Abstract
Pyocyanin (N-methyl-1-hydroxyphenazine), a redox-active virulence factor produced by the human pathogen Pseudomonas aeruginosa, is known to compromise mucociliary clearance. Exposure of human bronchial epithelial cells to pyocyanin increased the rate of cellular release of H2O2 3-fold above the endogenous H2O2 production. Real-time measurements of the redox-potential of the cytosolic compartment using the redox sensor roGFP1 showed that pyocyanin (100 μM) oxidized the cytosol from a resting value of -318 ± 5 mV by 48.0 ± 4.6 mV within 2 hours; a comparable oxidation was induced by 100 μM H2O2. While resting Cl- secretion was slightly activated by pyocyanin (to 10% of maximal currents), forskolin-stimulated Cl- secretion was inhibited by 86%. The decline was linearly related to the cytosolic redox potential (1.8% inhibition/mV oxidation). CF bronchial epithelial cells homozygous for ΔF508 CFTR failed to secrete Cl- in response to pyocyanin or H2O2 indicating that these oxidants specifically target CFTR and not other Cl- conductances. Treatment with pyocyanin also decreased total cellular glutathione levels to 62% and cellular ATP levels to 46% after 24 hours. We conclude that pyocyanin is a key factor that redox cycles in the cytosol, generates H2O2, depletes glutathione and ATP, and impairs CFTR function in Pseudomonas infected lungs.
Keywords: Pseudomonas aeruginosa, Pyocyanin, Hydrogen peroxide, Oxidative stress, Intracellular redox potential, CFTR, Chloride ion transport, Cystic fibrosis, Glutathione
INTRODUCTION
Pyocyanin (N-methyl-1-hydroxyphenazine) is a redox-active virulence factor secreted by Pseudomonsas aeruginosa, an opportunistic pathogen that causes pulmonary infections in hospitalized and immuno-compromised patients, and is a major pathogen associated with respiratory tract infections in cystic fibrosis (CF) [1]. Elevated levels of pyocyanin (as high as 130 μM) have been reported in pulmonary secretions of patients with CF and individuals with chronic bronchiectasis [2], as well as in ear secretions of patients with chronic otitis media [3]. Pyocyanin is secreted by most strains of P. aeruginosa, and its production is increased when the organism is at high density or in the biofilm form [4] as is the case in the lungs of CF patients [5]. Isolates of a CF epidemic strain of P. aeruginosa exhibited a widespread over-production of pyocyanin which was detectable during early exponential growth [3].
Pyocyanin is known to cause a variety of deleterious effects on airway physiology including the slow down of cilia beating [6] and mucociliary transport [7], disruption of epithelial integrity, modulation of immune protein expression [8], and an increase in IL-8 and ICAM-1 production [9]. Using a Saccharomyces cervisiae deletion library, 50 cellular targets for pyocyanin action were identified, including the V-type ATPase protein complex, as well as proteins involved in vesicle and protein transport, cell cycle, electron transport and respiration, and epidermal cell growth [10]. At neutral pH, pyocyanin exists as a zwitter ion that readily penetrates cell membranes, and its cytotoxic activity has been linked to its ability to generate reactive oxygen species (ROS) by redox cycling. The standard redox potential of pyocyanin (Eo’=-34 mV, [11]) is high enough to allow electron transfer from NAD(P)H (Eo’=-320 mV) and glutathione (Eo’=-240 mV) and reduced pyocyanin rapidly reacts with molecular oxygen to produce superoxide (O2.-) which is the precursor for H2O2 and other ROS.
The cystic fibrosis transmembrane conductance regulator (CFTR) protein plays a key role in controlling the mucociliary clearance process in human airways [12, 13] and is mutated in CF. CFTR functions as a cAMP-regulated chloride and HCO3 channel [14], is permeable to other small anions [15], and provides a pathway for the exit of reduced and oxidized glutathione [16, 17]. There is emerging evidence that reactive oxygen and nitrogen species target the activity of CFTR [18-21]. Patch clamp studies demonstrated that oxidizing conditions slow CFTR channel gating, presumably via cysteine residues that are present in the two nucleotide-binding domains and the regulatory domain of CFTR [19, 21]. Oxidized forms of glutathione inhibited CFTR channels by a mechanism that involved the glutathionylation of a cysteine residue at position Cys-1344 of the CFTR molecule [22]. Measurements of transepithelial Cl- secretion in Ussing chambers suggested that the CFTR-mediated Cl- secretion is affected by redox-active reagents, though some studies show activation by H2O2 [23-26], hypochlorus acid [27] and isoprostanes [28], whereas others report inhibition by H2O2 [26, 29], tert-butylhydroquinone [30], and cigarette smoke extract [31, 32]. A recent study dissected the dual effects of H2O2 and reported that secretion was stimulated by both apically and basolaterally applied H2O2 under resting conditions, whereas basolaterally but not apically applied H2O2 potentiated cAMP-stimulated anion secretion [26]. Other evidence for reduced CFTR activity by ROS comes from nasal potential difference measurements in smokers that suggested an acquired CFTR deficiency in the respiratory epithelium [31].
The role of Pseudomonas aeruginosa infection on CFTR has recently been studied and two reports suggested an inhibiton. A cell-free filtrate of P. aeruginosa (PA14) reduced CFTR Cl- transport by inhibiting the endocytic recycling of CFTR [33] and pyocyanin altered CFTR trafficking presumably through inhibition of the organelle H+ V-type ATPase [34].
We hypothesized that pyocyanin, through its ability to redox cycle and produce H2O2, would affect CFTR-mediated Cl- transport in bronchial epithelial cells. We have previously shown that the intracellular redox-state is not affected by the expression of wildtype CFTR [35]. In this study we determined the effects of pyocyanin on both CFTR Cl- transport and real-time oxidation of the cytosolic compartment by image-analysis of a redox-sensitive GFP (roGFP1; [35-37]). Combining these techniques yielded quantitative information about the impact of pyocyanin-induced oxidative stress on CFTR activity and intracellular redox potential. The involvement of CFTR was determined by comparing the Cl- secretory responses to pyocyanin and H2O2 in CFTR-corrected vs. parent CFBE41o- cells homozygous for ΔF508 CFTR as a negative control. Results obtained here suggest that pyocyanin exerted a small stimulatory effect on resting CFTR activity, and a large inhibitory effect on cAMP-stimulated CFTR activity with slow kinetics through a mechanism that involved intracellular oxidation and other factors. Exposure of the apical membrane of CFTR-corrected CFBE41o-monolayers to pyocyanin decreased total cellular GSH by 38% and intracellular ATP by 54% of untreated controls within 24 hours.
MATERIALS AND METHODS
Reagents
Unless otherwise specified, reagents and chemicals were obtained from Sigma (St. Louis, MO). The adenylate cyclase activator forskolin (Calbiochem, La Jolla, CA) was prepared in DMSO as a 20 mM stock solution and used at a final concentration of 20 μM added to the basolateral side; the CFTR-blocker GlyH101 [38] was kindly provided by Drs Nitin Sonawane and Alan Verkman (Univ. of California, San Francisco) or synthesized by ourselves (ADM & MJK) and was prepared as a 20 mM stock in DMSO. Pyocyanin was synthesized as described below or was obtained from Colour Your Enzyme (Toronto, Ontario, Canada), and used from a 10 or 100 mM stock solution in deionized water. Diphenylene iodonium (DPI) was made as a 5 mM stock and used at 1 μM.
Synthesis of pyocyanin
1-Hydroxyphenazine (0.100 g, 0.510 mmol, American Tokyo Kasie, Portland, OR) was added to methyl sulfate (0.579g, 4.918 mmol) and the solution was heated to 100 °C for 10 min in a flask fitted with a calcium chloride drying tube. The solution was allowed to cool to room temperature and dry ether was added (4 ml). A dark brown solid precipitated, was filtered, and washed with 3 portions of dry ether (10 ml). The solid was dissolved in water and made alkaline with several drops of a 10% sodium hydroxide solution. Once added, the solution changed color from a dark brown to a dark blue color. The aqueous layer was extracted exhaustively with chloroform until the organic layer was free of the blue pyocyanin compound. The chloroform extracts were combined and washed with three portions of a 5% hydrochloric acid solution (10 ml). The aqueous layer from the acid washes was made alkaline with 10% sodium hydroxide and extracted with chloroform exhaustively as described previously. The organic layer was dried over sodium sulfate and concentrated. Pyocyanin was recrystallized by dissolving the blue solid in the least amount of water possible and allowing the solution to cool in an ice bath. The crystals were collected and gave pyocyanin as a bright blue solid. Melting point was determined on a Fisher-Johns apparatus and was 131°C (reported 133°C [39]). 1H NMR and IR were recorded in CDCl3 with solvent signals used for reference at 7.26 ppm for 1H and 77.16 for 13C NMR and were consistent with published results [40]. Purity of pyocyanin was > 95% as determined by LCMS, which was accomplished using a Waters Alliance equipped with a Nova-Pak C18 column (3.9 × 150 mm).
Human bronchial epithelial cell culture
The parent CFBE41o- cell line was originally derived from a bronchial tissue isolate from a CF patient homozygous for the ΔF508 CFTR mutation and immortalized with the pSVori- plasmid that contained a replication-deficient simian virus 40 (SV40) genome. For the generation of corresponding CFTR-complemented line, the parental CFBE41o- cell line was transfected with an Epstein-Barr virus (EBV) based episomal expression vector pCEP4 (Invitrogen) containing normal CFTR cDNA via electroporation (Nucleofector II, Amaxa Biosystems). The construct comprised the entire ~6.2 kb CFTR cDNA that contained both the open reading frame and the 5’ and 3’ untranslated regions. CFTR-CFBE41o- cells were cultured in Minimum Essential Medium (Invitrogen) containing 10% fetal bovine serum (HyClone), 2 mM L-glutamine (UCSF cell culture facility), 100 unit/ml penicillin and 100 μg/ml streptomycin (Invitrogen), and were kept under selective pressure using 300 μg/ml hygromycin B (Invitrogen). The culture flasks and permeable filter inserts were coated with an extracellular matrix cocktail comprised of 0.01 mg/ml human fibronectin (BD Biosciences), 0.029 mg/ml Vitrogen (Cohesion, Inc.), and 0.1 mg/ml bovine serum albumin (Biosource/Biofluids). For transepithelial measurements, cells were seeded on Snapwell inserts (0.4-μm pore size, 1.1 cm2; Costar, Cambridge, MA) at a density of 5×105 cells/filter. Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2 and 95% air. Both the parental and CFTR-corrected CFBE41o- cell lines consistently maintained an epithelial phenotype and formed moderately tight epithelial monolayers, expressed Ca-activated Cl- currents, but no amiloride-sensitive Na absorption, and the CFTR-CFBE41o- clone maintained a consistently high level of transgene expression over all observed passages [41].
Measurement of cellular H2O2 production
H2O2 production was measured using the Amplex Red assay kit (Molecular Probes, USA). CFBE41o- cells were grown to confluency in 96-well plates (0.316 cm2 epithelial area, 100 μl fluid volume) and washed twice with glucose-supplemented PBS to remove the cell medium before the assay. Cells were exposed to increasing concentrations of H2O2 in glucose-supplemented PBS at a final volume of 100 μl. Amplex Red reacts in a 1:1 stoichiometry with H2O2 to produce resurofin, which was detected by a fluorescence plate reader (excitation, 530 ± 20 nm,; emission, 590 ± 10 nm, Wallac Victor2, Perkin Elmer). Fluorescence was measured every 30 min and calibrated against a standard curve generated from serial dilutions of H2O2. Rates of epithelial H2O2 production (in nmole·h-1·cm-2) were determined from slopes of calculated H2O2 concentrations vs. time by linear regression. Experiments were performed at least in quadruplicates.
Redox potential measurements using roGFP1 and imaging microscopy
Measurements of cytosolic redox potentials were performed in CFTR-CFB41o- cells transiently expressing redox-sensitive GFP mutant exactly as described [35]. Approximately 100,000 cells were exposed to 100 μM pyocyanin or increasing concentrations of H2O2 in a fluid volume of 100 μl. Briefly, cells were grown on cover glasses and transiently transfected with a plasmid coding for a redox-sensitive GFP mutant (roGFP1 [36, 37]) using Effectene transfection reagent (Qiagen). After 24 to 48 hours, roGFP1-expressing cells were mounted in a chamber on the stage of a Nikon Diaphot microscope with a 40x Neofluar objective (1.4 NA) and bathed in 100 μl Hepes-buffered Ringer’s solution. Ratiometric imaging was performed using a CCD camera, filter wheel (Lambda-10, Sutter Instruments) and Axon Imaging Workbench 4 (Axon CNS Molecular Devices) to collect emission (>510 nm) images during alternate excitation at 385 ± 5 nm and 474 ± 5 nm. Background-subtracted roGFP1 fluorescence ratios were recorded, and at the end of each experiment, maximal oxidation by treatment with 10 mM H2O2 and maximal reduction by treatment with 10 mM DTT was performed to determine the total dynamic range of the dye. Fluorescence ratios were averaged and converted to redox potentials (in mV) using a published in situ calibration curve [35].
Transepithelial short-circuit current measurements
CFBE41o- monolayers grown on Snapwell cell culture inserts were mounted via a slider into a modified Ussing chamber (Easy Mount Chamber Systems, Physiologic Instruments, San Diego, CA). Approximately 1 × 106 cells were exposed to 100 μM pyocyanin or H2O2 in a fluid volume of 5 ml. Transepithelial experiments were performed as previously described [42]. A serosal-to-mucosal Cl- gradient was used to increase the electrochemical driving force for Cl- secretion. The basolateral Ussing chamber solution contained (in mM): 120 NaCl, 20 NaHCO3, 5 KHCO3, 1.2 NaH2PO4, 5.6 glucose, 2.5 CaCl2, and 1.2 MgCl2. In the mucosal solution, all Cl- salts were exchanged for gluconate salts. Both hemi-chambers were constantly gassed with 5% CO2 /air and maintained at 35-37°C.
LC/MS/MS detection of GSH and GSSG
CFTR-CFBE41o- monolayers were grown to confluency on cell culture inserts (Falcon, 0.4- μm pore, 1.0 cm2; BD Labware, Franklin Lakes, NJ) and exposed to 1 ml cell culture medium containing 100 μM pyocyanin from the apical side for 24 hours. Intracellular GSH and GSSG were detected using LC/MS/MS following derivatization with isopropylchloroformate based on methods previously published by Husek et al. [43] with the following modifications. Membranes were cut from the inserts and placed into 500 μL volume of 10 % perchloric acid (PCA) containing 1 mM DTPA and frozen at −80°C. From these samples, the acid-soluble supernatants were collected following centrifugation at 14,000 rpm for 5 min. To the acidified supernatants, homoglutathione (10 μM) was added as an internal control. Subsequent to the addition of internal standards, solid phase extractions with a strong cation exchange resin (phenomenex) were performed to further enrich GSH and GSSG. GSH and GSSG were eluted by treating with 100 μl elution buffer consisting of 0.1 N NaOH, 40% N-propanol, and 10% pyridine. The eluted samples were mixed with 50 μl of derivatizing solution consisting of isooctane, chloroform, and isopropylchloroformate (75:40:10) and allowed to react for 2 min. The derivatized products were subsequently extracted with 200 μl of isooctane and dried under a constant stream of nitrogen. These samples were reconstituted with mobile phase consisting of methanol and water (80:20, v/v). Chromatographic separation of GSH, GSSG, and homoglutathione were performed on C18 reversed-phase column (25 × 2 mm, 0.3 μm) from Phenomenex (Torrance, Ca). The chromatographic system was Shimadzu LC-10AV separation module. The separation was performed under isocratic condition at a flow rate of 0.2 ml/min. Electrospray tandem mass spectrometric analysis was performed on Quattro Micro mass spectrometer from Micromass and analyzed using Masslinks software (3.1 version). Analytes were detected using the multiple reaction monitoring (MRM) scanning mode. Capillary voltage was set to 3 kV, source temperature to 150 C and nebulizer gas temperature to 400 C. The cone and evaporation gas flows were set at 200 and 800L/hr, respectively. Cone voltages for GSH, GSSG, and homoglutathione were set 35 V, 58 V, and 35 V, respectively. Collison energy for GSH, GSSG, and homoglutathione were all set at 20 e. Mass transition pairs monitored for GSH, GSSG and homoglutathione derivatives were 564.5>162.2, 953.5>836.5, and 578.5>162.2. Homoglutathione was used as internal standards for quantification of the GSH/GSSG redox couple. Absolute concentrations were calculated relative to cell number and a cell volume (5 pl per cell).
ATP measurements
Cellular ATP levels were determined using a a bioluminescent ATP assay kit according to the manufacturers instructions (EnzyLight™ ATP assay kit, BioAssay Systems, Hayward, CA). CFTR-corrected CFBE41o- cells were plated on a white, clear bottom 96-well plates (Costar 3610, Corning Incorporated, Corning, NY), grown to confluency and exposed to 100 μM pyocyanin in 100 μl cell culture medium for 24 hours. At the end of the incubation, cells were lysed with 90 μl assay buffer containing substrate (D-luciferin) and ATP enzyme (luciferase). ATP was determined by the amount of light emitted after the reaction of D-luciferin and ATP catalyzed by luciferase. The luminescence signal was measured within 20 minutes using the ATP luminometry mode of the plate reader (Wallac Victor2, Perkin Elmer) with an integration time of 1.0 sec and calibrated to a standard curve. ATP levels were reported in μM per well (0.316 cm2) and average values were calculated from n = 8 wells in each group.
Statistical analysis
Data are presented as original values or as mean ± SE; n refers to the number of experiments. Linear regressions and Hill-Michaelis-Menten fits as described in [42] were done with Sigmaplot (version 10, Systat Software). Effects of treatment were tested using t tests. Comparisons of multiple groups were done by ANOVAs followed by pairwise comparisons using the Holm-Sidak method. Statistical calculations were done using SigmaStat (version 3.5, Systat Software). Resulting p values are given and p<0.05 was considered significant.
RESULTS
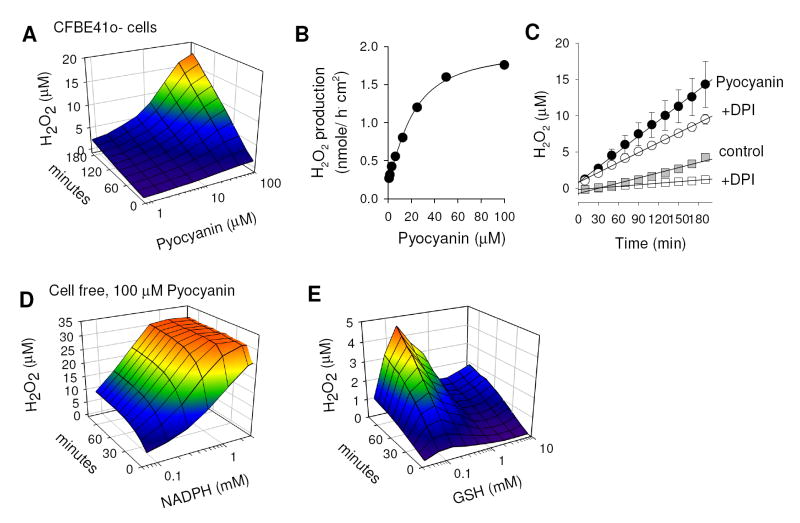
Pyocyanin enhances cellular production of H2O2 in bronchial epithelial cells
Pyocyanin is known to generate reactive oxygen species and H2O2 by its ability to redox cycle with cellular electron donors, such as intracellular NADPH and glutathione [44, 45]. First we tested basal and pyocyanin-induced H2O2 release by human CF bronchial epithelial cells (CFBE41o-). Cells grown in 96-well plates were treated with increasing concentrations of pyocyanin and the release of H2O2 into the extracellular buffer was measured using the Amplex Red reagent. Fig. 1A shows the time- and concentration-dependent effects of pyocyanin on cellular H2O2 production. These data were used to calculate rates of H2O2 release for every pyocyanin concentration by linear regression. Corresponding rates of pyocyanin-induced H2O2 release followed saturation kinetics (Fig. 1B) with a maximal rate of 1.67 ± 0.11 nmole·h-1·cm-2, and a half-maximal concentration of pyocyanin of 21.1 ± 2.0 μM. Because airway epithelial cells have their own mechanism to produce H2O2, which is governed by dual NADPH oxidases (Duox) [46], we used the NADPH oxidase blocker diphenylene iodinium (DPI, 1 μM) to distinguish Duox-derived from pyocyanin-induced H2O2 production. CFBE41o- cells generated H2O2 at a rate of 0.45 ± 0.03 nmole·h-1·cm-2 (Fig. 1C, grey squares), and 0.31 ± 0.03 nmole·h-1·cm-2 (n=25) were blocked by DPI (Fig. 1C, open squares). Treatment with pyocyanin (100 μM; Fig. 1C, filled circles) increased cellular H2O2 production 3-fold to 1.35 ± 0.03 nmole·h-1·cm-2, and 0.48 ± 0.03 nmole·h-1·cm-2 (n=3 were blocked by DPI (Fig. 1C, open circles). Effects of DPI were not different in pyocyanin-treated CFBE41o- cells.
Figure 1. Pyocyanin generates H2O2 in bronchial epithelial cells.
Production of H2O2 by pyocyanin was measured in CFBE41o- cells (A-C) or in cell-free experiments (D&E) using Amplex Red. (A) Time- and concentration-dependency of H2O2 release of CFBE41o- cells incubated with pyocyanin. Measurements were done with ~300,000 cells in 100 μl glucose-supplemented PBS. (B) Dose-dependency of pyocyanin-induced rates of H2O2 production (expressed as per 1 cm2 epithelial area). Line represents fit of data to Hill-Michaelis-Menten function [42]. Maximal rate of H2O2 production was 1.67 ± 0.11 nmole·h-1·cm-2, with a halfmaximal pyocyanin concentration of 21.1 ± 2.0 μM, a Hill coefficient of 1.36 ± 0.15, and a pseudo first-order rate of ~52 pmole·h-1·cm-2 per μM pyocyanin concentration; n = 8. (C) Comparison of H2O2 released from CFBE41o- cells in presence and absence of pyocyanin (100 μM) ± DPI (1 μM). Resulting rates (estimated by linear regression) were 0.45 ± 0.029 nmole·h-1·cm-2 (untreated, n=25), 0.14 ± 0.006 nmole·h-1·cm-2 (untreated + 1 μM DPI), 1.35 ± 0.025 nmole·h-1·cm-2 (pyocyanin, n=3), and 0.87 ± 0.015 nmole·h-1·cm-2 (pyocyanin + 1 μM DPI). (D&E) Time and concentration dependency of H2O2 production by NADPH (D) and GSH (E) in presence of pyocyanin (100 μM). Note different y axis scaling in D and E. A color gradient was added to panels A,D&E to visualize the H2O2 concentration in these plots.
Next we tested the ability of NADPH and GSH to redox cycle and produce H2O2 in presence of pyocyanin in cell free experiments. Both NADPH and GSH have previously been shown to serve as electron donors for pyocyanin [45] and this set of experiments was to quantify their relative contributions and verify their effects in our experimental system. Pyocyanin alone had no measurable effect on the Amplex Red signal in cell free experiments and required the presence of an extracellular reductant. Pyocyanin (100 μM) in presence of NADPH (150 μM) generated H2O2 at a rate of 3.49±0.37 nmole/h (n=4). For comparison, GSH (150 μM) generated H2O2 under the same conditions at a significantly lower rate of 0.115±0.064 nmole/h (n=4). The time-dependent and dose-dependent H2O2 production of NADPH and GSH with 100 μM pyocyanin in cell free experiments is shown in Figs. 1D&E. The H2O2 production in presence of NADPH increased with dose and time and saturated the detection within minutes at concentrations larger than 1 mM (Fig. 1D). GSH showed increased H2O2 production over time but the concentration dependence showed a peak at 150 μM. At higher concentrations the detected H2O2 concentrations decreased greatly (Fig. 1E). A possible explanation is that high millimolar concentrations of GSH reduce the concentration of H2O2 available for detection by Amplex Red (as similarly found for anthracyclines [47]). Despite this possible limitation of this assay, it is apparent that both NADPH and GSH redox cycle with pyocyanin at different rates.
These measurements indicated that i) exposure of CFBE41o- cells to pyocyanin enhanced the generation of H2O2 independent of and additive to the basal H2O2 production, and ii) the intracellular electron donors NADPH and GSH redox cycle with pyocyanin suggesting ready entry of pyocyanin into cells.
Real-time measurements of cytosolic oxidation by pyocyanin and H2O2
The acute effect of pyocyanin on intracellular oxidation was measured using a cytosolic redox-sensitive GFP mutant (roGFP1) that was recombinantly expressed in CFTR-corrected CFBE41o- cells (CFTR-CFBE41o-) and compared to effects of treatment with H2O2. Typical pseudocolor fluorescence ratio images of CFTR-CFB41o- cells expressing roGFP1 are shown in Figs. 2A-D. The resting redox potential of the cytosol was uniformly low and reduced (Fig. 2A,C) averaging -318 ± 5 mV (n = 6), similar to previous results obtained in the CF nasal epithelial cell line CF15 [35]. Exposure to pyocyanin (100 μM) oxidized the cytosol considerably after 2 hours (Fig. 2B), and H2O2 (100 μM) induced a similar oxidation already after 20 min (Fig. 2D). Fig. 2E shows the time-dependent oxidation of the cytosolic redox potential (ERedox) induced by 100 μM pyocyanin and by increasing concentrations of H2O2 from 10 μM to 500 μM. Notably, addition of defined concentrations of H2O2 resulted in rapid cytosolic oxidations that reached a relatively stable plateau within minutes, and step-wise increases of [H2O2] resulted in step-wise oxidations of redox potentials, consistent with the notion that H2O2 entered the cell and oxidized its targets. The stable ERedox attained after addition of H2O2 suggested that cellular reducing mechanisms were overwhelmed by the relatively large amounts of H2O2 added since we previously found in comparable measurements that cells readily reduced ERedox when H2O2 was removed [48, 49]. ERedox was maximally oxidized to about -255 mV at 500 μM H2O2, at which point roGFP1 saturates and is completely oxidized [35]. In contrast, addition of pyocyanin caused a slow and steady oxidation of the cytosol suggesting a constant redox cycling reaction and oxidation analogous to the continuous H2O2 release into the extracellular medium (Fig. 1A). These measurements showed that exposure to 100 μM pyocyanin for a period of 2 hours caused an oxidation of the cytosolic compartment by 48.0 ± 4.6 mV (n=3), a value that was similar to the effects of 100 μM H2O2 (48.1 ± 4.5 mM, n=3; Fig. 2F). This supports the notion that pyocyanin redox cycles with an intracellular electron donor resulting in intracellular oxidation and release of H2O2. These data also suggest that pyocyanin enters the cell quickly since both intracellular oxidation and extracellular H2O2 release is detected within minutes (compare Figs. 1C and 2E).
Figure 2. Oxidation of the cytosol of human bronchial epithelial cells by pyocyanin and H2O2.
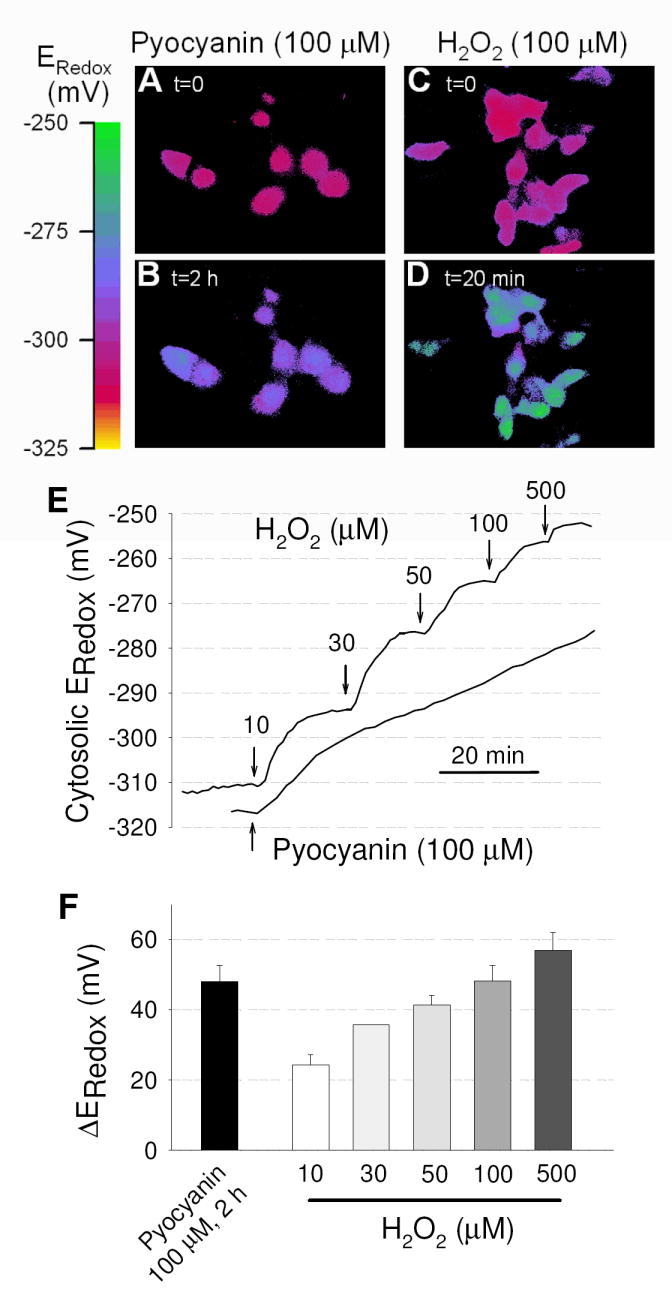
Cytosolic redox potential (Eredox) was measured in CFTR-CFB41o- cells by recombinantly expressing redox-sensitive roGFP1. (A-D) Pseudo-colored fluorescence ratio images of roGFP1-expressing cells during treatment with pyocyanin (A,B) or H2O2. (C,D) Colors correspond to the scale of redox potentials as shown by the scale bar. (E) Time-course of oxidation of the cytosol by pyoycanin (100 μM) in comparison to stepwise addition of 10-500 μM H2O2. Line represents average Eredox of >10 cells. (F) Average change in Eredox measured at steady state. Oxidation by 100 μM pyocyanin reached stable values after 2 hr treatment (black bar) and is compared to effects of increasing doses of H2O2 (open bars). Mean ± SE for 1-3 determinations at each concentration. Measurements were done with ~100,000 cells in 100-500 μl saline solution.
Activation of CFTR Cl- currents by pyocyanin in resting cells
CFTR is activated in cells by hormone- and neurotransmitter-induced cAMP signaling, and the protein kinase A-associated phosphorylation/dephosphorylation is the most thoroughly understood process that regulates CFTR activity. However, there is increasing evidence that other mechanisms modulate the activity of CFTR under certain cellular conditions. To examine the roles of pyocyanin and H2O2 on CFTR function we used the Ussing chamber assay and measured Cl- secretory responses in CF bronchial epithelial cells homozygous for ΔF508 CFTR (CFBE41o-) and CFTR-corrected CF bronchial epithelial cells (CFTR-CFBE41o-). Acute exposure of the apical membrane to pyocyanin (100 μM) activated resting Cl- currents in CFTR-corrected (Fig. 3A) but not in CF bronchial epithelial cells (Fig. 3B) suggesting that pyocyanin stimulated CFTR-mediated Cl- secretion. The pyocyanin-stimulated Cl- current (ICl) was completely blocked by the CFTR inhibitor GlyH101 in CFTR-corrected cells (Fig. 3A) but had no effect in CF cells (Fig. 3B). As a positive control, subsequent addition of ATP (500 μM) to the apical Ussing reservoir elicited a Cl- secretory response in both CFTR-corrected and CF cells, as well as in the presence of GlyH101 (Figs. 3A,B) indicating similar responsiveness of these cell lines to an unrelated agonist that activates the calcium-activated chloride conductance. Acute exposure of the apical membrane to H2O2 (100 μM) induced Cl- secretion in CFTR-corrected (Fig. 3C) but not in CF cells (Fig. 3D), and the H2O2-stimulated ICl was completely blocked by GlyH101. The pyocyanin-stimulated CFTR Cl- current averaged 7.3 ± 0.9 μA/cm2 (n=11) and was small compared to stimulation by 100 μM H2O2 (47.4 ± 5.8 μA/cm2, n=9) or the cAMP-elevating agonist forskolin (67.4 ± 5.3 μA/cm2, n=34) (Fig. 3E). For comparison, ATP-stimulated Cl- currents peaked at similar values for both CFTR-corrected and CF bronchial epithelial cells (on average, 26.5 ± 4.6 μA/cm2, n=23; Fig. 3E).
Figure 3. Activation of resting CFTR Cl- currents by pyocyanin and H2O2 in CFTR-corrected but not CF human bronchial epithelial cells (CFBE41o-).
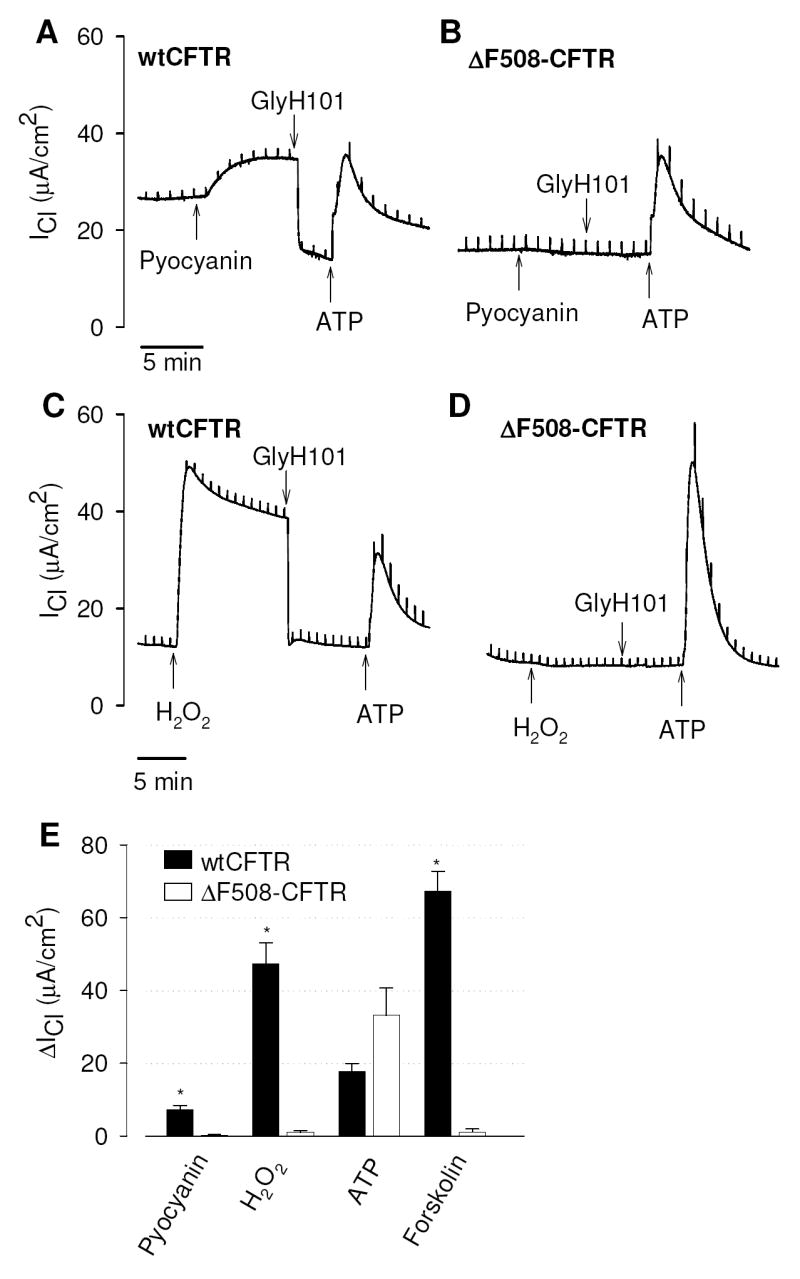
(A) Activation of Cl- currents (ICl) by pyocyanin (100 μM) in CFTR-corrected CF monolayers (wtCFTR) and inhibition of pyoycanin-stimulated ICl by the CFTR blocker GlyH101 (20 μM). (B) Pyocyanin had no effect on ICl in CF monolayers homozygous for ΔF508-CFTR (ΔF508-CFTR) whereas ATP elicited a chloride secretory response similar to wtCFTR-expressing cells. (C,D) Corresponding activation of resting ICl by H2O2 (100 μM) in CFTR corrected but not CF monolayers, and inhibition of H2O2-stimulated ICl by the CFTR blocker GlyH101 (20 μM). Note that both oxidants stimulated the CFTR-mediated but not the calcium-activated Cl- conductance. (E) Summary of stimulatory effects of pyocyanin (100 μM), H2O2 (100 μM), ATP (500 μM), and forskolin (20 μM) on ICl. Data are mean values ± SE (n = 8-34 experiments), * denotes significantly different from wtCFTR, p<0.05. Measurements were done with ~1 Mio cells in 5 ml saline solution.
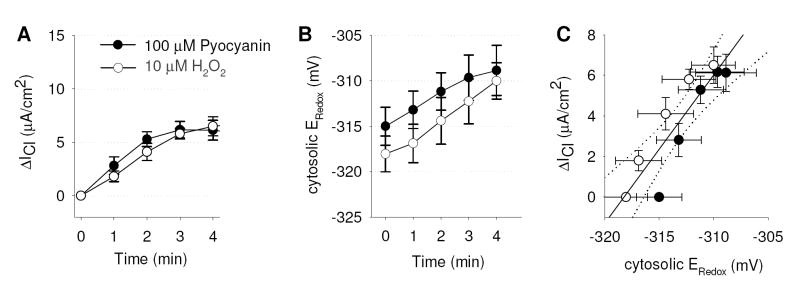
If the effects of pyocyanin on CFTR-dependent ICl were mediated by H2O2 production, it was expected that H2O2 would have similar stimulatory effects on ICl and ERedox. Fig. 4A&B show the acute (first four mins) effects of treatment of CFTR-corrected CF bronchial epithelial cells with 10 μM H2O2 or 100 μM pyocyanin on ICl measured in Ussing chambers (Fig. 4A) and on the cytosolic ERedox measured by fluorescence microscopy (Fig. 4B) using identical protocols for either experimental approach. Treatment of cells with 100 μM pyocyanin resulted in effects similar to those elicited by 10 μM H2O2 (Figs. 4A&B). Changes in cytosolic ERedox and ICl correlated tightly (Fig. 4C), resulting in a relation of 1.41 ± 0.05 μA/cm2 current stimulation per mV of cytosolic oxidation with no apparent difference between the regressions for the effects of H2O2 and pyocyanin (t test, p=0.21).
Figure 4. Short-term activation of CFTR Cl- transport and corresponding cytosolic redox potential changes by pyocyanin.
(A,B) Stimulation of Cl- currents (ΔICl) and intracellular redox potentials (Eredox) by 100 μM pyocyanin or 10 μM H2O2 in CFTR-corrected CFBE41o- monolayers plotted at 0, 1, 2, 3 and 4 minutes. Note that pyocyanin-induced ΔICl and ERedox were similar to those elicited by 10 μM H2O2. (C) Relationship between ΔICl and Eredox; line is regression line with slope = 1.41±0.05 μA·cm-2·mV-1, offset = 446±13.6 μA/cm2; dotted line is 95% confidence interval. Legend in panel A applies to all panels.
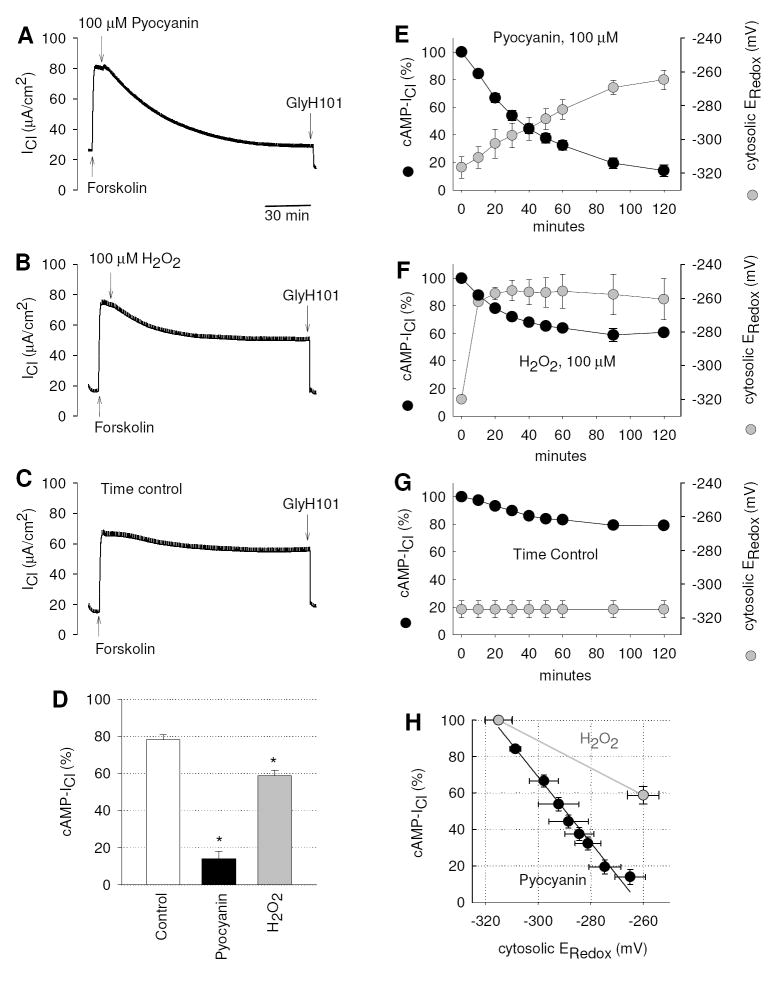
Inhibition of cAMP-stimulated CFTR Cl- currents by pyocyanin
Next we determined the effects of pyocyanin on CFTR Cl- currents under cAMP-stimulated conditions. CFTR Cl- currents were stimulated by the cAMP elevating agonist forskolin (20 μM), and subsequent addition of pyocyanin caused a gradual decline of the forskolin-stimulated ICl (cAMP-ICl) over the course of 2-3 hours (Fig. 5A). A small fraction of the remaining ICl was inhibited by GlyH101. Wash-out of pyocyanin after a 2-hr incubation partially restored CFTR Cl- currents to ~40% (not shown) suggesting that CFTR transport is not irretrievably damaged by pyocyanin. A similar inhibitory effect on cAMP-ICl was observed when 100 μM H2O2 was added to the apical reservoir (Fig. 5B). In a corresponding time control experiment the spontaneous decline of the cAMP-ICl was measured (Fig. 5C). In untreated monolayers cAMP-ICl remained stimulated during a 2-3 hours period, whereas exposure to either pyocyanin or H2O2 significantly inhibited cAMP-ICl by 86.0 ± 5.8% (n=8) and 40.0 ± 2.7% (n=4) respectively (Fig. 5D). A similar inhibitory effect of pyocyanin was observed on CFTR Cl- currents that were stimulated by the cAMP analogue 8-(4-chlorophenylthio)-adenosine (100 μM; 78±5% inhibition, n=2) or the CFTR activator genistein (20 μM; 74±4% inhibition, n=2) suggesting that the mode of inhibition dose not involve effects of proximal CFTR regulators. The observed pyocyanin-induced inhibition of CFTR has important implication for the restoration of CFTR function in infected airways of CF patients.
Figure 5. Long-term inhibition of forskolin-stimulated Cl- transport and corresponding cytosolic redox potential changes by pyocyanin.
Cl- current (ICl) was activated by the cAMP agonist forskolin (20 μM) across CFTR-corrected CFBE41o- monolayers, and subsequent exposure to mucosal (A) Pyocyanin (100 μM) or (B) H2O2 (100 μM) inhibited ICl. (C) Untreated time control experiment. (D) Relative amounts of cAMP-dependent Cl-currents (cAMP-ICl) measured 120 mins after addition of forskolin in time controls, and after treatment with pyocyanin or H2O2, * significantly different from control (ANOVA, p<0.001). (E) Pyocyanin-induced inhibition of forskolin-stimulated ICl (cAMP-ICl) occurs over the course of 2 hours and is paralleled by a gradual oxidation of the cytosol (ERedox) at an average rate of 0.57 mV/min. (F) H2O2-induced inhibition of cAMP-ICl levels off after 60 min and rapidly oxidizes ERedox at a rate of 5.9 mV/min. (G) Untreated time controls. (H) Relationship between inhibition of cAMP-ICl and time-matched corresponding ERedox for treatment with pyocyanin. For H2O2, data are plotted at time = 0 and after 120 min. Slopes were significantly different, H2O2, 0.75±0.07 %/mV, and pyocyanin, 1.81±0.1 %/mV (p<0.001, t test).
Time course comparisons of the inhibitory effects of pyocyanin and H2O2 (both 100 μM) on cytosolic oxidation and forskolin-stimulated ICl are shown in Figures 5E-G. The pyocyanin-induced inhibition of cAMP-ICl occurred with a slow time course similar to the oxidation of the cytosol, and the initial rate of oxidation averaged 0.57 mV/min (Fig. 5E). In contrast, H2O2 caused a ten times faster oxidation with an initial rate of 5.9 mV/min. ERedox stabilized within 20 minutes and the decline of cAMP-ICl by H2O2 reached stable values after 60 min (Fig. 5F). Both pyocyanin and H2O2 (at 100 μM each) elicited similar oxidations of the cytosolic redox potentials after two hours of treatment. However, the effect of pyoycanin on cAMP-ICl was significantly larger than that of H2O2 (ANOVA, p<0.001, Fig. 5D) suggesting that pyocyanin generated H2O2 and had additional effects that inactivated CFTR. This is further supported by the data shown in Fig. 5H, where the remaining portion of the cAMP-ICl is plotted vs. the corresponding ERedox, i.e., the time-independent relation between decline in forskolin-stimulated Cl- current and degree of cytosolic oxidation. The inhibition of cAMP-ICl shows significantly different dependencies on ERedox when treated with either pyocyanin or H2O2 (100 μM each). Treatment with pyocyanin blocked 1.81 ± 0.1% of ICl per mV change of ERedox, while treatment with H2O2 blocked 0.75 ± 0.07% of ICl per mV change of ERedox (p<0.001). This indicated a significantly stronger inhibition of cAMP-stimulated CFTR activity by pyocyanin than by H2O2 at comparably oxidized redox potentials.
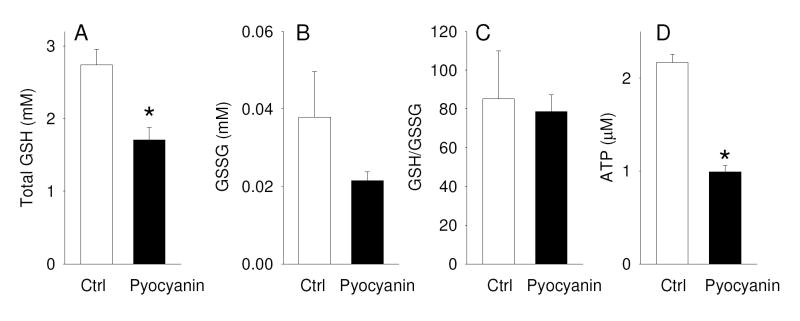
Chronic exposure to pyocyanin results in loss of total cellular GSH
Measurements of the cytosolic redox potential using RoGFP1 showed that chronic exposure to pyocyanin shifted ERedox towards oxidation by 48.0 ± 4.6 mV, and we further determined whether this change in ERedox caused alterations in the intracellular levels of glutathione (GSH) and glutathione disulfide (GSSG). Incubation of the apical cell membrane of CFTR-corrected CFBE41o- cells (grown on 1 cm2 filter inserts) with 1 ml cell culture medium containing 100 μM pyocyanin over a period of 24 hours resulted in a significant decrease of total cellular GSH levels to 62% of untreated control cells. Assuming a cell volume of 5 pl and unchanged volumes during pyocyanin exposure, we estimate that the total cellular GSH concentration dropped from 2.7 mM to 1.7 mM. (Fig. 6A). Determination of intracellular levels of GSSG showed that pyocyanin did not produce a significant increase in GSSG levels in CFTR-CFBE41o- cells (Fig. 6B) and the GSH/GSSG redox couple was not significantly changed (Fig. 6C) suggesting that pyoycanin depleted the intracellular antioxidant defense.
Figure 6. Effect of pyocyanin on GSH, GSSG, and ATP levels in CFBE41o- cells.
CFTR-corrected CFBE41o- monolayers grown on cell culture inserts (area: 1 cm2) were incubated with 1 ml cell culture medium in the absence (ctrl) or presence of 100 μM pyocyanin from the apical side for 24 h. (A) Total cellular GSH, (B) GSSG and (C) GSH/GSSG ratio were determined. Results shown are mean±SE, n=3. (D) Cells were grown in a 96-well plate (0.316 cm2) and incubated with 100 μl cell culture medium in the absence or presence of 100 μM pyocyanin for 24 h, following which intracellular ATP levels were determined (n=8). *, significantly different from control, t-test. Measured concentrations are reported for the lysate (~50,000 cell in 100 μl).
Because CFTR-mediated Cl secretion is dependent on intracellular ATP levels and pyocyanin is known to deplete cells of ATP [50, 51] we further investigated the effects of treatment of CFBE41o- cells with 100 μM pyocyanin on intracellular ATP levels. Fig. 6D illustrates that pyocyanin decreased intracellular ATP levels to 46% within 24 hours. A reduction in intracellualr ATP levels is expected to significantly impair CFTR function [52, 53]. Thus, treatment of cells with pyocyanin resulted in significant reduction in intracellular levels of both GSH and ATP.
DISCUSSION
The present study showed that physiologically relevant concentrations of pyocyanin resulted in 1) increased H2O2 production by bronchial epithelial cells to 3-fold above endogenous rates, 2) oxidation of the cytosol by −48 mV, 3) rapid stimulation of resting CFTR Cl- currents, 4) long-term inhibition of cyclic AMP-activated CFTR Cl- currents, 5) loss of ~1/3 of total cellular GSH, and 6) loss of ~1/2 of cellular ATP. The measured Cl- currents were identified as CFTR-mediated by comparing Cl- secretion in CF vs. CFTR-corrected bronchial epithelial cells and by inhibition with the CFTR blocker GlyH101. The observed stimulation and inhibition of CFTR by pyocyanin were kinetically distinct, of different magnitude, and showed differential dependence on the intracellular redox potential indicating that distinct cellular mechanisms were affected by pyocyanin.
Activation of CFTR by pyocyanin
Addition of pyocyanin to resting cells rapidly (maximal stimulation within 3-5 min) activated CFTR-mediated Cl- currents (7.3 ± 0.9 μA/cm2) to 9.3% of forskolin-stimulated currents (78 ± 2.5 μA/cm2). The initial effect of pyocyanin on the stimulation of CFTR and ERedox was mimicked by addition of 10 μM H2O2 suggesting that pyocyanin elicited its effects through cellular production of H2O2. The pyocyanin and H2O2-induced stimulation of CFTR-mediated Cl- currents in CFTR-corrected CF cells is consistent with a previous study in Calu-3 cells [24, 26] that reported a rapid DPC-blockable increase in transepithelial Cl- secretion by H2O2. Notably, the H2O2 concentrations previously used to stimulate CFTR Cl- currents in Calu-3 cells were 10 to 50-times higher when compared to CFTR-corrected CFBE41o- cells under similar experimental conditions (1-5 mM, [24, 26]). Since the recording conditions between these studies were similar, this suggests a cell type-dependent sensitivity to H2O2 treatment.
Oxidation of the cytosolic redox potential correlated tightly with activation of CFTR Cl- currents during the first few minutes of treatment with either pyocyanin or H2O2 (Fig. 4C). Taken together with data showing pyocyanin stimulated H2O2 production by cells, these data indicated that the effects of pyocyanin on CFTR resulted from pyocyanin-induced production of cellular H2O2 by redox cycling, which oxidized the cytosol and activated CFTR. Whether this is a direct or indirect effect on CFTR is currently unclear because both CFTR itself [20, 21] and its major regulators (PKA, phosphatases [54]) have been shown to be redox sensitive.
Inhibition of CFTR by pyocyanin
Unlike the rapid onset for the stimulation of CFTR activity by pyocyanin, its inhibition of forskolin-stimulated CFTR was characterized by its markedly slower kinetics, where maximal inhibitory effects were reached only after 2-3 hours. Some of the CFTR inhibition likely resulted from pyocyanin-induced oxidation, as shown by the inhibitory effects of H2O2-induced oxidation. Previous studies showed that, oxidative stress (by treatment with tert-butylhydroquinone) reduced CFTR expression by increasing the rate of mRNA degradation in T84 cells [30]. In addition, the V-type H+ ATPase in airway cells was inhibited by pyocyanin, resulting in reduced trafficking of CFTR to the plasma membrane [34]. The slow kinetics of CFTR inactivation that we have observed after pyocyanin treatment appears consistent with these observations.
Our data also suggest that the inhibitory effect of pyocyanin on CFTR Cl- currents includes an additional non-redox-mediated component: although both pyocyanin and H2O2 inhibited forskolin-stimulated CFTR, the effect of pyocyanin was larger (pyocyanin blocked 86% of CFTR current compared to 40% by H2O2, Fig. 5D), and its relation to the cytosolic redox potential was steeper (1.8% change of current per mV) compared to H2O2 (0.75% change of current per mV; Fig. 5H). Thus, the block of CFTR by pyocyanin was only partially mimicked by the addition of H2O2 despite similar effects on the cytosolic redox potential. This suggests that pyocyanin exerted inhibitory effects on CFTR Cl- currents that involved additional cellular targets, as proposed previously for the inhibition of catalase [55], ciliary beating [6] and cytoplasmic ATP concentrations [50]), although some of these observations may have resulted through indirect redox effects of pyocyanin. Previous determinations of whether an observed effect was redox-dependent or redox-independent have relied largely on experimental maneuvers that mimic oxidation (e.g., by addition of H2O2) or that inhibits oxidation (e.g., by addition of catalase). In our study we continuously measured cytosolic redox potentials and Cl- currents during the experimental manipulations, which allowed us to quantitatively relate the redox potentials to CFTR Cl- currents. Based on this approach (Fig. 5H) we conclude that <50% of pyocyanin-inhibition of CFTR occurred through an oxidation-mediated effect that was mimicked by H2O2-induced oxidation, while a large fraction (>50%) of pyocyanin’s inhibitory effect may be governed by other effects.
We found that treatment of CFBE41o- cells with pyocyanin results in a significant decrease of ATP concentrations, which was also previously noted [50]. In this regard, it is important to consider the involvement of the AMP-activated protein kinase (AMPK) which is a ubiquitous metabolic sensor that responds to small changes in intracellular ATP levels. Also, CFTR has the unique property that it requires ATP for its gating suggesting that its activity is coupled to the cellular metabolic state. AMPK was discovered to interact with CFTR and inhibited its activity in transepithelial experiments in airway epithelial cells [56]. CFBE41o- cells have been shown to express AMPK [57] and it is possible that pyocyanin caused an activation of AMPK thereby leading to an inhibition of CFTR activity.
Loss of total cellular GSH by pyocyanin
Reduced glutathione is the most abundant cellular thiol antioxidant and plays a critical role in the maintenance of the intracellular redox balance and redox-sensitive signaling events. A previous study showed that pyocyanin modulated the redox status in a complex manner [44]. By redox cycling with intracellular NADPH pyoycanin generated H2O2, which caused the formation of mixed disulfides by oxidation of protein and nonprotein thiol groups including GSH [44]. Since glutathione reductase must compete with pyocyanin for NADPH in order to reduce GSSG back to GSH, it became the rate limiting step that lowered intracellular levels of free sulfhydryl groups, including GSH. Our roGFP1 measurements showed that pyocyanin oxidized the cytosol by -48.0 ± 4.6 mV within 2-3 hours in CFTR-corrected CFBE41o- cells and a similar oxidation was reported in CF15 cells [48] suggesting that CFTR expression did not alter pyocyanin-induced oxidation of the cytosol. To complement these measurements we quantified the reduced and oxidized form of glutathione in cell lysates of CFTR-corrected CFBE41o- monolayers. Exposure to pyocyanin caused a significant decline of total cellular GSH to 62% of untreated monolayers within 24 hours. A similar value was reported in A549 lung epithelial cells and 16HBE14o- bronchial epithelial cells where total cellular GSH declined to 60% of untreated controls within 24 hours [45], and the cellular loss was accounted for by an efflux of GSH into the media [45]. Effects of pyocyanin on intracellular GSH levels have been detected as early as 30 min after exposure to pyocyanin [44]. We found that pyocyanin did not lead to a detectable increase in cellular GSSG in CFTR-corrected CFBE41o- cells, and a similar finding was previously reported in A549 lung epithelial cells [45] and HUVEC human endothelial cells, whereas 16HBE14o- bronchial epithelial cells showed a detectable increase in GSSG [45].
There are several possibilities that might have caused the loss of cellular GSH including the formation of H2O2 and mixed disulfides [44], a direct reaction between pyocyanin with cellular GSH (Fig. 1E and [45]), increased efflux of GSH from airway epithelial cells [45], as well as GSH conjugation [58], however no evidence was found for the conjugation of GSH with either the oxidized or reduced pyoycanin under cell-free conditions [44]. It is currently not known if pyocyanin decreases GSH by affecting the activity of enzymes involved in GSH synthesis.
The depletion of GSH by pyocyanin is of concern in chronic pseudomonal lung disease and in particular in patients with cystic fibrosis who are characterized by a systemic deficiency of GSH in the epithelial lining fluid in the lung [59]. The airways of most patients with CF become chronically infected with P. aeruginosa, and pyocyanin levels as high as ~130 μM have been measured in the sputum. A number of reports showed that pulmonary GSH levels are decreased in CF patients [59, 60] and several studies reported alterations in glutathione transport which were associated with the ΔF508 mutation of the CFTR chloride channel [16, 61]. Based on these reports we have previously measured the intracellular redox potential in CF and CFTR-corrected nasal epithelial cells and found that the cellular redox potential was not affected by CFTR expression. Based on these findings we consider that the presence of P. aeruginosa-derived pyocyanin could be the primary source that leads to oxidative stress and impairments in GSH status in the airways of CF patients in vivo.
Our transepithelial experiments showed that a decline in cellular GSH by 40% was associated with an inactivation of the cAMP-dependent CFTR Cl- transport by 86% in CFTR-corrected CF bronchial epithelial cells. These data suggest that intracellular GSH levels may be important for the proper function of the cAMP-dependent CFTR Cl- transport. CFTR possesses 18 cysteine residues that are possible targets for oxidation, and several oxidized forms of glutathione have been shown to glutathionylate and inhibit the CFTR channel [22]. In addition, the depletion of intracellular GSH was paralleled by a fall in intracellular ATP levels to 46% of untreated controls. It is well known that ATP is important for the proper gating of the CFTR channel, and therefore loss of cellular ATP might present a factor for the inhibition of CFTR activity by pyocyanin.
In conclusion, this study demonstrated that pathophysiological concentrations of pyocyanin adversely affect bronchial epithelial cell redox status and CFTR function indicating that the salt and water transport by the airway epithelium and, as a result, the composition of the airway surface liquid will be affected by the presence of pyocyanin in Pseudomonas aeruginosa-infected lungs. The major effect of pyocyanin, i.e., the substantial inhibition of active CFTR Cl- transport (Fig. 5D), is a critical component that will contribute to the impairments in epithelial fluid secretion and mucociliary clearance, and thus support the colonization of P. aeruginosa. In the CF lung, pyocyanin is a critical factor that needs to be considered for the outcome of clinical gene and drug therapy studies that are aimed at restoring CFTR function in the airway epithelium. The presence of high levels of pyocyanin in infected CF lungs may interfere with functional outcome measures for CFTR activity and hamper CF treatments.
Acknowledgments
KB was a recipient of a Summer Student Award from the Elizabeth Nash Foundation. This study was supported by Cystic Fibrosis Research Inc. (to EK, CS), Cystic Fibrosis Foundation (FISCHE07G0, MACHEN07G0, ILLEK08G0), Philip Morris USA Inc (to HF), and NIH grants AT0026020 (BI, JS) and HL86323 (HF).
LIST OF ABBREVIATIONS
- cAMP-ICl
forskolin-stimulated chloride current
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- CFBE41o-
cystic fibrosis bronchial epithelial cell line
- DPI
diphenyl iodinium
- GSH
reduced glutathione
- GSSG
glutathione disulfide
- ICl
transepithelial chloride current
- NADPH
nicotinamide adenine dinucleotide phosphate
- roGFP1
redox sensitive green fluorescent protein mutant
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen-Host Interactions in Pseudomonas aeruginosa Pneumonia. Am J Respir Crit Care Med. 2005;171:1209–1223. doi: 10.1164/rccm.200408-1044SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson R, Sykes DA, Watson D, Rutman A, Taylor GW, Cole PJ. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect Immun. 1988;56:2515–2517. doi: 10.1128/iai.56.9.2515-2517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reimer A, Edvaller B, Johansson B. Concentrations of the Pseudomonas aeruginosa toxin pyocyanin in human ear secretions. Acta Otolaryngol Suppl. 2000;543:86–88. doi: 10.1080/000164800454062. [DOI] [PubMed] [Google Scholar]
- 4.Schaber JA, Carty NL, McDonald NA, Graham ED, Cheluvappa R, Griswold JA, Hamood AN. Analysis of quorum sensing-deficient clinical isolates of Pseudomonas aeruginosa. J Med Microbiol. 2004;53:841–853. doi: 10.1099/jmm.0.45617-0. [DOI] [PubMed] [Google Scholar]
- 5.Lyczak JB, Cannon CL, Pier GB. Lung Infections Associated with Cystic Fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanthakumar K, Taylor G, Tsang KW, Cundell DR, Rutman A, Smith S, Jeffery PK, Cole PJ, Wilson R. Mechanisms of action of Pseudomonas aeruginosa pyocyanin on human ciliary beat in vitro. Infect Immun. 1993;61:2848–2853. doi: 10.1128/iai.61.7.2848-2853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munro NC, Barker A, Rutman A, Taylor G, Watson D, McDonald-Gibson WJ, Towart R, Taylor WA, Wilson R, Cole PJ. Effect of pyocyanin and 1-hydroxyphenazine on in vivo tracheal mucus velocity. J Appl Physiol. 1989;67:316–323. doi: 10.1152/jappl.1989.67.1.316. [DOI] [PubMed] [Google Scholar]
- 8.Denning GM, Iyer SS, Reszka KJ, O’Malley Y, Rasmussen GT, Britigan BE. Phenazine-1-carboxylic acid, a secondary metabolite of Pseudomonas aeruginosa, alters expression of immunomodulatory proteins by human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L584–592. doi: 10.1152/ajplung.00086.2003. [DOI] [PubMed] [Google Scholar]
- 9.Look DC, Stoll LL, Romig SA, Humlicek A, Britigan BE, Denning GM. Pyocyanin and Its Precursor Phenazine-1-Carboxylic Acid Increase IL-8 and Intercellular Adhesion Molecule-1 Expression in Human Airway Epithelial Cells by Oxidant-Dependent Mechanisms. J Immunol. 2005;175:4017–4023. doi: 10.4049/jimmunol.175.6.4017. [DOI] [PubMed] [Google Scholar]
- 10.Ran H, Hassett DJ, Lau GW. Human targets of Pseudomonas aeruginosa pyocyanin. Proc Natl Acad Sci USA. 2003;100:14315–14320. doi: 10.1073/pnas.2332354100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedheim E, Michaelis L. Potentiometric study of pyocyanine. J Biol Chem. 1931;91:355–368. [Google Scholar]
- 12.Boucher RC. Regulation of airway surface liquid volume by human airway epithelia. Pfluegers Arch. 2003;445:495–498. doi: 10.1007/s00424-002-0955-1. [DOI] [PubMed] [Google Scholar]
- 13.Pilewski JM, Frizzell R. Role of CFTR in Airway Disease. Physiol Rev. 1999;79:215–255. doi: 10.1152/physrev.1999.79.1.S215. [DOI] [PubMed] [Google Scholar]
- 14.Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci USA. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Illek B, Tam AW-K, Fischer H, Machen TE. Anion selectivity of apical membrane conductance of Calu 3 human airway epithelia. Pflugers Arch. 1999;437:812–822. doi: 10.1007/s004240050850. [DOI] [PubMed] [Google Scholar]
- 16.Linsdell P, Hanrahan JW. Glutathione permeability of CFTR. Am J Physiol. 1998;275:C323–326. doi: 10.1152/ajpcell.1998.275.1.C323. [DOI] [PubMed] [Google Scholar]
- 17.Kogan I, Ramjeesingh M, Li C, Kidd JF, Wang Y, Leslie EM, Cole SPC, Bear CE. CFTR directly mediates nucleotide-regulated glutathione flux. EMBO J. 2003;22:1981–1989. doi: 10.1093/emboj/cdg194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bebok Z, Varga K, Hicks JK, Venglarik CJ, Kovacs T, Chen L, Hardiman KM, Collawn JF, Sorscher EJ, Matalon S. Reactive oxygen nitrogen species decrease cystic fibrosis transmembrane conductance regulator expression and cAMP-mediated Cl- secretion in airway epithelia. J Biol Chem. 2002;277:43041–43049. doi: 10.1074/jbc.M203154200. [DOI] [PubMed] [Google Scholar]
- 19.Cotten JF, Welsh MJ. Covalent Modification of the Regulatory Domain Irreversibly Stimulates Cystic Fibrosis Transmembrane Conductance Regulator. J Biol Chem. 1997;272:25617–25622. doi: 10.1074/jbc.272.41.25617. [DOI] [PubMed] [Google Scholar]
- 20.Harrington MA, Gunderson KL, Kopito RR. Redox reagents and divalent cations alter the kinetics of cystic fibrosis transmembrane conductance regulator channel gating. J Biol Chem. 1999;274:27536–27544. doi: 10.1074/jbc.274.39.27536. [DOI] [PubMed] [Google Scholar]
- 21.Harrington MA, Kopito RR. Cysteine residues in the nucleotide binding domains regulate the conductance state of CFTR channels. Biophys J. 2002;82:1278–1292. doi: 10.1016/S0006-3495(02)75484-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Oliva C, Li G, Holmgren A, Lillig CH, Kirk KL. Reversible Silencing of CFTR Chloride Channels by Glutathionylation. J Gen Physiol. 2005;125:127–141. doi: 10.1085/jgp.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen T, Canada A. Modulation of human colonic T84 cell secretion by hydrogen peroxide. Biochem Pharmacol. 1994;47:403–410. doi: 10.1016/0006-2952(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 24.Cowley EA, Linsdell P. Oxidant stress stimulates anion secretion from the human airway epithelial cell line Calu-3: implications for cystic fibrosis lung disease. J Physiol (Lond) 2002;543:201–209. doi: 10.1113/jphysiol.2002.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soodvilai S, Jia Z, Yang T. Hydrogen peroxide stimulates chloride secretion in primary inner medullary collecting duct cells via mPGES-1-derived PGE2. Am J Physiol Renal Physiol. 2007;293:F1571–1576. doi: 10.1152/ajprenal.00132.2007. [DOI] [PubMed] [Google Scholar]
- 26.Ohashi T, Ito Y, Matsuno T, Sato S, Shimokata K, Kume H. Paradoxical Effects of Hydrogen Peroxide on Human Airway Anion Secretion. J Pharmacol Exp Ther. 2006;318:296–303. doi: 10.1124/jpet.106.102541. [DOI] [PubMed] [Google Scholar]
- 27.Venglarik CJ, Giron-Calle J, Wigley AF, Malle E, Watanabe N, Forman HJ. Hypochlorous acid alters bronchial epithelial cell membrane properties and prevention by extracellular glutathione. J Appl Physiol. 2003;95:2444–2452. doi: 10.1152/japplphysiol.00002.2003. [DOI] [PubMed] [Google Scholar]
- 28.Joy AP, Cowley EA. 8-iso-PGE2 Stimulates Anion Efflux from Airway Epithelial Cells via the EP4 Prostanoid Receptor. Am J Respir Cell Mol Biol. 2008;38:143–152. doi: 10.1165/rcmb.2006-0295OC. [DOI] [PubMed] [Google Scholar]
- 29.DuVall MD, Guo Y, Matalon S. Hydrogen peroxide inhibits cAMP-induced Cl- secretion across colonic epithelial cells. Am J Physiol Cell Physiol. 1998;275:C1313–1322. doi: 10.1152/ajpcell.1998.275.5.C1313. [DOI] [PubMed] [Google Scholar]
- 30.Cantin AM, Bilodeau G, Ouellet C, Liao J, Hanrahan JW. Oxidant stress suppresses CFTR expression. Am J Physiol Cell Physiol. 2006;290:C262–270. doi: 10.1152/ajpcell.00070.2005. [DOI] [PubMed] [Google Scholar]
- 31.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic Fibrosis Transmembrane Conductance Regulator Function Is Suppressed in Cigarette Smokers. Am J Respir Crit Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 32.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol. 2005;288:L894–902. doi: 10.1152/ajplung.00376.2004. [DOI] [PubMed] [Google Scholar]
- 33.Swiatecka-Urban A, Moreau-Marquis S, MacEachran DP, Connolly JP, Stanton CR, Su JR, Barnaby R, O’Toole GA, Stanton BA. Pseudomonas aeruginosa inhibits endocytic recycling of CFTR in polarized human airway epithelial cells. Am J Physiol Cell Physiol. 2006;290:C862–872. doi: 10.1152/ajpcell.00108.2005. [DOI] [PubMed] [Google Scholar]
- 34.Kong F, Young L, Chen Y, Ran H, Meyers M, Joseph P, Cho Y, Hassett D, Lau G. Pseudomonas aeruginosa pyocyanin inactivates lung epithelial vacuolar ATPase-dependent cystic fibrosis transmembrane conductance regulator expression and localization. Cell Microbiol. 2006;8:1121–1133. doi: 10.1111/j.1462-5822.2006.00696.x. [DOI] [PubMed] [Google Scholar]
- 35.Schwarzer C, Illek B, Suh JH, Remington SJ, Fischer H, Machen TE. Organelle redox of CF and CFTR-corrected airway epithelia measured with roGFP1. Free Radic Biol Med. 2007;43:300–316. doi: 10.1016/j.freeradbiomed.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanson GT, Aggeler R, Oglesbee D, Cannon M, Capaldi RA, Tsien RY, Remington SJ. Investigating Mitochondrial Redox Potential with Redox-sensitive Green Fluorescent Protein Indicators. J Biol Chem. 2004;279:13044–13053. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 37.Dooley CT, Dore TM, Hanson GT, Jackson WC, Remington SJ, Tsien RY. Imaging Dynamic Redox Changes in Mammalian Cells with Green Fluorescent Protein Indicators. J Biol Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 38.Muanprasat C, Sonawane ND, Salinas D, Taddei A, Galietta LJV, Verkman AS. Discovery of glycine hydrazide pore-occluding CFTR inhibitors: mechanism, structure-activity analysis, and In vivo efficacy. J Gen Physiol. 2004;124:125–137. doi: 10.1085/jgp.200409059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Budavari S, O’Neil MJ, Smith AE, Heckelman PE, Kinneary JF. The Merck Index. 12. Whitehouse Station, NJ: Merck Research Laboratories; 1996. [Google Scholar]
- 40.Funayama S, Eda S, Komiyama K, Omura S, Tokunaga T. Structure of phenazinomycin, a novel antitumor antibiotic. Tetrahedron Lett. 1989;30:3151–3154. [Google Scholar]
- 41.Illek B, Maurisse R, Wahler L, Kunzelmann K, Fischer H, Gruenert DC. Cl transport in complemented CF bronchial epithelial cells correlates with CFTR mRNA expression levels. Cell Physiol Biochem. 2008 doi: 10.1159/000149783. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Illek B, Lizarzaburu ME, Lee V, Nantz MH, Kurth MJ, Fischer H. Structural determinants for activation and block of CFTR-mediated chloride currents by apigenin. Am J Physiol Cell Physiol. 2000;279:C1838–C1846. doi: 10.1152/ajpcell.2000.279.6.C1838. [DOI] [PubMed] [Google Scholar]
- 43.Husek P, Matucha P, Vrankova A, Simek P. Simple plasma work-up for a fast chromatographic analysis of homocysteine, cysteine, methionine and aromatic amino acids. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;789:311–322. doi: 10.1016/s1570-0232(03)00104-1. [DOI] [PubMed] [Google Scholar]
- 44.Muller M. Pyocyanin induces oxidative stress in human endothelial cells and modulates the glutathione redox cycle. Free Radic Biol Med. 2002;33:1527–1533. doi: 10.1016/s0891-5849(02)01087-0. [DOI] [PubMed] [Google Scholar]
- 45.O’Malley YQ, Reszka KJ, Spitz DR, Denning GM, Britigan BE. Pseudomonas aeruginosa pyocyanin directly oxidizes glutathione and decreases its levels in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L94–103. doi: 10.1152/ajplung.00025.2004. [DOI] [PubMed] [Google Scholar]
- 46.Schwarzer C, Machen TE, Illek B, Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem. 2004;279:36454–36461. doi: 10.1074/jbc.M404983200. [DOI] [PubMed] [Google Scholar]
- 47.Reszka K, Wagner B, Burns C, Britigan B. Effects of peroxidase substrates on the Amplex red/peroxidase assay: antioxidant properties of anthracyclines. Anal Biochem. 2005;342:327–337. doi: 10.1016/j.ab.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 48.Schwarzer C, Fu Z, Fischer H, Machen TE. Redox-independent activation of NF-kB by P. aeruginosa pyocyanin in a CF airway epithelial cell line. J Biol Chem. 2008:M709693200. doi: 10.1074/jbc.M709693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schwarzer G, Kim E, Fischer H, Gruenert D, Ames B, Peden D, Machen T, Illek B. Dual effects of pyocyanin-induced oxidative stress on CFTR chloride transport. 2007;suppl.30:251–252. [Google Scholar]
- 50.O’Malley YQ, Abdalla MY, McCormick ML, Reszka KJ, Denning GM, Britigan BE. Subcellular localization of Pseudomonas pyocyanin cytotoxicity in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L420–430. doi: 10.1152/ajplung.00316.2002. [DOI] [PubMed] [Google Scholar]
- 51.Wilson R, Pitt T, Taylor G, Watson D, MacDermot J, Sykes D, Roberts D, Cole P. Pyocyanin and 1-hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. J Clin Invest. 1987;79:221–229. doi: 10.1172/JCI112787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winter MC, Sheppard DN, Carson MR, Welsh MJ. Effect of ATP concentration on CFTR Cl- channels: a kinetic analysis of channel regulation. Biophys J. 1994;66:1398–1403. doi: 10.1016/S0006-3495(94)80930-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quinton PM, Reddy MM. Control of CFTR chloride conductance by ATP levels through non-hydrolytic binding. Nature. 1992;360:79–81. doi: 10.1038/360079a0. see comments. [DOI] [PubMed] [Google Scholar]
- 54.Humphries KM, Pennypacker JK, Taylor SS. Redox Regulation of cAMP-dependent Protein Kinase Signaling: kinase versus phosphatase inactivation. J Biol Chem. 2007;282:22072–22079. doi: 10.1074/jbc.M702582200. [DOI] [PubMed] [Google Scholar]
- 55.O’Malley YQ, Reszka KJ, Rasmussen GT, Abdalla MY, Denning GM, Britigan BE. The Pseudomonas secretory product pyocyanin inhibits catalase activity in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1077–1086. doi: 10.1152/ajplung.00198.2003. [DOI] [PubMed] [Google Scholar]
- 56.Hallows KR, McCane JE, Kemp BE, Witters LA, Foskett JK. Regulation of Channel Gating by AMP-activated Protein Kinase Modulates Cystic Fibrosis Transmembrane Conductance Regulator Activity in Lung Submucosal Cells. J Biol Chem. 2003;278:998–1004. doi: 10.1074/jbc.M210621200. [DOI] [PubMed] [Google Scholar]
- 57.Hallows KR, Fitch AC, Richardson CA, Reynolds PR, Clancy JP, Dagher PC, Witters LA, Kolls JK, Pilewski JM. Up-regulation of AMP-activated Kinase by Dysfunctional Cystic Fibrosis Transmembrane Conductance Regulator in Cystic Fibrosis Airway Epithelial Cells Mitigates Excessive Inflammation. J Biol Chem. 2006;281:4231–4241. doi: 10.1074/jbc.M511029200. [DOI] [PubMed] [Google Scholar]
- 58.Chang M, Shi M, Forman HJ. Exogenous glutathione protects endothelial cells from menadione toxicity. Am J Physiol Lung Cell Mol Physiol. 1992;262:L637–643. doi: 10.1152/ajplung.1992.262.5.L637. [DOI] [PubMed] [Google Scholar]
- 59.Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency in glutathione in cystic fibrosis. J Appl Physiol. 1993;75:2419–2424. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- 60.Hudson V. Rethinking cystic fibrosis pathology: the critical role of abnormal reduced glutathione (GSH) transport caused by CFTR mutation. Free Radic Biol Med. 2001;30:1440–1461. doi: 10.1016/s0891-5849(01)00530-5. [DOI] [PubMed] [Google Scholar]
- 61.Gao L, Kim KJ, Yankaskas JR, Forman HJ. Abnormal glutathione transport in cystic fibrosis airway epithelia. Am J Physiol Lung Cell Mol Physiol. 1999;277:L113–118. doi: 10.1152/ajplung.1999.277.1.L113. [DOI] [PubMed] [Google Scholar]