Abstract
Objective
Examine the association between frailty status and change in cognitive function over time in older Mexican Americans.
Design
Data used are from the Hispanic Established Population for the Epidemiological Study of the Elderly (H-EPESE)
Setting
Five Southwestern states: Texas, New Mexico, Colorado, Arizona, and California.
Participants
1,370 non-institutionalized Mexican American men and women aged 65 and older with a Mini Mental State Examination (MMSE) ≥ 21 at baseline (1995−1996).
Measurements
Frailty defined as three or more of the following components: 1) unintentional weight-loss of > 10-lbs, 2) weakness (lowest 20% in grip-strength), 3) self-reported exhaustion, 4) slow walking speed (lowest 20% 16ft walk-time in seconds), and 5) low physical activity level (lowest 20% Physical Activity Scale for the Elderly (PASE) score). Socio-demographic factors, MMSE, medical conditions (stroke, heart-attack, diabetes, arthritis, cancer and hypertension), depressive symptoms and visual-impairment were obtained.
Results
Of the 1370 subjects, 684 (49.9%) were not-frail, 626 (45.7%) were pre-frail (1 − 2 components) and 60 (4.4%) were frail (≥3 components) in 1995/96. Using general linear mixed models, we found that frail subjects had greater cognitive decline over 10-years compared with non-frail subjects (Estimate = −0.67, SE = 0.13; p< .0001). This association remained statistically significant after controlling for potential confounding factors.
Conclusion
Frail status in older Mexican Americans with MMSE ≥ 21 at baseline is an independent predictor of MMSE score decline over a 10-year period. Future research is needed to establish pathophysiological components that can clarify the relationship between frailty and cognitive decline.
Keywords: Frailty, Cognitive decline, Mexican Americans
INTRODUCTION
Frailty is an important clinical and public health problem in older adults characterized by vulnerability to stressors, decreased strength, increased risk of morbidity, disability and mortality.1 The prevalence of frailty increases with age and varies by ethnicity and gender.1-3 Research shows that predictor of frailty in older Mexican Americans include sociodemographic variables (e.g. marital status and education), body mass index, cognitive function and disability.4
The relationship between frailty and cognition across different ethnic groups is unclear and has received little research attention.5 Some authors have reported significantly lower MMSE scores in Hispanics compared to non-Hispanic whites6,7. Several factors have been associated with cognitive decline in Hispanic older adults. Altered executive function was reported in 31.1% of older Mexican Americans based on results from a clock-drawing task.8 Slow gait speed and weak grip strength were also associated with decline in cognitive function in this population.9,10 Older age, lower education and living alone, along with diabetes, stroke, depression and visual alterations have been linked to impaired cognition in Mexican American older adults.11 There is however no information on the relationship between frailty status and subsequent change in cognitive function.
Given the expected growth in the population of Mexican American older adults in the United States, it is important to understand the relationship between frailty and health outcomes such as cognition, which increase health costs and decrease quality of life. The purpose of this study was to examine the relationship between frailty and cognition over ten years in a large sample of Mexican American older adults living in the community. We hypothesized that frail subjects would be at higher risk for cognitive decline over time, compared to persons identified as pre-frail or not frail.
METHODS
Sample and procedures
Data are from the Hispanic EPESE, a longitudinal study of Mexican Americans aged 65 and older, residing in Texas, New Mexico, Colorado, Arizona and California. The sample and its characteristics have been described elsewhere.12,13 The original probability-based sample was representative of approximately 500,000 older Mexicans Americans living in the Southwest in the mid-1990s. Five waves of data have been collected (1993−94, 1995−96, 1998−99, 2000−2001, and 2004−2005). The present study used data obtained at the 2nd, 3rd, 4th, and 5th wave (1995 to 2005). Information from the baseline (1st) interview was not used since the Physical Activity Scale for the Elderly (PASE),14,15 a component of the frailty index, was not administered. The PASE scale was only administered at the second and fifth waves. All other covariates and the frailty index components were collected at every wave (2nd to 5th). Of the 3,050 subjects interviewed at baseline (1993−1994) 2,438 subjects were re-interviewed (2,166 in person and 272 by proxy), 238 were confirmed dead through the National Death Index (NDI) and reports from relatives, 110 subjects refused to be re-interviewed, and 264 were lost to follow-up at the 2nd wave. We excluded subjects who were re-interviewed by proxy (N = 272) because they didn't complete the PASE scale.
Of the 2,166 subjects re-interviewed in person, 796 were subsequently excluded: Four-hundred and ninety-nine subjects had a MMSE score < 21 and 297 subjects had missing information on one of the components used to compute the frailty index (see description below) or the Center for Epidemiologic Studies Depression (CES-D) Scale.16 Of those with missing information for hand grip strength, 8 had surgery in the upper extremities; 60 refused to perform the task; 21 felt the procedure was unsafe; and 26 tried but were unable to complete the task. Of those with missing data for walking speed, 23 refused to perform the task; 27 felt the procedure was unsafe; and 4 tried but were unable to complete the task.
Proxy subjects (N = 272) were more likely to be older, to have a history of heart attack, stroke, near and distant vision impairment, and a lower level of education. Subjects excluded due to missing values in any of the frailty index components or covariates, and those who had a MMSE score < 21 (N = 796) were also more likely to be older, to have had a stroke, cancer, low body mass index (BMI), poorer performance in the 16-foot walk test, and high depressive symptoms.
The final sample consisted of 1,370 subjects who had a MMSE score ≥ 21, had complete information on the frailty index and covariates at the 2nd wave (1995/96), and were re-interviewed during the follow-up period. At the end of follow-up (year 2005−2006) 735 subjects were re-interviewed in person, 69 subjects refused to be re-interviewed, 107 subjects were lost to follow-up, and 459 subjects were confirmed dead through the National Death Index (NDI) and reports from relatives. Subjects who died were mostly older, men, had lower MMSE scores, BMI, and physical activity, poorer performance in hand grip strength and walk test, and reported more hypertension, stroke, diabetes and cancer when compared with those alive or those lost to follow-up. Subjects living and those lost to follow-up were younger, women, had high MMSE scores, elevated BMI, and performed better in hand grip strength and walk test when compared to those who died. Subjects lost to follow-up had the highest physical activity level, were less frail and had lower prevalence of heart attack, stroke and altered distant vision.
Measures
Frailty definition
Frailty was assessed using similar procedures to those developed by Fried et al.1 with the exception of physical activity where we used the PASE scale14,15instead of the Minnesota Leisure Activity Questionnaire. Following the procedures developed by Fried et al.,1 subjects with three or more components present were considered frail. Subjects with one or two components were considered pre-frail and those with zero components were considered not frail. The Frailty Index includes the following components (Table 1):
Table 1.
Percent of individuals for each frailty index component among older Mexican Americans with MMSE ≥ 21 (N=1370).
| Characteristic | Definition | N (%) |
|---|---|---|
| Weight Loss | Unintentional weight loss of > 10 pounds | 235 (17.2) |
| Grip Strength | Lowest 20% in grip strength (adjusted by gender and BMI) | 211 (15.4) |
| Men | ||
| Strength ≤ 21 for BMI ≤ 24.2 | ||
| Strength ≤ 24.5 for BMI 24.3 − 26.8 | ||
| Strength ≤ 25.4 for BMI 26.9 − 29.5 | ||
| Strength ≤ 25.5 for BMI > 29.5 | ||
| Women | ||
| Strength ≤ 13.5 Kg for BMI ≤ 24.7 | ||
| Strength ≤ 14.2 Kg for BMI 24.8 − 28.3 | ||
| Strength ≤ 15.0 Kg for BMI 28.4 − 32.1 | ||
| Strength ≤ 15.0 Kg for BMI > 32.1 | ||
| Exhaustion | Self-reported positive answer from either of two questions on CES-D Scale: I felt that everything I did was an effort, I could not get going | 143 (10.4) |
| Walk Time | Slowest 20% of walking time from 16-foot walk test adjusted by gender and height | 273 (19.9) |
| Men | ||
| Time ≥ 11.2 seconds for height ≤ 66 inches | ||
| Time ≥ 9.7 seconds for height > 66 inches | ||
| Women | ||
| Time ≥ 12.0 seconds for height ≤ 60.5 inches | ||
| Time ≥ 11.2 seconds for height > 60.5 inches | ||
| Physical | Lowest 20% of PASE Scale adjusted by gender | 124 (9.1) |
| Activity | Men ≤ 30 (range 0 − 342) | |
| Women ≤ 27.5 (range 0 − 306) |
MMSE=Mini Mental State Examination
BMI=Body Mass Index
CES-D=Center for Epidemiologic Studies Depression Scale
PASE=Physical Activity Scale for the Elderly
1) Weight loss
Calculated as the difference between weight measured at baseline interview and weight measured at 2nd wave. Subjects with unintentional weight loss of more than 10 pounds were categorized as positive for weight loss criterion (score = 1).
2) Grip strength
Assessed using a hand-held dynamometer (Jaymar Hydraulic Dynamo-meter model #5030J1- J.A. Corp) and measured in Kilograms (kg).17,18 The test was administered by a trained interviewer and two trials were performed. The higher of the two handgrip scores was used for scoring purposes. Subjects who were unable to perform the grip strength test and those in the lowest 20% adjusted for BMI and stratified by gender were categorized as positive for the weakness criterion (score = 1).
3) Exhaustion
Two items from the CES-D scale were used to assess exhaustion16. Number one: “I felt that everything I did was an effort” and number two: “I could not get going”. The items asked, “How often in the last week did you feel this way?” 0 = rarely or none of the time (< 1 day), 1 = some or little of the time (1−2 days), 2 = a moderate amount of the time (3−4 days), or 3 = most of the time (5−7 days). Subjects answering 2 or 3 to either of these two items were categorized as positive for the exhaustion criterion (score = 1).
4) Walk Time
Assessed over a 16-foot timed walk test. Subjects were asked to walk “as fast as felt safe.” Height and gender adjusted time points were used (gender-specific cutoff a medium height) (Table 1), with the slowest 20% and those unable to perform the test categorized as positive for the slowness criterion (score = 1).
5) Low physical activity
Assessed with the PASE scale.15 Subjects who scored in the lowest quintile adjusted by gender were categorized as positive for the low physical activity criterion (score = 1).
To compute the frailty index, e.g., frailty scores of 1 for grip strength, walking speed, and physical activity (PASE) were based on a score in the lowest quintile distribution. We did not use the actual cut point scores developed by Fried and others1 since the sample in their original study was younger than our baseline sample, and anthropometric values (weight and height), used to adjust for hand grip muscle strength and walking speed, are known to differ in Mexican Americans compared to the predominantly non-Hispanic white sample included in the original frailty study. 19-21
Modifying the cut point can be justified based on studies that have used different population groups. When analyzing frailty in samples different from those in the Cardiovascular Health Study, Fried and others,1,22 have used different cut-points to construct the frailty index. By changing the cut points of the frailty index, the risk for adverse effects due to frailty status could be modified. However, previous findings from the Hispanic EPESE have demonstrated that weight loss, and poor performance in hand grip strength and walking speed were associated with cognitive decline, disability, and mortality.9,10,17,18,23-25
Cognitive function
Cognitive function was assessed with the Mini Mental State Examination (MMSE).26 The English and Spanish versions of the MMSE were adopted from the Diagnostic Interview Scale (DIS) used in prior Hispanic community surveys.27 This Spanish version of the MMSE has met standard criteria for development of translated tests, including formal translation, back-translation, and consensus by committee for final item content. The Spanish MMSE version has been successfully used in community surveys of Mexican Americans.28 Scores range from 0 to 30, with lower scores indicating poorer cognitive ability. We divided the MMSE score based on two factors: the distribution in the 2,731 subjects at baseline assessments and the cut points from past aging research in minority populations.29 MMSE score was used as a dichotomized variable (< 21 for poor cognition vs. ≥ 21 for good cognition). This represents a cut-point frequently used in past studies in cognitive research among older populations with low educational attainment and low English literacy.27,30,31 In the present study, only subjects with MMSE scores ≥ 21 at baseline were included in the analysis.
Vision impairment
Near vision acuity was measured using a card with seven-digit “telephone numbers” of three different type sizes: 7-, 10-, and 23-point.32 Distance visual acuity was measured using a modified Snellen test employing directional “Es” at 4 m to estimate acuity from 20/40 to 20/200; if visual acuity was greater than 20/200 without correction, a subject was classified as being functionally blind.32 Subjects who could only read “Es” of 20/60 or greater were considered to have distance vision impairment and subjects who could read 20/40 or less were considered to have adequate distance vision.
Covariates
Socio-demographic variables included age, gender, marital status and years of formal education. The presence of various medical conditions was assessed with a series of questions asking subjects if they had ever been told by a physician that they had diabetes, heart attack, stroke, hypertension, arthritis or cancer. Depressive symptomatology was measured with the Center for Epidemiologic Studies Depression Scale (CES-D) with scores ranging from 0−60. We considered subjects with high depressive symptoms those with a score ≥ 16, as is common in the literature.16,33-35 Body Mass Index was computed as weight in kilograms divided by height in meters squared. Height was measured using a tape placed against the wall and weight was established using a Metro 9800 scale.
Statistical analysis
Chi square and ANOVA tests were used to examine the distribution of covariates for subjects by frailty status at the 2nd wave (1995−96). General linear mixed models using the MIXED procedure in SAS (SAS Institute, Cary, N.C.) were used to examine the factors associated with decline in cognitive function over a 10-year period as a function of frailty status. Mixed models provide a general outline for the analysis of repeated measures. This approach uses all available data on each subject, is not affected by random missing data, easily models time-effects and allows usage of realistic variance and correlation patterns.36 All the variables were analyzed as time-dependent covariates, except for age, gender, education, and frailty index. The mixed model approach was chosen for several reasons. First, it is the model which best accounts for missing or incomplete observations. Second, it allows for modeling of time-dependent change in our variables as well as time-dependent change in the magnitude of association between the variables. Finally, since the Hispanic EPESE data included repeated measures over 10 years, mixed models allowed flexibility in modeling the effects of time on our outcome.37,38
Three mixed models were constructed to test the relationship between frailty status and cognitive decline over a 10-year period. Model l included time, age, gender, marital status, years of formal education, frailty index, and time. In Model 2, an interaction term – frailty status * time - was used to estimate the effect of frailty status on the rate of change in cognitive function (slope) over time. In Model 3, stroke, heart attack, hypertension, diabetes, high depressive symptoms, arthritis, and near and distant vision impairment were added along with the variables in Model 1.
We also used the General Estimation Equations (GEE) to determine the odds ratio of having a MMSE < 21 over a 10-year period. The GEE methodology provides consistent estimators of the regression coefficients and of their variances under weak assumptions regarding the correlation among subject observations.37,38 This method relies on independence across subjects to consistently estimate the variance of the proposed estimators, even when the assumed working correlation structure is incorrect. All analyses were performed using the SAS System for Windows, Version 9.1.3 (SAS Institute, Cary, N.C.).
RESULTS
Slowness in walking speed and unintentional weight loss of 10 pounds or more were the criteria most frequently contributing to frailty status with 19.9% of the subjects qualifying as frail based on walking speed and 17.2% qualifying as frail based on weight loss. Table 1 lists the number and percent of persons meeting the criteria for each of the five components of the frailty index.
Table 2 includes the characteristics of the sample according to frailty status at 2nd wave (1995−96). The sample was divided in three groups: not frail [n = 684 (49.9%)]; pre-frail [n = 626 (45.7%)] and frail [n = 60 (4.4%)] using the frailty index described previously.1 Frail subjects were significantly more likely to be older (mean 73.2 years; standard deviation [SD] = 4.8), to have lower MMSE scores, lower level of education, lower BMI, poorer performance in hand grip and walk time tests, lower level of physical activity, higher depressive symptoms and higher exhaustion. Also, frail subjects were more likely to report hypertension, heart attack, stroke or cancer when compared with the pre-frail and not frail group.
Table 2.
Characteristics of the sample according to frailty status among older Mexican Americans with MMSE ≥ 21 (N=1370). Data collected in 1995−96.
| Predictor variables |
Not Frail N (%) |
Pre frail N (%) |
Frail N (%) |
|---|---|---|---|
| Total | 684 (49.9) | 626 (45.7) | 60 (4.4) |
| MMSE, mean (± SD) * | 26.1 (± 3.2) | 25.4 (± 3.2) | 24.8 (± 3.0) |
| Age, mean (± SD) * | 73.2 (± 4.8) | 74.5 (± 5.7) | 78.0 (± 7.0) |
| Gender (Female) | 405 (59.2) | 371 (59.3) | 32 (53.3) |
| Marital Status (Married) | 383 (56.0) | 361 (57.7) | 33 (55.0) |
| Education (years), mean (± SD) * | 5.9 (± 4.1) | 5.2 (± 3.8) | 5.6 (± 3.6) |
| Weight (pounds), mean ± SD * | 161.9 (± 29.9) | 156.8 (± 31.0) | 152.3 (± 36.1) |
| Height (inches), mean ± SD | 63.3 (± 3.7) | 63.1 (± 3.9) | 62.4 (± 4.0) |
| BMI (Kg/m2), mean ± SD † | 28.5 (± 4.8) | 27.8 (± 5.1) | 27.6 (± 6.3) |
| Hand grip strength (kg), mean ± SD * | |||
| Male | 34.7 (± 5.8) | 30.2 (± 7.5) | 20.8 (± 4.6) |
| Female | 22.0 (± 4.1) | 18.5 (± 5.0) | 13.6 (± 2.8) |
| Rapid walk test (min), mean ± SD * | 7.1 (± 1.8) | 9.8 (± 4.1) | 15.1 (± 6.8) |
| PASE, mean ± SD * | 120.0 (± 59.0) | 95.9 (± 59.6) | 34.5 (± 46.6) |
| CES-D, mean ± SD * | 3.8 (± 5.1) | 7.1 (± 8.3) | 10.0 (± 8.8) |
| Exhaustion * | 0 (0) | 121 (19.3) | 22 (36.7) |
| Hypertension * | 290 (42.4) | 298 (47.6) | 40 (66.7) |
| Heart * | 40 (5.9) | 65 (10.4) | 11 (18.3) |
| Stroke * | 26 (3.8) | 44 (7.0) | 12 (20.0) |
| Diabetes | 168 (24.6) | 180 (28.8) | 17 (28.3) |
| Arthritis | 295 (43.1) | 285 (45.5) | 31 (51.7) |
| Cancer† | 684(49.9) | 626(45.7) | 60(4.4) |
| Near vision impairment | 38 (5.6) | 33 (5.3) | 5 (8.3) |
| Distant vision impairment | 29 (4.2) | 32 (5.1) | 6 (10.0) |
BMI=Body Mass Index
MMSE=Mini Mental State Examination
PASE=Physical Activity Scale for the Elderly
CES-D=Center for Epidemiologic Studies Depression Scale
Chi-square test was used for categorical variables and ANOVA test for continuous variables
p value < .001
p value < .05
General linear mixed model estimates for MMSE score as a function of the frailty index over a 10-year period are shown in Table 3. In Model 1, the rate of decline in the MMSE score was 0.65 points per year. The association between pre-frail and frail status at baseline and MMSE score at each point of follow-up (Intercept of MMSE score) was −0.54 and −1.38 respectively, after adjusting for age, gender, education, marital status, and time. Model 2 tested the interaction between being pre-frail and frail and time of follow-up (slope of MMSE score over 10-years). There was a significant interaction between -frailty by time-, indicating that frail subjects had a significantly greater decline in MMSE score over time compared to subjects without frailty. The parameter estimate was −0.70 points per year (SE = 0.15; p<.0001). In Model 3, after adjusting for all covariates, the frailty by time interaction and decline in cognitive status over time remained statistically significant (−0.67 points per year; SE = 0.13; p<.0001). The time interaction represents the estimated effect of frailty status on the annual rate of change in cognitive function. Frailty status by time shows how the MMSE score declines over time if a subject is in the frail category. Cognitive decline over time remained greater in frail subjects after adjusting for all covariates. Hypertension, high depressive symptoms, and near and distant vision impairment were factors also associated with decline in the MMSE score.
Table 3.
General linear mixed model estimates for change in MMSE score over 10-year period as a function of frailty index among older Mexican Americans with MMSE ≥ 21 (N=1370).
| Explanatory variables |
Model 1 ß (SE) |
Model 2 ß (SE) |
Model 3 ß (SE) |
|---|---|---|---|
| Intercept | 30. (1.12) * | 30.87 (1.12) * | 30.93 (1.10)* |
| Time | − 0.65 (0.02) * | − 0.62 (0.03) * | −0.53 (0.04)* |
| Age (Years) | − 0.07 (0.01) * | − 0.07 (0.01) * | −0.07 (0.01)* |
| Gender (Female) | 0.23 (0.16) | 0.24 (0.16) | 0.31 (0.16) |
| Education (Years) | 0.27 (0.02) * | 0.27 (0.02) * | 0.27 (0.02) * |
| Marital Status (Married) | 0.35 (0.15) † | 0.36 (0.15) † | 0.23 (0.15) |
| Frailty Index | |||
| Not frail | Reference | Reference | Reference |
| Pre frail | −0.54 (0.16) * | −0.37 (0.21) | −0.10 (0.21) |
| Frail | −1.38 (0.39) * | 0.48 (0.56) | 0.94 (0.55) |
| Frailty Index*time | |||
| Not frail*time | Reference | Reference | |
| Pre frail*time | −0.05 (0.05) | −0.07 (0.04) | |
| Frail*time | −0.70 (0.15) * | −0.67 (0.13) * | |
| Diabetes | −0.05 (0.15) | ||
| Heart attack | 0.08 (0.10) | ||
| Depression | − 0.07 (0.01)* | ||
| Stroke | − 0.65 (0.26)* | ||
| Hypertension | 0.15 (0.13) | ||
| Arthritis | 0.07 (0.13) | ||
| Cancer | 0.29 (0.25) | ||
| Near Vision Impairment | −1.32 (0.33)* | ||
| Distant Vision Impairment | −0.64 (0.35) |
MMSE=Mini Mental State Examination
p value < .001
p value < .05
Generalized Estimation Equation (GEE) analysis was performed to estimate the odds ratio of having a MMSE < 21 over a 10-year period as a function of frailty at 2nd wave (baseline for this study). After adjusting for time and all covariates, the odds ratio of becoming cognitively impaired (MMSE < 21) over a 10-year period for frail subjects was 1.27 (95% CI 1.07−1.52) when compared with pre-frail subjects (OR=1.02; 95% CI, 0.95−1.08).
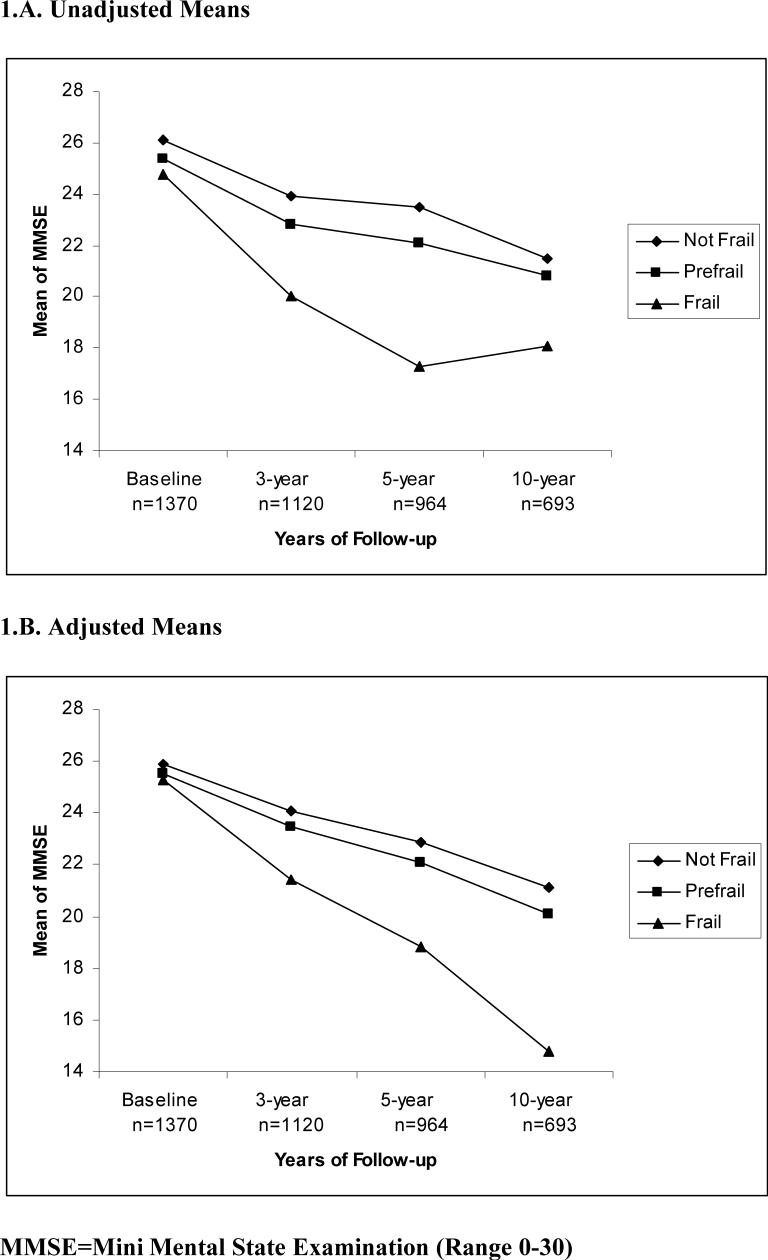
The decline in MMSE score by frailty status can be observed in Figures 1.A and 1.B. The unadjusted and adjusted mean distribution of MMSE score over time by frailty status at 2nd wave (1995/96) is shown. The slope of MMSE scores (Figure 1.A) decreased from baseline to the 5-year follow-up and then increased. However, after adjustment for all covariates (Figure 1.B), a continuing decline in the slope is observed from 5-year to 10-year of follow-up.
Figure 1.
Unadjusted and adjusted mean of MMSE over time by frailty status. 1.A. Unadjusted Means 1.B. Adjusted Means
DISCUSSION
As hypothesized, we found a statistically significant association between frailty and subsequent decline in cognitive function over a 10-year period in older Mexican Americans. The association remained significant after adjusting for potentially confounding variables such as age, education, gender, marital status, medical conditions, high depressive symptoms, and near and distant visual impairment. Subjects in the frail category had a faster and more severe decline in cognitive function than those in the pre-frail category; nevertheless, both groups showed a considerable decline.
In a recent study, Buchman et al.39 evaluated over 820 subjects for cognitive function and frailty status during a 3-year follow-up and found that the risk of developing Alzheimer's disease was 2.5 times higher when frailty was present at baseline. In our study, the risk of becoming cognitively impaired (MMSE < 21) for frail subjects was 1.3 times higher than of not frail subjects over a 10-year period. Also, the decline in cognition over time was more severe in frail subject compared to non-frail and pre-frail. Our findings are consistent with those reported by Buchman and colleagues, 39 but our results are based on a longer follow-up (10- years) and represent an underserved population with a lower education level. To our knowledge, this is the first study that focuses on frailty as a potential predictor of cognitive decline in older Mexican Americans. Our findings and those reported by Buchmann and colleagues 39 suggest cognitive decline and frailty could have common etiologic pathways. Altered gait, weight loss and muscle weakness have all been associated with the development of dementia and all are key components used to measure frailty.1,4,8,10
There are several potential pathways by which frailty could contribute to cognitive decline.39 Declining cognitive function is a longitudinal process that starts with minor alterations in a few domains and slowly progresses to changes in multiple domains.40 In some dementias, accumulation of neural plaques and fibrillary tangles is involved in the etiology of the decline.41,42 Primary and supplementary motor cortices, the substantia nigra and the striatum are often altered.41,42 Studies have shown that alterations in these areas of the brain are associated with modifications in the components of frailty such as weight loss and slow gait.43;44 These findings and the results from our analyses suggest the possibility that changes in neural systems that control motor function, metabolism, and fatigue may be present in frailty. More studies are necessary to establish the exact relationship and explain the biological and psychological processes by which frailty and cognition are related.
Another possible explanation for our findings is that the faster cognitive decline in the frail group reflects the slightly lower but normal baseline MMSE score in the frail group compared to the non-frail subjects. The lower, but normal, MMSE scores at baseline might represent a state of mild cognitive impairment (MCI) as indicated by the presence of measurable memory loss with no impairment in general cognitive and motor functioning.45 Adults with MCI have about 15% annual conversion rate to Alzheimer's dementia compared to 1% for those without MCI.45 We do not have data that indicates our subjects with low MMSE scores had MCI. Assessing the relationship between MCI and development of physical frailty in community-dwelling elders over time is an important topic for future research.
The relationship between cognition and frailty is complex and some investigators have argued that cognitive function should be included in the definition of frailty.46 In the meantime, rehabilitation and pharmacologic treatments targeted towards the different components of frailty should be considered simultaneously with the treatment for cognitive dysfunction. Any intervention targeting frail patients should also include a cognitive evaluation. Likewise, case management for a person with cognitive impairment should also include an assessment of frailty and an attempt to coordinate interventions to address both conditions.
The association between the pre-frail status and cognitive decline suggests the potential for preventive intervention oriented towards slowing the cognitive decline.47 The pre-frail status may be more easily modified by changes in diet, exercise, and medications.47 Research is required to identify factors associated with transitions into and out of the states of frailty and how these transitions are related to cognitive function.2
Our investigation includes a number of limitations. First, the MMSE has limited sensitivity to detect subtle changes in cognitive function in community living older persons. We did not have access to cerebral imaging information, neuropsychological testing or biomarkers. Our measure of cognitive function is particularly limited in assessing alterations in frontal lobe functions.48 Because depression and some cognitive disabilities are related to frontal system pathology, the association between frailty and cognitive decline could be mediated by other variables.49 Second, the information on medical conditions and comorbidities in our study is based on self-reports. Okura and others 39,50 have reported good agreement between self-reported medical conditions and actual medical diagnoses, but we did not have access to medical records to confirm subject self-reports. Third, subjects with MMSE < 21 at baseline, those deceased at follow-up, and those unwilling to be re-interviewed were excluded from the analyses and this may have introduced a bias. We did, however, include information on respondents in the analyses until their last follow-up. Subjects with more severe cognitive dysfunction (MMSE score <21) were excluded because establishing and interpreting their decline over time posed methodological and conceptual difficulties.
The strengths of our study include the large number of subjects from a well-defined and comprehensively studied sample representing an under-studied and under-served population. Other strengths include the prospective design, the 10-year follow-up period, and the use of a wide range of potential predictors related to cognitive decline.
In conclusion, we found that frailty in older Mexican Americans was an independent predictor of decline in MMSE scores over a 10-year follow-up period. Not frail and pre-frail subjects demonstrated less cognitive decline than subjects who were frail, suggesting a potential opportunity for early recognition and management. Future research is needed to better understand the pathophysiological, behavioral, and environmental variables contributing to the relationship between frailty and cognitive decline and to identify new interventions designed to improve quality of life and reduce frailty and cognitive disability in older adults.
ACKNOWLEDGMENTS
This study was supported by grants, AG10939 and AG17638, from the National Institute on Aging, US, and in part by the UTMB Center for Population Health and Health Disparities 1P50CA105631-02. Dr. Rafael Samper-Ternent is supported by a fellowship from the National Institute on Disability and Rehabilitation Research (H133P040003). Dr. Al Snih is supported by the Building Interdisciplinary Careers in Women's Health Research (BIRCWH) (Grant 5K12HD05023). Dr. Raji was supported in part by Public Health Service Grant No P30 – AG-024832-01 and by the Bureau of Health Professions’ Geriatric Academic Career Award K01 HP 00034−01. Dr. Ottenbacher is supported by an Independent Scientist Award (K02 AG019736).
Footnotes
Financial disclosure: None
Sponsor's role: Had no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
REFERENCES
- 1.Fried LP, Tangen CM, Walston JD, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Gill TM, Gahbauer EA, Allore HG, et al. Transitions between frailty states among community-living older persons. Arch Intern Med. 2006;166:418–423. doi: 10.1001/archinte.166.4.418. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch C, Anderson ML, Newman A, et al. The association of race with frailty: the Cardiovascular Health study. Ann Epidemiol. 2007;17:157–158. doi: 10.1016/j.annepidem.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Ottenbacher KJ, Ostir GV, Peek MK, et al. Frailty in older mexican americans. J Am Geriatr Soc. 2005;53:1524–1531. doi: 10.1111/j.1532-5415.2005.53511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Small GW, Rabins PV, Barry PP, et al. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer's Association, and the American Geriatrics Society. JAMA. 1997;278:1363–1371. [PubMed] [Google Scholar]
- 6.Hohl U, Grundman M, Salmon DP, et al. Mini-Mental State Examination and Mattis Dementia Rating Scale performance differs in Hispanic and non-Hispanic Alzheimer's disease patients. J Int Neuropsychol Soc. 1999;5:301–307. doi: 10.1017/s1355617799544019. [DOI] [PubMed] [Google Scholar]
- 7.Mulgrew CL, Morgenstern N, Shetterly SM, et al. Cognitive function and impairment among rural elderly Hispanic and non-Hispanic whites as assessed by the mini-mental state examination. J Gerontol B Psychol Sci Soc Sci. 1999;54B:223–230. doi: 10.1093/geronb/54b.4.p223. [DOI] [PubMed] [Google Scholar]
- 8.Royall DR, Espino DV, Polk MJ, et al. Prevalence and patterns of executive impairment in community dwelling Mexican Americans: results from the Hispanic EPESE study. Int J Geriatr Psychiatry. 2004;19:926–934. doi: 10.1002/gps.1185. [DOI] [PubMed] [Google Scholar]
- 9.Alfaro-Ancha A, Al Snih S, Raji MA, et al. Handgrip strength and cognitive decline in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2006;61:859–865. doi: 10.1093/gerona/61.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alfaro-Ancha A, Al Snih S, Raji MA, et al. Does 8-foot walk time predict cognitive decline in older Mexican Americans? J Am Geriatr Soc. 2007;55:245–251. doi: 10.1111/j.1532-5415.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen HT, Black SA, Ray LA, et al. Predictors of decline in MMSE scores among older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2002;57:M181–M185. doi: 10.1093/gerona/57.3.m181. [DOI] [PubMed] [Google Scholar]
- 12.Cornoni-Huntley J, Lafferty ME. Established populations for epidemiologic studies for the elderly, resource data book. National Institute on Aging; Bethesda, MD: 1986. National Institute on Aging. [Google Scholar]
- 13.Markides KS, Stroup-Benham CA, BS . The health of Mexican-American elderly: selected findings from the Hispanic EPESE. In: Wykle M, Ford A, editors. Serving Minority Elderly in the 21st Century. Springer; New York: 1999. [Google Scholar]
- 14.Taylor HL, Jacobs DR, Jr., Schucker B, et al. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 15.Washburn RA, Smith KW, Jette AM, et al. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 16.Radloff LS. 'The CES-D scale: A self report depression scale for research in the general population'. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 17.Al Snih S, Markides KS, Ray LA, et al. Hand grip strength and mortality in older Mexican Americans. J Am Geriatr Soc. 2002;50:1250–1256. doi: 10.1046/j.1532-5415.2002.50312.x. [DOI] [PubMed] [Google Scholar]
- 18.Al Snih S, Markides KS, Ottenbacher KJ, et al. Hand grip strength and incident ADL disability in elderly Mexican Americans over a seven-year period. Aging Clin Exp Res. 2004;16:481–486. doi: 10.1007/BF03327406. [DOI] [PubMed] [Google Scholar]
- 19.Gillum RF, Sempos CT. Ethnic variation in validity of classification of overweight and obesity using self-reported weight and height in American women and men: the Third National Health and Nutrition Examination Survey. Nutr J. 2005;4:27. doi: 10.1186/1475-2891-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuczmarski MF, Kuczmarski RJ, Najjar M. Descriptive anthropometric reference data for older Americans. J Am Diet Assoc. 2000;100:59–66. doi: 10.1016/S0002-8223(00)00021-3. [DOI] [PubMed] [Google Scholar]
- 21.Ryan AS, Roche AF, Kuczmarski RJ. Weight, stature, and body mass index data for Mexican Americans from the third national health and nutrition examination survey (NHANES III, 1988−1994). Am J Hum Biol. 1999;11:673–686. doi: 10.1002/(SICI)1520-6300(199909/10)11:5<673::AID-AJHB10>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 22.Boyd CM, Xue QL, Simpson CF, et al. Frailty, hospitalization, and progression of disability in a cohort of disabled older women. Am J Med. 2005;118:1225–1231. doi: 10.1016/j.amjmed.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 23.Amador LF, Al Snih S, Markides KS, et al. Weight change and mortality among older Mexican Americans. Aging Clinical and Experimental Research. 2006;18:196–204. doi: 10.1007/BF03324649. [DOI] [PubMed] [Google Scholar]
- 24.Raji MA, Kuo YF, Al Snih S, et al. Cognitive status, muscle strength, and subsequent disability in older Mexican Americans. J Am Geriatr Soc. 2005;53:1462–1468. doi: 10.1111/j.1532-5415.2005.53457.x. [DOI] [PubMed] [Google Scholar]
- 25.Al Snih S, Markides KS, Ray LA, et al. Body mass index and seven-year incidence of disability and mortality among older Mexican Americans. J Am Geriatr Soc. 2005;53:S84. [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. Mini-Mental State” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Bird HR, Canino G, Rubio-Stipec M, et al. Use of the mini-mental state examination in a probability sample of a Hispanic population. J Nerv Ment Dis. 1987;175:731–737. doi: 10.1097/00005053-198712000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Escobar J, Burnman A, Karno M, et al. Use of the mini-mental status examination (MMSE) in community population of mixed ethnicity: cultural and linguistic artifacts. J Nerv Ment Dis. 1986;174:607–614. doi: 10.1097/00005053-198610000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Raji MA, Al Snih S, Ray LA. Cognitive status and incident disability in older Mexican Americans. Ethn Dis. 2004;14:26–31. [PubMed] [Google Scholar]
- 30.Leveille SG, Guralnik JM, Ferruci L, et al. Black/white differences in the relationship between MMSE scores and disability: the Women's Health and Aging study. The journals of gerontology Series B, Psychological sciences and social sciences. 1998;53:201–208. doi: 10.1093/geronb/53b.3.p201. [DOI] [PubMed] [Google Scholar]
- 31.Uhlmann RF, Larson EB. Effect of education on the mini-mental state examination as a screening test for dementia. J Am Geriatr Soc. 1991;39:876–880. doi: 10.1111/j.1532-5415.1991.tb04454.x. [DOI] [PubMed] [Google Scholar]
- 32.Salive ME, Guralnik J, Christen W, et al. Functional blindness and visual impairment in older adults for three communities. Ophthalmology. 1992;99:1840–1847. doi: 10.1016/s0161-6420(92)31715-4. [DOI] [PubMed] [Google Scholar]
- 33.Haringsma R, Engels GI, Beekman AT, et al. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004;19:558–563. doi: 10.1002/gps.1130. [DOI] [PubMed] [Google Scholar]
- 34.Heikkinen RL, Kauppinen M. Depressive symptoms in late life: a 10-year follow-up. Arch Gerontol Geriatr. 2004;38:239–250. doi: 10.1016/j.archger.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Smarr KL. Measures of Depression and Depressive Symptoms. Arthritis Rheum. 2003;49:S134–S146. doi: 10.1002/acr.24191. [DOI] [PubMed] [Google Scholar]
- 36.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 37.Liang KY, Zeger SL. Longitudinal Data-Analysis Using Generalized Linear-Models. Biometrika. 1986;73:13–22. [Google Scholar]
- 38.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 39.Buchman AS, Boyle PA, Wilson RS, et al. Frailty is associated to with incident Alzheimer's disease and cognitive decline in elderly. Psychosom Med. 2007;69:483–489. doi: 10.1097/psy.0b013e318068de1d. [DOI] [PubMed] [Google Scholar]
- 40.Atkinson HH, Cesari M, Kritchevsky SB, et al. Predictors of combined cognitive and physical decline. J Am Geriatr Soc. 2005;53:1197–1202. doi: 10.1111/j.1532-5415.2005.53362.x. [DOI] [PubMed] [Google Scholar]
- 41.Burns JM, Galvin JE, Roe CM, et al. The pathology of the substantia nigra in Alzheimer's disease with extrapyramidal signs. Neurology. 2005;64:368–374. doi: 10.1212/01.WNL.0000158423.05224.7F. [DOI] [PubMed] [Google Scholar]
- 42.Wolf DS, Gearing M, Snowdown DA, et al. Progression of regional neuropathology in Alzheimer disease and normal elderly: findings from the Nun study. Alzheimer Dis Assoc Disord. 1999;13:226–231. doi: 10.1097/00002093-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Marquis S, Moore MM, Howieson DB, et al. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59:601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- 44.Camicoli R, Howieson D, Oken B, et al. Motor slowing precedes cognitive impairment in the oldest old. Neurology. 1998;50:1496–1498. doi: 10.1212/wnl.50.5.1496. [DOI] [PubMed] [Google Scholar]
- 45.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 46.Ferruci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2007;2004:625. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 47.Faber MJ, Bosscher RJ, Paw MJCA, et al. Effects of exercise programs on falls and mobility in frail and pre-frail older adults: A multicenter randomized controlled trial. Archives of physical medicine and rehabilitation. 2006;87:885–896. doi: 10.1016/j.apmr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 48.Wind AW, Schellevis FG, Van Staveren G, et al. Limitations of the Mini-Mental state examination in diagnosing dementia in general practice. Int J Geriatr Psychiatry. 1997;12:101–108. doi: 10.1002/(sici)1099-1166(199701)12:1<101::aid-gps469>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 49.Royall DR. Executive cognitive impairment: a novel perspective on dementia. Neuroepidemiology. 2000;19:293–299. doi: 10.1159/000026268. [DOI] [PubMed] [Google Scholar]
- 50.Okura Y, Urban LH, Mahoney DW, et al. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57:1096–1103. doi: 10.1016/j.jclinepi.2004.04.005. [DOI] [PubMed] [Google Scholar]