol. 1975;99:312–317. [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Franco D, deBoer PA, deGier-de Vries C, Lamers WH, Moorman AF. Methods on in situ hybridization, immunohistochemistry and beta-galactosidase reporter gene detection. Eur J. Morphol. 2001;39:169–191. doi: 10.1076/ejom.39.3.169.4670. [DOI] [PubMed] [Google Scholar]
- Franco D, Icardo JM. Molecular characterization of the ventricular conduction system in the developing mouse heart: topographical correlation in normal and congenitally malformed hearts. Cardiovascular Res. 2001;49:417–429. doi: 10.1016/s0008-6363(00)00252-2. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Oriori A, Simon H, Lai C, Klein R, Lemek G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. PNAS. 2002;99:2878–2883. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneline LS, Li X, Ciccone SLM, Hong P, Yang Y, Lee S-H, Orazi A, Srour EF, Clapp DW. Retroviral-mediated expression of recombinant Fancc enhances the repopulating ability of Fancc -/- hematopoietic stem cells and decreases the risk of clonal evolution. Blood. 2003;101:1299–1307. doi: 10.1182/blood-2002-08-2404. [DOI] [PubMed] [Google Scholar]
- Harvey RP, Biben C, Elliott DA. Transcriptional control and pattern formation in the developing vertebrate heart: studies on NK-2 class homeodomain factors. In: Harvey RP, Rosenthal N, editors. Heart Development. Academia press; 1999. pp. 111–129. [Google Scholar]
- Icardo JM. The growing heart: An anatomical perspective. In: Zak R, editor. Growth of the heart in health and disease. Raven Press; 1984. pp. 41–79. [Google Scholar]
- Ichida F, Tsubata S, Bowles KR, Haneda N, Uese K, Miyawaki T, Dreyer WJ, Messina J, Li H, Bowles NE, Towbin JA. Novel gene mutations in patients with left ventricular noncompaction or Bath Syndrome. Circ. 2001;103:1256–1263. doi: 10.1161/01.cir.103.9.1256. [DOI] [PubMed] [Google Scholar]
- Kim RY, Robertson EJ, Solloway MJ. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol. 2001;235:449–466. doi: 10.1006/dbio.2001.0284. [DOI] [PubMed] [Google Scholar]
- Kochilas LK, Li J, Jin F, Buck CA, Epstein JA. p57kip2 expression is enhanced during mid-cardiac murine development and is restricted to trabecular myocardium. Pediatric Res. 1999;45:635–642. doi: 10.1203/00006450-199905010-00004. [DOI] [PubMed] [Google Scholar]
- Lee KF, Simon H, Chen H, Bates B, Hung MC, Hauser C. Requirement for neuregulin receptor ErbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- Liberatore CM, Searcy-Schrick RD, Vincent EB, Yutzey KE. Nkx-2.5 gene induction in mice is mediated by a Smad concensus regulatory region. Dev Biol. 2002;244:243–256. doi: 10.1006/dbio.2002.0604. [DOI] [PubMed] [Google Scholar]
- Lien C-L, McAnally J, Richardson JA, Olson EN. Cardiac-specific activity of an NKX2-5 enhancer requires an evolutionarily conserved Smad binding site. Dev Biol. 2002;244:257–266. doi: 10.1006/dbio.2002.0603. [DOI] [PubMed] [Google Scholar]
- Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Massague J, Chen YG. Controlling TGF-beta signaling. Genes & Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–46. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Moorman AFM, Lamers WH. Development of the conduction system of the vertebrate heart. In: Harvey RP, Rosenthal N, editors. Heart Development. Academia press; 1999. pp. 195–207. [Google Scholar]
- Nakayama T, Cui Y, Christian JL. Regulation of BMP/Dpp signaling during embryonic development. Cell. Mol. Life Sci. 2000;57:943–956. doi: 10.1007/PL00000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP) Anat Rec. 2000;258:119–127. doi: 10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Neuhaus H, Rosen V, Thies RS. Heart specific expression of mouse BMP-10 a novel member of the TGF-β superfamily. Mech. Dev. 1999;80:181–184. doi: 10.1016/s0925-4773(98)00221-4. [DOI] [PubMed] [Google Scholar]
- Palmer S, Groves N, Schindeler A, Yeoh T, Biben C, Wang C-C, Sparrow DB, Barnett L, Jemkins NA, Copeland NG, Koentgen F, Mohum T, Harvey RP. The small muscle-specific protein Csl modifies cell shape and promotes myocyte fusion in an insulin-like growth factor 1-dependent manner. J. Cell Biol. 2001;153:985–997. doi: 10.1083/jcb.153.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentschler S, Zander J, Meyers K, France D, Levine R, Porter G, Rivkees SA, Morley GE, Fishman GI. Neuregulin-1 promotes formation of the murine cardiac conduction system. PNAS. 2002;99:10464–10469. doi: 10.1073/pnas.162301699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumyantsev PP. Growth and hyperplasia of cardiac muscle cells. In: Carlson BM, editor. Soviet Medical Reviews Supplement series. Harwood Acad. Publishers; 1991. [Google Scholar]
- Schneider MD, Gaussin V, Lyon KM. BMP signals for cardiac morphogenesis. Cytokine & growth factor. 2003;14:1–4. doi: 10.1016/s1359-6101(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Schott JJ, Benson DW, Basson CT, Pease W, Silberbach GM, Moak JP, Maron BJ, Seidman CE, Seidman JG. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998;281:108–111. doi: 10.1126/science.281.5373.108. [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, Burch JB, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11:451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- Shin CH, Liu Z-P, Passier R, Zhang C-L, Wang D-Z, Harris TM, Yamagishi H, Richardson JA, Childs G, Olson EN. Modulation of cardiac growth and development by HOP, an unusual homeodomain protein. Cell. 2002;110:725–735. doi: 10.1016/s0092-8674(02)00933-9. [DOI] [PubMed] [Google Scholar]
- Shou W, Aghdasi B, Armstrong DL, Guo Q, Bao S, Charng M-J, Mathews LS, Schneider MD, Hamilton SL, Matzuk MM. Cardiac defects and altered ryanodine receptor function in mice lacking FKBP12. Nature. 1998;391:489–492. doi: 10.1038/35146. [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Robertson EJ. Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development. 1999;126:1753–1768. doi: 10.1242/dev.126.8.1753. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature. 2000;407:221–226. doi: 10.1038/35025190. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–80. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Wang T, Donahoe PK, Zervos AS. Specific interaction of type 1 receptor of the TGF-β family with the immunophilin FKBP-12. Science. 1994;265:674–676. doi: 10.1126/science.7518616. [DOI] [PubMed] [Google Scholar]
- Wang T, Li B-Y, Danielson PD, Shah PC, Rockwell S, Lechleider RJ, Martin J, Manganaro T, Donahoe PK. The immunophilin FKBP12 functions as a common inhibitor of the TGFβ family type 1 receptors. Cell. 1996;86:435–444. doi: 10.1016/s0092-8674(00)80116-6. [DOI] [PubMed] [Google Scholar]
- Winnier GE, Kume T, Deng K, Rogers R, Bundy J, Raines C, Walter MA, Hogan BL, Conway S. Roles for the winged helix transcription factors MF1 and MFH1 in cardiovascular development revealed by nonalletic noncomplementation of null alleles. Dev. Biol. 1999;213:418–431. doi: 10.1006/dbio.1999.9382. [DOI] [PubMed] [Google Scholar]
- Yan Y, Frisen J, Lee MH, Massague J, Barbacid M. Ablation of the CDK inhibitor p57kip2 results in increased apoptosis and delayed differentiation during development. Genes Dev. 1997;11:973–983. doi: 10.1101/gad.11.8.973. [DOI] [PubMed] [Google Scholar]
- Zhang P, Liegeois NJ, Wong C, Finegold M, Hou H, Thompson JC, Silverman A, Harper JW, DePinho RA, Elledge SJ. altered cell differentiation and proliferation in mice lacking p57kip2 indicates a role in Beckwith-Wiedemann syndrome. Nature. 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]
- Zhang P, Wong C, DePinho RA, Harper JW, Elledge SJ. Cooperation between the Cdk inhibitors p21kip1 and p57kip2 in the control of tissue growth and development. Genes Dev. 1998;12:3162–3167. doi: 10.1101/gad.12.20.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Wong C, Liu D, Finegold M, Harper JW, Elledge SJ. p21cip1 and p57kip2 control muscle differentiation at the myogenin step. Genes Dev. 1999;13:213–224. doi: 10.1101/gad.13.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]