Abstract
Objectives
Nonalcoholic fatty liver disease (NAFLD) is a chronic liver disease associated with obesity that is now the most common liver disease in the United States. Cytokeratin 18 (CK18) is an intracellular protein released into the blood by both necrosis and apoptosis of hepatocytes. Levels of CK18 have not been previously reported in children with NAFLD.
Methods
In a cross sectional analysis of 62 children (28 normal weight, 14 obese and 20 suspected NAFLD) we measured CK18 levels as well as alanine aminotransferase, fasting glucose, fasting insulin and TNFα.
Results
CK18 was significantly elevated in the children with suspected NAFLD compared to obese controls and normal weight controls (median 424 U/L compared to 243 and 214 respectively, p <.001). In multiple logistic regression analysis, CK18 was the best single predictor of suspected NAFLD (prediction accuracy 84.1%).
Conclusions
CK18 is elevated in children with suspected NAFLD and should be investigated as a potential diagnostic marker of NAFLD.
Keywords: Cytokeratin 18, fatty liver, NAFLD, obesity, children
Introduction
Nonalcoholic fatty liver disease (NAFLD) is associated with obesity and closely linked with insulin resistance and the metabolic syndrome[1,2]. It is an important pediatric health problem because the rate of obesity in the childhood populations of developed countries has risen dramatically over the past several decades[3, 4], and up to 35% of obese children have NAFLD[5-7]. NAFLD and the presumed risks thereof have been defined primarily by the findings on liver biopsy[8-10]. Simple fatty liver (steatosis) forms one end of the NAFLD spectrum and is thought to be a relatively benign condition, though that is not known with certainty. Hepatic steatosis can be detected by ultrasound and quantitated by magnetic resonance imaging (MRI). Thus, it is not difficult to determine if an obese child has fatty liver. Nonalcoholic steatohepatitis (NASH), a lesion characterized by balloon degeneration of hepatocytes, inflammation and fibrosis, is considered to be a form of chronic hepatitis with the potential for progressing to cirrhosis and end-stage liver disease. It is important, therefore, to determine among the spectrum of patients with NAFLD those with simple fatty liver and those with NASH. At present, only liver biopsy can determine with accuracy the presence of NASH, since no imaging can show sufficient detail of inflammation or fibrosis [11].
While findings on liver biopsy have defined the need to discriminate, it is impractical to use liver biopsy to do so in a pediatric population. Liver biopsy is an expensive and invasive procedure, and it has some inherent risk. Considering the magnitude of the problem, using liver biopsies to diagnose NAFLD in obese children and follow its evolution would require resources and impose risks likely in excess of the benefits accrued from doing so.
An autopsy-based population study from southern California demonstrated that almost one-third of children with NAFLD had NASH[12]. No other study has reported liver biopsy findings in an unselected population of obese children. Available data are biased by selection of children for biopsy according to evidence of liver disease beyond simple steatosis, most commonly by elevation of serum alanine aminotransferase (ALT). These data show that, if anything, children have more aggressive and severe liver disease than adults with NAFLD[9, 10]. Adult studies demonstrate that NAFLD is associated with increased morbidity and a significant incidence of cirrhosis, presumably having progressed from NASH[13, 14]. It seems clear that knowing which obese children have NAFLD with the potential to progress is important in understanding their future health risks. Moreover, it would be important to know if those with NASH respond to therapy.
The current paradigm for assessing obese children for NAFLD/NASH is inadequate. It consists of identifying children at risk by BMI ≥95th percentile. Clinical findings of liver disease such as abdominal pain and hepatomegaly have not been adequately assessed in regard to detecting those children with significant liver disease. Imaging may be used to determine if steatosis exists. Assessing insulin sensitivity is useful in that there is a close link between NAFLD and insulin resistance, but this is not of proven value in discriminating pediatric patients with NASH from those with simple steatosis. Measuring serum ALT is a common approach for detecting NASH among pediatric patients with NAFLD[15]. However, the sensitivity of serum ALT elevation for detecting and its specificity for excluding NASH have not been tested in children. Because of these limitations, it is important to develop useful biomarkers that could better discriminate between simple fatty liver and NAFLD complicated by NASH.
Wiechkowska, et al. reported using caspase-cleaved cytokeratin 18 (CK18) fragments as a marker for adult NASH[16]. CK18 is an intracellular protein expressed at high levels by many cells of epithelial origin including hepatocytes. Because the protein is presumably released into the blood only after disintegration of the plasma membranes of cells containing it, plasma levels have been proposed as a measure of cell death. Caspase cleavage of CK18 (primarily by caspase-9, but also -3 and -7) occurs during apoptotic cell death and produces a fragment with the neo-epitope CK18-Asp396 identifiable by immunoassay[17]. Both CK18 and CK18-Asp396 have been used to quantitate tumor cell lysis in vivo and in vitro [15]. The ratio of the two has been proposed as a method for assessing the relative contributions of apoptosis and necrosis to cell death during therapy[18]. In a study of adult patients with NAFLD/NASH the concentration of plasma CK18-Asp396 fragment correlated with disease stage on biopsy and thus was proposed as a biomarker for NASH[16]. In this study we measured plasma CK18 levels in normal weight children and obese children with and without NAFLD.
Materials and Methods
This study was conducted at the University of Louisville and the Children's Memorial Hospital (Chicago). Plasma samples were obtained from obese children during a screening study and from subspecialty clinics as reported[19,20]. Healthy normal-weight children were recruited from the same clinics. Informed consent, adolescent assent and HIPAA waivers were obtained for each subject. Plasma samples from 62 children enrolled from October 2003 to May 2006 were analyzed for CK18. CK18-Asp 396 fragment levels were measured in only 44 children because of insufficient plasma in 18 subjects. One normal control had been previously identified as an outlier (had a very high tumor necrosis factor (TNFα)) and in this analysis he had a very high CK18 and CK 18-Asp 396 (650 and 939 U/L respectively). The data were included in the analysis but not displayed on the figures.
Subjects were weighed using a standard calibrated digital scale while wearing light clothing without shoes, and height was measured using a digital stadiometer. Body mass index (BMI) percentile and a BMI Z score were calculated based on the Centers for Disease Control, National Center for Health Statistics, standard curves for gender and age. Fasting, early morning blood specimens were obtained by standard phlebotomy and blood samples were stored at -80°C until assayed. ALT, AST and glucose were measured by a commercial laboratory. Soluble CK18 and caspase-cleaved CK18 ASP396 fragments were determined using an enzyme-linked immunosorbent assay (ELISA, Peviva, Bromma, Sweden). Fasting insulin levels and TNFα were determined by commercially-available ELISA (Biosource, Camarillo, CA).
Any subject with suspicion of liver disease to be included must have had a negative serologic work up for autoimmune liver disease (ANA, anti-smooth muscle antibody, antiliver/kidney microsomal antibody) and have normal ceruloplasmin, alpha-1-antitrypsin level and phenotype, and hepatitis B and C serology.
Data analysis
The establishment of groups in such a study as this is difficult because of the poor ability to detect NAFLD in obese subjects and to discriminate between subjects with simple fatty liver and those with NASH among those with NAFLD. The following definitions of the groups used for comparison are as follows:
Group I - 28 normal-weight subjects: children with a BMI <85th percentile without known chronic or acute diseases.
Group II - 14 children with BMI >95th percentile for age and gender (correlates with a BMI Z score ≥1.65) without chronic or acute diseases and no clinical indicator of NAFLD (see group III). Subjects in this group had normal serum ALT, but did not have imaging studies and therefore are not proven to have normal livers.
Group III - 20 children with BMI >95th percentile for age and gender with suspected or known NAFLD. The criteria for inclusion in this group were obese children who, for whatever reason, underwent evaluation for NAFLD. To be included, the subject must have had hepatic steatosis as determined by ultrasound or CT and elevated serum ALT [>40 IU/L].
Group IIIa - 6 children from group III who underwent liver biopsy and all had biopsy-proven NASH. As not all subjects in group III underwent liver biopsy, it is important to include these children in that group. Yet, as a distinct subset with proven NASH they constitute a group worthy of separate comparison.
Results of normally-distributed parameters are presented as means and standard deviations, whereas CK 18 and CK18-Asp 396 fragments are described using medians with 95% confidence intervals (CI) because of non-normal distributions. Differences of normally-distributed parameters between any two groups were assessed using t-tests, whereas differences across groups were assessed by analysis of variance and chi-squared tests as appropriate. Results of CK18 and CK18-Asp 396 fragments were compared between groups using a Wilcoxon test. Spearman's rank correlation coefficient was calculated to assess degree of association for CK18, CK18-ASP 396 fragments, ALT and age. All statistical analyses were performed in the R statistical environment (www.r-project.org).
Results
Table 1 shows the characteristics of the 62 children. There were more boys than girls though gender did not differ among groups. Race was represented as follows: 18 (29%) black, 34 (55%) white and 10 (16%) Hispanic. Hispanics were disproportionately represented in group III (NAFLD). Descriptive parameters of the 3 groups are shown (Table 1). Significant differences between the 3 groups were found in age, BMI, blood pressure, ALT, insulin, and glucose. Importantly, only ethnicity and ALT (by definition of the groups) were significantly different between groups II and III (Table 1). No subject in any group had clinical diabetes.
Table 1.
Baseline characteristics of subjects in the normal group, healthy obese group and the suspected NAFLD group. For continuous variables, the mean is reported ± standard error. Abbreviations: Black (B), White (W), Hispanic (H), Blood pressure (BP)
| Normal Controls (N=28) |
Obese (N=14) |
NAFLD (N=20) |
p for 3 groups |
p for obese compared to NAFLD |
|
|---|---|---|---|---|---|
| Age | 11 ± .49 | 12 ± .69 | 13 ± .60 | .04 | .23 |
|
Gender (male:female) |
15:13 | 9:5 | 12:8 |
X2 =0.4854 p = .785 |
.80 |
|
Ethnicity (N of B/W/H) |
8/17/3 | 8/6/0 | 2/11/7 |
X2= 14.03 p = .007 |
.003 |
| BMI Z Score | .28 ± .17 | 2.3 ± .08 | 2.1 ± .11 | <.001 | .26 |
| Systolic BP | 110 ± 2.3 | 130 ± 4.7 | 126 ± 2.6 | <.001 | .48 |
| Diastolic BP | 67 ± 2.0 | 73 ± 2.5 | 73 ± 2.6 | .14 | .48 |
| ALT (IU/L) | 26 ± 1.8 | 23 ± 3 | 79 ± 11 | <.001 | <.001 |
| Glucose (mg/dl) | 85 ± 1.0 | 91 ± 1.0 | 96 ± 2.6 | <.001 | .13 |
| Insulin (μU/ml) | 9.2± 1.2 | 25 ± 3.8 | 36 ± 6.2 | <.001 | .17 |
| TNFα (pg/mL) | 2.5± .13 | 2.9± .42 | 2.8± .43 | .59 | .92 |
Abbreviations: Black (B), White (W), Hispanic (H), Blood pressure (BP), Alanine aminotransferase (ALT), Tumor necrosis factor (TNF).
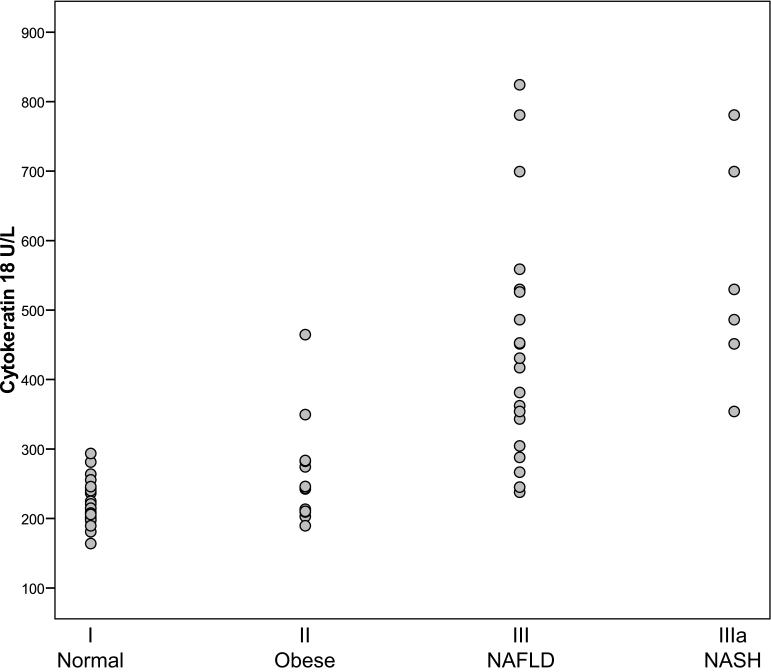
Plasma levels of CK18 are presented in Figure 1. Values overall ranged from 164 to 875 U/L. The median levels of CK18 were: 214 U/L (205-240, 95% CI) for group I; 243 U/L (209-283, 95% CI) group II; 424 U/L (343-526, 95% CI) for group III; and 508 U/L (354-780 95% CI) for group IIIa. Serum CK 18 values were significantly higher in group III (children with NAFLD) than in groups I (normal-weight children) and group II (overweight children without NAFLD), p<.001. The data shown in Figure 1 demonstrate that the CK 18 values for the subjects in group IIIa (NASH) ranked among the highest in group III, with a trend towards a significant increase (p = .06). In all subjects, serum CK18 correlated significantly with serum ALT values (rs = .58, p<.001).
Figure 1.
Comparison of Cytokeratin 18 levels in U/L for subjects for normal weight subjects (I), healthy obese subjects with normal ALT (II) and suspected NAFLD (III). Group IIIa represents the subjects from group III that had a liver biopsy demonstrating NASH. Median CK18 was significantly elevated in suspected NAFLD compared to normal and obese (p<0.001).
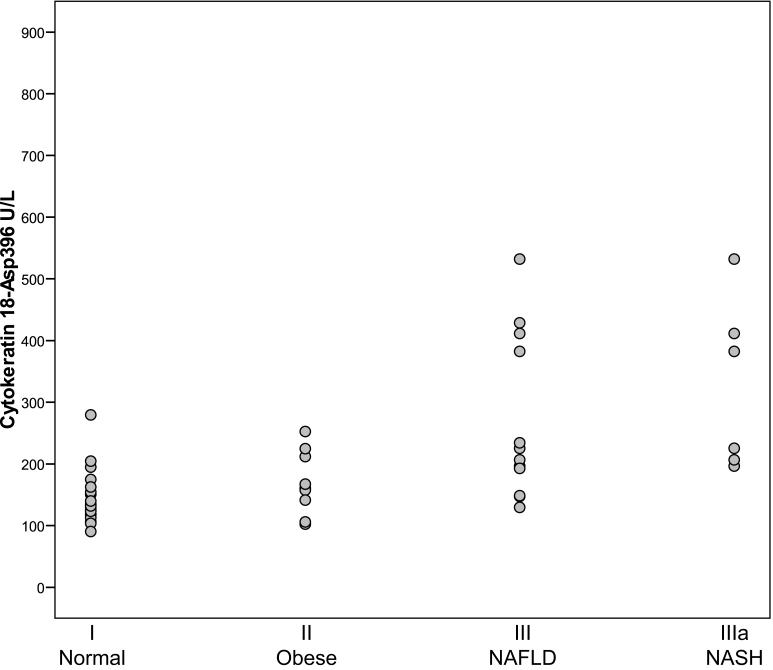
Plasma levels of CK18-Asp396 fragments are presented in Figure 2. Overall they ranged from 90 to 532 U/L. The median level of CK18-Asp396 fragments was 132 U/L (125-194, 95% CI) for group I, 161 U/L (106-225, 95% CI) for group II, 207 U/L (149- 412, 95% CI) for group III and 304 U/L (197-532, 95% CI) in group IIIa. The difference between suspected NAFLD compared to normal and obese was not significant (p = .08). In contrast to CK18, CK 18-Asp396 fragments did not significantly correlate with serum ALT (rs = .26, p = .08).
Figure 2.
Comparison of Cytokeratin 18-Asp 396 fragments in U/L for for normal weight subjects (I), healthy obese subjects (II) and suspected NAFLD (III). Group IIIa represents the subjects from group III that had a liver biopsy demonstrating NASH. No significant differences were found between groups.
Discussion
Our study demonstrates that plasma CK18 levels are elevated in children with a clinical diagnosis of NAFLD relative to normal-weight children and overweight children without NAFLD. This suggests that plasma CK18 may have potential as a serologic marker of NAFLD in children. That, by itself, may have some value in clinical medicine because it could help to identify children with obesity-related liver disease among those with obesity. Our data also suggest that among obese children with NAFLD, those with NASH and its attendant risks have exceptionally high plasma CK 18 levels. If it proves to be true that measuring CK 18 levels can contribute to a paradigm for distinguishing NASH from simple fatty liver, the importance of this finding cannot be over estimated. However, substantially more data will be needed to validate its use in this setting.
When we compared CK18 to other common clinically-used biomarkers of NAFLD, and as a single test, the plasma level of CK18 best predicted NAFLD (outside of serum ALT, which defined the groups). Fasting insulin and glucose are frequently tested in obese children and have potential as a clinical marker of disease risk. However, they did not differentiate children with NAFLD from obese children without NAFLD. On a cautionary note, plasma CK 18 is released during epithelial-cell necrosis and is not specific to hepatocytes. Thus, other inflammatory diseases or adenocarcinomas potentially could give rise to elevated levels. Rapid cell turnover during normal growth could result in elevated levels. Our data from a limited number of normal-weight children suggest that this will not be a major confounder when applying CK 18 measurements in studies of children. The design of our study precluded determining if CK18 is superior to ALT in assessing for NAFLD. Serum ALT is does not correlate well with level of inflammation in biopsies of NALFD/NASH patients, whereas Wiekowska et al demonstrated that CK18 fragments correlate with stage of disease[16]. In our study, CK18 fragment levels did not appear to be as predictive of NASH as did plasma CK 18. The median plasma CK 18 value of the 6 children with biopsy-proven NASH was higher than the other groups while the mean CK18 fragment levels was not. Critically, 3 of 6 with NASH fell well below the cut-off level of 395 U/L fro CK 18 fragments proposed by Wiekowska et al[16] for an adult cohort. It is unclear if this difference in findings is because we studied children or an artifact of our small sample size of NASH patients. We suggest that total CK 18 should be further investigated along with CK 18 fragment levels in the quest for a serologic test for severity of NAFLD in obese children.
Because of the limitations in this study, it should be considered only suggestive of potential value for measuring plasma CK 18 in the context of obesity. Children with suspected NAFLD do not routinely undergo liver biopsy. Thus, we did not directly assess the relationship between plasma CK18 levels and liver biopsy findings. Furthermore, our normal-weight subjects and overweight subjects did not undergo imaging for hepatic steatosis. It is unlikely that the normal-weight children had NAFLD since just 5% of normal-weight children had steatosis in an autopsy study[12]. Some of children in group II may well have had NAFLD with normal ALT. Finally, Hispanic children were over represented in the NAFLD group. It is possible that there is a genetic difference in plasma CK18 levels and this may be a confounder. Further studies will be required to sort out these and other issues.
In summary, this study demonstrated that plasma CK18 levels are significantly elevated in children with NAFLD and generally low in normal-weight children. Further studies will be needed to assess if plasma CK18 can be used clinically as a diagnostic test in children with NAFLD.
Acknowledgments
Supported, in part, by a clinical research grant from The American College of Gastroenterology (MV), Ruth Kirstein NRSA F32 (MV); the NIH (SB, CM), Emory University Faculty Distinction Award (JC) and the VA (CM) as well as the Northwestern University General Clinical Research Center.
References
- 1.Sanyal A, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 2.Neuschwander-Tetri B. Fatty liver and the metabolic syndrome. Curr Opin Gastroenterol. 2007;23:193–98. doi: 10.1097/MOG.0b013e32801421a9. [DOI] [PubMed] [Google Scholar]
- 3.Hedley AA, Ogden CL, Johnson CL, et al. Prevalence of Overweight and Obesity Among U.S. Children, Adolescents, and Adults. JAMA. 2004;291:2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 5.Louthan MV, Theriot JA, Zimmerman E, et al. Decreased prevalence of nonalcoholic fatty liver disease in black obese children. J Pediatr Gastroenterol Nutr. 2005;41:426–29. doi: 10.1097/01.mpg.0000177314.65824.4d. [DOI] [PubMed] [Google Scholar]
- 6.Schwimmer JA, McGreal N, Deutch R, et al. Influence of gender, race and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561–e565. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 7.Strauss R, Barlow S, Dietz W. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136:727–33. [PubMed] [Google Scholar]
- 8.Brunt EM, Neuschwander-Tetri BA, Oliver D, et al. Nonalcoholic Steatohepatitis: Histologic features and clinical correlations with 30 blinded biopsy specimens. Hum Pathol. 2004;35:1070–82. doi: 10.1016/j.humpath.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Xanthakos S, Miles L, Bucuvalas J, et al. Histologic spectrum of nonalcoholic fatty liver disease in morbidly obese adolescents. Clin Gastroenterol and Hepatology. 2006;4:226–232. doi: 10.1016/s1542-3565(05)00978-x. [DOI] [PubMed] [Google Scholar]
- 10.Schwimmer JA, Behling C, Newbury R, et al. Histopathology of Pediatric Nonalcoholic Fatty Liver Disease. Hepatology. 2005;42:641–49. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 11.Saadeh S, Younossi Z, Remer E, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–50. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 12.Schwimmer J, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 13.Ekstedt M, Franzen L, Mathiesen U, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–73. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 14.Adams L, Lymp J, Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population based cohort study. Gastroenterology. 2005;129:113–21. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Schwimmer J, Deutsch R, Rauch J, et al. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–05. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 16.Wieckowska A, Zein N, Yerian L, et al. In Vivo Assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27–33. doi: 10.1002/hep.21223. [DOI] [PubMed] [Google Scholar]
- 17.Schutte B, Henfling M, Kolgen W, et al. Keratin 8/18 breakdown and reorganization during apoptosis. Exp Cell Res. 2004;297:11–26. doi: 10.1016/j.yexcr.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 18.Kramer G, Erdal H, Mertens H, et al. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin. Cancer Res. 2004;64:1751–56. doi: 10.1158/0008-5472.can-03-2455. [DOI] [PubMed] [Google Scholar]
- 19.Louthan M, Lafferty-Oza M, Smith E, et al. Diagnosis and treatment frequency for overweight children and adolescents at well child visits. Clin Pediatr. 2005;44:57–61. doi: 10.1177/000992280504400107. [DOI] [PubMed] [Google Scholar]
- 20.Louthan MV, Joshi-Barve SJ, et al. Decreased serum adiponectin: an early event in pediatric nonalcoholic fatty liver disease. J Pediatr. 2005;147:835–38. doi: 10.1016/j.jpeds.2005.07.030. [DOI] [PubMed] [Google Scholar]