Abstract
Dysregulation of dopamine (DA) receptors is believed to underlie Parkinson’s disease pathology and L-DOPA-induced motor complications. DA receptors are subject to regulation by G protein-coupled receptor kinases (GRKs) and arrestins. DA lesion with 6-hydroxydopamine caused multiple protein- and brain region-specific changes in the expression of GRKs. In the globus pallidus, all four GRK isoforms (GRK2, 3, 5, 6) were reduced in the lesioned hemisphere. In the caudal caudate-putamen (cCPu) three GRK isoforms (GRK2, 3, 6) were decreased by DA depletion. The decrease in GRK proteins in globus pallidus, but not cCPu, was mirrored by reduction in mRNA. GRK3 protein was reduced in the rostral caudate-putamen (rCPu), whereas other isoforms were either unchanged or up-regulated. GRK6 protein and mRNA were up-regulated in rCPu and nucleus accumbens. L-DOPA (25 mg/kg, twice daily for 10 days) failed to reverse changes caused by DA depletion, whereas D2/D3 agonist pergolide (0.25 mg/kg daily for 10 days) restored normal levels of expression of GRK5 and 6. In rCPu, GRK2 protein was increased in most subcellular fractions by L-DOPA but not by DA depletion alone. Similarly, L-DOPA up-regulated arrestin3 in membrane fractions in both regions. GRK5 was down-regulated by L-DOPA in cCPu in the light membrane fraction, where this isoform is the most abundant. The data suggest that alterations in the expression and subcellular distribution of arrestins and GRKs contribute to pathophysiology of Parkinson’s disease. Thus, these proteins may be targets for antiparkinsonian therapy.
Keywords: 6-hydroxydopamine, arrestin, G protein-coupled receptor kinase, L-DOPA, Parkinson’s disease, pergolide
Parkinson’s disease (PD) is a neurodegenerative disorder caused by degeneration of dopaminergic neurons that provide dopamine (DA) to the striatum. DA replacement therapy with L-DOPA, although still the best treatment available, often results in motor complications such as dyskinesia (Cotzias et al. 1969; Stocchi et al. 1997). Loss of DA in the basal ganglia alters signaling via DA receptors in a complex manner. In particular, numerous signaling pathways in the DA-depleted basal ganglia show exaggerated responses to dopaminergic stimulation (Gerfen 2000; Gerfen et al. 2002; Brown et al. 2005; Sgambato-Faure et al. 2005; Bychkov et al. 2007). Chronic treatment with L-DOPA suppresses supersensitivity of the MAPK pathway (Bezard et al. 2005; Brown et al. 2005; Bychkov et al. 2006; Kim et al. 2006), while augmenting supersensitivity of others (Sgambato-Faure et al. 2005; Bychkov et al. 2006). The molecular mechanisms of signaling alterations caused by DA depletion remain largely unknown, and even the extent of these changes is not yet fully appreciated. Abnormal signaling via DA receptors in the basal ganglia caused by DA depletion and subsequent dopaminergic therapy undoubtedly play an important role in the pathophysiology of motor disturbances in PD and L-DOPA-induced dyskinesia. Therefore, the studies of signaling pathways in the DA-depleted striatum may prove critical for the understanding of the pathology of PD and dyskinesia and for the success of the antiparkinsonian and/or antidyskinetic therapy.
Dopamine receptors belong to the superfamily of G protein-coupled receptors (GPCRs). Upon persistent stimulation, many GPCRs undergo desensitization because of activation-dependent receptor phosphorylation by G protein-coupled receptor kinases (GRKs) followed by arrestin binding. The arrestin binding ‘arrests’ signaling via G proteins and induces receptor internalization (Gurevich and Gurevich 2006; DeWire et al. 2007). GPCR signaling is heavily dependent on the rate and extent of the desensitization process. In cultured cells and in vivo, an increase in arrestin and/or GRK concentration facilitates GPCR desensitization, whereas the reduction of arrestin/GRK levels leads to deficits in GPCR desensitization and internalization and to exaggerated G protein-mediated signaling via affected receptors (Menard et al. 1997; Xu et al. 1997; Iaccarino et al. 1998b; Bohn et al. 1999; Gainetdinov et al. 1999; Willets et al. 1999, 2004; Kim et al. 2001; Bohn et al. 2003; Gainetdinov et al. 2003; Pan et al. 2003). Arrestins, in addition to their role in quenching G protein-mediated signaling, also act as multifunctional adaptors redirecting the GPCR signaling to multiple alternative pathways (Shenoy and Lefkowitz 2003; Gurevich and Gurevich 2006; DeWire et al. 2007). The up-regulation of arrestins reduces G protein-mediated signaling while enhancing arrestin-dependent extracellular signal–regulated kinase (ERK) activation (Ahn et al. 2003, 2004a). Arrestins bind and redistribute signaling molecules among subcellular compartments (Ahn et al. 2004b; Song et al. 2006; Wang et al. 2006; Hanson et al. 2007) affecting not only the intensity but also the direction and timing of the signaling. Therefore, changes in concentrations of arrestins and GRKs and their subcellular localization may contribute to perturbations in dopaminergic signaling caused by loss of DA alone or in combination with L-DOPA therapy.
Two out of four arrestins, arrestin2 and arrestin3, and five out of seven GRKs, GRK2, 3, 4, 5, and 6, are expressed in the brain (Arriza et al. 1992; Gurevich et al. 2002, 2004; Bezard et al. 2005; Bychkov et al. 2006) and appear to interact with multiple GPCRs. Studies with genetically engineered mice and in living cells suggest that in vivo receptors may be preferentially phosphorylated by specific GRKs and differentially interact with arrestin isoforms (Koch et al. 1995; Rockman et al. 1996; Iaccarino et al. 1998b,a; Gainetdinov et al. 1999; Oakley et al. 2000; Kohout et al. 2001). In neostriatal neurons, DA D1 receptor interacts mostly with arrestin3 (Macey et al. 2005), whereas D2 receptor prefers arrestin2 (Macey et al. 2004). Recent studies indicate that phosphorylation of GPCRs by different GRKs have distinct functional consequences for both G protein-mediated and G protein-independent signaling (Kim et al. 2005; Ren et al. 2005). Arrestin isoforms differentially bind, activate, or move signaling proteins (McDonald et al. 2000; Scott et al. 2002; Wang et al. 2003; Witherow et al. 2004; Song et al. 2006). Thus, GPCR signaling is regulated in a complex manner by cellular complement of arrestin and GRKs isoforms.
The expression of arrestins and GRKs can be regulated in vitro and in vivo by persistent stimulation, blockade, or lack of stimulation of specific GPCRs (Iaccarino et al. 1998a; Ozaita et al. 1998; Hurle 2001; Diaz et al. 2002; Fan et al. 2002; Bezard et al. 2005; Iaccarino et al. 2005; Rubino et al. 2006; Salim et al. 2007). Here we tested the hypothesis that loss of DA in PD and/or excessive stimulation of DA receptors during L-DOPA therapy induce specific changes in the level of expression of arrestin or GRK subtypes. Importantly, as arrestins and GRKs interact with multiple GPCRs, changes in their expression caused by DA depletion would affect signaling via many receptors. We have previously described the up-regulation of arrestins and GRKs in the MPTP-treated monkeys reversed by chronic L-DOPA treatment (Bezard et al. 2005). Here we report that depletion of DA by the 6-hydroxydopamine lesion of dopaminergic neurons causes multiple region- and subcellular compartment-specific changes in the concentrations of arrestins and GRKs in the rat basal ganglia. Chronic treatment with L-DOPA failed to restore normal arrestin and/or GRK levels but in some cases caused additional alterations. In contrast, treatment with the long-lived DA agonist pergolide, which has lower propensity to elicit dyskinesia in humans and sensitization in animals (Foley et al. 2004; Stocchi and Olanow 2004; Stocchi et al. 2005), tended to normalize the expression of these regulatory proteins.
Materials and methods
Animals, surgery, and drug treatment
Adult Sprague–Dawley rats (Charles Rivers, Wilmington, MA, USA) were used for these experiments. The animals were housed at the Vanderbilt University’s animal facility with 12/12 light/dark cycle and had free access to food and water. All procedures followed National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee. Rats were deeply anesthetized with pentobarbital (50 mg/kg i.p.) and mounted on stereotaxis. Rats were treated with desipramine (25 mg/kg i.p.) 30 min prior to infusion of 6-hydroxydopamine (6-OHDA). 6-OHDA (8 μg in 4 μL of 0.05% ascorbic acid) was infused unilaterally into the medial forebrain bundle at coordinates A = −4.3 mm, L = 1.2 mm, H = −8.5 mm. After 3 weeks the animals were tested for rotational response to apomorphine (0.05 mg/kg s.c.) in a plexiglass cylinder 30 cm in diameter for 1 h using an automated rotometer (AccuScan Instruments, Columbus, OH, USA). Both ipsilateral and contralateral 360° turns were recorded, and only rats with a net rotational asymmetry (contralateral minus ipsilateral turns) of six turns per minute, which corresponds to more than 90% loss of DA, were used. The animals were randomly assigned to one of the three experimental groups: saline, L-DOPA (25 mg/kg i.p. twice daily), and pergolide (0.25 mg/kg, i.p. daily). The drug treatment continued for 10 days. The rats were tested for rotations for 1 h every day after the morning injections starting 15 min after the drug injection.
Tissue preparation
Upon completion of the drug administration schedule, the rats were decapitated under pentobarbital anesthesia, brain were collected and rapidly frozen on dry ice. The brain areas of interest, rostral caudate-putamen [rCPu; collected at the level of the island of Calleja magna, plates 13–15 in Paxinos and Watson (1998)], caudal caudatoputamen [cCPu; at the level of the globus pallidus (GP), plates 21–23], nucleus accumbens (Acb; includes both shell and core; plates 13–15), GP (plates 21–23), and prefrontal cortex (PFC; plates 8–10; includes prelimbic and infralimbic cortices), were outlined precisely on the precut 100 μm-thick coronal sections, and the tissue was scraped into 200 μL of lysis solution (Ambion, Austin, TX, USA). The lysis solution effectively lyses the tissue while inhibiting all enzymatic activity. It is suitable for the RNAse protection assay (RPA), which can be performed on the same samples used for western blots. Protein concentration in the samples was measured with Bradford reagent (Bio-Rad, Hercules, CA, USA). Samples were stored at −80°C until needed.
Subcellular fractionation
Subcellular fractions were prepared essentially as described (Dunah and Standaert 2001). Briefly, approximately 25 mg of fresh rostral or caudal striatum tissue (pooled from two rats, the intact and lesioned hemispheres separately) was homogenized in 10 volume of ice-cold HEPES-buffered sucrose (0.32 mol/L sucrose, 4 mmol/L HEPES pH 7.4, 1 mmol/L EGTA) containing protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO, USA) in glass-teflon homogenizer. Homogenate was centrifuged at 1000 g for 10 min at 4°C to remove nuclei and large debris. Supernatant 1 was centrifuged at 10 000 g for 15 min to obtain crude synaptosomal fraction and supernatant 2. The synaptosomal pellet was lysed by hypo-osmotic shock in nine volume of ice cold HEPES-buffer with protease inhibitor cocktail for 30 min. The lysate was centrifuged at 25 000 g for 20 min at 4°C to obtain synaptosomal membrane fraction (LP1) and crude synaptic vesicle fraction (LS1). Supernatant 2 was centrifuged at 165 000 g for 2 h to obtain cytosolic fraction (S3) and light membrane fraction (P3). Protein concentration in the samples was measured with Bradford reagent (Bio-Rad). Samples were then precipitated with 90% (v/v) methanol. The protein was pelleted by centrifugation (10 000 g, 10 min at 22°C), washed with 1 mL of 90% methanol, dried, and dissolved in sodium dodecyl sulfate sample buffer at the final concentration of 0.25 μg/μL.
Quantitative western blotting
To prepare samples for western blotting, proteins were precipitated from Lysis buffer with 90% (v/v) methanol. The proteins were then pelleted by centrifugation (10 000 g, 10 min at 22°C), washed with 1 mL of 90% methanol, dried, and dissolved in sodium dodecyl sulfate sample buffer at the final concentration of 0.25 μg/μL. Electrophoresis and transfer onto Immobilon-P (Millipore, Bedford, MA, USA) membrane were performed essentially as described in Bezard et al.(2005) using Criterion cells (Bio-Rad) with 26 wells per gel. This allowed us to run more samples on the same blot. Equal amounts of protein from each animal were loaded on the gel (0.625–5 μg). Membranes were blocked with 5% non-fat dry milk (Carnation brand; Nestle, Solon, OH, USA) in Tris-buffered saline containing 0.1% Tween-20 for 60 min at 37°C. The membranes were then washed with Tris-buffered saline with 0.1% Tween buffer, and proteins were detected with appropriate antibodies.
Arrestins were detected with arrestin2- (Mundell et al. 1999) (1 : 9000) or arrestin3-specific (Orsini and Benovic 1998) (1 : 900) affinity-purified rabbit polyclonal antibodies. We used rabbit polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) to quantify GRK2 (1 : 500), GRK3 (1 : 300), GRK5 (1 : 500), and GRK6 (1 : 300). To estimate the degree of DA denervation, we measured tyrosine hydroxylase (TH) concentration in the basal ganglia subdivisions and PFC using rabbit polyclonal antibody (Chemicon, Temecula, CA, USA) at 1 : 10 000. To test for the purity of subcellular fractions, we measured synaptophysin (mouse monoclonal 1 : 400; Sigma-Aldrich), SNAP-25 (mouse monoclonal 1 : 500; Chemicon), and PSD-95 (mouse monoclonal 1 : 250; BD Biosciences, San Jose, CA, USA). Blots were incubated overnight at 4°C with appropriate primary antibodies followed by horseradish peroxidase-conjugated goat anti-rabbit or rabbit anti-mouse secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at 1 : 25 000 to 1 : 10 000 dilution (1 h at 22°C) and SuperSignal enhanced chemiluminescence reagent WestPico (Pierce, Rockford, IL, USA). Upon development, the blots were exposed to X-ray film for appropriate periods of time.
For arrestins, dilutions of standards containing 1 : 1 mix of Escherichia coli-expressed purified bovine arrestin2 and arrestin3 (Gurevich et al. 2002) in sample buffer were loaded onto each gel along with samples. For quantification of GRKs, we used bovine GRK2 and GRK3 (Kim et al. 1993) and human GRK5 (Kunapuli et al. 1994) and GRK6 (Loudon and Benovic 1994), purified as described in the given references. Appropriate dilutions of GRK proteins were loaded onto each gel alongside the samples to generate calibration curves.
RNAse protection assay
The probes for the RPA were labeled with [32P]UTP in a standard in vitro transcription reaction. All four GRKs were analyzed in the same hybridization reaction. The probe for GRK2 was 183 bp in length (nucleotides 2094–2276, accession # NM_012776), for GRK3 – 225 bp (nucleotides 921–1146, accession # NM_012897), for GRK5 – 258 bp (1775–2033; accession # NM_030829), and for GRK6 – 277 bp (1708–1431; accession # NM_031657). RPA was performed essentially as described (Gurevich et al. 2002, 2004) using Direct Protect Kit (Ambion). Calibration curves were constructed using unlabeled full-length sense mRNA (0.625–20 pg/reaction) for arrestins and GRKs synthesized in vitro with full size cDNAs as templates. A series of calibration samples was included in each experiment and run alongside experimental samples on each gel.
Data analysis
For western blots and RPA autoradiograms, the gray values of the bands were measured on X-ray film using Versadoc imaging system (Bio-Rad). Calibration curves were fitted to linear equations using Prism 4.0 (GraphPad Software, San Diego, CA, USA) to ensure that all the samples were in the linear range. For the statistical analysis StatView software (SAS Institute, Cary, NC, USA) was used. The western blot data were analyzed separately for each group (saline, L-DOPA, pergolide) by repeated measure ANOVA with Hemisphere (intact vs. lesioned) as repeated measure factor. To compare the groups, the values for the lesioned hemisphere expressed as percent from the intact hemisphere were analyzed by one-way ANOVA with Group as main factor followed by Bonferroni/Dunn post hoc test with correction for multiple comparison. The value of p < 0.05 was considered significant.
Results
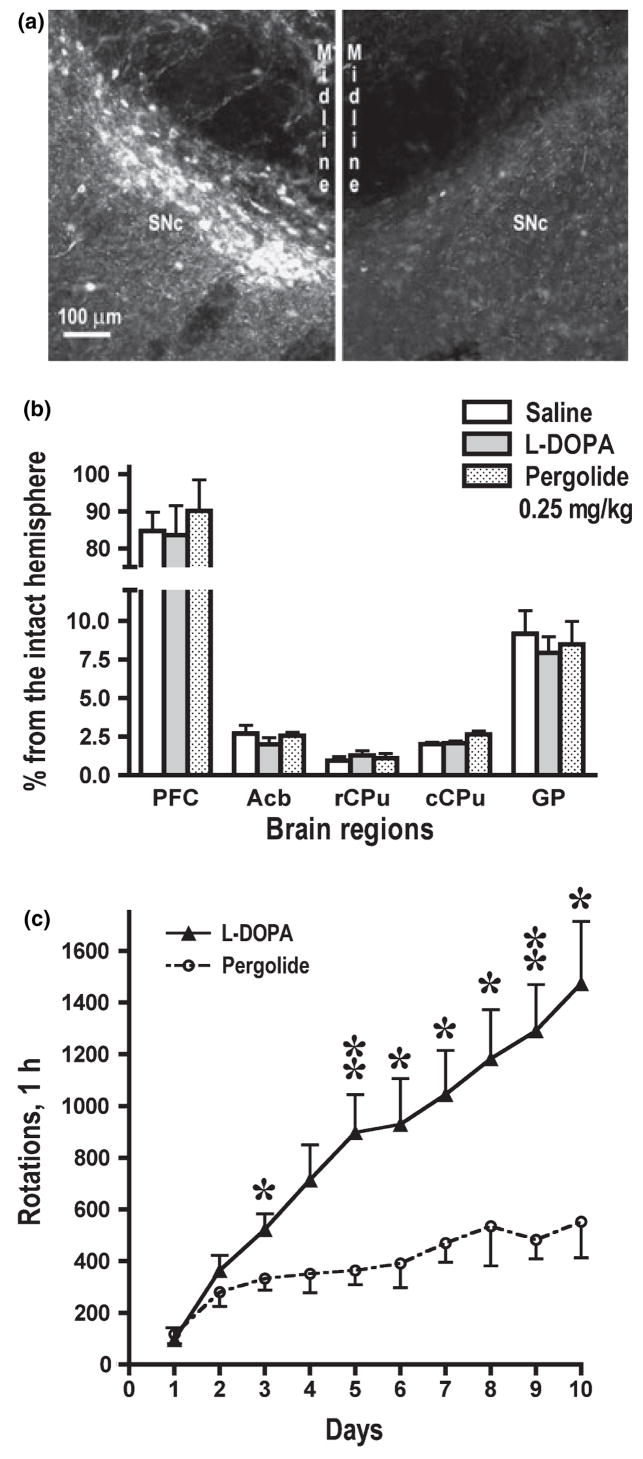
The lesion of the medial forebrain bundle brought about a very extensive loss of dopaminergic cells in the substantia nigra (Fig. 1a) and dopaminergic fibers in the striatum. Quantitative western blotting for TH revealed an almost complete loss in rCPu and cCPu (< 5% of the values in the intact hemisphere) (Fig. 1b). The TH depletion was somewhat less extensive in GP and minimal in PFC (Fig. 1b). We have tested all animals for rotational behavior following each drug injection. In agreement with previous reports (Bordet et al. 1997; Sgambato-Faure et al. 2005; Bychkov et al. 2007), chronic treatment with L-DOPA induced progressive increase in the rotation frequency indicative of the development of behavioral sensitization (Fig. 1c). Rats treated with pergolide showed a significantly slower increase in the rotation frequency during the entire treatment period (Fig. 1c).
Fig. 1.
Characterization of the 6-hydroxydopamine-induced dopaminergic denervation and rotational behavior. (a) High power photomicrograph of a representative nigral section immunostained for TH. Left image shows the substantia nigra in the intact hemisphere, right image – in the hemisphere lesioned with 6-hydroxydopamine. The photograph illustrates almost complete loss of TH-positive dopaminergic neurons on the lesioned side. (b) Quantification of TH immunoreactivity in the striatal regions and prefrontal cortex by western blot. The striatum and nucleus accumbens show severe depletion of dopaminergic fibers, whereas the loss of TH immunoreactivity in the globus pallidus is less pronounced. Minimal loss of TH immunoreactivity is observed in the prefrontal cortex. There were no significant differences among the three experimental groups (saline, L-DOPA, and pergolide) in the degree of dopaminergic lesion. (c) The number (mean ± SEM) of contralateral rotations during the first hour after daily L-DOPA administration. The rotation frequency in the L-DOPA- and pergolide-treated group was compared for each day by ANOVA with Group as main factor. *p < 0.05, **p < 0.01 to the corresponding values in the pergolide-treated group. The increase in the rotation frequency in the course of the treatment (behavioral sensitization) was significant in both groups: F(9108) = 11.28 p < 0.0001, and F(9,90) = 8.21, p < 0.0001, for the L-DOPA and pergolide group, respectively, according to repeated measure ANOVA with day repeated measure factor.
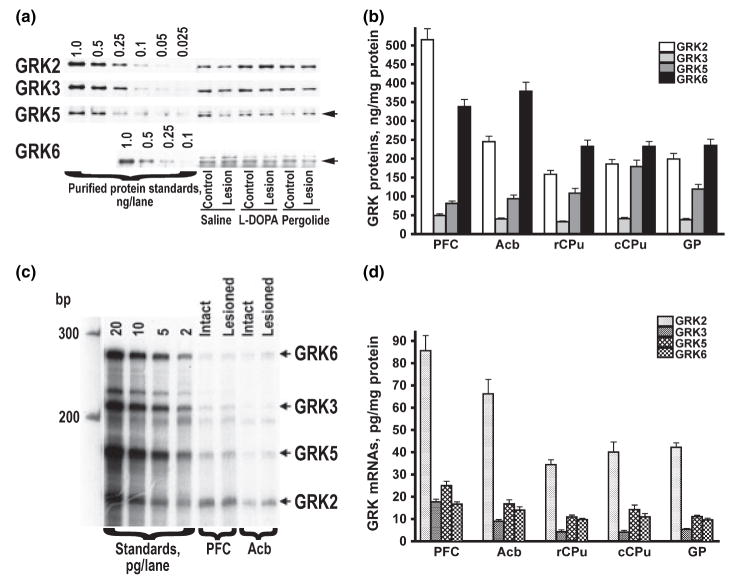
We demonstrated previously that the antibodies to arrestins (Gurevich et al. 2002, 2004) selectively label respective arrestin subtypes in the rat brain. Arrestin2 is the major arrestin subtype in the rat brain, with more than 10-fold excess over arrestin3 in the protein concentration in various subdivision of the basal ganglia (Gurevich et al. 2002, 2004). Antibodies to GRKs selectively labeled respective rat GRK subtypes (Fig. 2a), similarly to what we have reported previously for the monkey and human brain (Bezard et al. 2005; Bychkov et al. 2006). The expression levels of various GRK subtypes differed significantly among brain regions. GRK2 was the most abundant in PFC, whereas in the striatal regions GRK6 was the most abundant at the protein level followed by GRK2 (Fig. 2b). GRK3, a member of the GRK2 subfamily, is expressed at the level 4–10-fold lower than that of GRK2 and was the least abundant GRK subtype in all brain regions (Fig. 2b). GRK5, a member of the same GRK4 subfamily as GRK6, was expressed at a lower level than GRK6, but the difference was less substantial than that between GRK2 and GRK3. Interestingly, the concentration of the GRK2 mRNA was several fold higher than that of any other GRK in all brain regions, whereas the levels of GRK6 mRNA was quite low (Fig. 2c and d). These data suggest rapid turnover of GRK2 and much higher stability of GRK6.
Fig. 2.
(a) Representative western blots demonstrating antibody specificity and the expression of GRK isoforms in the rat striatum. To detect GRK2, 2.5 μg protein per lane was used; for the GRK3 and 5 –5 μg protein per lane, and 10 μg protein per lane for GRK6. (b) Comparison of concentrations of GRK protein levels in the normal rat brain. Bar graph represents the mean ± SE of the amount (ng/mg protein) of GRK isoforms in the striatal subterritories and prefrontal cortex of the control rat brain. The GRK concentrations were measured by quantitative western blot as described in Materials and methods. (c) Representative autoradiogram of RNAse protection assay measuring the concentrations of GRK mRNAs in the rat brain. mRNAs of four GRK isoforms were measured in the single reaction using specific probes of different size. Left four lanes show protected fragments generated from different amounts of GRK mRNAs synthesized in a in vitro transcription reactions using full-length GRK cDNA as templates. These standard samples were included in all experiments and were used to generate calibration curves to convert relative values into absolute concentrations of GRK mRNAs. (d) Comparison of concentrations of mRNA of GRK subtypes in the control rat brain. Bar graph represents the mean ± SE of the amount (pg/mg protein) of GRK mRNAs in the striatal subterritories and prefrontal cortex of the control rat brain. The GRK concentrations were measured by RNAse protection assay as described in Materials and methods.
DA depletion and dopaminergic treatment alter the expression of GRK subtypes
Effects of DA depletion on the expression of GRKs
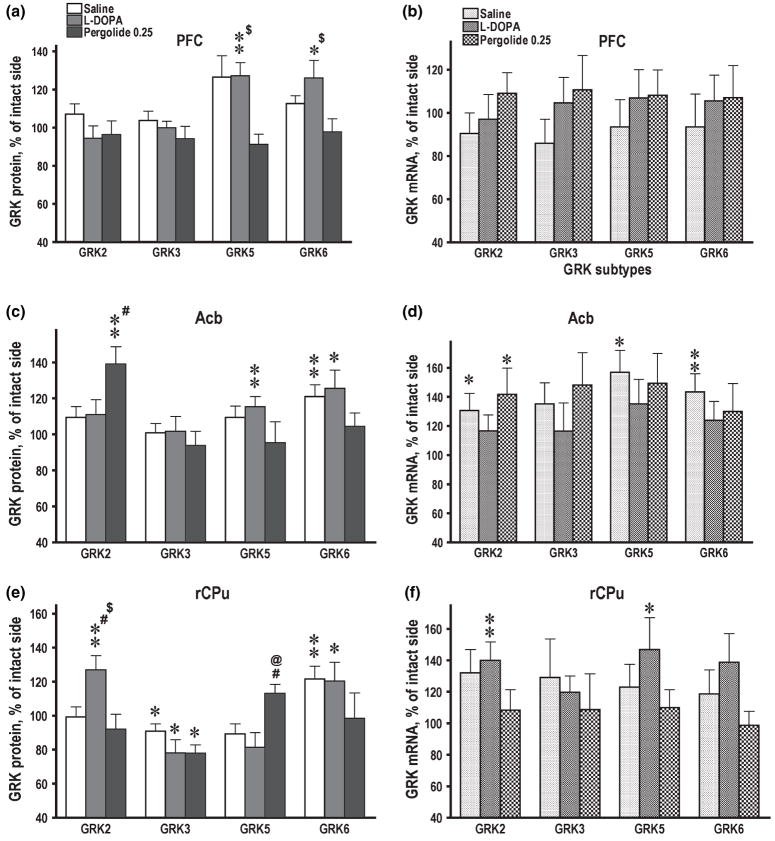
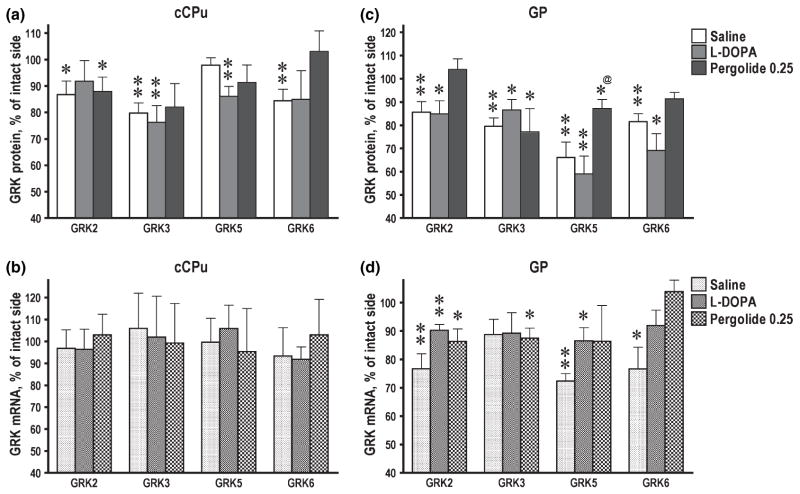
Neither 6-OHDA lesion nor subsequent treatment with L-DOPA or pergolide affected the expression of arrestins in the rat basal ganglia or PFC (data not shown). In contrast, GRK subtypes exhibited multiple region- and protein-specific changes in their expression following DA depletion and treatment with dopaminergic drugs. No significant changes in the concentrations of proteins or mRNAs following DA depletion were seen in PFC (Fig. 3a and b). In Acb, no significant changes were seen in GRK2, 3, or 5 following DA depletion, whereas the level of GRK6 was significantly increased in the lesioned as compared to the intact hemisphere (Fig. 3c). The increase in the GRK6 protein concentration in the lesioned hemisphere was mirrored by a similar increase in the concentration of GRK6 mRNA (Fig. 3d). Similarly, in rCPu, GRK6 was the only subtype up-regulated by the lesion (Fig. 3e). The concentration of GRK2 and GRK5 mRNAs were also elevated by DA depletion in Acb (Fig. 3d), although no corresponding increase was detected at the protein level (Fig. 3c). There was a small but significant decrease in the concentration of GRK3 protein, but not mRNA, in the lesioned rCPu (Fig. 3e and f), but not in Acb or PFC. In contrast to the rostral basal ganglia, in cCPu three out of four GRK subtypes (GRK2, 3, and 6) were decreased in the lesioned hemisphere (Fig. 4a). In GP, all four GRKs were down-regulated by DA depletion (Fig. 4c). In cCPu, down-regulation of GRK proteins was not accompanied by corresponding decrease in the concentration of their mRNAs (Fig. 4b), whereas in GP all mRNAs except GRK3 were reduced in the lesioned hemisphere (Fig. 4d).
Fig. 3.
Expression of GRK isoforms in the control and lesioned hemisphere in 6-hydroxydopamine-lesioned rats treated with saline, L-DOPA, or pergolide. Bar graphs show the concentrations of GRK proteins (a, c, e) and mRNA (b, d, f) in the prefrontal cortex (PFC) (a, b), nucleus accumbens (Acb) (c, d) and rostral caudate-putamen (rCPu) (e, f) in the lesioned hemisphere as percent of the corresponding values in the intact hemisphere (means ± SE). The GRK proteins were measured by western blot and mRNAs by RNAse protection assay as described in Materials and methods. The data were statistically analyzed separately for individual brain groups by repeated-measure one-way ANOVA with Hemisphere as a factor. *p < 0.05, **p < 0.01 to the values in the intact hemisphere. #p < 0.05 to the control group; @p < 0.05 to the L-DOPA group; $p < 0.05 to the pergolide group according to one-way ANOVA with Group as main factor followed by Bonferroni/Dunn post hoc comparison.
Fig. 4.
Expression of GRK isoforms in the control and lesioned hemisphere in 6-hydroxydopamine-lesioned rats treated with saline, L-DOPA, or pergolide. Bar graphs show the concentrations of GRK proteins (a, c) and mRNA (b, d) in the caudal caudate-putamen (cCPu) (a, b) and globus pallidus (c, d) in the lesioned hemisphere as percent of the corresponding values in the intact hemisphere (means ± SE). The GRK proteins were measured by western blot and mRNAs by RNAse protection assay as described in Materials and methods. The data were statistically analyzed separately for individual brain groups by repeated-measure one-way ANOVA with Hemisphere as a factor. *p < 0.05, **p < 0.01 to the values in the intact hemisphere. @p < 0.05 to the L-DOPA group according to one-way ANOVA with Group as main factor followed by Bonferroni/Dunn post hoc comparison.
Effects of chronic treatment with dopaminergic drugs on the expression of GRKs
We have compared the effects of L-DOPA and the long-lived DA agonist pergolide on the expression of arrestins and GRKs in the DA-depleted basal ganglia and PFC. In PFC, the L-DOPA-treated group was the only group exhibiting increased expression of GRK5 and 6 proteins (Fig. 3a) in the lesioned hemisphere without changes in corresponding mRNAs (Fig. 3b). In Acb, L-DOPA elevated the GRK5 protein concentration in the lesioned hemisphere (Fig. 3c). In rCPu, L-DOPA elevated GRK2 protein and mRNA concentrations in the lesioned as compared with the control hemisphere, whereas DA depletion alone had no effect (Fig. 3e and f). In cCPu, L-DOPA treatment reduced the GRK5 concentration but otherwise caused no additional alterations in the expression of GRK at the protein or mRNA level in cCPu or GP as compared with DA depletion alone (Fig. 4).
Pergolide, but not L-DOPA, reduced the concentrations of GRK6 protein in Acb and rCPu (Fig. 3c and e) elevated by DA depletion, so that the intact and lesioned hemispheres were no longer different. Unlike L-DOPA, pergolide did not increase the GRK5 expression in PFC and Acb (Fig. 3a and c). In Acb, GRK6 mRNA, up-regulated by the 6-OHDA lesion, was down-regulated by both dopaminergic drugs, whereas the GRK6 protein was reduced only by pergolide (Fig. 3c and d). Pergolide increased the GRK2 protein and mRNA in Acb (Fig. 3c and d), whereas DA depletion alone up-regulated GRK2 mRNA but not protein. Interestingly, in rCPu, pergolide, unlike L-DOPA, did not up-regulate GRK2 protein or mRNA (Fig. 3e and f). In cCPu and GP, where GRK6 protein was decreased following DA depletion, pergolide restored normal expression (Fig. 4a and c). The down-regulation of GRK2 protein, but not mRNA, in GP (but not cCPu) was also reversed by pergolide (Fig. 4c and d). Similarly to L-DOPA, pergolide caused minimal changes in the expression of GRK3. In particular, pergolide was unable to counteract the decrease in the GRK3 concentration in the lesioned rCPu, cCPu, and GP (Figs 3c, 4a and c).
Differential alterations in the levels of arrestins and GRKs in subcellular fractions
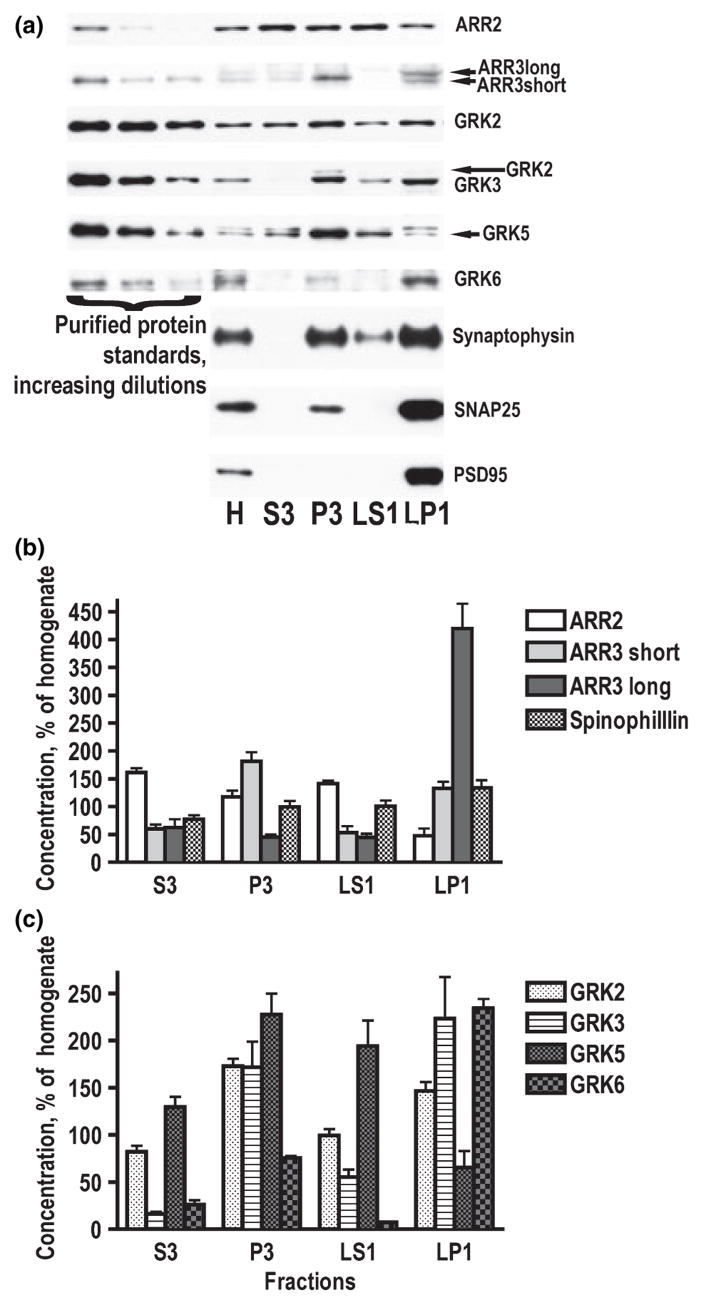
Arrestin and GRK subtypes differed significantly in their subcellular distribution in the normal striatum. Arrestin2 was distributed relatively evenly among fraction, with the exception of LP1 (synaptic membrane) fraction, where it was less abundant (Fig. 5a and b). Arrestin3 has two splice variants, short and long. Short variant is more abundant in the brain than the long isoform. However, the two arrestin splice isoforms have differential subcellular distribution. Both arrestin3 splice variants are enriched in membrane fractions, with arrestin3 short being particularly high in light membranes and long variant concentrated almost exclusively in synaptic membranes (LP1) (Fig. 5a and b). Membrane fractions (P3 + LP1) accounted for 60% of total arrestin3 (both splice variants) as compared to 24% of arrestin2. We have also analyzed subcellular distribution of spinophillin (neurabin II), the protein that has been shown to act as an antagonist of arrestins in their interaction with GPCRs (Wang et al. 2004). Spinophillin was evenly distributed in subcellular compartments, with only very slight enrichment in LP1 (Fig. 5a and b). GRK2 was only slightly enriched in both membrane fractions, whereas GRK3 was highly concentrated in the membrane fractions, particularly in synaptic membranes (Fig. 5a and c). Membrane fractions contained 80% of total GRK3 as opposed to 52% of GRK2. GRK5 and 6 had contrasting distributions. GRK5 was most abundant in P3 and LS1 fractions and relatively low in LP1 fraction (Fig. 5a and c). GRK6 was concentrated in synaptic membranes (LP1), present in P3, and barely detectable in the S3 and LS1 (Fig. 5a and c). Over 85% of total GRK6 localized to membrane fractions as compared to 42% of total GRK5.
Fig. 5.
Subcellular distribution of arrestin and GRK isoforms in the rat striatum. The striatal tissue was fractioned as described in Materials and methods. (a) Representative western blots showing the distribution of arrestins and GRKs in subcellular fractions. Corresponding purified proteins were used to identify bands. Only long splice variant of arrestin2 was detected. The most abundant splice variant of arrestin3 is short, but long variant predominated in the synaptic membrane fraction. (b) Quantification of western blot results showing relative concentrations (mean ± SEM) of arrestins and spinophillin in subcellular fractions (homogenate – 100%). (c) Subcellular distribution of GRK isoforms expressed as mean ± SEM (homogenate – 100%).
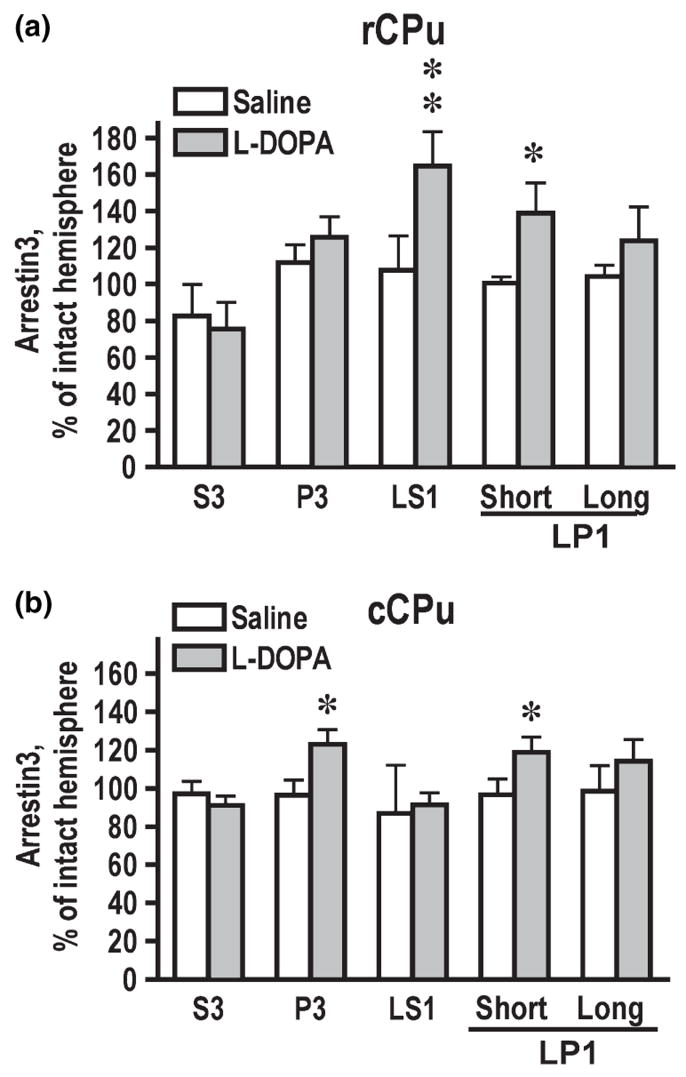
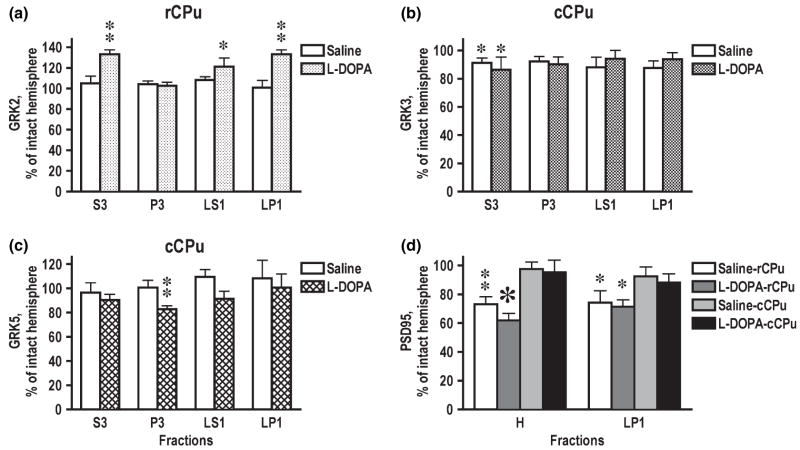
Next, we investigated changes in the concentrations of arrestins and GRKs in subcellular fractions after DA depletion and chronic treatment with dopaminergic drugs. We did not detect any changes in the arrestin concentrations in the total tissue lysate following DA depletion or drug treatment. However, we found that in the lesioned rCPu L-DOPA treatment significantly increased the arrestin3 concentration in LS1 (synaptic cytosol containing synaptic vesicles), where arrestin3 concentration is normally quite low, and LP1 (synaptosomal membranes), where arrestin3 is enriched (Fig. 6a). In the lesioned cCPu, arrestin3 level was significantly increased by L-DOPA in P3 and in LP1 fractions (Fig. 6b). As long arrestin3 is restricted to LP1 fraction, we only analyzed the expression of arrestin3 short in all fractions except LP1, where we measured both splice variants. In LP1, arrestin3 long showed a tendency to increase in the lesioned hemisphere but there were no statistically significant differences (Fig. 6a and b). The GRK2 concentration in the total tissue lysate of rCPu was elevated by L-DOPA treatment (Fig. 3e). We detected L-DOPA-induced increase in the GRK2 concentration in the homogenate and in all fractions except P3 in the lesioned as compared to the intact hemisphere (Fig. 7a). In cCPu, the lesion-induced loss of GRK3 observed in total homogenate was because of a decrease in soluble form of the protein in S3 (Fig. 7b), which was unaltered by L-DOPA treatment. Conversely, L-DOPA reduced the level of GRK5 in cCPu primarily by decreasing its concentration in P3 fraction (Fig. 7c). We have detected significant reduction in the concentration of PSD-95 in the lesioned hemisphere that was confined to rCPu and was not reversed by L-DOPA treatment (Fig. 7d). The data suggest that DA depletion and subsequent treatment with dopaminergic drugs not only alter the level of the arrestins and GRKs expression but also modulate their subcellular distribution.
Fig. 6.
Changes in the concentration of arrestin3 in the subcellular fractions in the rostral (rCPu) (a) and caudal caudate-putamen (cCPu) (b) caused by dopamine depletion and L-DOPA treatment (shown as percent of values for the intact hemisphere; mean ± SEM). The data were statistically analyzed for individual brain regions, groups, and subcellular fractions by repeated-measures one-way ANOVA with Hemisphere as a factor. *p < 0.05, **p < 0.01 to the values in the intact hemisphere.
Fig. 7.
Changes in the concentration of GRKs in the subcellular fractions in the rostral (rCPu) or caudal caudate-putamen (cCPu) caused by dopamine depletion and L-DOPA treatment (shown as percents of values for the intact hemisphere; mean ± SEM). (a) GRK2; (b) GRK3; (c) GRK5; (d) PSD-95. The data were statistically analyzed for individual brain regions, groups, and subcellular fractions by repeated-measure one-way ANOVA with Hemisphere as a factor. *p < 0.05, **p < 0.01 to the values in the intact hemisphere.
Discussion
Our data demonstrate that loss of DA and/or subsequent treatment with L-DOPA or DA agonist pergolide altered the expression of arrestin and GRK subtypes in the 6-OHDA animal model of PD (Table 1). Curiously, the directions of the changes elicited by DA depletion were opposite in the rostral and caudal subdivisions of the basal ganglia: in Acb and rCPu the GRK concentrations were either unchanged or elevated by the loss of DA and/or by DA drugs, whereas in cCPu and GP they were mostly reduced. GRK3 was the only subtype not increased in any brain region in any experimental group, but still there was a rostrocaudal gradient: GRK3 concentration was unchanged in Acb (and PFC) but reduced everywhere else. This reduction in the GRK concentration in the caudal basal ganglia appears specific for the hemiparkinsonian rat model, as we detected no decrease in the concentration of any arrestin or GRK protein in any region of the basal ganglia in a different PD model, MPTP-treated non-human primates (Bezard et al. 2005). Comparative biochemical characterization of different models is necessary to identify common elements likely associated with DA deficit as potential therapeutic targets, as neither model recapitulates every aspect of human PD. Importantly, the increase in the levels of GRK6 caused by DA depletion in the rat rostral basal ganglia is similar to the up-regulation of GRK6 in MPTP-treated monkey we reported previously (Bezard et al. 2005). These data together lend further support to the idea that GRK6 isoform is particularly important for the DA signaling (Gainetdinov et al. 2003, 2004) and suggest that up-regulation of GRK6 is induced by DA depletion via mechanisms common for the rat and primate striatum.
Table 1.
Summary of changes in the expression levels of arrestins and GRKs in the rat basal ganglia and prefrontal cortex following the 6-hydroxydopamine lesion and subsequent dopaminergic treatment
| PFC |
Acb |
rCPu |
cCPu |
GP |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protein | mRNA | Protein | mRNA | Protein | mRNA | Protein | mRNA | Protein | mRNA | |
| ARR2 | ⇔a | ⇔ | ⇔ | ⇔ | ⇔ | |||||
| ARR3 | ⇔ | ⇔ | ↑DOPA (LS1, LP1)b | ↑DOPA (P3, LP1) | ⇔ | |||||
| GRK2 | ⇔ | ⇔ | ↑Perg | ↑Co ↑Perg | ↑DOPA | ↑Co | ↓Co | ⇔ | ↓Co | ↓Co |
| ↑DOPA | ↓Perg | ↓DOPA | ↓DOPA | |||||||
| ↓Perg | ||||||||||
| GRK3 | ⇔ | ⇔ | ⇔ | ⇔ | ↓Co | ⇔ | ↓Co | ⇔ | ↓Co | ↓Perg |
| ↓DOPA | ↓DOPA | ↓DOPA | ||||||||
| ↓Perg | ↓Perg | |||||||||
| GRK5 | ↑DOPA | ⇔ | ↑DOPA | ↑Co | ⇔ | ↑Co | ↓DOPA | ⇔ | ↓Co | ↓Co |
| ↑DOPA | ↓DOPA | ↓DOPA | ||||||||
| ↓Perg | ||||||||||
| GRK6 | ↑DOPA | ⇔ | ↑Co | ↑Co | ↑Co | ↑Co | ↓Co | ⇔ | ↓Co | ↓Co |
| ↑DOPA | ↑DOPA | ↑DOPA | ↓DOPA | |||||||
Empty cells mean that values were not measured.
The symbol indicates that there were no significant differences between the control and lesioned hemispheres in any experimental group.
Indicates that the expression level of a protein (here, arrestin3) was significantly higher in the lesioned as compared to control hemisphere in a specific experimental group (here, L-DOPA-treated group) in indicated subcellular fractions (in parentheses, here LS1 and LP1).
The reason for such a specific regional pattern of changes in the expression of arrestins and GRKs is unclear. Striatal subterritories, although sharing many cytoarchitectural and molecular features, have specific connectivity and functions. In particular, Acb receives massive projections from PFC, whereas the bulk of cortico-striatal projection to the dorsolateral CPu (cCPu) comes from motor and sensorimotor cortical areas (Alexander and Crutcher 1990) Anterior CPu (rCPu) receives projections from both limbic, such as PFC, and motor cortical areas. Interestingly, GRK6 was up-regulated by L-DOPA in PFC, Acb, and rCPu and by dopaminergic lesion alone in Acb and PFC. GRK5 was also increased by L-DOPA treatment in both PFC and Acb. Such similar neurochemical alterations in closely connected brain structures suggest the possibility that downstream plastic changes caused by DA depletion and drug treatment involving specific brain circuitry are responsible for these effects rather than simply loss of DA. This explanation is supported by our previous findings of distinctive signaling alterations in each subdivision of the rat basal ganglia following DA depletion and drug treatment, although the differences were quantitative rather than qualitative (Bychkov et al. 2007). Additionally, the signaling pathways in the rat (Bychkov et al. 2007) and monkey PFC (Bezard et al. 2005) were affected by the dopaminergic lesion and L-DOPA treatment, although loss of dopaminergic innervation in PFC was minimal. Interestingly, in agreement with the previous report (Nash et al. 2005), we detected down-regulation of PSD-95, which was also confined to the rCPu. PSD-95 has recently been implicated in the DA-induced striatal synaptic plasticity (Yao et al. 2004). PSD-95 was decreased in mice strains with reduced striatal DA levels (norepinephrine transporter knockout and vesicular monoamine transporter hemizygous mice) but also in mice lacking DA transporter that have elevated extracellular DA levels (Yao et al. 2004). This indicates a complex mode of regulation of PSD-95 expression by DA. Therefore, differential changes in the PSD-95 availability in the rostral and caudal striatum observed in this study point to differential modifications of the plastic signaling mechanisms in these brain regions. One possibility is that reduced PSD-95 concentration produces supersensitivity of D1 DA receptors in the rostral striatum, as PSD-95 reduces surface expression and signaling via D1 receptors (Zhang et al. 2007). It is conceivable that differential modifications of signaling responses in the subregions of the basal ganglia and brain structures connected to them ultimately give rise to specific deficits in regulation of arrestin/GRK synthesis, degradation, and/or subcellular targeting.
Down-regulation of the GRK expression following lesion of dopaminergic neurons may be, at least partially, explained by the loss of GRK proteins localized to dopaminergic terminals. In particular, in cCPu, where there were no transcriptional changes, the loss of GRKs in the dopaminergic terminals might account for apparent decrease in the concentrations of GRKs. However, the decrease of all but one GRK in GP is mirrored by loss of mRNA suggesting that the down-regulation occurred in GP neurons. The concentration of GRK3 is decreased in rCPu, cCPu, and GP without corresponding reduction in mRNA. As GRK3 is highly expressed in the substantia nigra (Arriza et al. 1992) and, as we showed, is enriched in the fraction of synaptic membranes, it is conceivable that loss of GRK3 was largely because of degeneration of dopaminergic terminals. In this light, it is understandable why neither L-DOPA nor pergolide restored the GRK levels. As we measured total concentrations of GRK proteins in the basal ganglia structures, the changes we detected were most likely the result of the regulation of the GRK expression in striatal neurons combined with losses of GRK proteins located in nigrostriatal terminals.
Another important finding is that L-DOPA had little effect on the arrestin/GRK expression (Table 1). In most cases, L-DOPA failed to restore normal GRK levels altered by the loss of DA. L-DOPA caused an up-regulation of the GRK2 protein expression in rCPu, which was not seen after DA depletion alone. Additionally, L-DOPA increased the concentration of arrestin3 in specific subcellular fractions, whereas DA depletion alone caused no changes. In contrast, pergolide reversed the lesion-induced changes in the GRK expression more successfully than L-DOPA (Table 1). GRK6 and 5 responded to the pergolide treatment particularly well. The sensitization of the rotational response to pergolide was much less severe than to L-DOPA. Reduced behavioral sensitization to pergolide as compared to L-DOPA and the drug’s low propensity to induced dyskinesia in humans (Foley et al. 2004) may be linked with its ability to restore normal expression of GRKs in the DA-depleted basal ganglia. A reduction in the expression of arrestins and/or GRK in the lesioned striatum may be viewed as a compensatory response to the loss of DA designed to preserve signaling via DA receptors, as decreased availability of arrestins and GRKs invariably impedes receptor desensitization and enhances G protein-mediated signaling (Bohn et al. 1999; Mundell et al. 1999; Willets et al. 1999; Horie and Insel 2000; Kohout et al. 2001; Ahn et al. 2003; Gainetdinov et al. 2003). Upon degeneration of dopaminergic neurons, numerous pre- and post-synaptic compensatory mechanisms are engaged aiming at preserving dopaminergic signaling [reviewed in Bezard and Gross (1998) and Bezard et al.(2003)]. Simplistic logic would suggest that chronic administration of L-DOPA and/or pergolide should elevate the concentration of arrestins and/or GRKs to the pre-lesion level or even above it as a protective measure against excessive stimulation. It is well proven that higher concentration of arrestins and GRKs facilitates desensitization and reduces signaling via GPCRs (Ménard et al. 1996; Iaccarino et al. 1998a; Mundell et al. 1998; Iaccarino et al. 1999; Kim et al. 2001; Pan et al. 2003). This is likely the case with the elevation of the arrestin3 and GRK2 concentration caused by L-DOPA.
As we reported previously, L-DOPA treatment restored normal levels of the arrestin and GRK expression in the MPTP-lesioned monkey (Bezard et al. 2005), whereas in the rat only pergolide, but not L-DOPA, was moderately effective. One possible explanation might be a much longer duration of L-DOPA treatment in the monkey (45 vs. 10 days). Additionally, the treatment paradigm we used in the study is designed to rapidly elicit behavioral sensitization to L-DOPA with a high dose of L-DOPA. In contrast, monkeys were treated with the tailor-made individual doses designed to reverse parkinsonian symptoms in each animal, similar to the treatment of human patients. Low concentrations of arrestins and GRKs in the striatum of human PD patients treated with L-DOPA for years (Bychkov et al. 2006) are consistent with the notion that the long-term L-DOPA treatment down-regulates the arrestin/GRK expression in the DA-depleted striatum. The fact that pergolide restored normal levels of specific GRK isoforms in selected brain regions suggests that the arrestin/GRK expression in the lesioned basal ganglia is amenable to appropriate dopaminergic treatment. The 6-hydroxydopamine-lesioned rat and MPTP-lesioned monkey are widely used as animal models of PD and, as such, undoubtedly share some molecular features, but they are unlikely to be identical. For obvious reasons, rats are more commonly used for neurochemical studies, and many findings in rats have never been reproduced in monkeys. Therefore, it is still unclear to what extent these models are similar at the neurochemical level. Our studies highlighted the up-regulation of GRK6 in the rostral basal ganglia as a common mechanism associated with the loss of DA in both primate and rodent model of PD. In conclusion, general pattern of changes in the expression of arrestins and GRKs in the brain does not follow the pattern of DA depletion and does not fit the idea of a simple compensatory response to the DA depletion or drug treatment. Instead, the data suggest that the isoform- and brain region-specific alterations in the expression of arrestins and GRKs detected in this study arise from complex and widespread plastic changes in signaling mechanisms induced by loss of DA and/or subsequent treatment with dopaminergic drugs.
It is also possible that DA is important for the regulation of the GRK expression and/or degradation. Unfortunately, very little is known about the regulation of expression or degradation of GRK isoforms, It has been demonstrated that the concentration of GRK2 in cells is under elaborate control of multiple factors both at the transcription and degradation levels [reviewed in Penela et al.(2006)]. GRK2 is a short-lived protein with half-life about 90 min and is rapidly degraded by the proteasome system. Phosphorylation by c-Src (Penela et al. 2001) and ERK (Elorza et al. 2003) or ubiquitination by E3 Ubiquitin ligase Mdm2 (Salcedo et al. 2006) enhances the GRK2 degradation rate. Both processes are facilitated by arrestins and modulated by various signaling pathways activated by GPCRs or other receptors (Penela et al. 2001; Elorza et al. 2003; Salcedo et al. 2006). Rapid turnover of GRK2 is consistent with high mRNA : protein ratio observed in this work. Stimulation of alpha2- and beta2- adrenoreceptors is reported to up-regulates GRK3 in neuronal cell lines in an ERK1/2-dependent manner, possibly, via activation of Sp-1 and Ap-2 transcription factors (Salim et al. 2007). The interaction of GRK3 with Hsp90 protected the kinase from proteasome-mediated degradation, whereas increase in Ca2+ promoted the degradation of GRK3 by calcium-dependent proteases (Salim and Eikenburg 2007). It is likely that complex signaling mechanisms are involved in the regulation of the synthesis and degradation of other GRK subtypes. Therefore, signaling effects of the DA depletion and drug treatment could translate into altered transcription and/or degradation of GRKs leading to changes in their availability.
Our results demonstrated that arrestin and GRK subtypes have distinctive subcellular distribution and that alterations in the arrestin/GRK concentrations caused by loss of DA or drug treatment are often restricted to specific subcellular fractions. The subcellular distribution of arrestin2 and GRK2 in the brain are in good agreement with that of previous reports (Murga et al. 1998; Ozaita et al. 1998). The detailed subcellular distribution of other GRK isoforms and arrestin3 in the brain are reported here for the first time. About 50% of GRK2 was membrane-associated, whereas over 80% of GRK3, which is the closest relative of GRK2 (Ribas et al. 2007), was found in membrane fractions. Based on in vitro studies with heterologous expression systems, both GRK2 and 3 were expected to be primarily cytosolic and targeted to the plasma membrane in a receptor activation-dependent manner by virtue of their interaction with the membrane-bound Gβγ released upon G-protein interaction with GPCRs [reviewed in Willets et al.(2003)]. In vitro studies found that GRK5 and 6 might be membrane associated via interaction with membrane phospholipids (GRK5) or post-translational lipidation (palmitoylation) (GRK6) (Willets et al. 2003). Indeed, we found that over 85% of GRK6 was membrane-associated with the large proportion localized to synaptic membranes. These data agrees with previous observation that in the human brain 95% of GRK6 is membrane-associated (Grange-Midroit et al. 2002). Given an overall high level of GRK6 expression, GRK6 appears to be the major kinase at synapses and may play a critical role in the DA-induced synaptic plasticity.
The two non-visual arrestin subtypes differ in their subcellular distribution: arrestin2 is primarily cytosolic, with < 25% of total arrestin2 found in membrane fractions. Conversely, more than half of arrestin3 is associated with membranes, with the short splice variant enriched in the P3 and the long variant found almost exclusively in the synaptic membranes. Interestingly, the concentration of arrestin3, although unchanged in total cell lysates, was up-regulated by L-DOPA in both membrane fractions, where arrestin3 is most abundant. Arrestins are ubiquitous multifunctional molecules that, in addition to acting as adaptors for the GPCR desensitization and trafficking, serve as activators of multiple signaling cascades (Gurevich and Gurevich 2006; DeWire et al. 2007). Recently, arrestin3 was found to mediate behavioral effects of DA via coupling of DA D2 receptors with the protein kinase B/glycogen synthase kinase 3 (Akt/GSK3) pathway (Beaulieu et al. 2004, 2005, 2007). Akt (a.k.a. protein kinase B) is activated by phosphorylation at multiple regulatory residues (Chan et al. 1999). DA acting via D2 receptors inhibits Akt phosphorylation and activates glycogen synthase kinase 3, which is negatively regulated by Akt-dependent phosphorylation. Arrestin3 acts as a scaffold bringing together Akt and phosphatase 2A that dephosphorylates Akt (Beaulieu et al. 2005). We have recently reported that DA depletion caused a supersensitive Akt phosphorylation response to acute administration of a low dose of apomorphine (Bychkov et al. 2007). Moreover, hemiparkinsonian rats chronically treated with L-DOPA, but not pregolide, displayed constitutively elevated Akt phosphorylation in the lesioned hemisphere that was not further enhanced by apomorphine administration. As arrestin3 is an obligatory intermediary in the DA-induced Akt deactivation, elevated concentration of arrestin3 should be expected to reduce the Akt activity. It is tempting to speculate that up-regulation of arrestin3 by L-DOPA treatment might be a compensatory response aimed at reducing the level of constitutive Akt phosphorylation. If this is the case, then over-expression of arrestin3 in the DA-depleted striatum may inhibit behavioral sensitization to L-DOPA and, possibly, L-DOPA-induced dyskinesia.
Our understanding of functional consequences of changes in the arrestin/GRK expression caused by DA depletion and drug treatment is severely hampered by paucity of information about specific roles played in the brain by individual arrestin and GRK isoforms. GRK2 and 3 belong to the same GRK subfamily and are very similar in sequence and biochemical properties (Willets et al. 2003). GRK2 is expressed at a considerably higher level throughout the brain than GRK3 (Gurevich et al. 2004; Bychkov et al. 2006), with largely overlapping distribution (Arriza et al. 1992; Gurevich et al. 2004). Recent in vivo studies have demonstrated that each isoform has unique function in regulating sensitivity of different GPCRs (Gainetdinov et al. 2004; Premont and Gainetdinov 2007), although in vitro they tend to have similar effects (Ahn et al. 2004a; Kim et al. 2005). GRK5 and 6 (along with GRK4) belong to another GRK subfamily (Premont and Gainetdinov 2007; Ribas et al. 2007). Sequence homology between GRK5 and 6 is lower than that between GRK2 and 3, and each undergoes specific post-translational modifications (Willets et al. 2003). The experiments in cultured cells provided evidence that GRK2/3 and 5/6 isoforms may regulate the sensitivity and signaling of GPCRs in a reciprocal manner (Ahn et al. 2004a; Kim et al. 2005; Ren et al. 2005). GRK5 knockout mice show super-sensitivity to the stimulation of M2 muscarinic receptors (Gainetdinov et al. 2003), whereas mice lacking GRK6 are supersensitive to dopaminergic stimulation (Gainetdinov et al. 1999). Arrestin3 null mice are supersensitive to μ-opioid receptor stimulation (Bohn et al. 1999), whereas deletion of much more abundant arrestin2 has minimal effect (Gainetdinov et al. 2004). It remains unclear how two ubiquitous arrestins and four GRKs can regulate signaling via several hundreds GPCRs with any degree of specificity. Accumulating evidence suggests that functions of arrestin/GRK isoforms may be different in different cell types (Premont and Gainetdinov 2007). Our fractionation data suggest that differential subcellular localization of arrestins and GRKs may contribute to their functional specificity. The functional roles of arrestin and GRK isoforms may differ in different species. Further studies are required to uncover critical information that would make it possible to fully appreciate the functional role of the arrestin and GRK-mediated signaling in PD.
Acknowledgments
We are grateful to Dr R. J. Lefkowitz (Duke University) for the generous gift of purified GRK3. Full-length rat GRK2 and GRK3 clones were gifts from Dr F. Mayor (Centro de Biologia Molecular ‘Severo Ochoa’, Madrid, Spain), the GRK5 clone was provided by Dr Y. Nagayama (Nagasaki University, Japan), and rat GRK6 clone – by Dr J.-M. Elalouf (Commissariat a l’Energie Atomique Saclay, France). This work was supported by National Institutes of Health grants MH62654 and NS045117 (to EVG), EY11500 and GM77561 (to VVG), GM47417 and GM068857 (to JLB).
Abbreviations used
- Acb
nucleus accumbens
- Akt
protein kinase B
- cCPu
caudal caudate-putamen
- DA
dopamine
- ERK
extracellular signal-regulated kinase
- GP
globus pallidus
- GPCR
G protein-coupled receptor
- GRK
G protein-coupled receptor kinase
- LP1
synaptosomal membrane fraction
- LS1
crude synaptic vesicle fraction
- OHDA
hydroxydopamine
- P3
light membrane fraction
- PD
Parkinson’s disease
- PFC
prefrontal cortex
- rCPu
rostral caudate-putamen
- S3
cytosolic fraction
- TH
tyrosine hydroxylase
References
- Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. Desensitization, internalization, and signaling functions of beta-arrestins demonstrated by RNA interference. Proc Natl Acad Sci USA. 2003;100:1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Wei H, Garrison TR, Lefkowitz RJ. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by beta-arrestins 1 and 2. J Biol Chem. 2004a;279:7807–7811. doi: 10.1074/jbc.C300443200. [DOI] [PubMed] [Google Scholar]
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004b;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Dawson TM, Simerly RB, Martin LJ, Caron MG, Snyder SH, Lefkowitz RJ. The G-protein-coupled receptor kinases bARK1 and bARK2 are widely distributed at synapses in rat brain. J Neurosci. 1992;12:4045–4055. doi: 10.1523/JNEUROSCI.12-10-04045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE. Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog Neurobiol. 1998;55:93–116. doi: 10.1016/s0301-0082(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE, Brotchie JM. Presymptomatic compensation in Parkinson’s disease is not dopamine-mediated. Trends Neurosci. 2003;26:215–221. doi: 10.1016/S0166-2236(03)00038-9. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE, Qin L, Gurevich VV, Benovic JL, Gurevich EV. L-DOPA reverses the MPTP-induced elevation of the arrestin2 and GRK6 expression and enhanced ERK activation in monkey brain. Neurobiol Dis. 2005;18:323–335. doi: 10.1016/j.nbd.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA, Caron MG. Enhanced rewarding properties of morphine, but not cocaine, in beta(arrestin)-2 knock-out mice. J Neurosci. 2003;23:10265–10273. doi: 10.1523/JNEUROSCI.23-32-10265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet R, Ridray S, Carboni C, Diaz J, Sokoloff P, Schwartz JC. Induction of dopamine D3 receptor expression as a mechanism of behavioral sensitization to levodopa. Proc Natl Acad Sci USA. 1997;94:3363–3367. doi: 10.1073/pnas.94.7.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A, Deutch AY, Colbran RJ. Dopamine depletion alters phosphorylation of striatal proteins in a model of Parkinsonism. Eur J Neurosci. 2005;22:247–256. doi: 10.1111/j.1460-9568.2005.04190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bychkov ER, Gurevich VV, Joyce JN, Benovic JL, Gurevich EV. Arrestins and two receptor kinases are upregulated in Parkinson’s disease with dementia. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bychkov ER, Ahmed MR, Dalby KN, Gurevich EV. Dopamine depletion and subsequent treatment with L-DOPA, but not the long-lived dopamine agonist pergolide, enhances activity of the Akt pathway in the rat striatum. J Neurochem. 2007;102:699–711. doi: 10.1111/j.1471-4159.2007.04586.x. [DOI] [PubMed] [Google Scholar]
- Chan TO, Rittenhouse SE, Tsichlis PN. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- Cotzias GC, Papavasiliou PS, Gellene R. Modification of Parkinsonism by chronic treatment with L-dopa. N Engl J Med. 1969;280:337–345. doi: 10.1056/NEJM196902132800701. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Diaz A, Pazos A, Florez J, Ayesta FJ, Santana V, Hurle MA. Regulation of mu-opioid receptors, G-protein-coupled receptor kinases and beta-arrestin 2 in the rat brain after chronic opioid receptor antagonism. Neuroscience. 2002;112:345–353. doi: 10.1016/s0306-4522(02)00073-8. [DOI] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci. 2001;21:5546–5558. doi: 10.1523/JNEUROSCI.21-15-05546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elorza A, Penela P, Sarnago S, Mayor F. MAPK-dependent degradation of G protein-coupled receptor kinase 2. J Biol Chem. 2003;278:29164–29173. doi: 10.1074/jbc.M304314200. [DOI] [PubMed] [Google Scholar]
- Fan X, Zhang J, Zhang X, Yue W, Ma L. Acute and chronic morphine treatments and morphine withdrawal differentially regulate GRK2 and GRK5 gene expression in rat brain. Neuropharmacology. 2002;43:809–816. doi: 10.1016/s0028-3908(02)00147-8. [DOI] [PubMed] [Google Scholar]
- Foley P, Gerlach M, Double KL, Riederer P. Dopamine receptor agonists in the therapy of Parkinson’s disease. J Neural Transm. 2004;111:1375–1446. doi: 10.1007/s00702-003-0059-x. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Bohn LM, Walker JK, Laporte SA, Macrae AD, Caron MG, Lefkowitz RJ, Premont RT. Muscarinic supersensitivity and impaired receptor desensitization in G protein-coupled receptor kinase 5-deficient mice. Neuron. 1999;24:1029–1036. doi: 10.1016/s0896-6273(00)81048-x. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Bohn LM, Sotnikova TD, et al. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron. 2003;38:291–303. doi: 10.1016/s0896-6273(03)00192-2. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal function. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Dopamine-mediated gene regulation in models of Parkinson’s disease. Ann Neurol. 2000;47(Suppl):S42–S50. [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2 kinase. J Neurosci. 2002;22:5042–5054. doi: 10.1523/JNEUROSCI.22-12-05042.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange-Midroit M, García-Sevilla JA, Ferrer-Alcón M, La Harpe R, Walzer C, Guimón J. G protein-coupled receptor kinases, beta-arrestin-2 and associated regulatory proteins in the human brain: postmortem changes, effect of age and subcellular distribution. Brain Res Mol Brain Res. 2002;101:39–51. doi: 10.1016/s0169-328x(02)00144-4. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Gurevich VV. Arrestins: ubiquitous regulators of cellular signaling pathways. Genome Biol. 2006;7:236. doi: 10.1186/gb-2006-7-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich EV, Benovic JL, Gurevich VV. Arrestin2 and arrestin3 are differentially expressed in the rat brain during post-natal development. Neuroscience. 2002;109:421–436. doi: 10.1016/s0306-4522(01)00511-5. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Benovic JL, Gurevich VV. Arrestin2 expression selectively increases during neural differentiation. J Neurochem. 2004;91:1404–1416. doi: 10.1111/j.1471-4159.2004.02830.x. [DOI] [PubMed] [Google Scholar]
- Hanson SM, Cleghorn WM, Francis DJ, Vishnivetskiy SA, Raman D, Song X, Nair KS, Slepak VZ, Klug CS, Gurevich VV. Arrestin mobilizes signaling proteins to the cytoskeleton and redirects their activity. J Mol Biol. 2007;368:375–387. doi: 10.1016/j.jmb.2007.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie K, Insel PA. Retrovirally mediated transfer of a G protein-coupled receptor kinase (GRK) dominant-negative mutant enhances endogenous calcitonin receptor signaling in chinese hamster ovary cells. GRK inhibition enhances expression of receptors and receptor mRNA. J Biol Chem. 2000;275:29433–29440. doi: 10.1074/jbc.M003413200. [DOI] [PubMed] [Google Scholar]
- Hurle MA. Changes in the expression of G protein-coupled receptor kinases and beta-arrestin 2 in rat brain during opioid tolerance and supersensitivity. J Neurochem. 2001;77:486–492. doi: 10.1046/j.1471-4159.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by b-adrenergic receptor stimulation and blockade. Circulation. 1998a;98:1783–1789. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Rockman HA, Shotwell KF, Tomhave ED, Koch WJ. Myocardial overexpression of GRK3 in transgenic mice: evidence for in vivo selectivity of GRKs. Am J Physiol. 1998b;275:1298–1306. doi: 10.1152/ajpheart.1998.275.4.H1298. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Lefkowitz RJ, Koch WJ. Myocardial G protein-coupled receptor kinases: implications for heart failure therapy. Proc Assoc Am Physicians. 1999;111:399–405. doi: 10.1111/paa.1999.111.5.399. [DOI] [PubMed] [Google Scholar]
- Iaccarino G, Barbato E, Cipolletta E, De Amicis V, Margulies KB, Leosco D, Trimarco B, Koch WJ. Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur Heart J. 2005;26:1752–1758. doi: 10.1093/eurheartj/ehi429. [DOI] [PubMed] [Google Scholar]
- Kim CM, Dion SB, Onorato JJ, Benovic JL. Expression and characterization of two beta-adrenergic receptor kinase isoforms using the baculovirus expression system. Receptor. 1993;3:39–55. [PubMed] [Google Scholar]
- Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG. Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and beta-arrestins. J Biol Chem. 2001;276:37409–37414. doi: 10.1074/jbc.M106728200. [DOI] [PubMed] [Google Scholar]
- Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. Functional antagonism of different G protein-coupled receptor kinases for beta-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Palmiter RD, Cummins A, Gerfen CR. Reversal of supersensitive striatal dopamine D1 receptor signaling and extracellular signal-regulated kinase activity in dopamine-deficient mice. Neuroscience. 2006;137:1381–1388. doi: 10.1016/j.neuroscience.2005.10.054. [DOI] [PubMed] [Google Scholar]
- Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. Beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci USA. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunapuli P, Onorato JJ, Hosey MM, Benovic JL. Expression, purification, and characterization of the G protein-coupled receptor kinase GRK5. J Biol Chem. 1994;269:1099–1105. [PubMed] [Google Scholar]
- Loudon RP, Benovic JL. Expression, purification, and characterization of the G protein-coupled receptor kinase GRK6. J Biol Chem. 1994;269:22691–22697. [PubMed] [Google Scholar]
- Macey TA, Gurevich VV, Neve KA. Preferential interaction between the dopamine D2 receptor and arrestin2 in neostriatal neurons. Mol Pharmacol. 2004;66:1635–1642. doi: 10.1124/mol.104.001495. [DOI] [PubMed] [Google Scholar]
- Macey TA, Liu Y, Gurevich VV, Neve KA. Dopamine D1 receptor interaction with arrestin3 in neostriatal neurons. J Neurochem. 2005;93:128–134. doi: 10.1111/j.1471-4159.2004.02998.x. [DOI] [PubMed] [Google Scholar]
- McDonald PH, Chow CW, Miller WE, Laporte SA, Field ME, Lin FT, Davis RJ, Lefkowitz RJ. Beta-arrestin 2: a receptor-regulated MAPK scaffold for the activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- Menard L, Ferguson SS, Zhang J, Lin FT, Lefkowitz RJ, Caron MG, Barak LS. Synergistic regulation of beta2-adrenergic receptor sequestration: intracellular complement of beta-adrenergic receptor kinase and beta-arrestin determine kinetics of internalization. Mol Pharmacol. 1997;51:800–808. [PubMed] [Google Scholar]
- Ménard L, Ferguson SS, Barak LS, Bertrand L, Premont RT, Colapietro AM, Lefkowitz RJ, Caron MG. Members of the G protein-coupled receptor kinase family that phosphorylate the b2-adrenergic receptor facilitate sequestration. Biochemistry. 1996;35:4155–4160. doi: 10.1021/bi952961+. [DOI] [PubMed] [Google Scholar]
- Mundell SJ, Luty JS, Willets J, Benovic JL, Kelly E. Enhanced expression of G protein-coupled receptor kinase 2 selectively increases the sensitivity of A2A adenosine receptors to agonist-induced desensitization. Br J Pharmacol. 1998;125:347–356. doi: 10.1038/sj.bjp.0702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell SJ, Loudon RP, Benovic JL. Characterization of G protein-coupled receptor regulation in antisense mRNA-expressing cells with reduced arrestin levels. Biochemistry. 1999;38:8723–8732. doi: 10.1021/bi990361v. [DOI] [PubMed] [Google Scholar]
- Murga C, Penela P, Zafra F, Mayor F. The subcellular and cellular distribution of G protein-coupled receptor kinase 2 in rat brain. Neuroscience. 1998;87:631–637. doi: 10.1016/s0306-4522(98)00145-6. [DOI] [PubMed] [Google Scholar]
- Nash JE, Johnston TH, Collingridge GL, Garner CC, Brotchie JM. Subcellular redistribution of the synapse-associated proteins PSD-95 and SAP97 in animal models of Parkinson’s disease and L-DOPA-induced dyskinesia. FASEB J. 2005;19:583–585. doi: 10.1096/fj.04-1854fje. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, barrestin1, and barrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- Orsini MJ, Benovic JL. Characterization of dominant negative arrestins that inhibit beta-2-adrenergic receptor internalization by distinct mechanisms. J Biol Chem. 1998;273:34616–34622. doi: 10.1074/jbc.273.51.34616. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Escriba PV, Ventayol P, Murga C, Mayor FJ, Garcia-Sevilla JA. Regulation of G protein-coupled receptor kinase 2 in brains of opiate-treated rats and human opiate addicts. J Neurochem. 1998;70:1249–1257. doi: 10.1046/j.1471-4159.1998.70031249.x. [DOI] [PubMed] [Google Scholar]
- Pan L, Gurevich EV, Gurevich VV. The nature of the arrestin × receptor complex determines the ultimate fate of the internalized receptor. J Biol Chem. 2003;278:11623–11632. doi: 10.1074/jbc.M209532200. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Penela P, Elorza A, Sarnago S, Mayor FJ. Beta-arrestin-and c-Src-dependent degradation of G-protein-coupled receptor kinase 2. EMBO J. 2001;20:5129–5138. doi: 10.1093/emboj/20.18.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penela P, Murga C, Ribas C, Tutor AS, Peregrin S, Mayor FJ. Mechanisms of regulation of G protein-coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovasc Res. 2006;69:46–56. doi: 10.1016/j.cardiores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein-coupled receptor kinases govern G protein and beta-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas C, Penela P, Murga C, Salcedo A, García-Hoz C, Jurado-Pueyo M, Aymerich I, Mayor F. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. Biochim Biophys Acta. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- Rockman HA, Choi DJ, Rahman NU, Akhter SA, Lefkowitz RJ, Koch WJ. Receptor-specific in vivo desensitization by the G protein-coupled receptor kinase-5 in transgenic mice. Proc Natl Acad Sci USA. 1996;93:9954–9959. doi: 10.1073/pnas.93.18.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Viganò D, Premoli F, Castiglion IC, Bianchessi S, Zippel R, Parolaro D. Changes in the expression of G protein-coupled receptor kinases and beta-arrestins in mouse brain during cannabinoid tolerance: a role for RAS-ERK cascade. Mol Neurobiol. 2006;33:199–213. doi: 10.1385/MN:33:3:199. [DOI] [PubMed] [Google Scholar]
- Salcedo A, Mayor F, Penela P. Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2. EMBO J. 2006;25:4752–4762. doi: 10.1038/sj.emboj.7601351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim S, Eikenburg DC. Role of 90-kDa heat shock protein (Hsp 90) and protein degradation in regulating neuronal levels of G protein-coupled receptor kinase 3. J Pharmacol Exp Ther. 2007;320:1106–1112. doi: 10.1124/jpet.106.114835. [DOI] [PubMed] [Google Scholar]
- Salim S, Standifer KM, Eikenburg DC. Extracellular signal-regulated kinase 1/2-mediated transcriptional regulation of G-protein-coupled receptor kinase 3 expression in neuronal cells. J Pharmacol Exp Ther. 2007;321:51–59. doi: 10.1124/jpet.106.116921. [DOI] [PubMed] [Google Scholar]
- Scott MG, Le Rouzic E, Perianin A, Pierotti V, Enslen H, Benichou S, Marullo S, Benmerah A. Differential nucleocytoplasmic shuttling of beta-arrestins. Characterization of a leucine-rich nuclear export signal in beta-arrestin2. J Biol Chem. 2002;277:37693–37701. doi: 10.1074/jbc.M207552200. [DOI] [PubMed] [Google Scholar]
- Sgambato-Faure V, Buggia V, Gilbert F, Levesque D, Benabid AL, Berger F. Coordinated and spatial upregulation of arc in striatonigral neurons correlates with L-dopa-induced behavioral sensitization in dyskinetic rats. J Neuropathol Exp Neurol. 2005;64:936–947. doi: 10.1097/01.jnen.0000186922.42592.b7. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling. Biochem J. 2003;375:503–515. doi: 10.1042/BJ20031076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Raman D, Gurevich EV, Vishnivetskiy SA, Gurevich VV. Visual and both non-visual arrestins in their ‘inactive’ conformation bind JNK3 and Mdm2 and relocalize them from the nucleus to the cytoplasm. J Biol Chem. 2006;281:21491–21499. doi: 10.1074/jbc.M603659200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocchi F, Olanow CW. Continuous dopaminergic stimulation in early and advanced Parkinson’s disease. Neurology. 2004;62 (Suppl 1):S56–S63. doi: 10.1212/wnl.62.1_suppl_1.s56. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Nordera G, Marsden CD. Strategies for treating patients with advanced Parkinson’s disease with disastrous fluctuations and dyskinesias. Clin Neuropharmacol. 1997;20:95–115. doi: 10.1097/00002826-199704000-00001. [DOI] [PubMed] [Google Scholar]
- Stocchi F, Vacca L, Ruggieri S, Olanow CW. Intermittent vs. continuous levodopa administration in patients with advanced Parkinson disease: a clinical and pharmacokinetic study. Arch Neurol. 2005;62:905–910. doi: 10.1001/archneur.62.6.905. [DOI] [PubMed] [Google Scholar]
- Wang P, Wu Y, Ge X, Ma L, Pei G. Subcellular localization of beta-arrestins is determined by their intact N domain and the nuclear export signal at the C terminus. J Biol Chem. 2003;278:11648–11653. doi: 10.1074/jbc.M208109200. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhao J, Brady AE, Feng J, Allen PB, Lefkowitz RJ, Greengard P, Limbird LE. Spinophilin blocks arrestin actions in vitro and in vivo at G protein-coupled receptors. Science. 2004;304:1940–1944. doi: 10.1126/science.1098274. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lu R, Zhao J, Limbird LE. Arrestin serves as a molecular switch, linking endogenous alpha2-adrenergic receptor to SRC-dependent, but not SRC-independent, ERK activation. J Biol Chem. 2006;281:25948–25955. doi: 10.1074/jbc.M605415200. [DOI] [PubMed] [Google Scholar]
- Willets JM, Parent JL, Benovic JL, Kelly E. Selective reduction in A2 adenosine receptor desensitization following antisense-induced suppression of G protein-coupled receptor kinase 2 expression. J Neurochem. 1999;73:1781–1789. doi: 10.1046/j.1471-4159.1999.0731781.x. [DOI] [PubMed] [Google Scholar]
- Willets JM, Challiss RA, Nahorski SR. Non-visual GRKs: are we seeing the whole picture? Trends Pharmacol Sci. 2003;24:626–633. doi: 10.1016/j.tips.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Willets JM, Nash MS, Challiss RA, Nahorski SR. Imaging of muscarinic acetylcholine receptor signaling in hippocampal neurons: evidence for phosphorylation-dependent and -independent regulation by G-protein-coupled receptor kinases. J Neurosci. 2004;24:4157–4162. doi: 10.1523/JNEUROSCI.5506-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc Natl Acad Sci USA. 2004;101:8603–8607. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Dodd RL, Makino CL, Simon MI, Baylor DA, Chen J. Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature. 1997;389:505–509. doi: 10.1038/39068. [DOI] [PubMed] [Google Scholar]
- Yao WD, Gainetdinov RR, Arbuckle MI, Sotnikova TD, Cyr M, Beaulieu JM, Torres GE, Grant SG, Caron MG. Identification of PSD-95 as a regulator of dopamine-mediated synaptic and behavioral plasticity. Neuron. 2004;41:625–638. doi: 10.1016/s0896-6273(04)00048-0. [DOI] [PubMed] [Google Scholar]
- Zhang J, Vinuela A, Neely MH, Hallett PJ, Grant SG, Miller GM, Isacson O, Caron MG, Yao WD. Inhibition of the dopamine D1 receptor signaling by PSD-95. J Biol Chem. 2007;282:15778–15789. doi: 10.1074/jbc.M611485200. [DOI] [PMC free article] [PubMed] [Google Scholar]