Abstract
Objective
Human multipotent stromal cells (MSCs) readily form single-cell derived colonies when plated at clonal densities. However, the colonies are heterogeneous since the cells from a colony form new colonies that vary in size and differentiation potential when re-plated at clonal densities. The experiments here tested the hypothesis that the cells in the inner regions of colonies are partially differentiated but the differentiation is reversible.
Materials and Methods
Cells were separately isolated from the dense inner regions (IN) and less dense outer regions (OUT) of single-cell derived colonies. The cells were then compared by assays of their transcriptomes and proteins, and for clonogenicity and differentiation.
Results
The IN cells expressed fewer cell-cycle genes and higher levels of genes for extracellular matrix than the OUT cells. When transferred to differentiation medium, differentiation of the colonies occurred primarily in the IN regions. However, the IN cells were indistinguishable from OUT cells when re-plated at clonal densities and assayed for rates of propagation and clonogenicity. Also, the colonies formed by IN cells were similar to colonies formed by OUT cells in that they had distinct IN and OUT regions. Cultures of IN and OUT cells remained indistinguishable through multiple passages (30-75 population doublings), and both cells formed colonies that were looser and less dense as they were expanded.
Conclusions
The results demonstrated that the cells in the inner region of single-derived colonies are partially differentiated but the differentiation can be reversed by re-plating the cells at clonal densities.
Keywords: Adult bone marrow stem cells, Mesenchymal stem cells (MSCs), Real-time RT-PCR, Clonal assays, Colony formation, Marrow stromal cells, Microarray, Gene expression
Introduction
There is currently interest in both the biology and the potential therapeutic uses of the adult stem/progenitor cells from bone marrow referred to in initial reports as fibroblastic colony forming units, then in the hematological literature as marrow stromal cells subsequently as mesenchymal stem cells, and most recently as multipotent mesenchymal stromal cells or MSCs [1]. Although there is general agreement that the cells can be isolated as the plastic adherent cells from human marrow, there is no consensus as to how the cells should be expanded in culture. In particular, there has been no consensus as to whether cells in culture are homogeneous [2-4].
Human MSCs (hMSCs) that are expanded as confluent cultures appear homogeneous [3;5] and several antibodies have been developed to surface epitopes on confluent cultures of MSCs [6-15]. In contrast to the studies on confluent cultures of hMSCs, a number of reports emphasized the heterogeneity of earlier stage cultures. Early investigators observed that the first cells that appear in low density cultures are spindle shaped and they are replaced by much larger and flat cells as the cultures mature [16]. The evidence for heterogeneity of MSCs was subsequently emphasized by expansion of the cells at low density. When adherent cells initially isolated by plating mononuclear cells from bone marrow were re-plated at low densities of about 1 to 100 cells per cm2, the cells underwent dramatic changes in both morphology, rates of proliferation, and patterns of gene expression as they expand in culture [17]. The MSCs from low density cultures were readily cloned as single-cell-derived colonies [2;4;5;18-20], and up to 90 % of the MSCs generated colonies when tested by a single-cell CFU-F assay [21]. Some of the single-cell-derived colonies differentiated into osteoblasts, adipocytes, or chondrocytes in culture, but some of the colonies were less multipotential. The low density cultures contained both small, rapidly self-renewing cells (RS-MSCs) and larger, more slowly replicating cells (SR-MSCs). Cultures enriched for RS-MSCs cells had a greater potential to differentiate than cultures of the large, mature cells. Also, RS-MSCs engrafted more efficiently in immunodeficient mice [22]. The confluent cultures of the large, mature cells continued to secrete a number of growth factors, an observation consistent with their ability to serve as feeder layers for hematopoietic stem/progenitor cells. Remarkably, if the cultures were harvested before they reach confluency and replated at low density, the sequence of events repeated itself in that the cells initially were RS-MSCs and these gradually were replaced by SR-MSCs. The sequence repeated itself through several passages until the cells approach senescence.
Here we report that single-cell derived colonies are heterogeneous in that the cells in the inner regions (IN) differed from cells in the outer regions (OUT) in terms of morphology and their commitment to differentiation. However, the differences disappear if the cells are re-plated at clonal densities.
Materials and Methods
Human MSC isolation and expansion
Human MSCs were obtained from the Tulane Center for the Preparation and Distribution of Adult Stem Cells (http://www.som.tulane.edu/gene_therapy/distribute.shtml). The human MSCs were isolated and expanded as described previously [23]. The preparations were standardized in that they demonstrated tri-lineage differentiation in culture, were negative for hematopoietic markers (less than 1% for CD-34, -36, -45 and -117), and positive for several general markers for MSCs (over 95% for CD-29, -44, -49c, -59 -90, -105, -147, and -166). For experiments here, a frozen vial of MSCs (donor 5064, 7012 or 240) was thawed and the cells were plated in a 145 cm2 culture dish (Nunc) in complete culture medium (CCM): α-minimal essential medium (αMEM, GIBCO/BRL, Carlsbad, CA), 17% fetal bovine serum (FBS) lot selected for rapid growth of MSCs (Atlanta Biologicals, Norcross, GA), 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mM L-glutamine (GIBCO/BRL). After 24 hour incubation at 37°C and 5% CO2, the medium was removed, the cells were washed with PBS, and adherent viable cells were harvested with 0.25% trypsin and 1 mM EDTA for 5 min at 37°C as recovered passage 1 cells.
Time-lapse microscopy
Recovered passage 1 MSCs (donor 5064) were plated onto 57 cm2 dishes (Nunc) at 0.5 cells/cm2 and incubated at 37°C and 5% CO2 without medium change. After 2 hours, adherent single cells on a plate were marked with a scratch on the bottom of the plate under light microscopy. Pictures of partially overlapping fields of view around initial cell location were taken every 24 hours for 11 days by phase contrast (Nikon digital camera DXM 1200F; Kawasaki, Japan, attached to Nikon Eclipse microscope TE200; Japan). Multiple images were combined into one image using Adobe Photoshop 6.0.1. (Adobe Systems Inc., San Jose, CA)
Laser capture microdissection of inner and outer regions of colonies
Recovered passage 1 MSCs (donor 5064) were plated at 2 cells/cm2 on laser capture microdissection and pressure catapulting (LMPC) slides (PALM MembraneSlides NF, P.A.L.M. Microlaser Technologies GmbH; Bernried, Germany) that were placed into 57 cm2 plastic dishes (Nunc). Cells were cultured for 10 to 12 days in CCM at 37°C and 5% CO2 without medium change until dense colonies that were at least 4 mm in diameter were observed. The slides were washed with PBS, fixed with ice-cold 100% ethanol and dried. Colonies with distinct OUT and IN regions were outlined and laser microdissected (P.A.L.M. MicroBeam System with UV laser, P.A.L.M. RoboMover, driven by Palmwin software 2.2 and Zeiss Axiovert S-100 microscope; Carl Zeiss MicroImaging GmbH, Oberkochen, Germany). Dissected OUT or IN cells from two colonies were catapulted separately into one cap, filled with 20 μl of extraction buffer (PicoPure™ RNA Isolation Kit; Molecular devices, Sunnyvale, CA). The cells were spun down at 800 × g for 2 minutes, mixed by pipetting, and incubated at 42°C for 30 minutes before cooling to 4°C and extracting total RNA.
Isolation of live cells from OUT or IN regions of colonies
Recovered passage 1 MSCs (donors 5064, 7012, and 240) were plated onto 57 cm2 plastic dishes (Nunc) at 2 cells/cm2 and incubated at 37°C and 5% CO2 without medium change for 10 to 12 days. Colonies that were selected for the isolation were similar in size (at least 4 mm in diameter), round morphologies, distinct dense IN and less dense OUT regions with readily distinguishable cell phenotypes in each region. The cell phenotypes of the OUT regions were thin, spindle-shaped cells rarely contacting with each other; while the major population of the IN region composed of bigger cells, that were in contact with each other but not overlapping and that formed linear streams of cells, oriented towards OUT region.
Before isolation, 5 to 12 OUT regions or IN regions were outlined with marker. To isolate the OUT region of the colony, the central region was destroyed with pipette tip. The cells from the IN region of the colony were isolated after scraping the OUT region with a cell scraper (Corning, Lowell, MA). The dishes were washed with PBS, and cloning cylinders (Bel-Art Products, Pequannock, NJ) with grease (Dow Corning 7 Release Compound, Dow Corning, MI) were placed over the outlined region (either IN or OUT of the colony). Cells in the inside of the cloning cylinder were harvested with trypsin/EDTA. From 8 to 12 colonies from one or more plates were pooled for OUT region sample, while 5 to 8 were pooled for IN region sample. Samples were centrifuged at 1000g for 6 minutes and washed with PBS followed by centrifugation. Cells were either used for RNA isolation or used for growth curve, CFU-F, or subcloning assays.
RNA isolation and quality
IN and OUT cells were isolated from colonies of MSCs from donor 5064 by LMPC and from donors 7012 and 240 by live cell isolation method. Total RNA was extracted (PicoPure™ RNA Isolation Kit) with DNase treatment (RNase-Free DNase Set, Qiagen, Germantown, MD). The amount and quality of isolated total RNA was measured either by spectrophotometric assay (SmartSpec 3000, Bio-Rad, Hercules, CA) or capillary electrophoresis (Agilent 2100 Bioanalyzer; Agilent Technologies, Waldbronn, Germany) using RNA 6000 Pico kit (Agilent) and RNA 6000 Ladder (Ambion, Austin, TX). The 18S and 28S ribosomal RNA peaks were identified on the electrophorogram, and RNA Integrity Numbers (RINs) were calculated using the Bioanalyzer software. Six samples, three from the IN region (1IN, 2IN, and 5IN) of colonies and three from the OUT region (2OUT, 4OUT, and 5OUT) of colonies (donor 5064), with at least 20 ng of total RNA and RIN of at least 8.5, were chosen for microarrays.
Microarray assays
Ten nanograms of total RNA from each sample (3 IN and 3 OUT samples) were used in the amplification and labeling steps according to manufacturer's instructions (Ovation™ Biotin RNA Amplification and Labeling System, NuGEN Technologies, San Carlos, CA). The amount of biotin labelled cDNA was assayed by spectrophotometer (SmartSpec 3000).
Biotin labeled cDNA (1.6 to 2.2 μg) from the six separate samples were hybridized on the microarrays (HG-U133 Plus 2.0; Affymetrix, Santa Clara, CA) and processed as previously [23]. The scanned images were transferred to the dChip program [24-26]. Arrays were normalized using the invariant set normalization method and model based expression values were calculated using the signal from perfect match and mismatch probes. Negative values were assigned a value of one. Genes were considered significantly different in expression between IN and OUT samples if they (a) had at least a 2-fold difference (with 90% confidence), (b) were present in all the samples where the gene was up-regulated, and (c) had a t-test p-value of 0.05 or lower between groups (IN vs. OUT). The 199 transcripts (155 non-redundant genes based on Gene ID) that were significantly different were used in hierarchical clustering of the samples and genes [27;28]. The resulting two patterns of expression from the IN and OUT regions were examined for Gene Ontology (GO) term enrichment [29]. Next, p-values were calculated for each GO term based on the exact hyper-geometric distribution, to compare the frequencies of GO terms within each pattern to their frequencies across the entire microarray (p-values < 0.01 were considered significant). Microarray data will be available at the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/).
Real-time RT-PCR
Total RNA (40 ng) samples from IN and OUT regions of colonies from the three donors were converted into cDNA (High-Capacity cDNA Reverse Transcription Kit; Applied Biosystems, Foster City, CA). A custom designed card (TaqMan® Low Density Array; Applied Biosystems) was used to perform real-time RT-PCR [23]. Sample preparation and analysis was performed as described previously [23].
Immunocytochemistry
Recovered passage 1 MSCs (donor 5064) were plated onto 57 cm2 plastic dishes or 6 well plates (Nunc) at 2 cells/cm2 and incubated at 37°C and 5% CO2 without medium change. On day 12, cultures were washed with PBS, fixed in ice-cold 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) for 15 minutes, and washed again.
For labeling with MKI67 (antigen identified by monoclonal antibody Ki-67), the cells were permeabilized with 0.5% Triton X-100 (Sigma, Saint Louis, MO) in PBS for 15 minutes at room temperature. The remaining samples were not permeabilized. The cells were washed and blocked with 3% bovine serum albumin (BSA) (Albumin bovine fraction V; Sigma) in PBS for 1 hour at room temperature. After blocking step, areas of interest were outlined, sealed with aqua-hold barrier pen (Scientific Device Laboratory, Des Plaines, IL), and incubated with 400 μl of primary antibody in PBS and 1% BSA for 20 hours at 4°C in a humidified chamber. The buffer for anti-MKI67 also contained 0.025% Trition X-100. The primary antibodies were: 10 μg/ml mouse monoclonal anti-human-KI67 (IgG1 clone Ki-S5; Chemicon International, Inc., Temecula, CA); 2 μg/ml mouse monoclonal anti-human-VCAM1 [IgG1 clone BBIG-V1(4B2); R&D Systems, Minneapolis, MN]; and 4 μg/ml goat polyclonal antihuman–PODXL (podocalyxin-like) (IgG1; R&D Systems). Mouse monoclonal IgG1 (clone 11711; R&D Systems) in the same concentrations as the primary antibodies was used as the isotype control.
After incubation with the primary antibody, slides were cut out of plastic dishes using heated blade. Slides were washed in PBS and incubated with 400 μl containing 2 μg/ml of the secondary fluorescence-conjugated antibody [Alexa fluor-488 goat anti-mouse IgG (H+L), or Alexa fluor 594 donkey anti goat IgG (H+L); Molecular Probes, Eugene, OR] in PBS with 1% BSA for 1 hour at room temperature. For permeabilized samples, the buffer contained 0.025% of Triton X-100.
After washing with PBS, slides were mounted with VECTASHIELD Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA), covered with coverslips and sealed. Analyzis was performed using Nikon Eclipse E800 microscope with attached SPOT color 2.2.0 digital camera (Diagnostic Instruments, Sterling Heights, MI) using SPOT version 3.1 software. The same settings were used to acquire control labelled images and all pictures on the slide. Subsequent image manipulations were made in Adobe Photoshop 6.0.1.
Differentiation within colonies
Recovered passage 1 MSCs (donor 7012) were plated onto 57 cm2 plastic dishes (Nunc) at 2 cells/cm2 and incubated at 37°C and 5% CO2 in CCM for 9 days without medium change. On day 9 the cultures were washed with PBS and the medium on different plates was replaced with either CCM (control), adipogenic medium or osteogenic medium. Adipogenic medium consisted of CCM supplemented with 0.5 μM dexamethasone (Sigma), 0.5 μM 3-Isobutyl-1-methylxanthine (Sigma), and 50 μM indomethacin (Sigma). Osteogenesis medium consisted of CCM supplemented with 1 nM dexamethasone, 20 mM β-glycerolphosphate, 50 μg/ml L-ascorbic acid-2-phosphate. CCM and differentiation media were changed every 3 days for 21 days.
After 21 days the cultures were washed with PBS and fixed in formalin at room temperature for 1 hour. To stain lipid droplets within the cells, adipogenic cultures were washed with PBS, incubated with 0.3% Oil-Red-O (Sigma) solution for 20 minutes at room temperature, and washed again. To stain the sites of mineralization, osteogenic cultures were washed with water, incubated with 1% Alizarin Red S solution (Sigma) for 20 min at room temperature, and washed again.
Growth curve assay
To study the growth kinetics, MSCs isolated from the whole colony and different colony regions (donor 5064) were plated in CCM at 100 cells/cm2 onto 6 well dishes (Nunc) in triplicate. Cells were incubated for 1 to 12 days with medium change every 3 days. For counting, cells on individual wells were washed with PBS, lifted with trypsin/EDTA, washed with CCM by centrifugation, and resuspended in 0.4% Trypan Blue (GIBCO/BRL) before counting in a hemacytometer.
Subcloning and CFU-F assays
To study clonogenicity, cells (donors 5064, 240, and 7012) initially isolated either from the OUT region or the IN region were plated at 2 cells/cm2 onto 57 cm2 dishes (Nunc) in CCM. After 10 to 12 days without medium change, IN regions were isolated from colonies generated from IN regions and replated. The same procedure was performed with colonies generated from OUT regions. Subcloning was continued until colonies were no longer compact with distinct IN and OUT regions for up to five cycles of isolation and re-plating.
CFU-F assays from the same cell preparations in each cycle were done in parallel and in triplicate. On day 14, plates were washed with PBS and stained with 3 % crystal violet (Sigma-Aldrich, Inc., Saint Louis, MO) in 100% methanol for 5 min, washed with water, and air dried. Stained colonies that were at least 2 mm in diameter were counted. Percentage of colonies per plate was obtained using total number of colonies and initial amount of the cells plated.
For densitometry, pictures of partially overlapping fields of colonies (donor 7012) at 40 X magnification were taken with ORCA digital camera (ORCA, ER, Hamamatsu, Japan) attached to an inverted microscope (Nikon Eclise, TE200 Nikon). Final image was obtained by combining multiple images into one using Image J 1.38× software. After conversion into 8-bit grayscale format and background subtraction, gray intensity values were obtained for each pixel through the diameter of the colony. The pixels were then plotted against their correspondent gray intensity values in Excel (Microsoft Corporation, Redmond, WA, USA). Sliding window of 50 pixels was used for smoothing.
Images of differentiated colonies were created by combining pictures of partially overlapping fields of view taken by phase contrast. Multiple images were combined into one using Adobe Photoshop 6.0.1. Linear image adjustments were performed by varying brightness and contrast in order to improve image visibility without changing original image structure.
Results
Expansion of a single-cell derived colony
To follow expansion of single cells into colonies, vials of frozen passage 1 MSCs (donor 5064) were thawed, plated overnight to recover viable adherent cells, re-plated at 0.5 cells per cm2 on 57 cm2 plastic dishes, and time lapse photomicrographs taken by phase microscopy. Single adherent cells that were spindle-shaped were detected within 2 hours (Figure 1). To follow expansion, the areas containing single cells were marked by scratches on the bottom of the plate. Expansion of a single cell into a typical colony is presented in Figure 1. Two cells were seen in the same area on Day 1, and the number increased until Day 12. Round, reflactile cells in mitosis were visible in the photomicrographs as white spots mostly in the periphery of the colony. On Days 4 to 6, the cells remained spindle shaped with a minimum of cell to cell contacts. By Day 12, the cells at the center of the colony were confluent, but non-confluent spindle-shaped cells were present around the periphery of the colony.
Figure 1. Expansion of a single-cell derived colony.
Recovered passage 1 human MSCs (donor 5064) were plated at 0.5 cells per cm2 and incubated for 12 days without medium change. Phase photomicrographs were taken approximately every 24 hours for 12 days. Location of single cell after 2 hour incubation was marked by right angle cuts on bottom of dish. Scale bar: 500 μm. Arrows: single cells after 2 hour and 1 day incubation.
Differences in mRNAs expressed by cells in the center and periphery of colonies
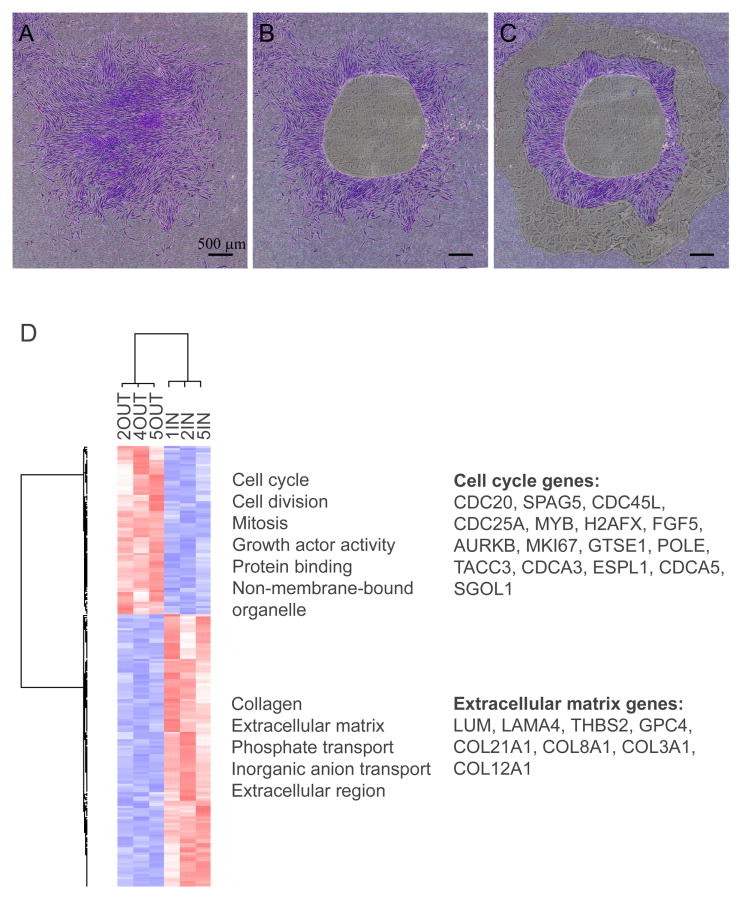
Separate samples of cells in the center (IN, 3 samples) and periphery (OUT, 3 samples) of colonies (donor 5064) were isolated by LMPC (Figure 2A, 2B, and 2C), RNA was extracted, amplified, and the expressed mRNAs assayed with microarrays. A capillary electrophoretic assay demonstrated that the extracted RNA was intact showing distinct 28S and 18S peaks without background (Supplemental Figure 1). Total of 199 differentially expressed transcripts (155 non-redundant genes) were identified and used in hierarchical clustering of the samples and genes (Figure 2D, Supplemental Table 1 and 2). The data clearly distinguished the OUT samples from the IN samples from a series of separately analyzed colonies. The cells derived from the IN cells of the colony expressed higher levels of genes for extracellular matrix and inorganic anion transport (p-value < 0.001) (Figure 2D). The OUT cells from the periphery expressed higher levels of genes for cell cycle and division, mitosis, growth factor activity, protein binding, and non-membrane-bound organelle (p-value < 0.001) (Figure 2D). In the IN cells, the extracellular matrix molecules up-regulated included thrombospondin 2 (THBS2), laminin alpha 4 (LAMA4), lumican (LUM), glypican 4 (GPC4), and alpha 1 chain of collagen III (COL3A1), VIII (COL8A1, XII (COL12A1) and XXI (COL21A1) (Supplemental Table 1). In the OUT cells, the cell division genes up-regulated included cell division cycle homologs 20 (CDC20) and 25 (CDC25), cell division cycle associated 3 (CDCA3) and 5 (CDCA5), aurora kinase B (AURKB), sperm associated antigen 5 (SPAG5), extra spindle pole bodies homolog 1 (ESPL1), centromere protein J (CENPJ), and shugoshin-like 1 (SGOL1) (Supplemental Table 2).
Figure 2. Isolation and microarray assays of IN and OUT samples.
MSCs (donor 5064) were incubated on laser microdissection slides at 2 cells per cm2 for 12 days without medium change. Cells from IN and OUT were isolated with LMPC. The colonies in the figure were stained with crystal violet for illustrative purposes only. (A) Intact colony. (B) Colony after IN was captured. (C) Colony after IN and OUT were captured. (D) Heat map of microarray data from IN and OUT samples using 199 differentially expressed genes. Enriched GeneOntology terms with p values < 0.001 are shown for both clusters. On the heat map, red indicates upregulation and blue downregulation based on gene-wise standardized values. Three OUT samples (2OUT, 4OUT, 5OUT) and three IN samples (1IN, 2IN, 5IN) were used in the microarray assays. Scale bar in A, B, and C: 500 μm. Abbreviations: AURKB, aurora kinase B; CDC, cell division cycle; COL21A1, collagen type 21 alpha 1; ESPL, extra spindle pole bodies homolog; FGF; fibroblast growth factor; GPC, glypican; GTSE, G-2 and S-phase expressed; H2AFX, H2A histone family member X; IN, inner region of a colony; LMPC, laser capture microdissection and pressure catapulting; LAMA, laminin alpha; LUM, lumican; MKI67, antigen identified by monoclonal antibody Ki-67; MYB, v-myb myeloblastosis viral oncogene homolog; OUT, outer region of a colony; POLE, polymerase epsilon; SGOL, shugoshin-like; SPAG, sperm associated antigen; TACC, transforming acidic coiled-coil containing protein; THBS, thrombospondin.
To confirm the microarray data, the same samples of RNA were assayed by real-time RT-PCR. The data identified 28 genes up-regulated in IN sample relative to the OUT sample and 15 genes showed significant up-regulation in the OUT sample compared to the IN sample (Figure 3A and B). Comparison of data from the real-time RT-PCR assays with data from the microarray data indicated similar differences in the genes expressed by IN and OUT cells (Figure 3A and 3B). However, the real-time RT-PCR assay was more sensitive to differences in that 16/28 IN genes and 4/15 OUT genes in the microarray assay did not reach the threshold level of a 2-fold difference. Based on the real-time RT-PCR assays the largest changes in the IN sample were vascular cell adhesion molecule 1 (VCAM1) (10.6-fold up-regulation), THBS2 (9.9-), LUM (9.0-), growth arrest-specific 1 (GAS1) (8.1-), and galactosidase beta 1 (GLB1) (6.7-). The largest changes in the OUT sample were the antigen identified by monoclonal antibody Ki-67 (MKI67 or Ki67) (32.5-fold up-regulation), hyaluronan mediated motility receptor (HMMR or CD168) (30.0-), AURKB (14.8-), podocalyxin-like (PODXL) (7.2-) and leukemia inhibitory factor (LIF) (7.2-).
Figure 3. Assays of mRNA from IN and OUT regions by microarrays and real-time RT-PCR.
Total RNA was isolated from IN and OUT cells obtained with LMPC (donor 5064) or live cell isolation method (donors 7012 and 240). Genes shown have significant changes in their expression based on real-time RT-PCR. (A) Genes upregulated in the OUT sample (donor 5064). (B) Genes upregulated in the IN sample (donor 5064). (C) Genes up-regulated in the OUT region of all three donors (5064, 7012, and 240). (D) Genes up-regulated in the IN region of all three donors. Values are fold changes. Asterisk: changes not significant in the microarray assays (< 2-fold). Error bars: 95 % confidence intervals; n=3. Abbreviations: AGC, aggrecan; ANGPT, angiopoietin; AURKB, aurora kinase B; CCND, cyclin D; CTNNB, catenin beta; CXCL12, CXC chemokine ligand 12; DCN, decorin; DKK, dickkopf homolog; E2F7, E2F transcription factor 7; FGF, fibroblast growth factor; FZD, frizzled homolog; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GAS, growth arrest-specific; GDF, growth differentiation factor; GLB, galactosidase beta; GPC, glypican; GSN, gelsolin; HMMR, hyaluronan-mediated motility receptor; IL, interleukin; IN, inner region of a colony; ITGA, integrin alpha; LIF, leukemia inhibitory factor; LMPC, laser capture microdissection and pressure catapulting; LPXN, leupaxin; LUM, lumican; MCM, minichromosome maintenance deficient; MKI67, antigen identified by monoclonal antibody Ki-67; MMP, matrix metalloproteinase; NOTCH, notch homolog; OUT, outer region of a colony; PODXL, podocalyxin-like; RT-PCR, reverse transcriptase polymerase chain reaction; RARB, retinoic acid receptor beta; RUNX, runt-related transcription factor; SOCS, suppressor of cytokine signaling; SOX, sex determining region Y-box; STAT, signal transducer and activator of transcription; SYNPO, synaptopodin; SYTL, synaptotagmin-like; THBS, thrombospondin; VCAM, vascular cell adhesion molecule; VIL, villin; WNT, wingless-type MMTV integration site; WWTR, ww domain containing transcription regulator.
To confirm the results, real-time RT-PCR assays were performed on colonies generated by MSCs from two additional donors (7012 and 240 in Figure 3C and 3D). As expected from previous reports [23], variations were observed with samples from different donors (Figure 3C and 3D), but the results were similar for 60% (9/15) of the up-regulated OUT genes and 32% (9/28) of the up-regulated IN genes (Figure 3C and 3D). Also, IN cells from all the donors expressed much higher levels of two genes for extracellular matrix proteins (THBS2 and LUM) and the growth arrest specific 1 protein (GAS1)
Differences Demonstrated by Immunocytochemistry
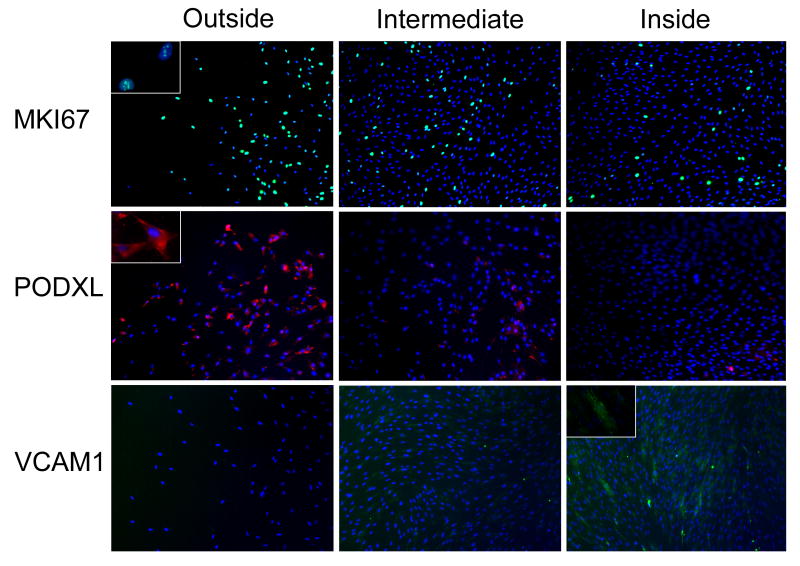
Immunocytochemistry of the colonies confirmed that OUT cells expressed higher levels of MKI67, a marker for proliferating cells, and PODXL, a marker for motile cells (Figure 4). Immunostaining also confirmed that the IN cells expressed higher levels of cell adhesion molecule VCAM1 (Figure 4). These results suggest that within colonies the OUT cells are proliferating more rapidly than the IN cells.
Figure 4. Immunocytochemistry of MKI67, PODXL, and VCAM1 in colonies.
Three regions of MSC colonies from donor 5064 are shown. Nuclear counterstain: DAPI. Insets: enlarged regions of the same slides. Magnification: 40X, 100X (insets). Abbreviations: MKI67, antigen identified by monoclonal antibody Ki-67; PODXL, podocalyxin-like; VCAM, vascular cell adhesion molecule.
Differentiation of colonies
To study the differentiation capability of IN and OUT cells within a colony, MSC colonies were cultured under adipogenic and osteogenic conditions for 21 days. Colonies grown under adipogenic conditions and stained with Oil-Red-O showed lipid droplets preferably in the IN cells (Figure 5). Similarly, colonies grown under osteogenic conditions and stained with Alizarin Red S showed sites of mineralization mainly in the IN region (Figure 5). The results further confirmed the observations that the IN cells were more committed to differentiation than OUT cells. The cells in the OUT region have the morphology of fibroblasts that is characteristic of MSC plated at a low density [16].
Figure 5. Differentiation of colonies.
Representative colonies (donor 7012) after adipogenic or osteogenic differentiation. Colonies were fixed with formalin and stained with either Oil-Red-O (adipogenesis) or Alizarin Red S (osteogenesis). Under both conditions, differentiation initiated in the IN regions of the colonies. Scale bar: 1 mm.
Similar Rates of Proliferation on IN and OUT Cells on Replating
To compare the proliferation potential of IN and OUT cells, MSCs were plated on plastic dishes and the two fractions separated by scraping away either the IN or OUT cells of colonies and lifting the remaining cells with a cloning cylinder. The cells were replated at 100 cells/cm2 and incubated for up to 12 days. The IN and OUT cells proliferated at essentially the same rate (Figure 6A). Also the doubling times as a function of days in culture were essentially the same with the peak of proliferation around day 3 (Figure 6A).
Figure 6. Expansion and clonogenicity of isolated IN and OUT cells.
MSCs (donors 5064, 7012, and 240) were plated at 2 cells per cm2 and incubated for 10 to 12 days. Cells (passage 2) were isolated either from the OUT or the IN and re-plated at 100 cells per cm2 for growth curve assay and at 2 cells per cm2 for CFU-F assay. (A) Growth curve assay for donor 5064. Inset: Fold changes per day. (B) CFU-F assays of cells from IN and OUT of all three donors. Subcloning was continued for up to five cycles of isolation and re-plating (passage 7). Error bars: standard deviations; n=3. Abbreviations: CFU-F, colony forming unit fibroblast; IN, inner region of a colony; OUT, outer region of a colony; P, passage.
Clonogenicity of IN and OUT cells
To compare their clonogenicity, the cells from all three donors were grown as previously, and the IN and OUT cells were isolated from passage 2 colonies and re-plated at low density. The IN and OUT cells from passage 3 were isolated and re-plated at low density. The sequence was repeated until the cells did not generate colonies with distinct IN and OUT regions (Supplemental Figure 2). The results were analyzed by both the number of colonies generated (CFUs) and the morphology of the colonies.
There were no significant differences in CFU values obtained for IN and OUT cells from passage 3 with MSCs from the three donors (Figure 6B). As expected [19], however, there was decrease in CFUs with further passages with a distinct decrease with some preparations of MSCs. Colonies with distinct IN and OUT regions were obtained until passage 7 with MSCs from donor 5064, and passage 6 with MSCs from donor 240, but only until passage 4 with MSCs donor 7012.
To assess the morphology of the colonies generated, we classified colonies with diameters of 4 mm or more with distinct IN and OUT regions as type I and smaller colonies or loose large colonies as type II (Figure 7A). The distinctions between the two types of colonies that were apparent by phase microscopy were readily confirmed by densitometry of dye-stained colonies (Supplemental Figure 3). With MSCs from all three donors, the fraction of type I colonies decreased upon subcloning while the fraction of type II increased (Figure 7B, 7C, and 7D). There were no apparent differences between the IN and OUT samples. Interestingly, type II colonies became majority at passage 6 for donor 5064 (Figure 7B), at passage 5 for donor 240 (Figure 7C), and at passage 4 for donor 7012 (Figure 7D). Similarly, the size distribution of colonies in all three donors shifted towards smaller diameter colonies upon passaging (Figure 8A, 8B, and 8C).
Figure 7. Changes in colony morphology upon subcloning.
MSC colonies were stained with crystal violet, measured, and classified either as type I or type II. Type I colonies were at least 4 mm in size with a dense IN. Type II colonies were either less than 4 mm in size with dense IN or larger but loose. (A) Representative large and small type I colonies (upper panel) and type II colonies (lower panel) from one culture dish of donor 240 MSCs (passage 3). See Supplemental Figure 3 for densitometry of stained type I and type II colonies. Distribution of type I and II colonies during subcloning for donor: (B) 5064, (C) 240, and (D) 7012. Error bars: standard deviations; n=3. Scale bar: 1 mm. Abbreviations: CFU-F, colony forming unit fibroblast; IN, inner region of a colony; OUT, outer region of a colony; P, passage.
Figure 8. Colony size distribution upon subcloning.
MSC colonies were divided into four groups based on their size (2.0-3.5 mm, 4.0-5.5 mm, 6.0-7.5 mm, and 8.0-9.5 mm). Size distribution of colonies derived from donor: (A) 5064, (B) 240, and (C) 7012. Error bars: standard deviations; n=3. Abbreviations: IN, inner region of a colony; OUT, outer region of a colony; P, passage.
Discussion
As originally noted by Friedenstein [30], one of the characteristic feature of MSC cultures is their clonal growth, i.e. their ability to form distinct single-cell derived colonies after plating at very low densities [3;5;30]. Previous reports demonstrated that the single cell derived colonies of MSCs vary in morphology, growth rates, and differentiation capacity [4;19;31]. In addition, MicroSAGE analysis of single-cell derived colonies showed that MSCs expressed mRNAs common to different mesenchymal cell lineages, suggesting heterogeneity within a colony [32]. In the present study we focused on the observation that most early culture single-cell derived colonies had very high density inner regions (IN), and less dense outer regions (OUT). The cells in the OUT region have the morphology of fibroblasts that is characteristic of MSC plated at a low density. Microarray assays demonstrated that the cells in the IN region expressed many genes for extracellular matrix, and the cells in the OUT region expressed genes involved in cell cycle and division. Validation of microarray data with real-time RT-PCR assays indicated a good correlation between these two methods. However, the real-time RT-PCR assays were more sensitive in that they detected changes that were not significant in the microarray assays. Also, the changes in several genes detected by realtime RT-PCR were much larger than microarray data suggested. The differences may in part be explained by the fact that the real-time RT-PCR assay did not require amplification of the RNA. We demonstrated differences in protein expression by immunocytochemistry of colonies confirming the mRNA data in that the IN cells expressed higher levels of the cell adhesion protein (VCAM1), while the OUT cells expressed higher levels of the cell cycle protein (MKI67) suggesting that within colonies the OUT cells are proliferating more rapidly than the IN cells. The OUT cells also expressed higher levels of an anti-adhesion protein (PODXL), which is a member of the CD34 family of sialomucins and has been identified in undifferentiated human embryonic and hematopoietic stem cells [33-36]. In addition, PODXL has been shown to increase the aggressive phenotype of malignant cells and thus could be important in MSC migration and survival in vivo [37]. Recently we identified higher expression of PODXL in early cultures of MSCs compared to late cultures, suggesting PODXL as a marker of highly proliferative early culture MSCs [23]. Interestingly, the OUT cells resemble the early culture MSCs in their gene expression patterns, while the IN cells are more similar to the late culture MSCs [23]. Therefore the results suggested that the IN cells were more committed to mesenchymal differentiation than the OUT cells. The suggestion was confirmed by transferring the colonies to differentiating in conditions in which, in agreement with previous observations [4], differentiation into both adipocytes and osteoblasts initially occurred in the IN regions of colonies. However, when IN cells were re-plated at clonal densities, the IN cells were indistinguishable from OUT cells in their rates of propagation, clonogenicity and the formation of colonies with distinct IN and OUT regions. Therefore the results indicated that the partial differentiation of the IN cells was reversible. The proliferation of MSCs is limited by density in culture and therefore probably in vivo under physiological and pathological conditions. The heterogeneity and the plasticity observed in the cultures suggest that the protocols used to expand human MSCs in culture may have a major influence on how efficiently the cells engraft and repair tissues in animal models and in patients [22].
Supplementary Material
Supplemental Figure 1. RNA quality assay of LMPC samples. Total RNA was extracted from the LMPC isolated cells and the quality and quantity of the RNA was checked with bioanalyzer. Virtual gels are shown for three OUT samples (2OUT, 4OUT, 5OUT) and three IN samples (1IN, 2IN, 5IN) with RNA ladder and bands for 28S and 18S rRNA. Abbreviations: IN, inner region of a colony; LMPC, laser capture microdissection and pressure catapulting; OUT, outer region of a colony; rRNA, ribosomal RNA.
Supplemental Figure 2. Subcloning of IN and OUT cells. Representative colonies from the subcloning experiment at passage 2 and 6 for donor 5064. Both the IN and OUT regions generated similar colonies. Scale bar: 1 mm. Abbreviations: IN, inner region of a colony; OUT, outer region of a colony; P, passage.
Supplemental Figure 3. Density analysis of type I and II colonies. Representative colonies from donor 7012 analyzed by staining with crystal violet and densitometry. Scale: 0-255 (black- white).
Acknowledgments
This work was supported in part by grants from the National Institute of Health (P01 RR 17447, P01 HL 075161) and the Louisiana Gene Therapy Research Consortium.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Sekiya I, Larson BL, Smith JR, et al. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–541. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 5.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 6.Barry F, Boynton R, Murphy M, et al. The SH-3 and SH-4 antibodies recognize distinct epitopes on CD73 from human mesenchymal stem cells. Biochem Biophys Res Commun. 2001;289:519–524. doi: 10.1006/bbrc.2001.6013. [DOI] [PubMed] [Google Scholar]
- 7.Barry FP, Boynton RE, Haynesworth S, et al. The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105) Biochem Biophys Res Commun. 1999;265:134–139. doi: 10.1006/bbrc.1999.1620. [DOI] [PubMed] [Google Scholar]
- 8.Dennis JE, Carbillet JP, Caplan AI, et al. The STRO-1+ marrow cell population is multipotential. Cells Tissues Organs. 2002;170:73–82. doi: 10.1159/000046182. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Pacheco JM, Oliver C, Kimatrai M, et al. Human decidual stromal cells express CD34 and STRO-1 and are related to bone marrow stromal precursors. Mol Hum Reprod. 2001;7:1151–1157. doi: 10.1093/molehr/7.12.1151. [DOI] [PubMed] [Google Scholar]
- 10.Gronthos S, Zannettino AC, Graves SE, et al. Differential cell surface expression of the STRO-1 and alkaline phosphatase antigens on discrete developmental stages in primary cultures of human bone cells. J Bone Miner Res. 1999;14:47–56. doi: 10.1359/jbmr.1999.14.1.47. [DOI] [PubMed] [Google Scholar]
- 11.Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- 12.Jones EA, Kinsey SE, English A, et al. Isolation and characterization of bone marrow multipotential mesenchymal progenitor cells. Arthritis Rheum. 2002;46:3349–3360. doi: 10.1002/art.10696. [DOI] [PubMed] [Google Scholar]
- 13.Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991;78:55–62. [PubMed] [Google Scholar]
- 14.Stewart K, Monk P, Walsh S, et al. STRO-1, HOP-26 (CD63), CD49a and SB-10 (CD166) as markers of primitive human marrow stromal cells and their more differentiated progeny: a comparative investigation in vitro. Cell Tissue Res. 2003;313:281–290. doi: 10.1007/s00441-003-0762-9. [DOI] [PubMed] [Google Scholar]
- 15.Yoo HJ, Yoon SS, Park S, et al. Production and characterization of monoclonal antibodies to mesenchymal stem cells derived from human bone marrow. Hybridoma (Larchmt) 2005;24:92–97. doi: 10.1089/hyb.2005.24.92. [DOI] [PubMed] [Google Scholar]
- 16.Mets T, Verdonk G. Variations in the stromal cell population of human bone marrow during aging. Mech Ageing Dev. 1981;15:41–49. doi: 10.1016/0047-6374(81)90006-3. [DOI] [PubMed] [Google Scholar]
- 17.Gregory CA, Ylostalo J, Prockop DJ. Adult bone marrow stem/progenitor cells (MSCs) are preconditioned by microenvironmental “niches” in culture: a two-stage hypothesis for regulation of MSC fate. Sci STKE. 2005;2005:e37. doi: 10.1126/stke.2942005pe37. [DOI] [PubMed] [Google Scholar]
- 18.Colter DC, Class R, DiGirolamo CM, et al. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow 6. Proc Natl Acad Sci U S A. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiGirolamo CM, Stokes D, Colter D, et al. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999;107:275–281. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 20.Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: Harnessing the power of adult stem cells to repair tissues. PNAS. 2003;100:11917–11923. doi: 10.1073/pnas.1834138100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith JR, Pochampally R, Perry A, et al. Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells. 2004;22:823–831. doi: 10.1634/stemcells.22-5-823. [DOI] [PubMed] [Google Scholar]
- 22.Lee RH, Hsu SC, Munoz J, et al. A subset of human rapidly self-renewing marrow stromal cells preferentially engraft in mice. Blood. 2006;107:2153–2161. doi: 10.1182/blood-2005-07-2701. [DOI] [PubMed] [Google Scholar]
- 23.Larson BL, Ylostalo J, Prockop DJ. Human multipotent stromal cells undergo sharp transition from division to development in culture. Stem Cells. 2008;26:193–201. doi: 10.1634/stemcells.2007-0524. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A. 2001;98:31–36. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Wong WH. DNA-Chip Analyzer (dChip) In: Parmigiani G, Garrett ES, Irizarry R, et al., editors. The Analysis of Gene Expression Data: Methods and Software. Springer; 2003. [Google Scholar]
- 26.Zhong S, Li C, Wong WH. ChipInfo: Software for extracting gene annotation and gene ontology information for microarray analysis. Nucleic Acids Res. 2003;31:3483–3486. doi: 10.1093/nar/gkg598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisen MB, Spellman PT, Brown PO, et al. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golub TR, Slonim DK, Tamayo P, et al. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 29.Ashburner M, Ball CA, Blake JA, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 31.Kuznetsov SA, Krebsbach PH, Satomura K, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 32.Tremain N, Korkko J, Ibberson D, et al. MicroSAGE analysis of 2,353 expressed genes in a single cell-derived colony of undifferentiated human mesenchymal stem cells reveals mRNAs of multiple cell lineages. Stem Cells. 2001;19:408–418. doi: 10.1634/stemcells.19-5-408. [DOI] [PubMed] [Google Scholar]
- 33.Furness SG, McNagny K. Beyond mere markers: functions for CD34 family of sialomucins in hematopoiesis. Immunol Res. 2006;34:13–32. doi: 10.1385/IR:34:1:13. [DOI] [PubMed] [Google Scholar]
- 34.Doyonnas R, Nielsen JS, Chelliah S, et al. Podocalyxin is a CD34-related marker of murine hematopoietic stem cells and embryonic erythroid cells. Blood. 2005;105:4170–4178. doi: 10.1182/blood-2004-10-4077. [DOI] [PubMed] [Google Scholar]
- 35.Choo AB, Tan HL, Ang SN, et al. Selection Against Undifferentiated Human Embryonic Stem Cells by a Cytotoxic Antibody Recognizing Podocalyxin-like Protein-1. Stem Cells. 2008 doi: 10.1634/stemcells.2007-0576. [DOI] [PubMed] [Google Scholar]
- 36.Takeda T, Go WY, Orlando RA, et al. Expression of podocalyxin inhibits cell-cell adhesion and modifies junctional properties in Madin-Darby canine kidney cells. Mol Biol Cell. 2000;11:3219–3232. doi: 10.1091/mbc.11.9.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sizemore S, Cicek M, Sizemore N, et al. Podocalyxin increases the aggressive phenotype of breast and prostate cancer cells in vitro through its interaction with ezrin. Cancer Res. 2007;67:6183–6191. doi: 10.1158/0008-5472.CAN-06-3575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. RNA quality assay of LMPC samples. Total RNA was extracted from the LMPC isolated cells and the quality and quantity of the RNA was checked with bioanalyzer. Virtual gels are shown for three OUT samples (2OUT, 4OUT, 5OUT) and three IN samples (1IN, 2IN, 5IN) with RNA ladder and bands for 28S and 18S rRNA. Abbreviations: IN, inner region of a colony; LMPC, laser capture microdissection and pressure catapulting; OUT, outer region of a colony; rRNA, ribosomal RNA.
Supplemental Figure 2. Subcloning of IN and OUT cells. Representative colonies from the subcloning experiment at passage 2 and 6 for donor 5064. Both the IN and OUT regions generated similar colonies. Scale bar: 1 mm. Abbreviations: IN, inner region of a colony; OUT, outer region of a colony; P, passage.
Supplemental Figure 3. Density analysis of type I and II colonies. Representative colonies from donor 7012 analyzed by staining with crystal violet and densitometry. Scale: 0-255 (black- white).