Abstract
OBJECTIVE
To review the definition, clinical presentation, and management of inflammatory breast cancer in primary care.
SOURCES OF INFORMATION
Relevant research and review articles, as well as personal experience of the authors practising in a specialized locally advanced breast cancer program at a comprehensive cancer centre. Evidence is levels II and III.
MAIN MESSAGE
Inflammatory breast cancer is a rare disease that typically presents with a rapidly enlarging erythematous breast, often with no discernable breast mass. Identification of warning signs and recognition of clinical symptoms are crucial to prompt diagnosis and appropriate referral. Management in the primary care setting includes treatment of symptoms, psychosocial support, regular surveillance and follow-up, as well as palliative care.
CONCLUSION
Family physicians are usually the entry point to the health care system and are well positioned to assess inflammation of the breast and recognize the warning signs of an underlying inflammatory breast cancer. They are also important members of the team that provides support for breast cancer patients and their families during treatment, follow-up, and end-of-life care.
RÉSUMÉ
OBJECTIF
Rappeler la définition, la présentation clinique et le traitement du cancer inflammatoire du sein en médecine primaire.
SOURCES DE L’INFORMATION
Recherche et articles de revue pertinents ainsi que l’expérience personnelle des auteurs qui exercent dans un programme local spécialisé pour le cancer du sein dans un centre de soins intégrés pour les personnes atteintes du cancer.
PRINCIPAL MESSAGE
Le cancer inflammatoire du sein est une affection rare qui se présente typiquement sous la forme d’un sein érythémateux qui grossit rapidement, souvent sans masse mammaire palpable. La reconnaissance des signes d’alerte et des symptômes cliniques est essentielle à un diagnostic précoce et à une demande de consultation appropriée. Dans un contexte de première ligne, la prise en charge comprend le traitement des symptômes, un support psycosocial, une surveillance régulière, un suivi ainsi que des soins palliatifs.
CONCLUSION
Étant habituellement le point d’entrée du système de santé, le médecin de famille est bien placé pour évaluer une inflammation mammaire et reconnaître les signes indicateurs d’un cancer inflammatoire du sein. C’est également un membre important de l’équipe qui assure le soutien aux patientes atteintes d’un cancer du sein et à leur famille au cours du traitement, le suivi et les soins en fin de vie.
The average family physician will see 2 new cases of breast cancer each year.1 Inflammatory breast cancer (IBC) is a rare subgroup of breast cancer that presents particular challenges. Although few women presenting with inflammation of the breast have cancer, family physicians must recognize warning signs and differentiate IBC from the more common benign disorders.
One of the main challenges of IBC is prompt recognition of disease symptoms. Inflammatory breast cancer often presents with diffuse erythema of the breast in the absence of a discrete breast lump, which might not be recognized by patients as a “warning sign” for aggressive breast cancer, thus delaying presentation.2 Clinicians might not recognize IBC warning signs if women are younger, pregnant, or breastfeeding.3 Because IBC is a rapidly progressing form of cancer, it is essential that family physicians be familiar with its clinical presentation and make timely referrals. Following diagnosis, family physicians play an important role in symptom control and supportive care.
In this article we will expound upon basic principles surrounding diagnosis and management of IBC in primary care.
Case 1
Callie is a 36-year-old who gave birth to her first child 2 months ago. Five days ago she noticed redness and tenderness in her left breast. She has no relevant personal past medical history; however, her mother and maternal aunt were both diagnosed with breast cancer in their 60s. Callie presents to your office with visible erythema of the left breast in the upper outer quadrant and concomitant tenderness. She is concerned about infection and breast-feeding on that side. Should you be concerned about IBC?
Case 2
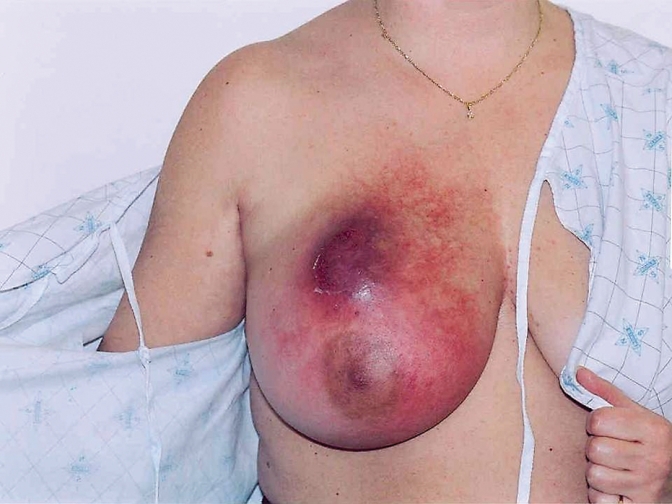
Edith is a 76-year-old widow. She presents to your office complaining of a 3-month history of enlargement of the right breast. Physical examination reveals that her right breast is enlarged and red, and the skin is firm, with pitting (Figure 1). She has palpable axillary lymph nodes. You suspect IBC. What are your next steps in diagnosis and management?
Figure 1.
Right breast is enlarged and red, and the skin is firm, with pitting
Sources of information
Data for this review were identified by a search of MEDLINE (January 1950 to April 2008) using search terms inflammatory breast cancer, detection, differential, risk factors, family physician, and survival. References were hand-searched for relevance and limited to English-language articles. Evidence is level II (epidemiologic studies) and III (reviews based on retrospective case series and expert opinions).
Definition
Inflammatory breast cancer is a clinical diagnosis. Patients typically present with a rapidly progressing, tender, firm, enlarged breast (Table 1). This presentation is due to the invasion of skin dermal lymphatics by breast cancer cells. The obstructed lymph channels produce characteristic skin changes—erythema, warmth, edema, and induration—that mimic an inflammatory process, except cancer cells rather than inflammatory infiltrate are seen under the microscope.4 These changes are sometimes, but not always, demonstrated on skin biopsy.
Table 1.
Clinical features of inflammatory breast cancer
| OBJECTIVE SIGNS | SYMPTOMS |
|---|---|
|
|
Epidemiology
Inflammatory breast cancer is not common, representing anywhere from 1% to 6% of all breast cancer diagnoses (level II evidence).5 A recent review suggested that the incidence of IBC could be rising; authors found an incidence of 2% from 1988 to 1990 and 2.5% from 1997 to 1999.5
Inflammatory breast cancer is more common among black women and is associated with younger age at diagnosis (mean age 50 to 58 years compared with 50 to 64 years among those diagnosed with noninflammatory breast cancer).6 Studies based on cases in North Africa, where for unknown reasons 50% of breast cancer cases present as IBC, have suggested an association with obesity and younger age at first giving birth.6 However, there are no validated risk factors that might aid in raising suspicion for IBC.6,7
Inflammatory breast cancer is commonly diagnosed in women who have never had breast cancer, but it can also develop in a breast that contains a known tumour or that has been previously treated. These “secondary” cases of IBC behave similarly to “primary” cases of IBC, and therefore a diagnosis of IBC cannot be excluded in a woman with a known history of breast cancer.8
Clinical presentation
Patients with IBC usually present with a complaint of colour change in 1 breast, usually pink that evolves into a darker red and spreads over the entire breast. The patient might describe a sensation of heat in the breast, and the breast itself enlarges rapidly over a period of only a few weeks (Table 1 and Figure 2). Patients usually do not experience fever.9
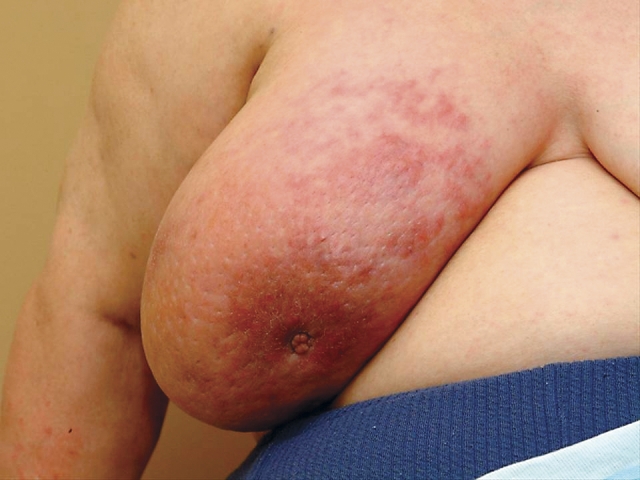
Figure 2. Patients with inflammatory breast cancer.
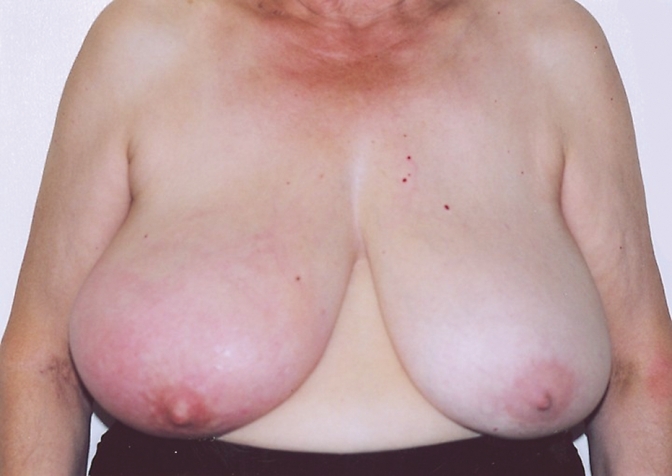
Patients usually present complaining of colour change in one breast, usually pink that evolves into a darker red and spreads over the entire breast. The patient might describe a sensation of heat in the breast, and the breast itself enlarges rapidly.
Physical examination should focus on assessing warmth, swelling, induration, erythema, and peau d’orange. Nipple changes such as crusting and retraction might be seen but are not criteria for diagnosis.9 Underlying tumour masses might be palpable in IBC, but up to 30% of patients will present with no underlying masses. Instead, such a patient will have a diffusely enlarged breast.9 Given the rapid spread of this type of tumour, it is not uncommon to find axillary adenopathy. Rarely, patients might have signs or symptoms of metastatic spread (Table 2).
Table 2.
Signs and symptoms of metastatic breast cancer
| SITE OF SPREAD | SYMPTOM | SIGN |
|---|---|---|
| Lymph nodes | Pain and swelling in axillary, supraclavicular, or infraclavicular areas | Axillary, supraclavicular, or infraclavicular adenopathy |
| Bone | Pain in bones out of keeping with patients’ usual pain, without previous trauma and not responding to medication | Tenderness on percussion of the spine |
| Liver | Abdominal pain, early satiety | Enlarged liver on palpation, with a firm liver edge |
| Lung | Shortness of breath | Auscultation and percussion of lungs compatible with pleural effusion or pulmonary edema |
| Brain | Morning headache, vomiting | Neurologic symptoms, papilledema |
Certain warning signs in a woman with a red breast increase the likelihood of IBC (Table 3). These include a personal history of breast cancer, nonlactational status, and palpable axillary adenopathy. Unless a clear alternative diagnosis is present, these women should be referred immediately to surgical oncologists.
Table 3.
Red-flag warning signs of inflammatory breast cancer
| WARNING SIGNS |
|---|
| Previous history of breast cancer |
| Symptoms of mastitis in a nonlactating woman |
| Palpable adenopathy |
Differential diagnoses
The differential diagnoses for inflammation of the breast include benign diseases and other malignancies, and can be stratified by lactation status (Table 4).
Table 4. Differential diagnoses of breast inflammation.
Entities in boldface are common.
| STATUS OF BREAST | BENIGN CONDITIONS | MALIGNANT CONDITIONS |
|---|---|---|
| Lactating | Acute mastitis | Inflammatory breast cancer |
| Abscess | ||
| Galactocele | ||
| Nonlactating | Duct ectasia | Inflammatory breast cancer |
| Lymphoma | ||
| Nonspecific | Generalized dermatitis (insect bite, sunburn, allergy) | Leukemia |
| Fat necrosis | ||
| Tuberculosis | ||
| Sarcoidosis | ||
| Cat-scratch disease | ||
| Syphilis |
Lactation mastitis occurs in up to 10% of lactating women and is associated with localized tenderness, fever, and leukocytosis—features that help to distinguish it from IBC.10 Mastitis develops rapidly over a few days. The erythema is associated with tenderness and occupies a wedge-shaped quadrant of the breast, and the patient feels unwell. Infection is usually caused by Staphylococcus aureus and improvement occurs within 24 to 48 hours of initiating antibiotics.
Duct ectasia is a benign entity of the nonlactating breast that affects perimenopausal and postmenopausal women. It occurs when ducts beneath the nipples become clogged with fatty material, producing a lump. There is frequently itching or burning around the nipple and a thick discharge.11 These changes are well demarcated and involve less than one-third of the breast.12 Duct ectasia is usually a self-limited disease but might lead to subareolar abscesses that require surgical resection.11
Other benign entities that can be confused with IBC include Mondor disease and fat necrosis. Mondor disease, or phlebitis of the thoracoepigastric vein, usually presents as a painful, possibly palpable, cord and is usually preceded by trauma.10 Because there is a possible association with malignancy, a mammogram is suggested for women older than 35 years of age. Treatment includes reassurance and nonsteroidal anti-inflammatory drugs. Fat necrosis can occur spontaneously or as a result of trauma.10 The usual presentation of fat necrosis is a palpable lump, and a biopsy might be required to differentiate it from malignancy. Neither Mondor disease nor fat necrosis will result in enlarged lymph nodes, another clue to diagnosis of IBC.11
Malignant entities that mimic IBC include leukemic infiltration of the breast. These patients are usually systemically unwell, and a peripheral blood smear usually confirms this rare diagnosis.
Generally, benign breast conditions will resolve with appropriate treatment. Most experts recommend waiting 7 days before initiating further investigations, unless the clinical suspicion of IBC is high (classic breast appearance, axillary nodes, no history of lactation or trauma).
Investigations
If clinical suspicion of IBC is high, prompt referral to a surgeon should be made. Otherwise the first investigation ordered is generally a mammogram. The classic changes that can be seen on a mammogram in IBC are skin thickening, trabecular and stromal thickening, and increased breast density.13 Inflammatory breast cancer, as mentioned, might not be associated with a breast mass, and concomitant high breast density can obscure masses as well; therefore, absence of suspicious masses on the mammogram is not helpful in ruling out IBC.
Ultrasound of the breast and axillary lymph nodes can be useful when IBC is suspected. Ultrasound imaging might detect masses that were not obvious on palpation or mammography, or reveal axillary adenopathy, and thereby facilitate biopsy for diagnosis.13
If results of these investigations do not suggest a benign diagnosis (such as fat necrosis), and the patient’s symptoms persist beyond 3 weeks despite appropriate investigations and conservative treatment (such as nonsteroidal anti-inflammatory drugs and antibiotics), prompt referral to a surgeon is necessary.
Management
Patients with IBC need to be treated promptly, ideally in a multidisciplinary breast oncology clinic that specializes in the treatment of IBC. This patient group requires coordination of medical, surgical, and radiation oncology care as well as nursing and other supportive disciplines. These clinics are usually only found within cancer centres affiliated with tertiary hospitals, and might require patients to travel substantial distances.
Management of IBC encompasses combined-modality therapy. Owing to the skin involvement of IBC, the risk of loco-regional and distant recurrence is too high to justify immediate mastectomy. Preoperative chemotherapy is therefore standard care.14 Before initiating chemotherapy, a core biopsy is performed to confirm the diagnosis and determine hormone-receptor and HER-2/neu status of the tumour. A metastatic workup completes staging. This workup includes bone scans, chest x-ray scans, and abdominal ultrasound scans (with more focused tests if there are any additional symptoms).
The purpose of chemotherapy up front (also known as neoadjuvant or preoperative chemotherapy) is to eliminate micrometastatic disease and reduce inflammation in the breast, making tumours amenable to surgery and radiation. Response to chemotherapy also tells oncologists about the aggressiveness of tumours, providing clues to patients’ ultimate prognoses. After chemotherapy, patients undergo mastectomy and axillary lymph node dissection, followed by radiation therapy to the chest wall and regional lymph nodes.15 The final component of treatment includes endocrine therapy and targeted therapy, such as trastuzumab, depending on receptor status. In total, the active treatment of IBC takes about 1 year.
Common side effects of chemotherapy include alopecia, nausea and vomiting, and risk of febrile neutropenia. Patients are advised to promptly visit the nearest emergency department if they experience a fever (higher than 38°C for at least 1 hour, or 1 reading above 38.2°C). Some patients receiving taxane chemotherapy experience bony pain or myalgias. This pain often responds to treatment with acetaminophen, but can be severe enough to require opioid treatment as well.16 Long-term effects include increased risk of cardiac damage (due to anthracycline therapy) and increased risk of leukemia.
Prognosis
When compared with other breast cancer patients presenting at the same stage, women with IBC have worse prognoses. Moreover, up to 25% of women present with metastatic, incurable disease.17 Median overall survival for IBC patients is 2.9 to 4.2 years,18,19 with lower survival rates among black women and those with estrogen receptor–negative tumours.5 These survival rates have not changed significantly over the past 30 years, emphasizing the aggressive nature of the disease.19 Newer biologic agents such as trastuzumab and lapatinib might improve outcomes for patients with IBC.14 Given the baseline poor prognosis of IBC, eligible patients are offered inclusion in clinical trials if possible.
Family physicians’ role in management
Family physicians are gatekeepers: they play a crucial role in identifying IBC and referring patients appropriately. The oncology team treats the cancer and related problems; however, the family doctor manages nononcologic diagnoses that were present before diagnosis of IBC. Some conditions might be affected by treatment; for instance, blood-glucose control in patients with diabetes often worsens during chemotherapy, owing to steroids given adjunctively. The family doctor often needs to provide psychological support to the patient and her family. Once treatment is finished, close clinical surveillance and annual mammography are shared responsibilities of the oncologist and family doctor. The family doctor also monitors for long-term side effects of treatment (Table 5).
Table 5. Family physician follow-up of women treated for breast cancer.
The goals of follow-up are to provide support and counseling, to detect metastatic disease and ensure appropriate treatment is initiated promptly, and to provide patient education as new information about breast cancer becomes available.
| FOLLOW-UP ACTIVITIES | RECOMMENDATIONS FOR EXAMINATION |
|---|---|
| Medical history | Bone pain |
| Dyspnea | |
| Weight loss | |
| Abdominal symptoms | |
| New nodules or masses | |
| Vaginal bleeding if taking tamoxifen* | |
| Physical examination | Breast and chest wall |
| Axilla | |
| Supraclavicular nodes | |
| Arm lymphedema | |
| Hepatomegaly | |
| Ascites | |
| Leg swelling if taking tamoxifen† | |
| Mammograms | Annually |
| Routine laboratory and radiological tests | As clinically indicated by signs or symptoms |
| Bone mineral density scans for women at risk of osteoporosis or starting aromatase inhibitors | At completion of adjuvant chemotherapy and before initiation of aromatase inhibitors |
| Repeat in 12-18 mo | |
| Advise lifestyle modification (reduce alcohol and caffeine intake, stop smoking, increase weight-bearing exercise, take calcium and vitamin D supplements) | |
| Consequences of disease or treatment | Menopausal symptoms |
| Cognitive functioning | |
| Fatigue | |
| Weight gain | |
| Sexual functioning | |
| Fertility | |
| Arthralgias | |
| Lymphedema | |
| Psychosocial distress |
If vaginal bleeding is present in patients taking tamoxifen, a trans-vaginal ultrasound is recommended. Refer to a gynecologist if the endometrial thickness is > 10 mm or if there is persistent bleeding with thickness > 5 mm.
If a patient experiences a thrombotic event while taking tamoxifen, a referral back to the oncologist is recommended.
Often the family physician is not only the patient’s doctor but also her whole family’s doctor. If the patient is the primary caregiver in the family, a shifting of roles needs to occur, and coping can be difficult. Previous relationship difficulties are often exacerbated by the stress of a new diagnosis. Children of any age are substantially affected.20 Family physicians should inquire about children’s health and development; some warning signs include somatic complaints, difficulties with school, depression, and anxiety. Some families might benefit from multidisciplinary support teams that include family physicians, oncologists, nurses, and social workers. Internet resources can also be helpful (Table 6).
Table 6.
Internet resources
| RESOURCE | WEBSITE | DESCRIPTION OF RESOURCE |
|---|---|---|
| General information | ||
| Canadian Cancer Society | www.cancer.ca | Information and resources across Canada |
| Canadian Breast Cancer Network | www.cbcn.ca | Database of support groups and information |
| National Cancer Institute | www.cancer.gov | US website with up-to-date clinical information for patients and health professionals, clinical trials |
| Support groups | ||
| Willow | www.willow.org | Support groups and support information across Canada |
| Princess Margaret Hospital Survivorship Program | www.caringvoices.ca | Support groups and support information |
| Families with children | ||
| Gilda’s Club | www.gildasclubtoronto.org | On-line resources for children |
| We Can Cope | http://wecancope.com | Information and frequently asked questions |
| Palliative care | ||
| Princess Margaret Hospital Palliative Care | www.caringtotheend.ca | Information for health professionals and patients |
Given the poor prognosis of IBC, family doctors might need to help with palliative care as well. Communication with a patient’s oncologist is essential for optimizing symptom management and organizing end-of-life care. Palliative care clinics affiliated with cancer centres are excellent referral centres that organize home nursing and social work, and they will liaise with family doctors to provide comprehensive and consistent care.
Conclusion
Family physicians are uniquely positioned to assure timely diagnosis of IBC, which is an aggressive form of breast cancer. Prompt recognition of symptoms and signs suggestive of IBC, referral to a multidisciplinary team for treatment, and support of women and their families throughout treatment and beyond assures optimal management of this potentially fatal disease.
Case 1 resolution
Callie has a low-grade fever, and her baby is not feeding properly from the affected breast. Examination reveals a focal area of pain, redness, and induration in the outer upper quadrant, with no palpable lymphadenopathy. Bloodwork results show leukocytosis and left shift. You instruct Callie about compresses, ibuprofen, and continued breastfeeding, and prescribe a 14-day course of oral cloxacillin.11 You expect improvement within 48 hours; if there is no response within 7 days you will reconsider the diagnosis, perform a mammogram and an ultrasound, and, depending on the results, refer Callie to a breast cancer centre.
Case 2 resolution
Edith has many worrisome features of IBC, and prompt mammogram and ultrasound are performed. She is immediately referred to a tertiary oncology centre when pathology from the ultrasound-guided lymph node biopsy reveals breast adenocarcinoma. You help manage her pain and symptoms throughout her ordeal, but despite optimal treatment her cancer spreads and she dies peacefully, pain-free, at a hospice.
Levels of evidence
Level I: At least one properly conducted randomized controlled trial, systematic review, or meta-analysis
Level II: Other comparison trials, non-randomized, cohort, case-control, or epidemiologic studies, and preferably more than one study
Level III: Expert opinion or consensus statements
EDITOR’S KEY POINTS
Inflammatory breast cancer (IBC) is a rare type of breast cancer, accounting for between 1% and 6% of all breast cancer diagnoses. Because IBC is aggressive, however, the prognosis is poorer than for women with other types of breast cancer. Up to 25% of women present with metastatic, incurable disease. Median overall survival for IBC patients is 2.9 to 4.2 years.
Patients will usually present with inflammation of the breast. Physical examination should focus on assessing warmth, swelling, induration, erythema, and peau d’orange. Nipple changes might be seen but are not criteria for diagnosis. Up to 30% of patients will not have underlying masses. It is not uncommon to find axillary adenopathy, and signs or symptoms of metastatic spread are rare.
Warning signs in a woman with a red breast include a personal history of breast cancer, non-lactational status, and palpable axillary adenopathy. Unless a clear alternative diagnosis is present, these women should be referred immediately to surgical oncologists.
POINTS DE REPÈRE DU RÉDACTEUR
Le cancer inflammatoire du sein (CIS) est une forme rare de cancer qui représente entre 1 et 6% de tous les diagnostics de cancer du sein. À cause de son caractère agressif, le CIS présente un moins bon pronostic que les autres types de cancer du sein. Jusqu’à 25% des femmes atteintes ont une maladie métastatique incurable au diagnostic. Globalement, la survie médiane des patientes présentant un CIS est de 2,9 à 4,2 ans.
Les patientes consultent généralement pour une inflammation du sein. L’examen physique devrait se concentrer sur la présence de chaleur, d’œdème, d’induration, d’érythème et de peau d’orange. Il se peut qu’il y ait des modifications du mamelon, mais il ne s’agit pas d’un critère diagnostique. Jusqu’à 30% des patientes n’auront pas de masse sous-jacente. Les adénopathies axillaires ne sont pas rares, mais les signes et symptômes d’un envahissement métastatique sont peu fréquents.
Les signes à vérifier chez une femme qui a une rougeur au sein sont entre autres des antécédents de cancer du sein, le fait de ne pas être en période d’allaitement et des ganglions axillaires palpables. À moins d’un autre diagnostic évident, ces femmes devraient être immédiatement aiguillées vers un chirurgien oncologiste.
Footnotes
Cet article a fait l’objet d’une révision par des pairs.
Contributors
Drs Molckovsky, Freedman, Heisey, and Clemons and Ms Fitzgerald contributed to the concept of the article, the literature search, the review of selected articles, and preparing the manuscript for submission
Competing interests
None declared
This article has been peer reviewed.
References
- 1.Watson DE, Katz A, Reid R, Bogdan B, Roos N, Heppner P. Family physician workloads and access to care in Winnipeg: 1991 to 2001. CMAJ. 2004;171(4):339–42. doi: 10.1503/cmaj.1031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bish A, Ramirez A, Burgess C, Hunter M. Understanding why women delay in seeking help for breast cancer symptoms. J Psychosom Res. 2005;58(4):321–6. doi: 10.1016/j.jpsychores.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Mason G, Johnson O. Inflammatory breast cancer: patient advocate view. Semin Oncol. 2008;35(1):87–91. doi: 10.1053/j.seminoncol.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Cariati M, Bennett-Britton TM, Pinder SE, Purushotham AD. Inflammatory breast cancer. Surg Oncol. 2005;14(3):133–43. doi: 10.1016/j.suronc.2005.07.004. Epub 2005 Sep 9. [DOI] [PubMed] [Google Scholar]
- 5.Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: the surveillance, epidemiology, and end results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97(13):966–75. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine PH, Veneroso C. The epidemiology of inflammatory breast cancer. Semin Oncol. 2008;35(1):11–6. doi: 10.1053/j.seminoncol.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Ferdous CS, Islam TM, Meza LA. Review of inflammatory breast cancer: a meta-analysis [abstract] J Clin Oncol. 2008;26(Suppl):1131. [Google Scholar]
- 8.Resetkova E. Pathologic aspects of inflammatory breast carcinoma: part 1. Histomorphology and differential diagnosis. Semin Oncol. 2008;35(1):25–32. doi: 10.1053/j.seminoncol.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Singletary SE, Cristofanilli M. Defining the clinical diagnosis of inflammatory breast cancer. Semin Oncol. 2008;35(1):7–10. doi: 10.1053/j.seminoncol.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Givens ML, Luszczak M. Breast disorders: a review for emergency physicians. J Emerg Med. 2002;22(1):59–65. doi: 10.1016/s0736-4679(01)00437-1. [DOI] [PubMed] [Google Scholar]
- 11.Marchant DJ. Inflammation of the breast. Obstet Gynecol Clin North Am. 2002;29(1):89–100. doi: 10.1016/s0889-8545(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 12.Dahlbeck SW, Donnelly JF, Theriault RL. Differentiating inflammatory breast cancer from acute mastitis. Am Fam Physician. 1995;52(3):929–34. [PubMed] [Google Scholar]
- 13.Le-Petross CH, Bidaut L, Yang WT. Evolving role of imaging modalities in inflammatory breast cancer. Semin Oncol. 2008;35(1):51–63. doi: 10.1053/j.seminoncol.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Chia S, Swain SM, Byrd DR, Mankoff DA. Locally advanced and inflammatory breast cancer. J Clin Oncol. 2008;26(5):786–9. doi: 10.1200/JCO.2008.15.0243. [DOI] [PubMed] [Google Scholar]
- 15.Buchholz TA, Tucker SL, Msaullo L. Predictors of local-regional recurrence after neoadjuvant chemotherapy and mastectomy without radiation. J Clin Oncol. 2002;20(1):17–23. doi: 10.1200/JCO.2002.20.1.17. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald B, Napolskikh J, Salvo N, Freedman O, Dranitsaris G, Ali V, et al. The incidence of taxane-induced myopathy in patients receiving chemotherapy for early stage breast cancer. Poster presented at: Canadian Association of Medical Oncologists Annual Scientific Meeting; May 2008; Toronto, ON. [Google Scholar]
- 17.Wingo PA, Jamison PM, Young JL, Gargiullo P. Population-based statistics for women diagnosed with inflammatory breast cancer (United States) Cancer Causes Control. 2004;15(3):321–3. doi: 10.1023/B:CACO.0000024222.61114.18. [DOI] [PubMed] [Google Scholar]
- 18.Low JA, Berman AW, Steinberg SM, Danforth DN, Lippman ME, Swain SM. Long-term follow-up for locally advanced and inflammatory breast cancer patients treated with multimodality therapy. J Clin Oncol. 2004;22(20):4067–74. doi: 10.1200/JCO.2004.04.068. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Angulo AM, Hennessy BT, Broglio K, Meric-Bernstam F, Cristofanilli M, Giordano SH, et al. Trends for inflammatory breast cancer: is survival improving? Oncologist. 2007;12(8):904–12. doi: 10.1634/theoncologist.12-8-904. [DOI] [PubMed] [Google Scholar]
- 20.Osborn T. The psychosocial impact of parental cancer and adolescents: a systematic review. Psychooncology. 2007;16(2):101–26. doi: 10.1002/pon.1113. [DOI] [PubMed] [Google Scholar]


