Abstract
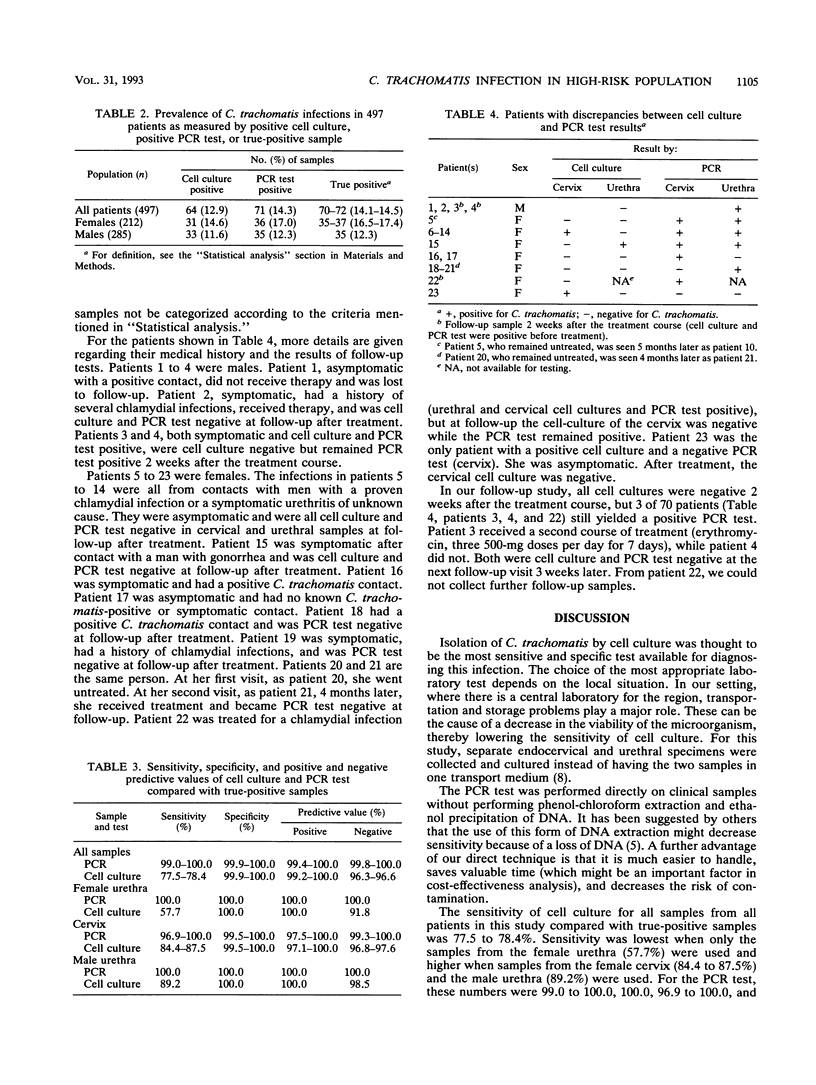
A study to compare the polymerase chain reaction (PCR) test with the cell culture method in diagnosing urogenital Chlamydia trachomatis infections was performed. From 497 patients (212 women, 285 men) attending an outpatient clinic for sexually transmitted diseases, a total of 814 samples (female patients, cervix and urethra; male patients, urethra) were collected. This total included follow-up samples from 35 women and 35 men positive for C. trachomatis by cell culture and/or PCR test, which were collected 2 weeks after treatment with doxycycline (two 100-mg doses per day for 7 days). The PCR test was performed directly on clinical samples without performing phenol-chloroform extraction and ethanol precipitation of DNA. The prevalence of C. trachomatis as measured by positive cell culture was 64 of 497 (12.9%) for all patients, 31 of 212 (14.6%) for women, and 33 of 285 (11.6%) for men. The prevalences as measured by positive PCR test were 71 of 497 (14.3%), 36 of 212 (17.0%), and 35 of 285 (12.3%), respectively. The sensitivities of the cell culture and the PCR test compared with that of true-positive samples were 77.5 to 78.4% and 99.0 to 100.0%, respectively. Discrepancies between cell culture and the PCR test were found for 23 of 497 patients (4.9%), 19 of 212 females (9.0%), and 4 of 285 males (1.4%). Nineteen pretreatment samples from 19 patients (4 female endocervical, 13 female urethral, and 2 male urethral samples) were cell culture negative and PCR test positive, while 1 pretreatment female endocervical sample was cell culture positive and PCR test negative. The posttreatment samples from all patients were cell culture negative, but the PCR test remained positive for 3 of 70 patients (1 female endocervical and 2 male urethral samples). One of these samples became spontaneously negative in three more weeks. The medical history of the individual patient and the negative PCR tests after treatment for nearly all patients support our hypothesis that the positive PCR test results were clinically relevant for the cell culture-negative but PCR test-positive but PCR test-positive patients of the population studied.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarnaes S. L., Peterson E. M., De La Maza L. M. The effect of media and temperature on the storage of Chlamydia trachomatis. Am J Clin Pathol. 1984 Feb;81(2):237–239. doi: 10.1093/ajcp/81.2.237. [DOI] [PubMed] [Google Scholar]
- Barnes R. C. Laboratory diagnosis of human chlamydial infections. Clin Microbiol Rev. 1989 Apr;2(2):119–136. doi: 10.1128/cmr.2.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunham R. C., Maclean I. W., Binns B., Peeling R. W. Chlamydia trachomatis: its role in tubal infertility. J Infect Dis. 1985 Dec;152(6):1275–1282. doi: 10.1093/infdis/152.6.1275. [DOI] [PubMed] [Google Scholar]
- Claas H. C., Melchers W. J., de Bruijn I. H., de Graaf M., van Dijk W. C., Lindeman J., Quint W. G. Detection of Chlamydia trachomatis in clinical specimens by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 1990 Dec;9(12):864–868. doi: 10.1007/BF01967500. [DOI] [PubMed] [Google Scholar]
- Claas H. C., Wagenvoort J. H., Niesters H. G., Tio T. T., Van Rijsoort-Vos J. H., Quint W. G. Diagnostic value of the polymerase chain reaction for Chlamydia detection as determined in a follow-up study. J Clin Microbiol. 1991 Jan;29(1):42–45. doi: 10.1128/jcm.29.1.42-45.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. B., Katz B. P., van der Pol B., Caine V. A., Batteiger B. E., Newhall W. J. Effect of blind passage and multiple sampling on recovery of Chlamydia trachomatis from urogenital specimens. J Clin Microbiol. 1986 Dec;24(6):1029–1033. doi: 10.1128/jcm.24.6.1029-1033.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg J. A. Clinical and laboratory considerations of culture vs antigen assays for detection of Chlamydia trachomatis from genital specimens. Arch Pathol Lab Med. 1989 May;113(5):453–460. [PubMed] [Google Scholar]
- Kluytmans J. A., Niesters H. G., Mouton J. W., Quint W. G., Ijpelaar J. A., Van Rijsoort-Vos J. H., Habbema L., Stolz E., Michel M. F., Wagenvoort J. H. Performance of a nonisotopic DNA probe for detection of Chlamydia trachomatis in urogenital specimens. J Clin Microbiol. 1991 Dec;29(12):2685–2689. doi: 10.1128/jcm.29.12.2685-2689.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre J., Laperrière H., Rousseau H., Massé R. Comparison of three techniques for detection of Chlamydia trachomatis in endocervical specimens from asymptomatic women. J Clin Microbiol. 1988 Apr;26(4):726–731. doi: 10.1128/jcm.26.4.726-731.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony J. B., Chernesky M. A. Effect of swab type and storage temperature on the isolation of Chlamydia trachomatis from clinical specimens. J Clin Microbiol. 1985 Nov;22(5):865–867. doi: 10.1128/jcm.22.5.865-867.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard L., Birkelund S., Christiansen G. Use of polymerase chain reaction for detection of Chlamydia trachomatis. J Clin Microbiol. 1990 Jun;28(6):1254–1260. doi: 10.1128/jcm.28.6.1254-1260.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Markoff B. A., Schachter J., de la Maza L. M. The 7.5-kb plasmid present in Chlamydia trachomatis is not essential for the growth of this microorganism. Plasmid. 1990 Mar;23(2):144–148. doi: 10.1016/0147-619x(90)90033-9. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Oda R., Alexander R., Greenwood J. R., de la Maza L. M. Molecular techniques for the detection of Chlamydia trachomatis. J Clin Microbiol. 1989 Oct;27(10):2359–2363. doi: 10.1128/jcm.27.10.2359-2363.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer M. A., Prager V., Shalwitz J., Vaughan E., Moscicki B., Brown R., Wibbelsman C., Schachter J. Prevalence of urethral Chlamydia trachomatis and Neisseria gonorrhoeae among asymptomatic, sexually active adolescent boys. J Infect Dis. 1987 Jul;156(1):223–224. doi: 10.1093/infdis/156.1.223. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Rogers R. E., Katz B. P., Brickler J. F., Lineback P. L., Van der Pol B., Jones R. B. Diagnosis of chlamydial infection in women attending antenatal and gynecologic clinics. J Clin Microbiol. 1987 May;25(5):868–872. doi: 10.1128/jcm.25.5.868-872.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriprakash K. S., Macavoy E. S. Characterization and sequence of a plasmid from the trachoma biovar of Chlamydia trachomatis. Plasmid. 1987 Nov;18(3):205–214. doi: 10.1016/0147-619x(87)90063-1. [DOI] [PubMed] [Google Scholar]
- Stamm W. E. Diagnosis of Chlamydia trachomatis genitourinary infections. Ann Intern Med. 1988 May;108(5):710–717. doi: 10.7326/0003-4819-108-5-710. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Thomas B. J. Laboratory techniques for the diagnosis of chlamydial infections. Genitourin Med. 1991 Jun;67(3):256–266. doi: 10.1136/sti.67.3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. H., Lee M. F., Yin S. C., Yang D. M., Cheng S. F. Comparison of polymerase chain reaction, monoclonal antibody based enzyme immunoassay, and cell culture for detection of Chlamydia trachomatis in genital specimens. Sex Transm Dis. 1992 Jul-Aug;19(4):193–197. doi: 10.1097/00007435-199207000-00002. [DOI] [PubMed] [Google Scholar]


