Abstract
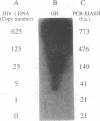
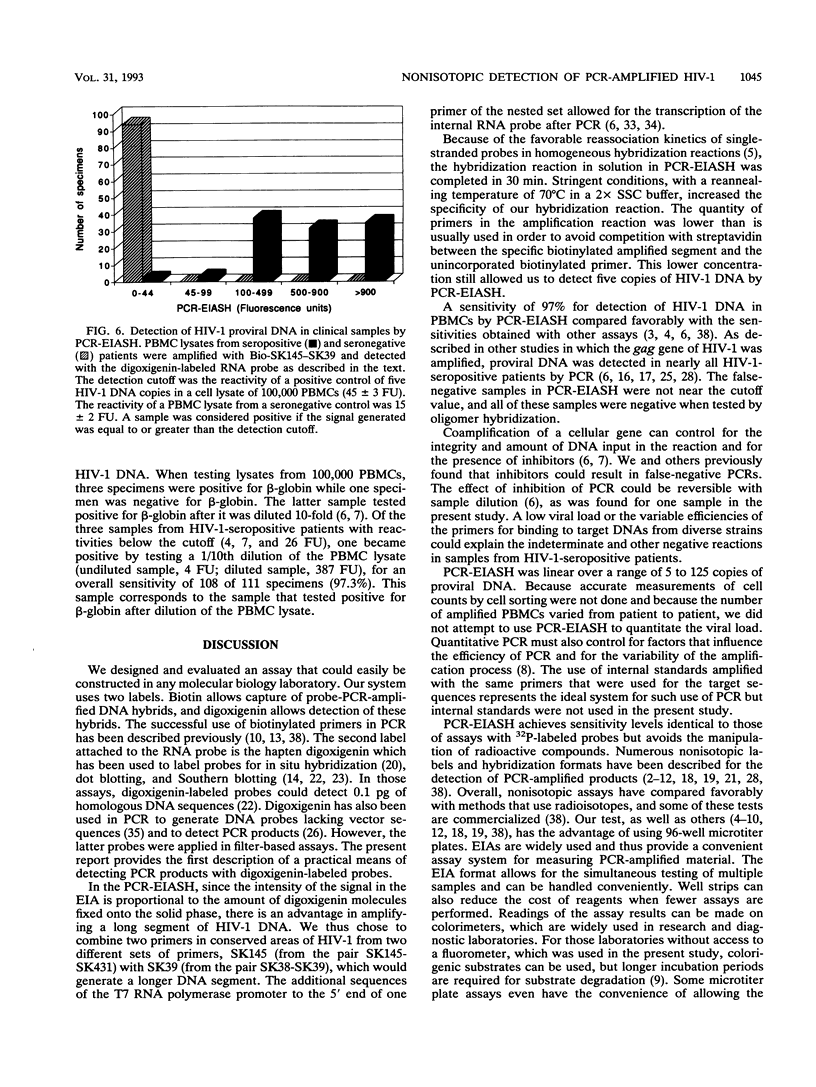
An enzyme-linked immunoassay (EIA) combined with a solution hybridization (SH) reaction was devised to detect human immunodeficiency virus type 1 (HIV-1) provirus amplified by the polymerase chain reaction (PCR). In this nonisotopic PCR assay, designated PCR-EIASH, a fragment of the HIV-1 gag gene from peripheral blood mononuclear cells (PBMCs) was first amplified with biotinylated primers. The biotinylated amplified DNA segment was reacted in solution with an internal RNA probe labeled with digoxigenin-11-UTP. Hybrids were captured in a microtiter plate coated with streptavidin. Specific bound hybrids were quantitated by the addition of an enzyme-labeled antibody against digoxigenin and a fluorogenic substrate. The hybridization, immunological, and amplification parameters of PCR-EIASH were optimized as follows: 12.5 pmol of each primer was used in the PCR; the reannealing reaction of amplified products with the RNA probe, which was used at 0.30 microgram/ml, was completed in 30 min at 70 degrees C in 2x SSC (1x SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Five copies of HIV-1 DNA diluted in a lysate of 100,000 PBMCs from a seronegative control could be detected by PCR-EIASH with a signal of 41 +/- 3 fluorescent units above a background noise of 13 +/- 2 fluorescent units. A total of 91 PBMC lysates from 91 seropositive patients sampled once and 20 PBMC lysates from 10 seropositive patients sampled twice were tested in duplicate in the PCR-EIASH; 107 samples were positive in duplicate tests, 1 sample was indeterminate, and 3 samples were negative. Of the latter three samples, one became positive by diluting the cell lysate, suggesting the presence of an inhibitor of Taq polymerase. The three samples negative for HIV-1 by PCR-EIASH were also negative when amplified with SK145-SK39 and detected with 32P-labeled SK102.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott M. A., Poiesz B. J., Byrne B. C., Kwok S., Sninsky J. J., Ehrlich G. D. Enzymatic gene amplification: qualitative and quantitative methods for detecting proviral DNA amplified in vitro. J Infect Dis. 1988 Dec;158(6):1158–1169. doi: 10.1093/infdis/158.6.1158. [DOI] [PubMed] [Google Scholar]
- Brandt C. D., Rakusan T. A., Sison A. V., Josephs S. H., Saxena E. S., Herzog K. D., Parrott R. H., Sever J. L. Detection of human immunodeficiency virus type 1 infection in young pediatric patients by using polymerase chain reaction and biotinylated probes. J Clin Microbiol. 1992 Jan;30(1):36–40. doi: 10.1128/jcm.30.1.36-40.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway B., Adler K. E., Bechtel L. J., Kaplan J. C., Hirsch M. S. Detection of HIV-1 DNA in crude cell lysates of peripheral blood mononuclear cells by the polymerase chain reaction and nonradioactive oligonucleotide probes. J Acquir Immune Defic Syndr. 1990;3(11):1059–1064. [PubMed] [Google Scholar]
- Conway B., Bechtel L. J., Adler K. A., D'Aquila R. T., Kaplan J. C., Hirsch M. S. Comparison of spot-blot and microtitre plate methods for the detection of HIV-1 PCR products. Mol Cell Probes. 1992 Jun;6(3):245–249. doi: 10.1016/0890-8508(92)90023-q. [DOI] [PubMed] [Google Scholar]
- Coutlee F., Viscidi R. P., Yolken R. H. Comparison of colorimetric, fluorescent, and enzymatic amplification substrate systems in an enzyme immunoassay for detection of DNA-RNA hybrids. J Clin Microbiol. 1989 May;27(5):1002–1007. doi: 10.1128/jcm.27.5.1002-1007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutlée F., Bobo L., Mayur K., Yolken R. H., Viscidi R. P. Immunodetection of DNA with biotinylated RNA probes: a study of reactivity of a monoclonal antibody to DNA-RNA hybrids. Anal Biochem. 1989 Aug 15;181(1):96–105. doi: 10.1016/0003-2697(89)90399-0. [DOI] [PubMed] [Google Scholar]
- Coutlée F., Saint-Antoine P., Olivier C., Vessous-Elbaz A., Voyer H., Berrada F., Bégin P., Giroux L., Viscidi R. Discordance between primer pairs in the polymerase chain reaction for detection of human immunodeficiency virus type 1: a role for taq polymerase inhibitors. J Infect Dis. 1991 Oct;164(4):817–818. doi: 10.1093/infdis/164.4.817. [DOI] [PubMed] [Google Scholar]
- Coutlée F., Saint-Antoine P., Olivier C., Voyer H., Kessous-Elbaz A., Berrada F., Bégin P., Giroux L., Viscidi R. Evaluation of infection with human immunodeficiency virus type 1 by using nonisotopic solution hybridization for detection of polymerase chain reaction-amplified proviral DNA. J Clin Microbiol. 1991 Nov;29(11):2461–2467. doi: 10.1128/jcm.29.11.2461-2467.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutlée F., Viscidi R. P., Saint-Antoine P., Kessous A., Yolken R. H. The polymerase chain reaction: a new tool for the understanding and diagnosis of HIV-1 infection at the molecular level. Mol Cell Probes. 1991 Aug;5(4):241–259. doi: 10.1016/0890-8508(91)90046-m. [DOI] [PubMed] [Google Scholar]
- Dahlén P. O., Iitiä A. J., Skagius G., Frostell A., Nunn M. F., Kwiatkowski M. Detection of human immunodeficiency virus type 1 by using the polymerase chain reaction and a time-resolved fluorescence-based hybridization assay. J Clin Microbiol. 1991 Apr;29(4):798–804. doi: 10.1128/jcm.29.4.798-804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I. The polymerase chain reaction. A new method of using molecular genetics for medical diagnosis. N Engl J Med. 1990 Jan 18;322(3):178–183. doi: 10.1056/NEJM199001183220307. [DOI] [PubMed] [Google Scholar]
- Holodniy M., Katzenstein D. A., Sengupta S., Wang A. M., Casipit C., Schwartz D. H., Konrad M., Groves E., Merigan T. C. Detection and quantification of human immunodeficiency virus RNA in patient serum by use of the polymerase chain reaction. J Infect Dis. 1991 Apr;163(4):862–866. doi: 10.1093/infdis/163.4.862. [DOI] [PubMed] [Google Scholar]
- Höltke H. J., Kessler C. Non-radioactive labeling of RNA transcripts in vitro with the hapten digoxigenin (DIG); hybridization and ELISA-based detection. Nucleic Acids Res. 1990 Oct 11;18(19):5843–5851. doi: 10.1093/nar/18.19.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltke H. J., Seibl R., Burg J., Mühlegger K., Kessler C. Non-radioactive labeling and detection of nucleic acids. II. Optimization of the digoxigenin system. Biol Chem Hoppe Seyler. 1990 Oct;371(10):929–938. doi: 10.1515/bchm3.1990.371.2.929. [DOI] [PubMed] [Google Scholar]
- Jackson J. B., Kwok S. Y., Sninsky J. J., Hopsicker J. S., Sannerud K. J., Rhame F. S., Henry K., Simpson M., Balfour H. H., Jr Human immunodeficiency virus type 1 detected in all seropositive symptomatic and asymptomatic individuals. J Clin Microbiol. 1990 Jan;28(1):16–19. doi: 10.1128/jcm.28.1.16-19.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jason J., Ou C. Y., Moore J. L., Lawrence D. N., Schochetman G., Evatt B. L. Prevalence of human immunodeficiency virus type 1 DNA in hemophilic men and their sex partners. Hemophilia-AIDS Collaborative Study Group. J Infect Dis. 1989 Nov;160(5):789–794. doi: 10.1093/infdis/160.5.789. [DOI] [PubMed] [Google Scholar]
- Keller G. H., Huang D. P., Manak M. M. A sensitive nonisotopic hybridization assay for HIV-1 DNA. Anal Biochem. 1989 Feb 15;177(1):27–32. doi: 10.1016/0003-2697(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Keller G. H., Huang D. P., Manak M. M. Detection of human immunodeficiency virus type 1 DNA by polymerase chain reaction amplification and capture hybridization in microtiter wells. J Clin Microbiol. 1991 Mar;29(3):638–641. doi: 10.1128/jcm.29.3.638-641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp D. J., Smith D. B., Foote S. J., Samaras N., Peterson M. G. Colorimetric detection of specific DNA segments amplified by polymerase chain reactions. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2423–2427. doi: 10.1073/pnas.86.7.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler C., Höltke H. J., Seibl R., Burg J., Mühlegger K. Non-radioactive labeling and detection of nucleic acids. I. A novel DNA labeling and detection system based on digoxigenin: anti-digoxigenin ELISA principle (digoxigenin system). Biol Chem Hoppe Seyler. 1990 Oct;371(10):917–927. doi: 10.1515/bchm3.1990.371.2.917. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Lifson A. R., Stanley M., Pane J., O'Malley P. M., Wilber J. C., Stanley A., Jeffery B., Rutherford G. W., Sohmer P. R. Detection of human immunodeficiency virus DNA using the polymerase chain reaction in a well-characterized group of homosexual and bisexual men. J Infect Dis. 1990 Mar;161(3):436–439. doi: 10.1093/infdis/161.3.436. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Tomita N., Ohtsuki Y., Kitajima K. Detection of provirus in an HTLV-II producer CD8+ T cell line by polymerase chain reaction combined with digoxigenin-ELISA method. Jpn J Cancer Res. 1990 Apr;81(4):313–316. doi: 10.1111/j.1349-7006.1990.tb02567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Ou C. Y., McDonough S. H., Cabanas D., Ryder T. B., Harper M., Moore J., Schochetman G. Rapid and quantitative detection of enzymatically amplified HIV-1 DNA using chemiluminescent oligonucleotide probes. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1323–1329. doi: 10.1089/aid.1990.6.1323. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schnittman S. M., Greenhouse J. J., Psallidopoulos M. C., Baseler M., Salzman N. P., Fauci A. S., Lane H. C. Increasing viral burden in CD4+ T cells from patients with human immunodeficiency virus (HIV) infection reflects rapidly progressive immunosuppression and clinical disease. Ann Intern Med. 1990 Sep 15;113(6):438–443. doi: 10.7326/0003-4819-113-6-438. [DOI] [PubMed] [Google Scholar]
- Schowalter D. B., Sommer S. S. The generation of radiolabeled DNA and RNA probes with polymerase chain reaction. Anal Biochem. 1989 Feb 15;177(1):90–94. doi: 10.1016/0003-2697(89)90019-5. [DOI] [PubMed] [Google Scholar]
- Stoflet E. S., Koeberl D. D., Sarkar G., Sommer S. S. Genomic amplification with transcript sequencing. Science. 1988 Jan 29;239(4839):491–494. doi: 10.1126/science.3340835. [DOI] [PubMed] [Google Scholar]
- Stürzl M., Oskoui K. B., Roth W. K. 'Run-off' polymerization with digoxigenin labelled nucleotides creates highly sensitive and strand specific DNA hybridization probes: synthesis and application. Mol Cell Probes. 1992 Apr;6(2):107–114. doi: 10.1016/0890-8508(92)90054-2. [DOI] [PubMed] [Google Scholar]
- Viscidi R. P., Yolken R. G. Molecular diagnosis of infectious diseases by nucleic acid hybridization. Mol Cell Probes. 1987 Mar;1(1):3–14. doi: 10.1016/0890-8508(87)90003-x. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Sonigo P., Danos O., Cole S., Alizon M. Nucleotide sequence of the AIDS virus, LAV. Cell. 1985 Jan;40(1):9–17. doi: 10.1016/0092-8674(85)90303-4. [DOI] [PubMed] [Google Scholar]
- Whetsell A. J., Drew J. B., Milman G., Hoff R., Dragon E. A., Adler K., Hui J., Otto P., Gupta P., Farzadegan H. Comparison of three nonradioisotopic polymerase chain reaction-based methods for detection of human immunodeficiency virus type 1. J Clin Microbiol. 1992 Apr;30(4):845–853. doi: 10.1128/jcm.30.4.845-853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]