Abstract
Purpose:
To evaluate quality adjusted survival (QAS) of patients with locally advanced squamous cell carcinoma of the head and neck treated with four different radiation fractionation schedules.
Methods and Materials:
Quality-adjusted survival was calculated using the quality-adjusted time without toxicity or relapse (Q-TWiST) methodology. Utilities (patient preferences for certain health states) were obtained by threshold analysis. Q-TWiST therefore equaled TWiST + [(weight for toxicity) × (time spent in toxicity)] + [(weight for relapse) × (time spent in relapse)]
Results:
A statistically significant increase in QAS existed for patients treated with hyperfractionated radiotherapy (HFX) compared to standard fractionated radiotherapy (SFX) with a toxicity utility ≥ 0.57 and relapse utility ≤ 0.72. No statistically significant difference was observed for patients treated with the other two fractionation schedules compared to SFX.
Conclusions:
Q-TWiST analysis identified patient groups that would benefit from more aggressive therapy. Further investigation with patient generated utilities is needed.
Keywords: Quality-adjusted survival, head and neck cancer, RTOG 9003
Introduction
The Radiation Therapy Oncology Group (RTOG) has incorporated quality of life (QOL) studies into a number of clinical trials and has published the effects of treatment on the different facets of quality of life. (1,2,3,4,5)
Quality of life analysis, however, can be limited in its applicability. Comparison of quality of life results of head and neck cancer treatment with lung cancer treatments can be difficult if different QOL instruments are used. In addition, cancer specific quality of life instruments may not be sensitive enough to detect all of the effects of treatment on patients.
The RTOG has recently embarked on determining quality-adjusted survival by means of Q-time and quality-adjusted time without toxicity or relapse (Q-TWiST) analyses. (6,7) The end result is survival adjusted for the toxic effects of treatment and time spent in a state of relapse. This allows comparison of treatments of different types of cancers and in a broader sense, treatments of different conditions. It also allows the ability for further analysis, including economic analysis.
We report quality-adjusted survival using a Q-TWiST analysis for patients undergoing treatment on RTOG protocol 90-03.
Material and Methods
RTOG 90-03 was a phase III clinical trial evaluating various radiotherapy fractionation schedules in the treatment of locally advanced squamous cell carcinomas of the head and neck. (8) The schema for this protocol has been reported elsewhere but patients were treated with either standard fractionation radiotherapy, hyperfractionated radiotherapy, accelerated hyperfractionated radiotherapy with split, or accelerated fractionated radiotherapy with concomitant boost. (8,9)
Quality adjusted survival was measured by the Q-TWiST (quality-adjusted time without toxicity or relapse) methodology (10). Time without toxicity or relapse (TWiST) was calculated by means of the following formula: TWiST = Survival −[Time in Toxicity (Tox)] − [Time in relapse (Rel)]. This method equates time spent with relapse or toxicity to death, and TWiST is considered of equal importance to all patients regardless of co-morbidities. Survival was truncated beyond 24 months to provide a restricted mean. (11) Patients were deemed to be in a toxicity state when there was recorded toxicity in the skin (≥ grade 2), mucosa (≥ grade 2), salivary gland (≥ grade 2), esophagus (≥ grade 2), larynx (≥ grade 3), upper gastrointestinal (≥ grade 2), spinal cord (≥ grade 2), bone (≥ grade 3), or joint (≥ grade 3). Time in toxicity was measured from onset of toxicity to time below toxicity threshold.
Glasziou et al. defined Q-TWiST as the weighted average of relapse and toxicity added to TWiST representing the quality-adjusted survival time (12). Patients selecting higher values of utility for toxicity, i.e. they preferred or chose states of increased morbidity, had higher quality adjusted survival compared to patients with lower utility values for the toxicity with the utility for relapse states kept constant. Utilities are measures of patient preferences. Utilities are measured and scored from 0, meaning very little preference for that health state, to 1, high preference for the health state. A value of 1 for the utility would equate to perfect health while 0 would equate to a death. Utility values of 0.8 for toxicity and 0.5 for relapse were used to test for quality adjusted survival differences between the treatment arms. As utilities are a measure of patient preference, threshold analysis (6) was also used to identify other combinations of toxicity and relapse utility values for which there is a statisticially significant improvement for one treatment over another. The average of Q-TWiST time is calculated using the product-limit method (Kaplan-Meier) which is the standard approach for estimating survival. Time in relapse (Rel) was calculated from the onset of disease progression until death with utilities varying from 0.0 to 1.0 in increments of 0.001, at each point examining the difference between the two treatment regimens. Q-TWiST therefore equaled TWiST + [(weight for Tox) × (Tox)] + [(weight for Rel) × (Rel)].
Results
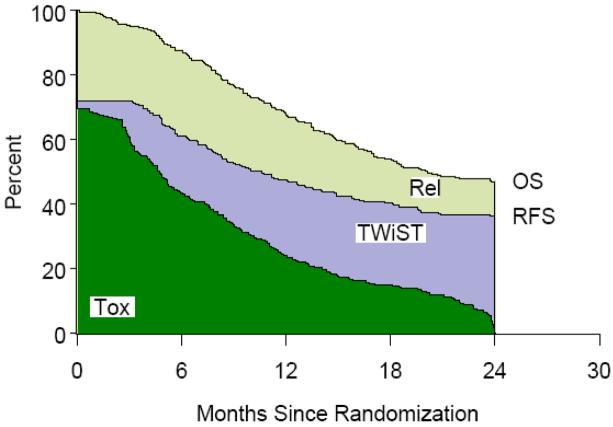
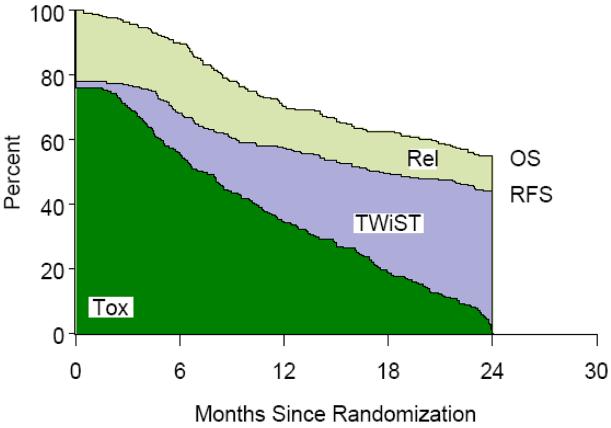
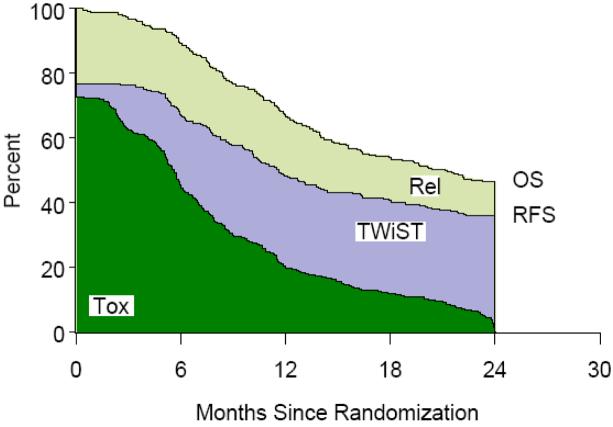
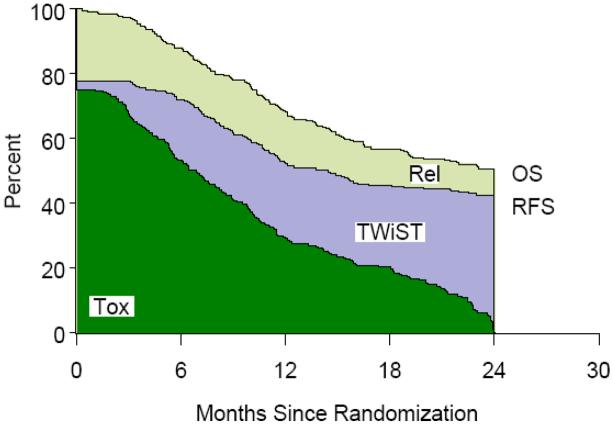
Table 1 lists the partitioned mean survival times by treatment group and partitioned survival graphs are shown in Figures 1,2,3,4. No statistically significant difference was noted between groups in mean TWiST or mean Rel survival times but a statistical difference was noted in time spent in Tox between standard fractionated radiotherapy and hyperfractionated radiotherapy treatment arms (p=0.0037). Mean quality adjusted survival by treatment group is shown in Table 2. Threshold analysis determined patients would favor hyperfractionated radiotherapy over standard fractionated radiotherapy if they were less risk averse and had utility for toxicity ≥ 0.57 and a utility ≤ 0.72 for relapse, as indicated by the yellow section of Figure 5. In addition, threshold analysis found no advantage (or disadvantage) of either accelerated hyperfractionated radiotherapy with split or accelerated fractionated radiotherapy with concomitant boost as compared to SFX (Figures 6 & 7).
Table 1.
Mean Survival partitioned into TwiST, Tox, and Rel (Months)
| Treatment Arm | TwiST | Tox | Rel |
|---|---|---|---|
| SFX (n=266) |
4.9 | 6.9 | 5.0 |
| HFX (n=261) |
5.2 | 9.0 | 3.8 |
| AHFXS (n=274) |
5.8 | 7.1 | 4.1 |
| AFXC (n=267) |
5.2 | 7.1 | 3.8 |
SFX- Standard Fractionated Radiotherapy; HFX-Hyperfractionated radiotherapy; AHFXS-Accelerated Hyperfractionated Radiotherapy with Split; AFXC-Accelerated Fractionated Radiotherapy with Concomitant Boost; Twist- Time without toxicity or relapse; Tox- Toxicity; Rel-Relapse
Figure 1.
Standard Fractionation Radiotherapy (SFX) Partitioned Survival
Figure 2.
Hyperfractionation Radiotherapy (HFX) Partitioned Survival
Figure 3.
Accelerated Hyperfractionated Radiotherapy with Split (AHFXS) Partitioned Survival
Figure 4.
Accelerated Fractionated Radiotherapy with Concomitant Boost (AFXC) Partitioned Survival
Table 2.
Quality Adjusted Survival for Utility of 0.8 for Tox and 0.5 for Rel
| Treatment Arm | Mean Quality Adjusted Survival (Months) |
p-value* |
|---|---|---|
| SFX (n=266) |
12.99 | ------ |
| HFX (n=261) |
14.27 | 0.057 |
| AHFXS (n=274) |
13.55 | 0.38 |
| AFXC (n=267) |
13.60 | 0.36 |
Comparing mean QAS to SFX arm. SFX- Standard Fractionated radiotherapy; HFX-Hyperfractionated radiotherapy; AHFXS- Accelerated hyperfractionated radiotherapy with split; AFXC- Accelerated fractionated radiotherapy with concomitant boost; Tox-Toxicity; Rel- Relapse
Figure 5. Hyperfractionated Radiotherapy (HFX) versus Standard Radiotherapy (SFX) Threshold Plot.
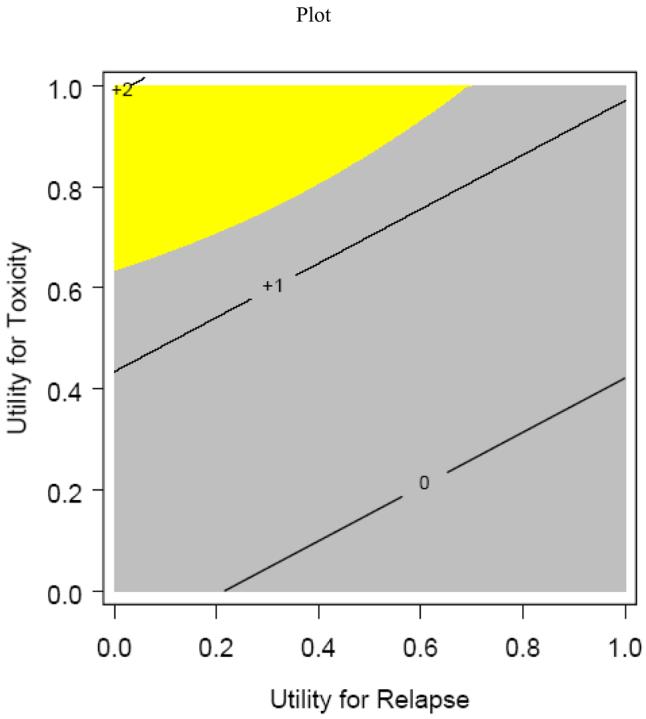
This is a threshold analysis for determining utilities between Standard Radiotherapy (SFX) and Hyperfractionated Radiotherapy (HFX). Patients with a combination of utilities above the line would prefer HFX.
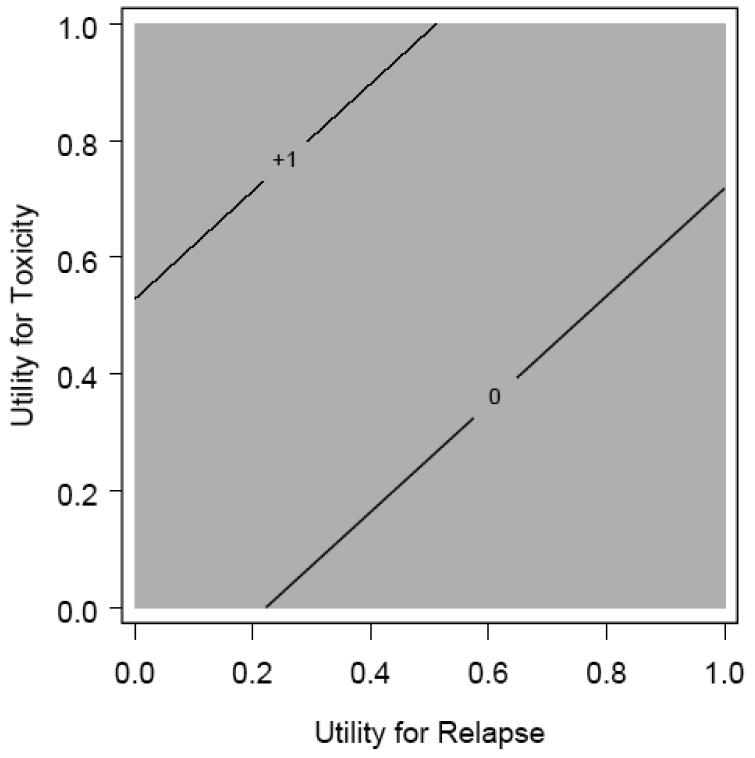
Figure 6. Accelerated Hyperfractionated Radiotherapy with Split versus Standard Fractionated Radiotherapy Threshold Plot.
Threshold analysis comparing Accelerate Hyperfractionated radiotherapy with split (AHFXS) compared to standard radiotherapy (SFX). There is no combination of utilities that would result in AHFXS being superior to SFX.
Figure 7. Accelerated Fractionate Radiotherapy with Concomitant Boost versus Standard Fractionated Radiotherapy Threshold Plot.
Threshold analysis comparing Accelerate Fractionated Radiotherapy with Concomitant boost (AFXC) compared to standard radiotherapy (SFX). There is no combination of utilities that would result in AFXC being superior to SFX.
The Q-TWiST analysis results in patients who are risk averse, i.e. low utility scores for Tox, would have overall lower quality adjusted survival because the time spent in Tox would be devalued. Patients who are less risk averse, i.e. high utility scores for Tox, would have higher quality adjusted survival because the time spent in Tox would not be devalued to the same degree as the more risk averse patients. This is illustrated in Table 2.
Discussion
Physicians and patients are becoming acutely aware of the importance of selecting the treatment that not only gives the highest probability of cure or local control but also maintains the highest quality of life. A number of quality of life instruments are available for physician and patients to use to assess the effect of treatment on QOL. But the plethora of instruments also makes comparing the results of treatment for similar cancers let alone trying to compare the effects of treatment of head and neck cancer with that for diabetes mellitus difficult.
Weymuller et al. reported on their experience using a novel quality of life instrument measuring the quality of life of 549 patients with head and neck cancer. (13) They used an in-house quality of life instrument developed and validated at the University of Washington. They found stage and primary tumor site influenced composite QOL scores. Their instrument was not able to determine QOL differences between patients with advanced primary tumors undergoing either surgery or organ preservation. They also could not detect any differences when global health-related QOL was used as an outcome. (13) As the authors correctly point out no single QOL instrument exists that is appropriate for all studies. (13) Ringash and Bezjak also agree that no one instrument is ideal for all patients and consideration should be given to disease subsite, treatment, timing of assessment, clinical setting, study purpose and research question. (14)
Interestingly, Paleri et al. also published a study comparing laryngectomy alone versus combined therapy using the University of Michigan Head and Neck Quality of Life questionnaire showing no significant difference between groups. (15) This mirrors the results of Weymuller et al. (13)
Major et al. reported no statistical differences were found between patients receiving chemoradiation and surgery for locally advanced laryngeal and hypopharyngeal cancers except for role limitations attributable to physical health. (16) The absence of any difference may have been due to the small numbers of patients in the study. Major et al. used the Health Status Questionnaire-12 (HSQ-12) administered retrospectively by mail and with phone follow-up.
The RTOG has also published the effect of treatment on QOL of patients with squamous cell carcinoma of the head and neck. Johnson et al. published the results of RTOG 9607; a phase II study evaluating the use of Misoprostol in the protection of oral and pharyngeal mucosa. (3) They also used the University of Washington QOL/symptom scale. They found a decrease in quality of life and function in all areas covered by the questionnaire at 6 weeks with recovery by 12 weeks.
Fisher et al. on the other hand used the Head and Neck Performance Status Scale (HNPSS) and the Functional Assessment of Cancer Therapy (FACT-H & N), version 2 to assess QOL in the analysis of outcome of patients treated on RTOG 90-03. (5) They reported pretreatment patient and tumor characteristics impact on QOL prior to the initiation of therapy. They also found intensification of radiotherapy, marital status, tumor site, and nutritional support all affect QOL during the first year after therapy. (5).
Unfortunately, it is very difficult to compare these two previous studies since they used two different QOL instruments. The resulting number cannot be used to determine how much the misoprostol affected QOL in comparison to the detriment in QOL exhibited by the patients treated on RTOG 90-03.
The use of the Q-TWiST methodology allows for identification of patient subgroups that might benefit for more aggressive therapy. Threshold analysis showed less risk averse patients, i.e. those with higher utility values for Tox, would benefit from more aggressive therapy. The Q-TWiST analysis would result in greater QAS by having a higher utility value for Tox.
Q-TWiST determination of QAS also allows comparison of treatments for entirely different diseases. Arriving at a single number for quality adjusted survival gives physicians and other health care professionals the ability to determine which treatments utilize resources more efficiently. The treatment resulting in the highest quality adjusted survival would be the preferred treatment and could be used in societies with limited medical resources. Treatments resulting in the highest quality adjusted survival would be utilized while treatments falling below a certain level would not. This analysis could also be used to generate quality adjusted survival for use in cost-utility analysis.
Conclusions
QAS analysis using the Q-TWiST methodology can assist patients, physicians, and health care policy makers determine the most appropriate treatment for individual patients. Less risk averse patients would benefit from the most aggressive treatments. Further analysis using patient derived utilities should be performed to validate these results.
The Q-TwiST methodology provides a standardized way of arriving at a result that can be used across not only treatments of head and neck cancers but other diseases.
References
- 1.Regine W, Scott C, Murray K, et al. Neurocognitive Outcome in Brain Metastasis Patients Treated with Accelerated Fractionation (AF) vs. Accelerated Hyperfractionation (AH) Whole Brain Radiotherapy (WBRT): An Analysis from Radiation Therapy Oncology Group (RT)G) Study 91-04. Int J Radiat Oncol Biol Phys. 2000;48:158–159. doi: 10.1016/s0360-3016(01)01676-5. [DOI] [PubMed] [Google Scholar]
- 2.Choucair A, Scott C, Urtasun R, et al. Quality of Life and Neuropsychological Evaluation for Patients with Malignant Astrocytomas. RTOG 91-14. Int J Radiat Oncol Biol Phys. 1997;38(1):9–20. doi: 10.1016/s0360-3016(97)00223-x. [DOI] [PubMed] [Google Scholar]
- 3.Johnson D, Scott C, Marks J, et al. Assessment of Quality of Life and Oral Function of Patients Participating in a Phase II Study of Radioprotection of Oral and Pharyngeal Mucosa by the Prostaglandin E1 Analoge Misoprostol (RTOG 96-07) Int J Radiat Oncol Biol Phys. 2001;51:97. doi: 10.1016/s0360-3016(02)03750-1. [DOI] [PubMed] [Google Scholar]
- 4.Scott C, Stern J, Asbell S, et al. Age and Marital Status Linked to Quality of Life of Long Term Survivors of Head and Neck or Prostate Cancer: Report from a Survey of Radiation Therapy Oncology Group Patients. Int J Radiat Oncol Biol Phys. 2001;51:98. [Google Scholar]
- 5.Fisher J, Scott C, Fu K, et al. Treatment Patient and Tumor Characteristics Impact Quality of Life (QOL) in Patients with Locally Advanced Squamous Cell Cancer of The Head and Neck: Report of The Radiation Therapy Oncology Group (RTOG) Trial 90-03. Int J Radiat Oncol Biol Phys. 2001;51:98. [Google Scholar]
- 6.Murray KJ, Nelson DF, Scott C, et al. Quality-Adjusted Survival Analysis of Malignant Glioma. Patients Treated with Twice-Daily Radiation (RT) and Carmustine: A Report of Radiation Therapy Oncology Group (RTOG) 83-02. Int J Radiat Oncol Biol Phys. 1995;31:453–459. doi: 10.1016/0360-3016(95)93160-9. [DOI] [PubMed] [Google Scholar]
- 7.Movsas B, Scott C, Sause W, et al. The Benefit of Treatment Intensification is Age and Histology-Dependent in Patients with Locally Advanced Non-Small Cell Lung Cancer (NSCLC): A Quality-Adjusted Survival Analysis of Radiation Therapy Oncology Group (RTOG) Chemo-Radiation Studies. Int J Radiat Oncol Biol Phys. 1999;45(5):1143–1149. doi: 10.1016/s0360-3016(99)00325-9. [DOI] [PubMed] [Google Scholar]
- 8.Fu K, Pajak T, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: First report of RTOG 90-03. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 9.Konski A, Scott C, Won M, et al. Cost-Utility Analysis of Radiation Therapy Oncology Group (RTOG) 90-03: Phase III Randomized Study Comparing Altered Fractionation to Standard Fractionation Radiotherapy for Locally Advanced Head and Neck Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys. doi: 10.1002/hed.20949. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelber RD, Gelman RS, Goldhirsch A. A Quality-of Life-Oriented Endpoint for Comparing Therapies. Biometrics. 1989;45:781–795. [PubMed] [Google Scholar]
- 11.Gelber RD, Cole BF, Gelber S, Goldehirsch A. Comparing Treatments Using Quality-Adjusted Survival: The Q-TwiST Method. Amer Stat. 1995;49:161–169. [Google Scholar]
- 12.Glasziou PP, Simes RJ, Gelber RD. Quality Adjusted Survival Analysis. Stat Med. 1990;9:1259–1276. doi: 10.1002/sim.4780091106. [DOI] [PubMed] [Google Scholar]
- 13.Weymuller EA, Yuch B, Deleyiannis WB, et al. Quality of Life in Patients with Head and Neck Cancer. Arch Otolaryngol Head Neck Surg. 2000;126:329–336. doi: 10.1001/archotol.126.3.329. [DOI] [PubMed] [Google Scholar]
- 14.Ringash J, Bezjak A. A Structured Review of Quality of Life Instruments for Head and Neck Cancer Patients. Head Neck. 2001;23(3):201–13. doi: 10.1002/1097-0347(200103)23:3<201::aid-hed1019>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Paleri V, Stafford FW, Leontsinis TG, Hildreth AJ. Quality of Life in Laryngectomees: A Post-treatment Comparison of Layrngectomy Alone Versus Combined Therapy. J Laryngol Otol. 2001;115(6):450–4. doi: 10.1258/0022215011908144. [DOI] [PubMed] [Google Scholar]
- 16.Major MS, Bumpous JM, Flynn MB, Schill K. Quality of Life after Treatment for Advanced Laryngeal and Hypopharyngeal Cancer. Laryngoscope. 2001;111(8):1379–82. doi: 10.1097/00005537-200108000-00012. [DOI] [PubMed] [Google Scholar]