Abstract
BACKGROUND
Knowledge of the shock potential gradient (∇V) and postshock activation is limited to internal defibrillation of short-duration ventricular fibrillation (SDVF).
OBJECTIVE
The purpose of this study was to determine these variables after external defibrillation of long-duration VF (LDVF).
METHODS
In six pigs, 115–20 plunge needles with three to six electrodes each were inserted to record throughout both ventricles. After the chest was closed, the biphasic defibrillation threshold (DFT) was determined after 20 seconds of SDVF with external defibrillation pads. After 7 minutes of LDVF, defibrillation shocks that were less than or equal to the SDVF DFT strength were given.
RESULTS
For DFT shocks (1632 ± 429 V), the maximum minus minimum ventricular voltage (160 ± 100 V) was 9.8% of the shock voltage. Maximum cardiac ∇V (28.7 ± 17 V/cm) was 4.7 ± 2.0 times the minimum ∇V (6.2 ± 3.5 V/cm). Although LDVF did not increase the DFT in five of the six pigs, it significantly lengthened the time to earliest postshock activation following defibrillation (1.6 ± 2.2 seconds for SDVF and 4.9 ± 4.3 seconds for LDVF). After LDVF, 1.3 ± 0.8 episodes of spontaneous refibrillation occurred per animal, but there was no refibrillation after SDVF.
CONCLUSION
Compared with previous studies of internal defibrillation, during external defibrillation much less of the shock voltage appears across the heart and the shock field is much more even; however, the minimum ∇V is similar. Compared with external defibrillation of SDVF, the biphasic external DFT for LDVF is not increased; however, time to earliest postshock activation triples. Refibrillation is common after LDVF but not after SDVF in these normal hearts, indicating that LDVF by itself can cause refibrillation without requiring preexisting heart disease.
Keywords: External defibrillation, Ventricular fibrillation, Electrical mapping, Shock, Defibrillators, Potential gradient
Introduction
Most individuals with sudden cardiac arrest have had ventricular fibrillation (VF) for several minutes by the time responders arrive.1 Although VF lasting more than 1 minute (long-duration VF [LDVF]) differs from short-duration VF (SDVF),2,3 no mapping studies have examined defibrillation after LDVF. While transthoracic biphasic defibrillation usually halts LDVF,4 spontaneous refibrillation frequently occurs.5 A better understanding of transthoracic defibrillation and spontaneous refibrillation after LDVF is essential for improvement in therapies for patients with sudden cardiac arrest.
The first aim of this study was to determine the voltages and potential gradient (∇V) field created throughout the ventricles during transthoracic defibrillation. These data have not previously been recorded for SDVF or LDVF, except at a single cardiac site.6 Changes in extracellular ion concentrations and interstitial fluid volume as well as alterations in the geometry of the heart caused by LDVF could affect cardiac ∇V for shocks after LDVF. The defibrillation threshold (DFT) is different for SDVF and LDVF.7–9 There are two possible explanations for this finding, which are not mutually exclusive. First, the ∇V field during SDVF and LDVF could be different. Second, the response of the heart to the same ∇V field during SDVF and LDVF could be different. We tested the hypothesis that LDVF alters ∇V and, hence, defibrillation efficacy.
The second aim of this study was to determine the relationship, if any, between the ∇V field generated by transthoracic defibrillation and early postshock electrical activity after LDVF. Earliest postshock activation occurs in regions with low ∇V after defibrillation of SDVF with internal electrodes that create a highly uneven cardiac ∇V field.10–12 We tested the hypothesis that because ∇V is much more uniform with external defibrillation than with internal defibrillation or because the mechanism of defibrillation differs after SDVF and LDVF, early postshock activation sites after LDVF do not cluster in low-∇V regions.
The third aim was to evaluate the frequency and nature of spontaneous refibrillation after LDVF in previously normal hearts. It is not known whether refibrillation is caused by the disease process that caused the initial VF episode or whether LDVF itself has detrimental effects that can reinitiate VF. We tested the hypothesis that, in contrast to SDVF, in which refibrillation is uncommon, refibrillation occurs frequently within the first 1–2 minutes after LDVF in previously normal hearts.
Methods
Animal preparation
Pigs obtained from the University of Alabama at Birmingham Animal Resource Program were managed in accordance with guidelines in the Position of the American Heart Association on Research Animal Use.13 The University of Alabama at Birmingham Institutional Animal Care and Use Committee approved the experimental protocol.
Six healthy mixed-breed swine (36.9 ± 8.8 kg) of either sex were anesthetized with intramuscular atropine (0.04 mg/kg), zolazepam-tiletamine (4.4 mg/kg), and xylazine (4.4 mg/kg). Animals were mechanically ventilated, and anesthesia was maintained by inhalation of isoflurane (1.75 to 2.0%) in 100% oxygen. Animals were given intravenous 0.9% saline. Lead II electrocardiogram and systemic arterial blood pressure were continuously monitored.
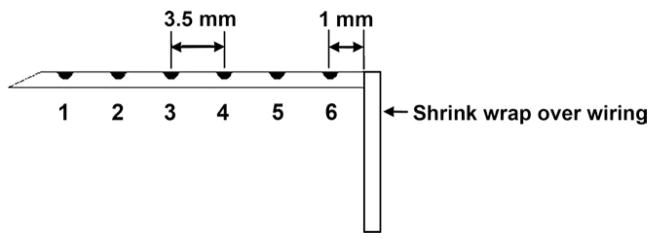
The heart was exposed through a median sternotomy and supported in a pericardial cradle. Sixty plunge needles, each containing four unipolar electrodes, were inserted throughout the left ventricle (LV), and 45–50 plunge needles, each containing three unipolar electrodes, were placed throughout the right ventricle (RV). The needles were 1–1.5 cm apart. Ten longer needles with six unipolar electrodes were inserted into the septum from the anterior and posterior interventricular grooves (Figure 1). Each needle was made of epoxy fiberglass strands (0.7 mm diameter in the heart and 0.6 mm diameter before the right angle) and had 50-μm diameter silver electrodes spaced 3.5 mm apart, with the most epicardial electrode 1 mm beneath the surface.14
Figure 1.
Diagram of a septal plunge needle. Black dots represent electrodes with the most endocardial electrode labeled 1 and the most epicardial labeled 6.
Since chest compression could not be performed without dislodging the plunge needles, the aortic root and right atrial appendage were cannulated for cardiopulmonary bypass. The chest was then closed in multiple layers, and bilateral chest tubes were placed to evacuate air and fluid. Intravenous heparin (100 U/kg) was administered before placement of the cannulae and repeated every hour (50 U/kg). Animals were defibrillated closed chest with Physio-Control Quik-Combo EDGE (Physio-Control Inc, Redmond, WA) defibrillation electrodes located on the chest wall in a left-to-right lateral configuration. The medial edges of both electrodes were approximately 2 cm lateral to the sternum with their cranial edge extending approximately to the second intercostal space.
Defibrillation protocol
After 20 seconds of electrically induced SDVF, a 150- or 200-J biphasic shock was given (LifePak 12, Physio-Control Inc, Redmond, WA). If it failed, energy was increased in 50- or 60-J increments up to 360 J. If the initial shock was successful, VF was reinitiated after 4 minutes and a shock 30 or 50 J lower was given. This process was repeated until a shock failed. The lowest shock strength that defibrillated was called the SDVF DFT.
VF was then reinitiated, and after 7 minutes a shock of the SDVF DFT strength was delivered. A shock was considered successful if no VF was observed within 5 seconds after the shock. If unsuccessful, repeat shocks with escalation of energy by 50-J increments were delivered until maximum defibrillator energy (360 J) was delivered or defibrillation success occurred. As previously described by Chen et al,15 type A successful shocks were defined as shocks that terminated VF with no postshock activity recorded for >130 ms after the shock. Type B successful shocks were defined as shocks that terminated VF with earliest postshock electrical activity recorded <130 ms after the shock. Because type A success occurred in the first four animals, animals 5 and 6 were initially given a shock 50 J below the SDVF DFT to see whether a type B success would occur.
No cardiopulmonary support was performed during VF; however, 30 seconds after termination of LDVF, the animal was placed on cardiopulmonary bypass at 1 L/min. Cardiopulmonary bypass was necessary because of poor cardiac function after the prolonged ischemia of LDVF. The animal was observed for refibrillation for 15 minutes after defibrillation.
Data acquisition
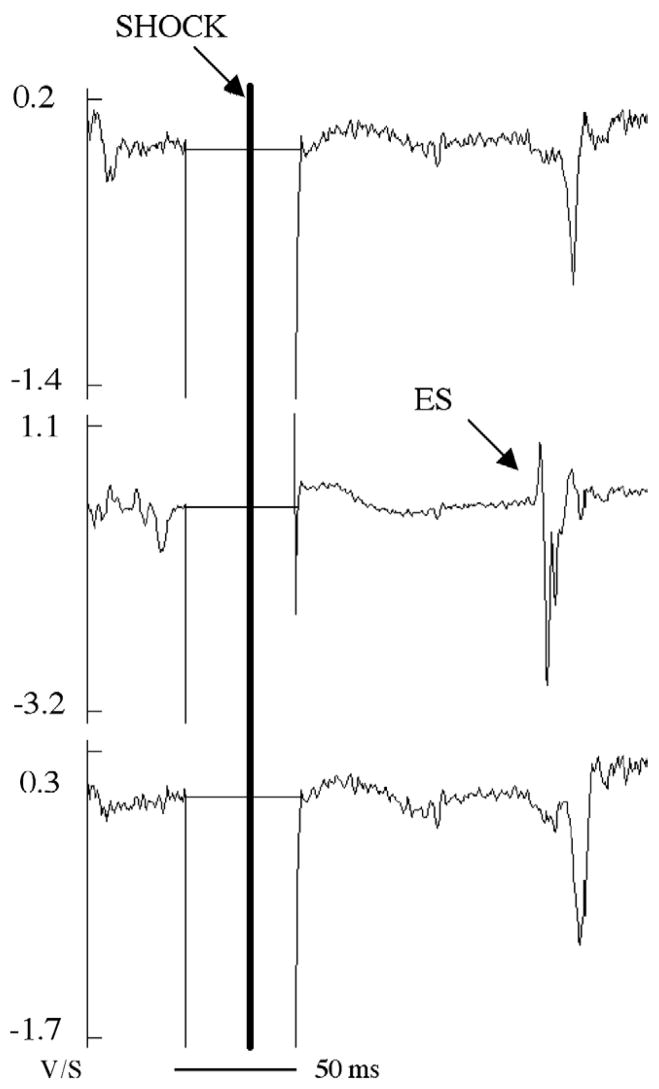
Unipolar potentials were recorded with a 528-channel mapping system16 as follows: 2-kHz sample rate, 0.5- to 500-Hz bandpass filter, 50× gain, with reference and ground electrodes closely approximated at the aortic root. An external device signaled the mapping system 10 ms before the shock to switch voltage attenuators on, change amplifier coupling from AC to DC, and decrease the gain of each channel.17 Most channels recovered within 30 ms after the end of the shock (Figure 2).
Figure 2.
The dV/dt of the recordings from a three-electrode RV plunge needle during a successful defibrillation shock after SDVF. Earliest activation after the isoelectric window is indicated by ES. The flat horizontal line represents time during which the gain was decreased during the shock. Approximately 50 ms was allowed owing to uncertainty in triggering of the defibrillator.
The animal was euthanized by induction of VF, and the heart and plunge needles were removed. Plunge needle locations were marked with color-coded pins. A three-dimensional (3D) digitizer was used to determine the spatial location of each needle and the axial position of each electrode with respect to the epicardial insertion point of the needle, as described elsewhere.18
Data analysis
The investigators viewed a 3D computer display in which recorded activations were animated. Activation in each unipolar electrogram was identified when dV/dt computed using a 5-point digital filter was ≤−0.5 V/s.19 The time of each local activation was taken at the maximum negative dV/dt. The site of earliest postshock activation was defined as the site of the first electrode that registered activation that propagated to initiate the first postshock activation cycle.17 Early sites were defined as the earliest site as well as the sites of electrodes in which activation was detected within 4 ms of the earliest site that did not directly propagate from the earliest site. The heart was divided into anterior, lateral, and posterior thirds and into apical, middle, and basal thirds, and each needle electrode was assigned to its proper divisions in the 3D digitized map. The innermost electrode on the needles was defined as endocardial, and the outermost electrode was defined as epicardial. The interior one (RV) or two (LV) electrodes were defined as midwall.
The ∇V was calculated from the potentials recorded during the shock. To smooth background noise, the potentials at all electrodes 2 ms after the leading edge of the first phase of the shock were fit to a second-order polynomial in all three dimensions to obtain a calculated potential.18 The spatial derivative of the calculated potential at each electrode was obtained to represent the ∇V magnitude. Sites of early activation after successful defibrillation of SDVF and LDVF were related to the shock ∇V at that site. The charge voltage (V) of the LifePak 12 was calculated using a formula supplied by Physio-Control Corporation: V = (shock energy [J]/7.87 × 10−5)1/2.
Refibrillation was defined as VF occurring spontaneously more than 5 seconds after defibrillation of LDVF. The animated display of dV/dt was used to determine the earliest site of activation during refibrillation and the path of wave front propagation away from this site. Early activity was defined as reentrant if an electrode recorded activation more than once during a single propagation wave front.20 In the absence of reentry, early activation was described as focal.
Statistical analysis
Values are given as mean ± standard deviation. The square of the linear regression coefficient (R2) was used to evaluate correlation. Student’s t-tests were used to identify statistical differences among groups. P <.05 was considered statistically significant.
Results
Potential gradient fields
Each animal received three to eight shocks during the SDVF DFT determination and after LDVF. The SDVF DFT energies ranged from 70 to 360 J (Table 1). DFT energy correlated with animal size (R2 = 0.81). The shock strength required for LDVF defibrillation (1660 ± 446 stored V) was not significantly different than the SDVF DFT (1632 ± 429 stored V).
Table 1.
Potential recordings and ∇V calculated for DFT strength shocks after SDVF
| Shock
|
Potential
|
∇V
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Animal | Energy, J | Voltage, V | Maximum, V | Minimum, V | Range, V | % | Maximum, V/cm | Minimum, V/cm | Ratio | Mean, V/cm |
| 1 | 200 | 1594 | 51.1 | −61.4 | 112.5 | 7.1 | 19.3 | 3.3 | 5.8 | 10.9 |
| 2 | 250 | 1782 | 89.3 | −142.5 | 231.8 | 13.0 | 36.0 | 4.6 | 7.8 | 23.9 |
| 3 | 70 | 943 | 10.0 | −56.3 | 66.3 | 7.0 | 8.8 | 4.8 | 1.8 | 5.5 |
| 4 | 150 | 1381 | 11.7 | −48.5 | 60.2 | 4.4 | 12.9 | 3.4 | 3.8 | 8.8 |
| 5 | 360 | 2139 | 81.3 | −232.4 | 313.7 | 14.7 | 45.5 | 9.7 | 4.7 | 25.2 |
| 6 | 300 | 1952 | 72.1 | −105.3 | 177.4 | 9.1 | 49.9 | 11.4 | 4.4 | 17.8 |
| Mean | 221.7 ± 105 | 1632 ± 429 | 52.6 ± 35 | −107.7 ± 71 | 160.3 ± 100 | 9.8 | 28.7 ± 17 | 6.2 ± 3.5 | 4.7 ± 2.0 | 15.4 ± 8.2 |
Maximum = maximum value measured in the heart. Minimum = minimum value measured in the heart.
For DFT strength shocks, the difference between the measured maximum and minimum ventricular voltage (160 ± 100 V) was only 9.8% of the stored voltage. Maximum cardiac ∇V was 28.7 ± 17 V/cm, and minimum ∇V was 6.2 ± 3.5 V/cm. The mean ratio of the highest to lowest ∇V was 4.7 ± 2.0 (Table 1).
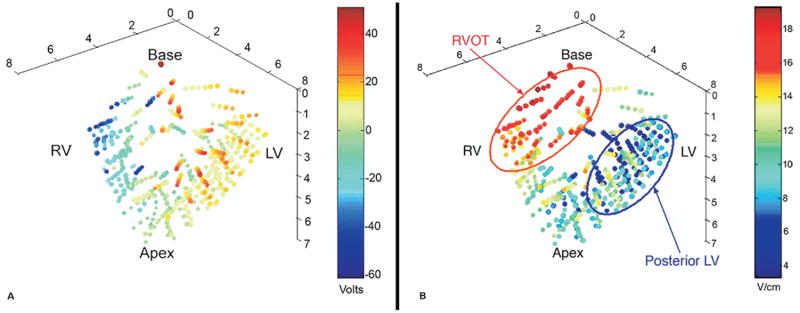
The potential distributions generated by shocks of the same voltage during SDVF and LDVF were similar in the same animal (R2 = 0.81–0.98). The minimum ∇V required for defibrillation was positively correlated with the size of the animal (R2 = 0.75, P = .03) as well as with the DFT voltage (R2 = 0.80, P = .02). There was no significant correlation between the mean ∇V required for defibrillation and animal size (R2 = 0.51, P = .11). ∇V was higher (1) in the RV than in the LV (P <.05), (2) in the anterior than in the posterior third of the ventricles (P <.05), and (3) for epicardial than for endocardial electrodes (P <.05; Table 2). Figure 3 shows an example of the fitted potentials (Figure 3A) and ∇V (Figure 3B).
Table 2.
Mean ∇V (V/cm) distribution by region after a DFT strength shock during SDVF
| Animal | Endocardium | Epicardium | Posterior | Anterior | LV | RV |
|---|---|---|---|---|---|---|
| 1 | 11.0 ± 3.5 | 11.7 ± 4.4 | 7.5 ± 2.4 | 13.3 ± 2.6 | 9.3 ± 2.9 | 13.8 ± 3.6 |
| 2 | 23.5 ± 5.9 | 25.7 ± 7.8 | 17.3 ± 5.1 | 28.5 ± 4.0 | 22.2 ± 6.8 | 27.7 ± 5.7 |
| 3 | 8.8 ± 2.3 | 9.1 ± 3.0 | 7.7 ± 2.5 | 8.9 ± 2.4 | 7.5 ± 2.5 | 11.0 ± 1.2 |
| 4 | 5.5 ± 0.8 | 5.6 ± 1.0 | 5.0 ± 0.1 | 5.9 ± 0.9 | 5.1 ± 0.3 | 6.2 ± 1.1 |
| 5 | 24.6 ± 7.1 | 26.3 ± 8.6 | 17.8 ± 4.0 | 30.3 ± 5.7 | 22.4 ± 4.9 | 29.0 ± 9.3 |
| 6 | 18.0 ± 5.0 | 18.0 ± 5.9 | 15.3 ± 2.2 | 20.0 ± 6.4 | 15.0 ± 2.0 | 21.1 ± 5.9 |
| Mean | 15.2 ± 4.1a | 16.1 ± 5.1a | 11.8 ± 2.7b | 17.8 ± 3.7b | 13.6 ± 3.2c | 18.1 ± 4.5c |
Denotes statistical difference between endocardial and epicardial regions (P <.05).
Denotes statistical difference between posterior and anterior regions (P <.05).
Denotes statistical difference between LV and RV regions (P <.05).
Figure 3.
A 3D map of fitted potentials (A) and ∇V (B) recorded during a 200-J shock in one animal. The top of the heart represents the base, and the bottom represents the apex. The right side contains the LV needles, and the left side contains the RV needles. Color bars indicate the scale used to display the recorded voltage (A) and ∇V (B). The most positive potentials are on the right, and the most negative potentials are on the left, corresponding to the right and left lateral defibrillation pad locations. The ∇V is lowest in the posterior LV and highest in the right ventricular outflow tract (RVOT).
Early postshock activity after defibrillation of SDVF
After SDVF, the interval from the beginning of the shock until earliest recorded postshock activation (the isoelectric interval) for DFT strength shocks ranged from 71 to 5723 ms (1.6 ± 2.2 seconds). Only one type B success occurred. The first postshock cycle was a sinus beat in only two animals after defibrillation of SDVF. The ∇V at the earliest site was more than 1 standard deviation below the mean (low-∇V area) in two animals, more than 1 standard deviation above the mean (high-∇V area) in one animal, and within 1 standard deviation of the mean in the other three animals.
For SDVF failed shocks one step below the DFT, the isoelectric interval ranged from 30 to 78.5 ms (58.2 ± 19.2 ms). The number of early sites ranged from one to five (with an average of two). Only five animals could be analyzed at one step below the DFT because of technical problems with the recordings in one animal. Earliest activity arose from the LV in all five animals analyzed. It arose from the epicardium in three animals and from the midwall in the remaining two animals. There was no difference in the basal-to-apical location of the earliest sites (two basal, two apical, one mid). Two animals had earliest activity arising from the posterior third of the LV, and three had earliest activity arising from the lateral LV. All earliest activity appeared to be focal in origin. There was no consistent correlation between the sites of early activation and local voltage gradients, even though they were rare in high-voltage gradient areas. The ∇V at the earliest site was within 1 standard deviation of the mean in three animals. The earliest site of activity arose from an area >1 standard deviation below the mean (low-∇V area) in one animal and from an area >1 standard deviation above the mean (high-∇V area) in one animal.
Early postshock activity after defibrillation of LDVF
After LDVF, all animals were defibrillated: four with the first shock, either the SDVF DFT (three animals) or 50 J less than the SDVF DFT (one animal). Two animals required two shocks to be defibrillated. One received an SDVF DFT strength shock that failed, followed by a successful shock 50 J greater. The other received a shock 50 J below the SDVF DFT that failed, followed by a successful shock of SDVF DFT strength. All successful shocks were type A, with an isoelectric window ranging from 400 ms to >10 seconds (4.9 ± 4.3 seconds). The shock episode with an isoelectric window >10 seconds was followed by asystole and was given a value of 11 seconds for calculation of the mean and standard deviation of the isoelectric window. The two failed shocks also had long isoelectric intervals: 131 and 155 ms. No type B defibrillation occurred.
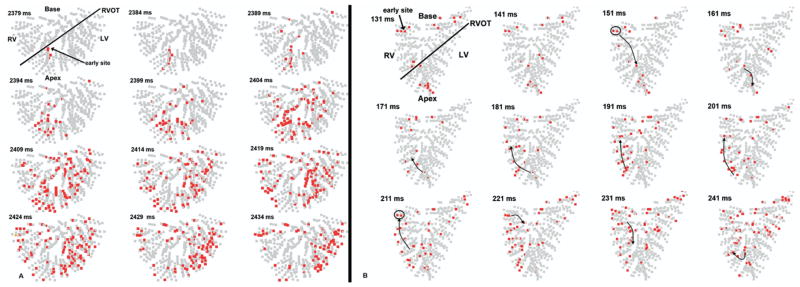
The isoelectric window for both successful and failed episodes was significantly longer for LDVF (3845 ± 4511 ms) than for SDVF (841 ± 1739 ms; P <.05). Despite these long isoelectric intervals, only one animal had a sinus beat immediately after defibrillation of LDVF. The number of early activation sites ranged from zero (prolonged asystole) to five, with a mean of 1.7. Focal spread of early activation occurred after six of the eight LDVF shocks (Figure 4A), while one failed shock was followed by reentry (Figure 4B) and one successful shock produced asystole. The earliest site was recorded by the anterior septal needles in four animals. After successful LDVF shocks without asystole, earliest activity arose from the epicardium in three, the midwall in one, and the endocardium in one. Earliest activation after both failed shocks arose from the posterior endocardium in regions with ∇V <1 standard deviation below the mean.
Figure 4.
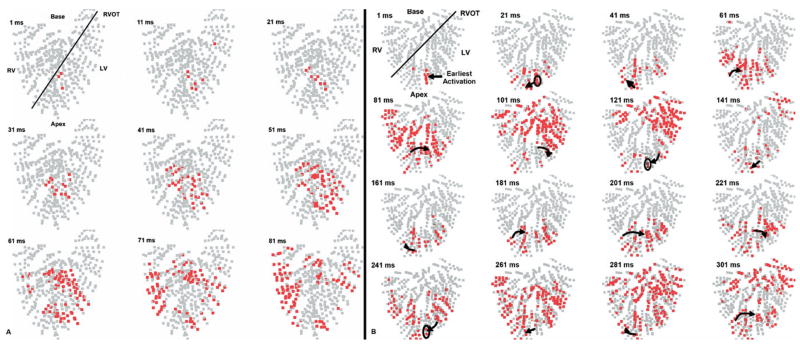
Example of postshock activity after defibrillation of LDVF. In A, each panel shows in red electrode sites at which dV/dt is ≤−0.5 V/s at any time during a 5-ms interval. Frames advance in 5-ms increments from left to right (time stamp represents time from successful shock delivery). The heart is oriented in the anatomical position with the base at the top and the apex at the bottom. The black line separates RV and LV. A: Focal activity after successful defibrillation. Earliest activity arose focally within the anterior septum and spread throughout the ventricles. In B, the panels represent 10-ms time steps. B: Reentry after a failed defibrillation shock. The earliest activity arose in the posterior RV endocardium and formed a reentrant circuit within the posterior RV (black arrows).
Refibrillation
At least one refibrillation episode followed LDVF in five animals; however, no episodes of refibrillation followed SDVF. The mean time to the first refibrillation episode was 71 seconds (range 5.2–139 seconds). Three animals had a second episode of spontaneous VF occurring 100, 166, and 182 seconds after defibrillation of LDVF. The earliest sites of origin of spontaneous VF occurred in the LV in three episodes, in the RV in three episodes, and in the septum in two episodes. They arose from the endocardium in three episodes, the epicardium in two episodes, and the midwall in three episodes. Seventy-five percent of episodes began in the posterior third of the heart. Six (75%) arose focally (Figure 5A), while two (25%) arose by reentry (Figure 5B). Seventy-five percent of the episodes were immediately preceded by premature ventricular beats (PVCs) arising focally from the posterior RV base. Half of these PVCs arose from the endocardium and half from the midwall.
Figure 5.
Example of spontaneous refibrillation after LDVF. In A, each panel shows in red electrode sites at which dV/dt is ≤−0.5 V/s at any time during a 10-ms interval. Frames advance in 10-ms increments from left to right. The earliest activity arises from a focus in the LV near the septum and spreads outward in all directions. In B, the panels represent 20-ms time steps. The earliest activity arises from the apical LV followed by reentry within this region.
Discussion
The major findings of this study are as follows. (1) Only about 10% of the shock voltage for external defibrillation appears across the heart. (2) The ratio of maximum-to-minimum cardiac ∇V for external defibrillation (4.7) is much less than that reported for internal defibrillation (>20).10,11 (3) The minimum ∇V for external defibrillation (6.2 V/cm) is within the range reported for internal defibrillation (2.7–10.9 V/cm).10,11,21 (4) Sites of earliest postshock activation after external defibrillation do not consistently cluster in regions of low ∇V for either SDVF or LDVF. (5) The biphasic DFT is not increased by VF duration; however, the time to earliest postshock activation triples after LDVF. (6) Spontaneous refibrillation is common after LDVF but not after SDVF and usually arises focally in the posterior third of the ventricles.
Shock voltage appearing across the heart
Although defibrillation with electrodes on or in the heart is successful with energies of 5–30 J,22 external defibrillation requires significantly more energy (100–360 J).23 Possible reasons for this increased energy are that (1) only a small amount of the shock voltage appears across the heart or (2) a higher minimum ∇V is required to defibrillate with transthoracic than with internal electrodes. We found that the minimum ∇V required for external defibrillation was within the range of that reported for internal defibrillation, suggesting that a higher minimum ∇V is not required for external defibrillation. Our study found that only 9.8% of the delivered voltage appears across the heart. This is somewhat smaller than the estimate obtained by a modeling study that suggested that approximately 4% of shock current traverses the heart during transthoracic defibrillation in humans.24 The most likely cause for such a small amount of an external shock appearing within the heart is that much of the current is shunted through the skeletal muscle in the rib cage.24,25 However, it has been suggested that the modeling study overestimated the current shunted by the rib cage.26 In our study, larger animals required increased voltage delivery to obtain the minimum ∇V required for defibrillation.
Distribution of ∇V
The ∇V correlates with the DFT for defibrillation electrodes on or in the heart with a minimum ∇V of 2.7–10.9 V/cm required for a biphasic waveform.10,11,21 The cardiac ∇V field is highly uneven after defibrillation using transvenous and epicardial electrode configurations, with a ratio of the highest to lowest ∇V of 15–27:1.10,11,15 Modeling of transthoracic defibrillation of humans predicted that this ratio is much smaller, ~4–5:1, indicating a more even ∇V field.25 Our experimental results confirm this prediction; the mean ratio of highest to lowest ∇V was 4.7. Because the spatial rate of change of ∇V is greatest adjacent to the source electrodes and decreases with distance from them, the main reason that the ∇V distribution is more even for external than for internal defibrillation is probably that the electrodes are farther away from the heart with external defibrillation.
High ∇V can cause damage. Shocks cause temporary inexcitability of normal myocardium, where ∇V is >60 V/cm,27 which is roughly 10 times greater than the minimum ∇V needed to defibrillate. If this same relationship is present during LDVF, then, even if the shock is twice as large in voltage (4 times larger in energy) as needed to defibrillate, it is unlikely to cause this damaging effect during external defibrillation. However, recent evidence suggests that electroporation can occur with cardiac ∇V as low as 25 V/cm,28 a value that could occur during external defibrillation.
Theoretical analysis indicates that both ∇V and its derivative may play a role in defibrillation.29 If the derivative of ∇V is more important, then the more even ∇V field for external electrodes should be less efficient for defibrillation than the more uneven ∇V generated by internal electrodes. However, our finding that the minimum ∇V for external defibrillation is within the range for internal defibrillation suggests that ∇V is more important than its derivative. If so, then the more even ∇V field for external defibrillation wastes much less shock energy than internal defibrillation in cardiac regions in which ∇V is greater than needed to defibrillate. This interpretation is supported by our finding that a mean of only 160 V is needed across the heart for biphasic defibrillation with external electrodes, while approximately 270 V is needed with internal electrodes.11
Relationship of ∇V and sites of early postshock activation
After SDVF, earliest activation arises from areas of low ∇V after failed internal shocks near the DFT in strength.11 In our study, the location of earliest postshock activity did not cluster in the low-∇V region. In the study of internal defibrillation by Wharton et al,11 87% of the early sites of postshock activation occurred in regions in which ∇V was <15 V/cm, a value almost 3 times the minimum ∇V. Because the shock field with the internal electrodes used in that study was so uneven, with a mean maximum ∇V ranging from 106 to 182 V/cm, the region with ∇V <15 V/cm was small. However, because of the much more even field in our study in which the mean ∇V in the LV was 13.6 V/cm, the region with ∇V <15 V/cm was over half the LV. Defibrillation likely involves other factors in addition to a low ∇V, such as, the electrophysiological state of the cells when the shock is given.
The effect of LDVF on defibrillation
Our results indicate that the decrease in the LDVF compared with the SDVF biphasic DFT9,30 is not caused by a significant change in the ∇V field. Therefore, the decreased DFT for LDVF compared with SDVF is caused by an altered response of the myocardium to this field. One marked change in this response is the isoelectric window. Compared with SDVF, the isoelectric window significantly lengthened after LDVF. It is possible that those first post-shock cycles appearing seconds after the shock are not caused by the shock itself but are idioventricular escape beats independent of the shock. This could be an additional explanation for why their sites of origin were not closely related to the ∇V distribution.
Spontaneous refibrillation
While spontaneous refibrillation after SDVF in patients undergoing VF induction during implantation of an ICD is rare, refibrillation occurs frequently after defibrillation of LDVF in individuals with sudden cardiac arrest.5 In our study, spontaneous VF was common after defibrillation of LDVF but not of SDVF in previously healthy swine, indicating that refibrillation does not require preexisting cardiac disease but can be caused solely by the detrimental effects of LDVF. All refibrillation in our study occurred during the first 3 minutes after defibrillation with no refibrillation episodes during the next 12 minutes. Refibrillation has previously been shown to occur within the first minute after reperfusion of regional ischemia in dogs, arising from the border of the ischemic reperfused region and traveling across nonischemic tissue.31 It may be that reperfusion after global ischemia produced by LDVF causes similar reperfusion arrhythmias.
We found that most refibrillation episodes began as focal activity within the posterior third of the ventricles and were preceded by a PVC that originated in the posterior basal RV. Targeting this focal activity and the preceding ventricular ectopy may decrease the incidence of refibrillation.
Limitations
This study was performed in swine and may be limited in its applicability to humans. Studies involving electrically induced VF may not completely represent VF found in clinical practice. Acutely opening and closing the chest and the presence of the plunge needles may have altered the ∇V field produced by the external shocks as well as the cardiac response to the ∇V field,32,33 although the small diameter of the plunge needles may have mitigated some of these effects. The cardiopulmonary bypass perfusion of 1 L/min used in this study may exceed that during cardiopulmonary resuscitation in humans. Cardiopulmonary bypass was not performed after SDVF to see whether it would cause refibrillation. We compared ∇V during external defibrillation to data reported previously for internal defibrillation10,11 but used a different method to calculate ∇V.18 We did not perform internal defibrillation for direct comparison because of the instability of the animal preparation after LDVF. This study is also limited by the small sample size.
Acknowledgments
The authors thank Dennis Rollins for technical assistance and Kate Sreenan for assistance with manuscript preparation.
This work was supported in part by National Heart, Lung, and Blood Institute grant nos. HL85370, HL28429, and HL42760. Dr. Walcott, Dr. Ideker, and Sharon Melnick receive research funding from Physio-Control Corp.
References
- 1.Cobb LA, Fahrenbruch CE, Walsh TR, et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. JAMA. 1999;281:1182–1188. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- 2.Wiggers CJ. The mechanism and nature of ventricular fibrillation. Am Heart J. 1940;20:399–412. [Google Scholar]
- 3.Allison JS, Qin H, Dosdall DJ, et al. The transmural activation sequence in porcine and canine left ventricle is markedly different during long-duration ventricular fibrillation. J Cardiovasc Electrophysiol. 2007;18:1306–1312. doi: 10.1111/j.1540-8167.2007.00963.x. [DOI] [PubMed] [Google Scholar]
- 4.Garcia LA, Allan JJ, Kerber RE. Interactions between CPR and defibrillation waveforms: effect on resumption of a perfusing rhythm after defibrillation. Resuscitation. 2000;47:301–305. doi: 10.1016/s0300-9572(00)00244-6. [DOI] [PubMed] [Google Scholar]
- 5.White RD, Russell JK. Refibrillation, resuscitation and survival in out-of-hospital sudden cardiac arrest victims treated with biphasic automated external defibrillators. Resuscitation. 2002;55:17–23. doi: 10.1016/s0300-9572(02)00194-6. [DOI] [PubMed] [Google Scholar]
- 6.Rosborough JP, Deno DC, Walker RG, et al. A percutaneous catheter-based system for the measurement of potential gradients applicable to the study of transthoracic defibrillation. Pacing Clin Electrophysiol. 2007;30:166–174. doi: 10.1111/j.1540-8159.2007.00645.x. [DOI] [PubMed] [Google Scholar]
- 7.Walcott GP, Melnick SB, Chapman FW, et al. Relative efficacy of monophasic and biphasic waveforms for transthoracic defibrillation after short and long durations of ventricular fibrillation. Circulation. 1998;98:2210–2215. doi: 10.1161/01.cir.98.20.2210. [DOI] [PubMed] [Google Scholar]
- 8.Tang W, Weil MH, Sun S, et al. The effects of biphasic and conventional monophasic defibrillation on postresuscitation myocardial function. J Am Coll Cardiol. 1999;34:815–822. doi: 10.1016/s0735-1097(99)00270-3. [DOI] [PubMed] [Google Scholar]
- 9.Wu TJ, Lin SF, Hsieh YC, et al. Early recurrence of ventricular fibrillation after successful defibrillation during prolonged global ischemia in isolated rabbit hearts. J Cardiovasc Electrophysiol. 2008;19:203–210. doi: 10.1111/j.1540-8167.2007.00979.x. [DOI] [PubMed] [Google Scholar]
- 10.Tang AS, Wolf PD, Afework Y, et al. Three-dimensional potential gradient fields generated by intracardiac catheter and cutaneous patch electrodes. Circulation. 1992;85:1857–1864. doi: 10.1161/01.cir.85.5.1857. [DOI] [PubMed] [Google Scholar]
- 11.Wharton JM, Wolf PD, Smith WM, et al. Cardiac potential and potential gradient fields generated by single, combined, and sequential shocks during ventricular defibrillation. Circulation. 1992;85:1510–1523. doi: 10.1161/01.cir.85.4.1510. [DOI] [PubMed] [Google Scholar]
- 12.Chen P-S, Wolf PD, Claydon FJ, III, et al. The potential gradient field created by epicardial defibrillation electrodes in dogs. Circulation. 1986;74:626–636. doi: 10.1161/01.cir.74.3.626. [DOI] [PubMed] [Google Scholar]
- 13.AHA Special Report. Position of the American Heart Association on research animal use. Circulation. 1985;71:849A–850A. [PubMed] [Google Scholar]
- 14.Rogers JM, Melnick SB, Huang J. Fiberglass needle electrodes for transmural cardiac mapping. IEEE Trans Biomed Eng. 2002;49:1639–1641. doi: 10.1109/TBME.2002.805483. [DOI] [PubMed] [Google Scholar]
- 15.Chen P-S, Shibata N, Dixon EG, et al. Activation during ventricular defibrillation in open-chest dogs: evidence of complete cessation and regeneration of ventricular fibrillation after unsuccessful shocks. J Clin Invest. 1986;77:810–823. doi: 10.1172/JCI112378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolf PD, Rollins DL, Simpson EV, et al. A 528 channel system for the acquisition and display of defibrillation and electrocardiographic potentials. Paper presented at Proceedings of Computers in Cardiology; Los Alamitos, CA. 1993. [Google Scholar]
- 17.Chattipakorn N, Fotuhi PC, Ideker RE. Prediction of defibrillation outcome by epicardial activation patterns following shocks near the defibrillation threshold. J Cardiovasc Electrophysiol. 2000;11:1014–1021. doi: 10.1111/j.1540-8167.2000.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 18.Barnette AR, Bayly PV, Zhang S, et al. Estimation of 3-D conduction velocity vector fields from cardiac mapping data. IEEE Trans Biomed Eng. 2000;47:1027–1035. doi: 10.1109/10.855929. [DOI] [PubMed] [Google Scholar]
- 19.Usui M, Callihan RL, Walker RG, et al. Epicardial sock mapping following monophasic and biphasic shocks of equal voltage with an endocardial lead system. J Cardiovasc Electrophysiol. 1996;7:322–334. doi: 10.1111/j.1540-8167.1996.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Skinner JL, Rogers JM, et al. The effects of acute and chronic amiodarone on activation patterns and defibrillation threshold during ventricular fibrillation in dogs. J Am Coll Cardiol. 2002;40:375–383. doi: 10.1016/s0735-1097(02)01942-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhou X, Daubert JP, Wolf PD, et al. Epicardial mapping of ventricular defibrillation with monophasic and biphasic shocks in dogs. Circ Res. 1993;72:145–160. doi: 10.1161/01.res.72.1.145. [DOI] [PubMed] [Google Scholar]
- 22.Olsovsky MR, Hodgson DM, Shorofsky SR, et al. Effect of biphasic waveforms on transvenous defibrillation thresholds in patients with coronary artery disease. Am J Cardiol. 1997;80:1098–1100. doi: 10.1016/s0002-9149(97)00615-2. [DOI] [PubMed] [Google Scholar]
- 23.Schneider T, Martens PR, Paschen H, et al. Multicenter, randomized, controlled trial of 150-J biphasic shocks compared with 200- to 360-J monophasic shocks in the resuscitation of out-of-hospital cardiac arrest victims. Optimized Response to Cardiac Arrest (ORCA) Investigators. Circulation. 2000;102:1780–1787. doi: 10.1161/01.cir.102.15.1780. [DOI] [PubMed] [Google Scholar]
- 24.Lerman BB, Deale OC. Relation between transcardiac and transthoracic current during defibrillation in humans. Circ Res. 1990;67:1420–1426. doi: 10.1161/01.res.67.6.1420. [DOI] [PubMed] [Google Scholar]
- 25.Camacho MA, Lehr JL, Eisenberg SR. A three-dimensional finite element model of human transthoracic defibrillation: paddle placement and size. IEEE Trans Biomed Eng. 1995;42:572–578. doi: 10.1109/10.387196. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt J, Gatlin B, Eason J, et al. Skeletal muscle grids for assessing current distributions from defibrillation shocks. Crit Rev Biomed Eng. 1992;20:121–139. [PubMed] [Google Scholar]
- 27.Yabe S, Smith WM, Daubert JP, et al. Conduction disturbances caused by high current density electric fields. Circ Res. 1990;66:1190–1203. doi: 10.1161/01.res.66.5.1190. [DOI] [PubMed] [Google Scholar]
- 28.Fedorov VV, Nikolski VP, Efimov IR. Effect of electroporation on cardiac electrophysiology. Methods Mol Biol. 2008;423:433–448. doi: 10.1007/978-1-59745-194-9_34. [DOI] [PubMed] [Google Scholar]
- 29.Sobie EA, Tung L. Postshock potential gradients and dispersion of repolarization in cells stimulated with monophasic and biphasic waveforms. J Cardiovasc Electrophysiol. 1998;9:743–756. doi: 10.1111/j.1540-8167.1998.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 30.Tovar OH, Jones JL. Electrophysiological deterioration during long-duration ventricular fibrillation. Circulation. 2000;102:2886–2891. doi: 10.1161/01.cir.102.23.2886. [DOI] [PubMed] [Google Scholar]
- 31.Ideker RE, Klein GJ, Harrison L, et al. The transition to ventricular fibrillation induced by reperfusion following acute ischemia in the dog: a period of organized epicardial activation. Circulation. 1981;63:1371–1379. doi: 10.1161/01.cir.63.6.1371. [DOI] [PubMed] [Google Scholar]
- 32.Beaudoin DL, Roth BJ. Effect of plunge electrodes in active cardiac tissue with curving fibers. Heart Rhythm. 2004;1:476–481. doi: 10.1016/j.hrthm.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Woods MC, Sidorov VY, Holcomb MR, et al. Virtual electrode effects around an artificial heterogeneity during field stimulation of cardiac tissue. Heart Rhythm. 2006;3:751–752. doi: 10.1016/j.hrthm.2005.11.003. [DOI] [PubMed] [Google Scholar]