Abstract
Objectives
A number of herbal dietary anti-oxidant supplements containing Indole-3 Carbinol (I3C) and Resveratrol (RE) have been established as anti-proliferative agents in cancer. These compounds have both similar as well as unique molecular targeting profiles. The purpose of this study is to analyze their mechanism of action when used individually and in combination in ovarian cancer.
Methods
SK-OV-3 ovarian cancer cells were treated with various doses of I3C, RE or I3C+RE. Alamar Blue dye assay was used to examine cell growth and proliferation. Cell cycle and apoptosis were analyzed by flow cytometry. Western blot was performed to determine the expression of the genes associated with cell cycle and apoptosis. CA-125, a functional marker of ovarian cancer, and nitric oxide, were analyzed by ELISA.
Results
I3C or RE inhibited cell proliferation, and caused cell contraction and apoptosis. Analysis of apoptosis-associated genes revealed an inhibition of Retinoblastoma protein (Rb) and Survivin (SVV) gene expression. This was accompanied by elevation of p21, a tumor suppressor. Cell cycle was inhibited at both G1 and G2/M by individual treatments, and accentuated by a combination. Alamar Blue assay revealed a clear synergistic action of I3C+RE. CA125 was inhibited by either I3C or RE treatments. In contrast, basal nitric oxide production was inhibited by I3C and I3C+RE but not RE alone.
Conclusions
This is the first evidence demonstrating the effects of I3C on ovarian cancer cells and its synergism with RE. Based on this model, our data indicate that combinations of compounds with different targeting properties will be more effective in chemoprevention and/or chemotherapy of ovarian and possibly other cancers.
Keywords: Ovarian Cancer, Indol-3-Carbinol, Resveratrol, Survivin (SVV), Apoptosis, Cell cycle
Introduction
Indole-3-carbinol (I3C) is a compound present in cruciferous vegetables such as broccoli, cabbage and cauliflower. Its ability to cause G1 arrest of cell cycle, induction of apoptosis and interfere with signal transduction pathways have been demonstrated in a variety of cancers, including that of prostate (1), melanoma (2) and breast cancer cell lines (3). It causes Akt inactivation in the PC-3 prostate cancer cell line (4) and human breast tumor cell line MDA-MB468 but not in the non-tumorigenic HBL-100 cell line (5). Ability of this compound to target multiple genes involved in cell cycle (cyclin D1, cyclin E, cyclin-dependent kinases CDK2, CDK4 CDK6), apoptosis (Bcl-2, Bcl-xl, surviving, inhibitor of apoptosis protein IAP, x chromosome linked IAP, proapoptotic gene Bax, activation of caspase-9 and caspase3, have been well documented (6). In addition it has been shown to inhibit activation of transcription factors including nuclear factor-kappa B, SP1, estrogen receptor, androgen receptor and nuclear factor-E2-related factor 2 (Nrf2). It has a strong hepatoprotective activity against various carcinogens. Due to its broad spectrum of activities combined with low toxicity has been acclaimed as a potent chemopreventive and anti-cancer agent (7). No information is available on its effects in ovarian cancer. Resveratrol (RE) is a powerful antioxidant present in grape skin and seeds. It has Cox-1 inhibitory activity (8,9) and causes G1 arrest (10,11), induces apoptosis by TRAIL sensitization and down regulates survivin expression (12). These two compounds belong to a group of potential chemopreventive agents of dietary origin. They are commercially available in the U.S as dietary nutritional supplements in the form of plant and seed extracts. In addition to their antioxidant activity, such chemopreventive agents are known to modulate drug metabolism by acting on liver enzymes (13,14) and show anti-inflammatory actions controlled thru inhibition of macrophage generated lipopolysaccharide- mediated nitric oxide (15). All these mechanisms may come into play in determining their chemopreventive and −/− or chemotherapeutic activities. Further, when used together in a mixture, there may be additive and / or synergistic actions of these compounds. In view of their overlapping and disparate activity profiles, we investigated the combined action of two such chemopreventive agents, viz. I3C and RE, on the human ovarian cancer cell line SK-OV-3.
Materials and Methods
Cell Culture and Alamar-Blue assay for growth inhibition
SK-OV-3 ovarian cancer cells (IZSBS BS TCL84: ATCC, Manassas, VA) were plated on 96 well plates (103 cells/well ) and cultured in McCoy’s 5A medium supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin (0.1 ml/well). Mesenchymal Stem Cells (MSCs) kindly provided by Dr. David Welsh (LSU Health Sciences Center, New Orleans) were cultured in MEM-Alpha (GIBCO Cat No. 12561) supplemented with 16.5% of Bovine Serum Albumin (Atlanta Biologicals, Catalogue No. S-11550) and 1% L-Glutamine (GIBCO, Catalogue No.25030). The cells were incubated at 37 °C and 5% CO2. I3C and RE (Sigma, St.Louis, MO) were dissolved in 70% ethanol at 10 mg/ml concentration and applied, at least in triplicates, to the wells to achieve final concentrations ranging from 0.5 to 100µg/ml. Control wells received 10 µl of ethanol, the maximum permissible dose of alcohol used in previous studies. The suppression of cell growth was determined using the alamarBlue assay (Alamar Biosciences, Sacramento, CA). AlamarBlue was added to the wells in an amount equal to 10% of the total culture medium volume. The oxidized form of this dye, which is non-toxic, is converted to the reduced form by mitochondrial enzyme activity of the viable cells. The shift in fluorescence was measured at 570 nm (excitation) and 600 nm (emission) in a Fluorometer (LabSystems Fluoreskan-II), 4 hours after addition of the dye and the results expressed as percent of controls. Culture medium (supplemented with treatment components and fresh alamarBlue) was changed daily and fluorescence readings were taken 4 hrs after each medium change. The cells were monitored daily over a 6 day period to gauge the shift in fluorescence. This method provides an accurate measure of the number of cells metabolizing the dye to a reduced state and is a sensitive and accurate means of monitoring cell proliferation(16).
In order to ascertain the specificity of observed effects mesenchymal stem cells MSCs) were used as an example of non-cancerous cell type. MSCs were treated with the highest dose of I3C (100 µg / ml) and RE (100µg / ml) individually or in combination, and then evaluated using the alamarBlue Assay, as described above.
Cell morphology
Morphological changes of SK-OV-3 cells were observed at various time intervals after treatment with either compound alone or in combination, using a phase-contrast tissue culture microscope. Cell morphology, contraction, breakdown and detachment from substratum were noted.
Cell cycle analysis
SK-OV-3 cells (106) were grown (in triplicates) in 60 mm Petri dishes with 2 ml DMEM medium supplemented with 10% fetal calf serum and 1% penicillin-streptomycin for 24 hrs. They were treated with I3C (100 µg/ml) or RE (100 µg/ml) alone or in combination, for various time intervals and harvested by trypsinization, washed with PBS, fixed in alcohol, DNA stained with propidium iodide after RNAse treatment according to standard protocol. Cell cycle analysis was accomplished by flow-cytometry (BD Biosciences). Cells in G0/G1, S, G2M and apoptotic phases were quantified and data expressed as percentage of cells in each phase. Conditioned media samples were frozen at −70 °C for analysis of nitric oxide and CA-125, as described below.
Nitric oxide synthase measurement
Total nitric oxide synthase activity was measured using a nitrate/nitrite colorimetric assay kit from Cayman Chemical Company (Ann Arbor, MI), according to the manufacturer’s instruction. This assay kit utilizes nitrate reductase to convert nitrate to nitrite in the culture medium and Griess reagents to convert nitrite into a deep purple azo compound which is measured at 550 nm. This assay has a range of 2 to 35 µM and can accurately measure nitric oxide activity in 0.1 ml of culture medium. Results were expressed as µmoles of total nitrate + nitrite produced by 106 cells over 24 hrs, when the cells were harvested for cell cycle analysis (mean of triplicates).
CA125 assay
CA125 is an antigen produced by ovarian epithelial carcinoma cells and has been utilized as a marker for this cancer. The level of CA-125 was measured in culture media by solid phase enzyme linked immunoassay (ELISA) using a commercially available kit (United Biotech, Mountain View, California). Briefly, this method depends on the capture of antigen on streptavidin coated 96 well plates using a biotinylated antibody (capture antibody). A second horse-radish peroxidase conjugated antibody (detection antibody) is added and bound peroxidase is measured using a chromogenic substrate. The amount bound is proportional to the concentration of the antigen present in the sample, which is quantified using a standard curve. For this study, the assay range was between 10 and 500 units/ml. Results were expressed as units of CA-125 produced by 106 cells over 24 hrs period (mean of triplicates).
Western blot analysis of molecular pathways affected by I3C and RE treatment
In order to explore potential molecular mechanisms that might be contributing to the observed synergistic response, we analyzed the components of molecular pathways involved in cell cycle and apoptosis. Because the SKOV-3 cell line used in our experiments do not express p16 and p53, we examined the lysates for cell cycle markers, p21 and Rb (retinoblastoma) proteins as well as Survivin, a marker of apoptosis.
SK-OV-3 cells were treated with 100µg/ml of I3C or RE alone and in combination and protein lysates were collected 24hrs post-treatment. Western blot analysis was performed as previously described (17). Proteins were extracted with a lysis buffer and precipitated using RIPA buffer. Protein samples (60µg) were boiled for 5 min in an equal volume of reducing buffer (5mM Tris/HCl pH 7.4, 4% (w/v) SDS, 20% (v/v) glycerol, 10% (v/v) mercaptoethanol, 0.2% (w/v) bromophenol blue), resolved on 12% polyacrylamide gels, and electroblotted onto nitrocellulose membranes. The antibodies used were monoclonal anti-Rb antibody (BD Pharmingen) and Survivin (SVV) antibody (Santa-Cruz Co., sc-10811). After probing with the secondary antibody, proteins were visualized using Supersignal horseradish peroxidase according to the manufacturer’s instructions (Pierce). Equal loading of the protein samples was assessed by re-probing the membrane with a 1:2000 dilution of the Actin antibody (Santa Cruz).
Statistic analyses
Data from triplicates were analyzed by Prizm software and Means were calculated and compared using t- test.
Results were presented as Mean ± S.E.M. of at least triplicates or replicates from three experiments and the data were analysed statistically using Newman–Keuls multiple comparison test and Student's t-test using GraphPad Prism 2.01. Differences with P < 0.05 were considered significant.
Results
Cytotoxicity assay for I3C and RE alone and in combination using alamarBlue dye
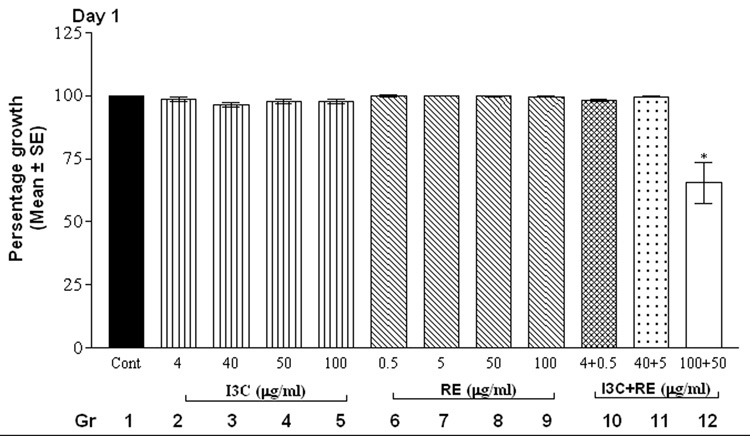
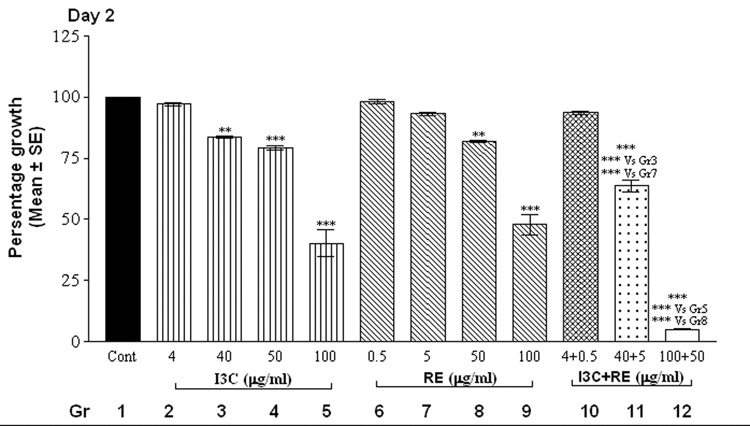
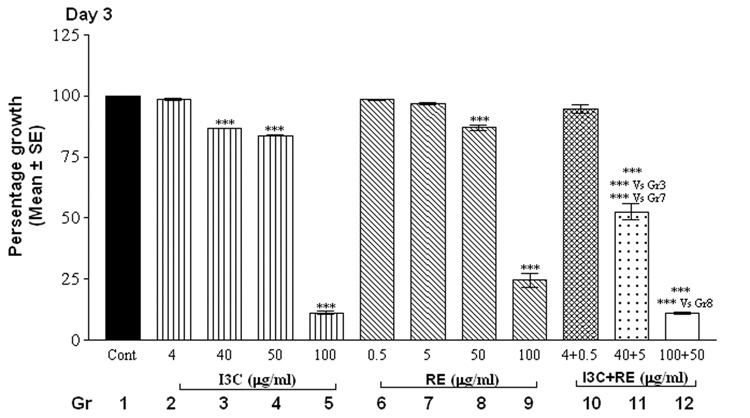
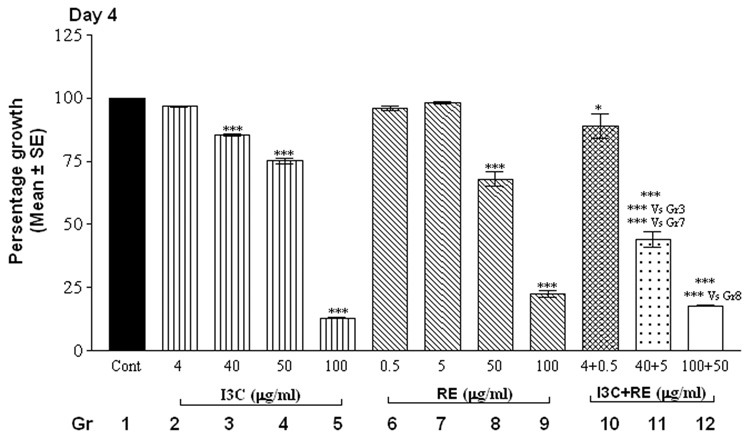
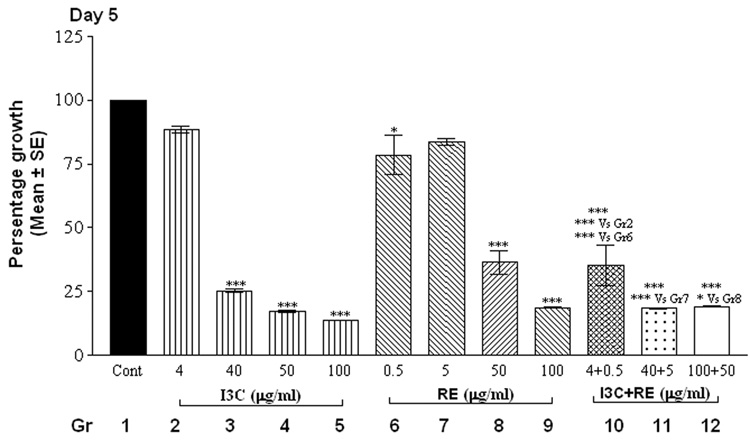
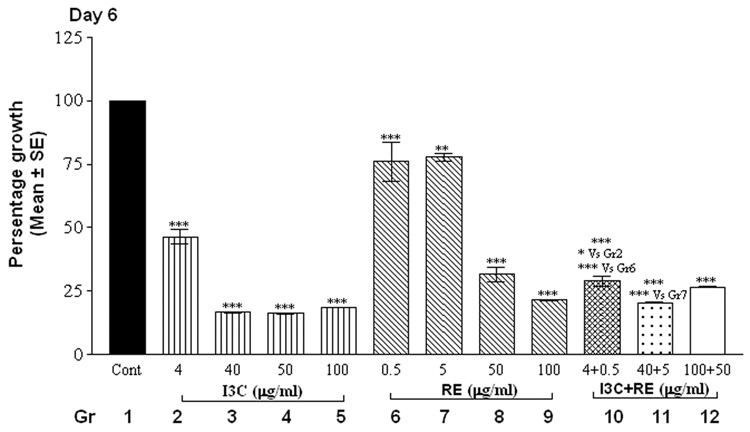
The alamarBlue assay revealed that cells in control groups (vehicle-treated SK-OV-3 cells) reduced the dye in a dose and time-dependent manner (Fig.1). Treatment with individual compounds (at all concentrations) did not differ significantly from controls at 24 hrs. However, combination treatment with 100 µg I3C and 50 µg RE resulted in a highly significant decrease in fluorescence by 24 hrs (Fig. 1, day 1), indicating inhibition of proliferation; this decrease was further accentuated by day 2 and remained at this low level through the remaining duration of the experiment. On day 2, the higher levels of I3C (40, 50 and 100 µg/ml) and RE (50 and 100 µg/ml) treatments resulted in significant inhibition individually as well as in combination (Fig.1, day 2). In contrast the lowest levels of 4 µg I3C or 0.5µg RE treatment did not result in inhibition till day 4. However combination treatment with these lowest levels showed inhibition of proliferation starting on day 4, which was further increased on days 5 and 6. In summary, the time taken for onset of inhibition increased as the dose of individual and combination treatments decreased. Synergistic response was maintained at all combinations over individual treatment groups, on the 1st day the response began. In contrast to the effects on SK-OV-3 cells, even the highest concentrations of 100µg I3C + 50µg RE had no effect on proliferation of MSCs ( data not shown), used as an example of non-cancerous cells, indicating the specificity of the effects of this combination.
Fig. 1.
Effects of RE, I3C and their combination on the metabolism of SK-OV-3 cells with alamarBlue dye. Cells were treated over a 6 day period and alamarBlue assay was performed daily as described under methods. The data is expressed as percent of growth ± SEM, as compared to vehicle control (100%), unless noted otherwise. Level of significance is denoted as follows: *, p <0.05; **, p<0.01; ***, p<0.001. Note a synergistic action of the two agents when used in combination in contrast to each one alone.
Cell morphology
Control SK-OV-3 cells treated with vehicle only exhibited a smooth epithelial pattern with large round nuclei. Concomitent with onset of alamarBlue reaction, cells started to contract, loose cell-cell contact and became crenated and detached from substratum. The higher doses caused extensive detachment of cells from substratum at 24 hrs and the lower doses took longer time for showing these deleterious effects. (data not shown)
Cell cycle analysis of the effects of I3C and RE alone and in combination
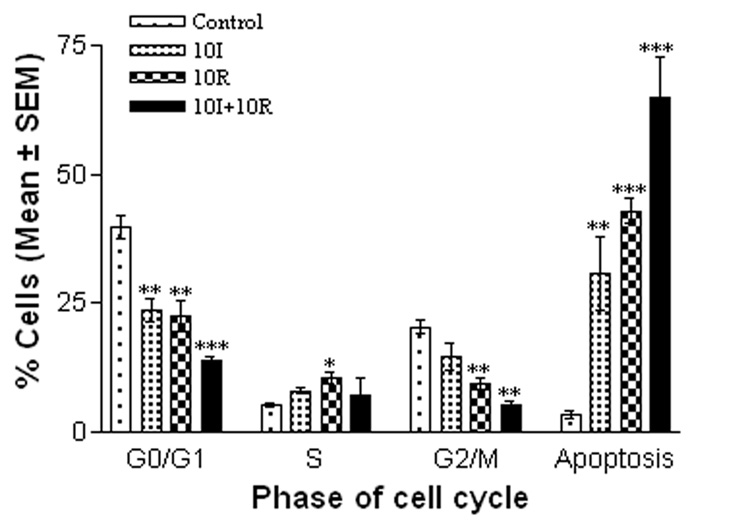
Treatment of cells with 100 µg/ml of I3C or RE alone resulted in cell cycle arrest at G0/G1 and increased apoptosis by 24 hrs, as compared to control groups (Fig.2). Treatment with both I3C and RE resulted in a further decrease in the percentage of cells in G0/G1 to less than 20% and increased the percentage of apoptotic cells to almost 70%. Interestingly, the effects of these two compounds seem to be different on G2/M phase of the cell cycle. While I3C had minimal inhibition on G2/M, RE exerted a highly significant inhibition of over 50% (p = <0.01). These effects were further accentuated when these two compounds were used in combination (p = <0.001). Flow cytometric profiles of control and treated cells are shown in Figure 3A – 3D. Height and areas under G0/G1, S phase and G2/M peaks are reduced and area under peak showing apoptosis increase as a result of combination treatment. However the ratios of G0/G1 vs G2/M were 1.94 (control), 1.60 (I3C), 2.37 (RE) and 2.53(I3C+RE), further substantiating a reduction in G2M with RE only or combination groups.
Fig. 2.
Induction of cell cycle arrest and apoptosis by I3C and RE treatment. SK-OV-3 cells were treated with 100µg/ml of I3C or RE alone and in combination. The cells were analyzed by flow cytometry. All values expressed as % cells (mean ± S.E.M.). Level of significance is denoted as follows: *, p < 0.05; **, p<0.01; ***, p < 0.001.
Fig. 3.
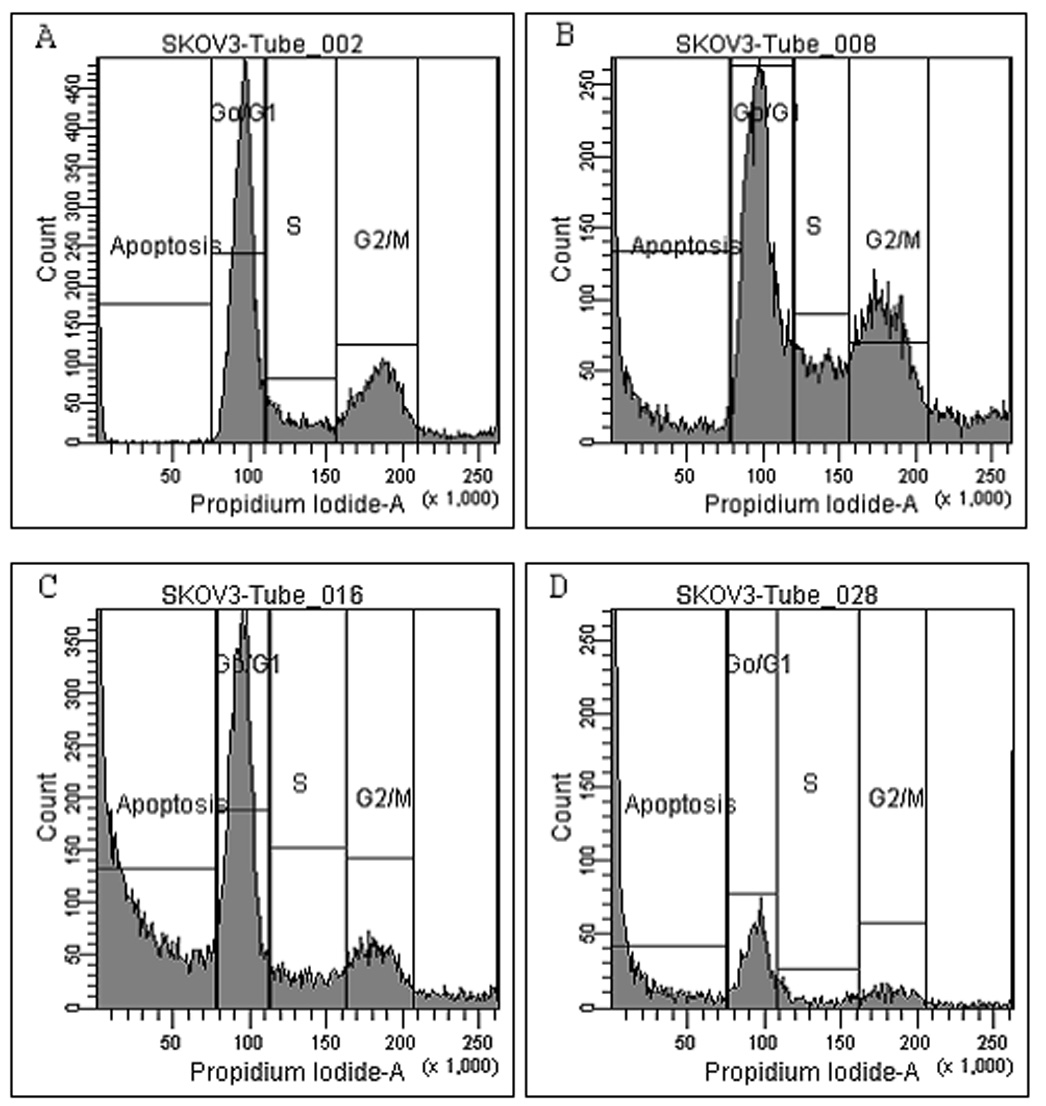
Flow cytometric pattern of SK-OV-3 cells treated with I3C, RE and combination: SK-OV-3 cells without treatment (controls) (A), treatment with 100 µg/ml I3C only (B), 100 µg RE only (C) or treated with a combination of I3C and RE (D), were examined by flow cytometry. Note drastic reduction in G0/G1, G2/M peaks and a dramatic increase in apoptosis (Sub G0) in the treatment groups.
Nitric Oxide production is affected by I3C and RE treatment
SK-OV-3 cells in the vehicle treated control group showed a basal nitric oxide synthase activity equal to 126 ± 5.4 µM of total nitrate + nitrite/106 cells over 24 hrs (mean ±STD). This was not significantly affected by treatment with RE (132 ± 6.3 µM of total nitrate + nitrite/ 106 cells over 24 hrs, mean of quadruplicates). In contrast, I3C completely inhibited the production of nitric oxide to undetectable levels.
Treatment of SK-OV-3 cell line with I3C and RE inhibits the secretion of CA-125
Control group of SK-OV-3 cells secreted CA-125 into the medium at the rate of 112.5 U ±25 / 106 cells over 24 hrs time-course culture (mean of quadruplicates). These levels were inhibited to undetectable values by treatment with either I3C or RE.
Effect of I3C and RE on apoptosis-related proteins
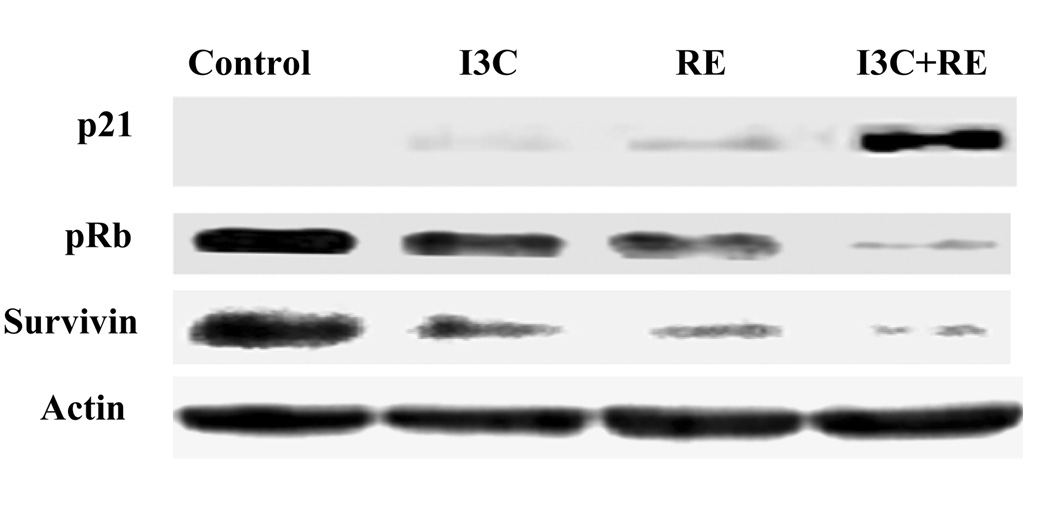
SK-OV-3 cells were treated with 100µg/ml of I3C or RE alone as well as in combination. Protein lysates collected 24hrs post-treatment were analyzed by western blot to examine the effect of I3C or RE alone or in combination on the proteins asociated with cell cycle (p21 and Rb) and apoptosis (SVV). Expression of the cell cycle inhibitor p21 increased by either treatment and was accentuated by combination treatment (Fig. 4). In contrast, Rb was inhibited by either treatment, and this inhibition was further accentuated by combination treatment. Similarly, expression of the anti-apoptotic protein SVV decreased with either treatment and was further accentuated by combination treatment. Interestingly, while no significant changes in Bcl-2/Bax ratio were detected (data not shown), expression of the anti-apoptotic protein SVV decreased with either treatment and was further accentuated by combination treatment. These molecular data correlate well with our observations described above, indicating inhibition of cell cycle and induction of apoptosis.
Fig. 4.
Synergistic up-regulation of tumor suppressor protein p21 and inhibition of Rb and Survivin expression by I3C and RE. Protein lysates were collected from cultured-treated SK-OV-3 and examined by western blot analysis as described under methods. Actin was used as loading control.
Discussion
The effects of RE on the cell cycle of a number of cancer cell types, including prostate and ovarian SK-OV-3 cells, has been previously demonstrated (8–12). Although I3C has been extensively investigated in prostate, breast and other cancer cell lines, its effects on ovarian cancer have not been demonstrated. Here we demonstrate for the first time effects of I3C on an ovarian cancer cell line and its synergism with RE in causing cell detachment and death by apoptosis. While both these compounds are potent phyto- anti-oxidants with overlapping activity profiles, they also have unique actions. We utilized the non-toxic alamarBlue dye to investigate the cell proliferation response, as this allows a longitudinal evaluation of the same culture samples over several days, in response to treatment. A dose-response / time course study was performed using the alamarBlue dye over a 6 day period (Fig.1). This study revealed that these two compounds administered individually or in combination resulted in inhibition of cell proliferation. Decreasing doses resulted in longer time periods being taken to achieve inhibition. Also the effects of combination treatment were greater than either individual treatment. This was specifically noticeable in the lowest dose level of 4 µg I3C + 0.5µg RE. This lowest level of combination was chosen as these levels represent the approximate bioavailable levels, achievable in vivo when these two compounds are administered orally (18). At the highest dose of 100 µg of either compound alone used in this present study, all the parameters tested (p21, Rb and SVV) showed a certain maximal change (inhibition or stimulation) which was always further accentuated by combination treatment. This indicates that the observed effects were specific and none of these doses were toxic in a nonspecific way. This also further indicates that these two compounds are possibly acting by different mechanisms and thus cause a greater combined effect. I3C induced G1 cell cycle arrest was first shown to be due to inhibition of cyclin-dependent kinase 6 and inhibition of Rb protein phosphorylation (19). While these two compounds have similar actions on G0/G1 phase of cell cycle and apoptosis, there seems to be a differential effect on G2/M phase. Thus RE had a highly significant effect on this phase with an observed inhibition of greater than 50%, as compared to controls (fig.2). Further, it was interesting to note that only I3C was capable of inhibiting basal nitric oxide production. These may be contributory factors in causing synergistic and/or additive effects when cells were given a combination treatment. In contrast, CA-125 levels were completely inhibited by either treatment, indicating that it may not be playing any role in causing this synergistic action. A systematic study of gene expression by these two compounds administered individually or together, is in progress to pinpoint the possible mechanisms underlying this observed synergism. RE is an inhibitor of Cox-1 and causes autophagy in ovarian cancer cells (20), in addition to activation of apoptotic pathways such as mitochondrial release of cytochrome-c, formation of apoptosome complex and caspase activation (21). Its known ability to suppress angiogenesis (22) may confer added advantage in its known ability to suppress tumor growth in animal models. I3C has been demonstrated to down regulate Bcl-2 levels in human melanoma cells (3), sensitizes prostate cancer cells to TRAIL-induced apoptosis (23) and caused Akt inactivation in PC-3 prostate cancer cells (4) Previous studies (3) using melanoma cell lines have shown an inhibition of Bcl-2 following treatment with I3C. However, in the present study using ovarian cells, no such inhibition was observed with any treatment. Interestingly, the anti-apoptotic protein, SVV showed a clear reduction with either treatment and an additive effect with the combination, suggesting that down regulation of SVV may be a common molecular link playing an important role in the induction of apoptosis. While this manuscript is being prepared, it was reported that RE induces apoptosis of prostate cell lines by upregulating pro-apoptotic proteins such as Bax, Bak, PUMA, Noxa, Bim, TRAIL-R1/DR4 and TRAIL-R2/DR5, and downregulates the expression of anti-apoptotic proteins including SVV (24). Also, I3C has been shown to induce apoptosis of prostate cell lines by targeting an array of signaling pathways including a dose-dependent inhibition of SVV (25). However, our study is the first to evaluate the combinatory effect of RE and I3C on SVV.
In our present investigation, we observed that the SK-OV-3 cells underwent profound morphological changes upon combination treatment. Initial contraction of the cells led to detachment and loss of cells by 24 hrs. This indicates a definitive effect on cell adhesion molecules. Their exact mechanism(s) of action in causing this synergism leading to cell detachment, cell cycle arrest and apoptosis needs to be further evaluated at the molecular level. This will provide a foundation for their use in combination for chemoprevention and / or chemotherapy of ovarian and possibly other cancers. It will be possible to include additional compounds of plant origin such as sulphoraphane, quercetin and curcumin etc. to simultaneously target additional molecular signal transduction pathways such as NF-kappa-B, to further potentiate action on tumorigenesis and tumor progression in vivo. Efficacy and toxicity of such combinations is being investigated using animal models with a view of utilizing them in human clinical trials. It is interesting to note that many of these phytochemicals are already commercially available, as dietary herbal supplements. Such supplements have not been FDA approved and not indicated for specific diseases, but are being sold simply as nutritional supplements for general health and immune function maintenance. This underscores an immediate need to further establish the efficacy and toxicity profiles of combination of active compounds and use them based on logically derived synergistic combinations.
Acknowledgements
Dr. Lan Nguyen and Dr. Ghazala A Azam participated in this project for partial fulfillment of OB-GYN residency research program. The authors wish to thank Dr. Beatrice Finkle, Flow Cytometric Core, Stanley S Scott Cancer Center of LSUHSC, for assistance with cell cycle analysis and Mr. Jamie Lopez with editing the manuscript. This work was supported in part from grants CA81125, CA91185, a training grant R25-CA47877 from NCI and Maternal and Child Health Development and Research MCHDR) program of NICHD. Dr. Abd Elmageed is supported by the Louisiana Cancer Research Consortium (LCRC) fund and in part by the Egyptian Ministry of Higher Education.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
References
- 1.Chinni SR, Li Y, Upadhyay S, Koppolu PK, Sarkar FH. Indole-3-Carbinol induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene. 2001;20:2927–2936. doi: 10.1038/sj.onc.1204365. [DOI] [PubMed] [Google Scholar]
- 2.Kim DS, Jeong YM, Moon SI, Kim SY, Kwon SB, Park ES, Youn SW, Park KC. Indole-3-carbinol enhances ultraviolet B-induced apoptosis by sensitizing melanoma cells. Cell.Mol.Life.Sci. 2006;63:2661–2668. doi: 10.1007/s00018-006-6306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YS, Milner JA. Targets for indole-3-carbinol in cancer prevention. J.Nutr.Biochem. 2005;16:65–73. doi: 10.1016/j.jnutbio.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Sreenivasa R, Chinni SR, Sarkar FH. Akt inactivation is a key event in indole-3-carbinol induced apoptosis in PC-3 cells. Clin.Canc.Res. 2005;8:1228–1236. [PubMed] [Google Scholar]
- 5.Howells LM, Gallacher-Horley B, Houghton CE, Manson MM, Hudson EA. Indole-3-carbinol inhibits Protein kinase B/Akt and induces apoptosis in the human breast tumor cell line MDA MB468 but not in the nontumorigenic HBL100 line. Mol.Cancer.Ther. 2002;1:1161–1172. [PubMed] [Google Scholar]
- 6.Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 7.Weng JR, Tsai CH, Kulp SK, Chen CS. Indole-3-carbinol as a chemopreventive and anti-cancer agent. Cancer Lett. 2008;262:153–163. doi: 10.1016/j.canlet.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang SH, Kim JS, Oh TJ, Kim MS, Lee SW, Woo SK, Cho HS, Choi YH, Kim YH, Rha SY, Chung HC, An SW. Genome-scale analysis of resveratrol induced gene expression profile in human ovarian cancer cells using cDNA microarray. Int.J.Oncol. 2003;22:741–750. [PubMed] [Google Scholar]
- 9.Notas G, Nifli AP, Kampa M, Vercauteren J, Kouroumalis E, Castanas E. Resveratrol exerts its Antiproliferative effect on HepG2 hepatocellular carcinoma cells, by inducing cell cycle arrest and NOS activation. Biochim.Biophys Acta. 2006;1760:1657–1666. doi: 10.1016/j.bbagen.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Lin HY, Tang HY, Keating T, Wu YH, Shih A, Hammond D, Sun M, Hercbergs A, Davis FB, Davis PJ. Resveratrol is pro-apoptotic and thyroid hormone is anti-apoptotic in glioma cells: Both actions are integrin and ERK-mediated. Carcinogenesis. 2008;29:62–69. doi: 10.1093/carcin/bgm239. [DOI] [PubMed] [Google Scholar]
- 11.Benitez DA, Pozo-Guisado E, Alvarez-Barrientos A, Fernandez-Salguero PM, Castellón EA. Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines. J Androl. 2007;28:282–293. doi: 10.2164/jandrol.106.000968. [DOI] [PubMed] [Google Scholar]
- 12.Fulda S, Debatin KM. Sensitization for tumor necrosis factor related apoptosis inducing ligand induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 2004;64:337–346. doi: 10.1158/0008-5472.can-03-1656. [DOI] [PubMed] [Google Scholar]
- 13.Gerhauser C, Klimo K, Heiss E, Neumann I, Gamal-Eldeen A, Knauft J, Liu GY, Sitthimonchai S, Frank N. Mechanism based in vitro screening of potential cancer chemopreventive agents. Mutat. Res. 2003;523–524:163–172. doi: 10.1016/s0027-5107(02)00332-9. [DOI] [PubMed] [Google Scholar]
- 14.Jang M, Pezzeugo JM. Cancer chemoprevention activity of resveratrol. Drugs Exp Clin Res. 1999;25:65–77. [PubMed] [Google Scholar]
- 15.Chen YH, Dai HJ, Chang HP. Suppression of inducible nitiric oxide production by indole and isothiocyanate derivatives from Brassica plants in stimulated macrophages. Planta Med. 2003;69:696–700. doi: 10.1055/s-2003-42790. [DOI] [PubMed] [Google Scholar]
- 16.Nocari MM, Shalev A, Benias P, Russo C. A novel one-step, highly sensitive fluorometric assay to evaluate cell-mediated cytotoxicity. J. Immunol. Methods. 1998;213:157–167. doi: 10.1016/s0022-1759(98)00028-3. [DOI] [PubMed] [Google Scholar]
- 17.Ouhtit A, Muller K, Davis D, Ullrich SE, McConkey D, Ananthaswamy HN. Temporal events in skin injury and the early adaptive responses in ultraviolet-irradiated mouse skin. Am J Pathol. 2000;156:201–207. doi: 10.1016/S0002-9440(10)64720-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howells LM, Moiseeva EP, Neal CP, Foreman BE, Andreadi CK, Sun Y, Hudson A, Manson MM. Predicting the physiological relevance of in vitro cancer preventive activities of phytochemicals. Acta Pharmacol Sin. 2007;28:1274–1304. doi: 10.1111/j.1745-7254.2007.00690.x. [DOI] [PubMed] [Google Scholar]
- 19.Cover CM, Hsieh SJ, Tran SH, Hallden G, Kim GS, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibits the expression of cyclin-dependent kinase-6 and induces a G1 cell cycle arrest of human breast cancer cells independent of estrogen receptor signaling. J Biol Chem. 1998;273:3838–3847. doi: 10.1074/jbc.273.7.3838. [DOI] [PubMed] [Google Scholar]
- 20.Opipari AW, Tan L, Boitano AE, Sorenson DR, Aurora A, Liu RJ. Resveratrol induced autophagocytosis in ovarian cancer cells. Cancer Res. 2004;64:696–703. doi: 10.1158/0008-5472.can-03-2404. [DOI] [PubMed] [Google Scholar]
- 21.Park JW, Choi YJ, Suh SI, Back WK, Shu MH, Jin IN, Min DS, Woo JH, Chang JS, Passaniti A, Lee YH, Kwon TK. Bcl-2 overexpression attenuates resveratrol induced apoptosis in U937 cells by inhibition of caspace 3 activity. Carcinogenesis. 2001;22:1633–1639. doi: 10.1093/carcin/22.10.1633. [DOI] [PubMed] [Google Scholar]
- 22.Brakenhielm E, Cao R, Cao Y. Suppression of angiogenesis, tumor growth and wound healing by Resveratrol, a natural compound in red wine and grapes. FASEB J. 2000;15:1798–1800. doi: 10.1096/fj.01-0028fje. [DOI] [PubMed] [Google Scholar]
- 23.Jeon K, Rih JK, Kim HJ, Lee YJ, Cho CH, Goldberg ID, Rosen EM, Bae I. Pretreatment with indole-3-carbinol augments TRAIL-induced apoptosis in a prostate cancer cell line, LNCaP. FEBS Lett. 2003;544:246–251. doi: 10.1016/s0014-5793(03)00473-3. [DOI] [PubMed] [Google Scholar]
- 24.Shankar S, Chen Q, Siddiqui I, Sarva K, Srivastava RK. Sensitization of TRAIL-resistant LNCaP cells by resveratrol (3, 4', 5 tri-hydroxystilbene): molecular mechanisms and therapeutic potential. J Mol Signal. 2007;2:7–24. doi: 10.1186/1750-2187-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weng JR, Tsai CH, Kulp SK, Wang D, Lin CH, Yang HC, Ma Y, Sargeant A, Chiu CF, Tsai MH, Chen CS. A potent indole-3-carbinol derived antitumor agent with pleiotropic effects on multiple signaling pathways in prostate cancer cells. Cancer Res. 2007;67:7815–7824. doi: 10.1158/0008-5472.CAN-07-0794. [DOI] [PubMed] [Google Scholar]