Abstract
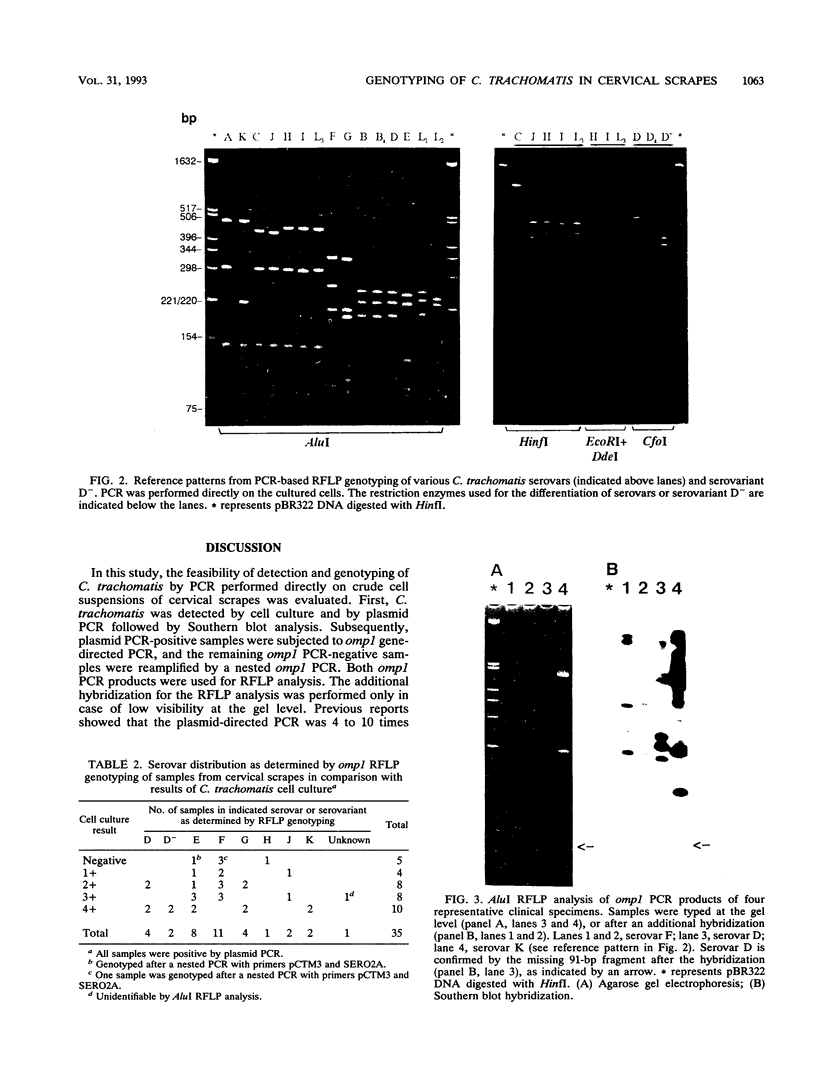
Detection and genotyping of Chlamydia trachomatis were optimized by using a polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis performed directly with crude cells of cervical scrapes. Different PCR pretreatment methods were evaluated on samples which were positive for C. trachomatis by cell culture. In comparison with DNA extraction and different proteolytic digestion methods, a simple pretreatment of 10 min of boiling appeared to be optimal for PCR amplification. Crude samples (n = 209) were first screened for C. trachomatis by both cell culture and plasmid PCR. Subsequently, positive samples found by plasmid PCR were subjected to a direct omp1 PCR-based RFLP analysis to differentiate C. trachomatis serovars A to K, Ba, Da, and L1 to L3 and serovariant D-. All cervical scrapes that were found positive for C. trachomatis by cell culture (n = 30) were also positive by plasmid PCR and omp1 PCR and could be easily genotyped. In addition, of the culture-negative group, eight samples were found positive by plasmid PCR. Five of these eight samples were also positive by omp1 PCR; of these five, two were positive by a nested omp1 PCR. Genotyping by RFLP analysis of the 35 omp1 PCR-positive samples showed that serovars D, E, and F are the most prevalent types found in cervical scrapes, while serovariant D- was also detected. This study shows that direct PCR and PCR-based RFLP analysis are feasible for detection and genotyping of C. trachomatis in cervical scrapes and are more sensitive than culture-based serotyping.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes R. C., Rompalo A. M., Stamm W. E. Comparison of Chlamydia trachomatis serovars causing rectal and cervical infections. J Infect Dis. 1987 Dec;156(6):953–958. doi: 10.1093/infdis/156.6.953. [DOI] [PubMed] [Google Scholar]
- Batteiger B. E., Lennington W., Newhall W. J., Katz B. P., Morrison H. T., Jones R. B. Correlation of infecting serovar and local inflammation in genital chlamydial infections. J Infect Dis. 1989 Aug;160(2):332–336. doi: 10.1093/infdis/160.2.332. [DOI] [PubMed] [Google Scholar]
- Claas H. C., Melchers W. J., de Bruijn I. H., de Graaf M., van Dijk W. C., Lindeman J., Quint W. G. Detection of Chlamydia trachomatis in clinical specimens by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 1990 Dec;9(12):864–868. doi: 10.1007/BF01967500. [DOI] [PubMed] [Google Scholar]
- Dean D., Patton M., Stephens R. S. Direct sequence evaluation of the major outer membrane protein gene variant regions of Chlamydia trachomatis subtypes D', I', and L2'. Infect Immun. 1991 Apr;59(4):1579–1582. doi: 10.1128/iai.59.4.1579-1582.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D., Schachter J., Dawson C. R., Stephens R. S. Comparison of the major outer membrane protein variant sequence regions of B/Ba isolates: a molecular epidemiologic approach to Chlamydia trachomatis infections. J Infect Dis. 1992 Aug;166(2):383–392. doi: 10.1093/infdis/166.2.383. [DOI] [PubMed] [Google Scholar]
- Frost E. H., Deslandes S., Veilleux S., Bourgaux-Ramoisy D. Typing Chlamydia trachomatis by detection of restriction fragment length polymorphism in the gene encoding the major outer membrane protein. J Infect Dis. 1991 May;163(5):1103–1107. doi: 10.1093/infdis/163.5.1103. [DOI] [PubMed] [Google Scholar]
- Griffais R., Thibon M. Detection of Chlamydia trachomatis by the polymerase chain reaction. Res Microbiol. 1989 Feb;140(2):139–141. doi: 10.1016/0923-2508(89)90047-8. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Holmes K. K., Grayston J. T. Immunotypes of Chlamydia trachomatis isolates in Seattle, Washington. Infect Immun. 1983 Aug;41(2):865–868. doi: 10.1128/iai.41.2.865-868.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näher H., Drzonek H., Wolf J., von Knebel Doeberitz M., Petzoldt D. Detection of C trachomatis in urogenital specimens by polymerase chain reaction. Genitourin Med. 1991 Jun;67(3):211–214. doi: 10.1136/sti.67.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde J. M., Rieffe M., Rozenberg-Arska M., Ossenkoppele P. M., Nawrocki R. P., van Loon A. M. Development and clinical evaluation of a polymerase chain reaction test for detection of Chlamydia trachomatis. J Clin Microbiol. 1992 Aug;30(8):2122–2128. doi: 10.1128/jcm.30.8.2122-2128.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard L., Traulsen J., Birkelund S., Christiansen G. Evaluation of urogenital Chlamydia trachomatis infections by cell culture and the polymerase chain reaction using a closed system. Eur J Clin Microbiol Infect Dis. 1991 Dec;10(12):1057–1061. doi: 10.1007/BF01984929. [DOI] [PubMed] [Google Scholar]
- Rodriguez P., Vekris A., de Barbeyrac B., Dutilh B., Bonnet J., Bebear C. Typing of Chlamydia trachomatis by restriction endonuclease analysis of the amplified major outer membrane protein gene. J Clin Microbiol. 1991 Jun;29(6):1132–1136. doi: 10.1128/jcm.29.6.1132-1136.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikku P., Puolakkainen M., Leinonen M., Nurminen M., Nissinen A. Cross-reactivity between Chlamydiazyme and Acinetobacter strains. N Engl J Med. 1986 Apr 3;314(14):922–923. doi: 10.1056/NEJM198604033141413. [DOI] [PubMed] [Google Scholar]
- Sayada C., Denamur E., Orfila J., Catalan F., Elion J. Rapid genotyping of the Chlamydia trachomatis major outer membrane protein by the polymerase chain reaction. FEMS Microbiol Lett. 1991 Sep 15;67(1):73–78. doi: 10.1016/0378-1097(91)90447-i. [DOI] [PubMed] [Google Scholar]
- Suchland R. J., Stamm W. E. Simplified microtiter cell culture method for rapid immunotyping of Chlamydia trachomatis. J Clin Microbiol. 1991 Jul;29(7):1333–1338. doi: 10.1128/jcm.29.7.1333-1338.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Thomas B. J., Osborn M. F. Evaluation of enzyme immunoassay (Chlamydiazyme) for detecting Chlamydia trachomatis in genital tract specimens. J Clin Pathol. 1987 Feb;40(2):194–199. doi: 10.1136/jcp.40.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenvoort J. H., Suchland R. J., Stamm W. E. Serovar distribution of urogenital Chlamydia trachomatis strains in The Netherlands. Genitourin Med. 1988 Jun;64(3):159–161. doi: 10.1136/sti.64.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. W., Tyler S. D., Giercke S., Pollard D. R., McNicol P., Rozee K. R. Comparison of polymerase chain reaction and chlamydiazyme for the detection of Chlamydia trachomatis in clinical specimens. Eur J Clin Microbiol Infect Dis. 1992 Mar;11(3):233–236. doi: 10.1007/BF02098085. [DOI] [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Watkins N. G., Caldwell H. D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989 Apr;57(4):1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]