Abstract
Apolipoprotein E (apoE) has been implicated in modulating the central nervous system (CNS) inflammatory response. However, the molecular mechanisms involved in apoE-dependent immunomodulation are poorly understood. We hypothesize that apoE alters the CNS inflammatory response by signaling via low-density lipoprotein (LDL) receptors in glia. To address this hypothesis, we used a small bioactive peptide formed from the receptor-binding domain of apoE, apoE peptide (EP), to study LDL receptor signaling in microglia. To model glial activation, we treated primary mouse microglia and the microglial cell line BV2 with lipopolysaccharide (LPS) and studied two inflammatory responses: an increase in nitric oxide production (NO) and a decrease in apoE production. We found that treatment of primary microglia and BV2 cells with EP attenuated LPS-induced NO accumulation and apoE reduction in a dose-dependent manner. Using the receptor associated protein to block ligand binding to members of the LDL receptor family, we found that EP attenuated both of these LPS-induced inflammatory responses via LDL receptors. We studied two intracellular signaling cascades associated with apoE: c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK). LPS induced both ERK and JNK activation, while EP induced ERK activation, but drastically reduced JNK activation. Inhibition of JNK with SP600125 reduced LPS-induced NO production and apoE reduction in a dose-dependent manner. Treatment of BV-2 cells with suboptimal EP in combination with JNK inhibitor enhanced attenuation of LPS-induced NO production. These data suggest that microglial LDL receptors regulate JNK activation, which is necessary for apoE modulation of the inflammatory response.
Keywords: apolipoprotein E, LPS, Alzheimer's disease, signal transduction, nitric oxide
INTRODUCTION
Apolipoprotein E (apoE) is a 34-kDa glycosylated protein whose primary function is to transport lipids between cells and throughout the circulatory system. In humans, apoE exists in three isoforms, termed E2, E3, and E4, which differ by the amino acids at residues 112 and 158: E2 (Cys112Cys158); E3 (Cys112Arg158); and E4 (Arg112Arg158) (Weisgraber 1994). Carriers of the APOE ε4 allele have poor prognosis with central nervous system (CNS) diseases, most notably Alzheimer's disease (AD) (Strittmatter et al. 1993). Inflammation is a hallmark of many of these CNS diseases. Increasing evidence suggests that apoE down regulates CNS inflammation in an isoform specific manner and application of apoE or apoE mimetic peptides, formed from the receptor-binding region of apoE, modulates the inflammatory response (Aderem and Ulevitch 2000; Laskowitz et al. 1997; Laskowitz et al. 2000; Laskowitz et al. 1998; Laskowitz et al. 2001; Lynch et al. 2001; Lynch et al. 2003; Mace et al. 2007; Ophir et al. 2005; Vitek et al. 2007).
While apoE modulates the CNS inflammatory response, apoE expression itself is also regulated by neuronal injury and glial activation. ApoE is upregulated in the CNS after neuronal injury (Poirier et al. 1991) and down-regulated when macrophages or glia are activated by an endotoxin such as lipopolysaccharide (LPS) (Dory 1993; Gafencu et al. 2007; Menju et al. 1989; Mouchel et al. 1995; Saura et al. 2003; Zuckerman and O'Neal 1994). ApoE is expressed mainly in glial cells (Boyles et al. 1985; Murakami et al. 1988) and maintenance of apoE is vital to maintain cholesterol transport required for membrane repair and recycling in the CNS.
Though the mechanisms by which apoE modulates the CNS immune responses have not been elucidated, some evidence suggests that members of the low-density lipoprotein (LDL) receptor family can modulate the glial inflammatory response (LaDu et al. 2000; LaDu et al. 2001; Laskowitz et al. 2001). ApoE is bound and internalized via receptor-mediated endocytosis by these receptors, including the low-density lipoprotein receptor (LDLR), very-low density lipoprotein receptor (VLDLR), apolipoprotein E receptor 2 (ApoEr2) and LDL receptor-related protein-1 (LRP1) in the CNS. Neurons primarily express ApoEr2, LRP1 and VLDLR (Christie et al. 1996; Kim et al. 1996; Rebeck et al. 1993). Glial cells are known to express LDLR, LRP1 and VLDLR, but not ApoEr2 (Christie et al. 1996; Rebeck et al. 1993). The neuronal receptors have been extensively implicated in various signaling processes, including neurite outgrowth, calcium homeostasis, kinase activation, and cell migration (Beffert et al. 2004). However, little is known about the signaling properties of these receptors in glia.
The aims of our present study were to address how LDL receptors regulate the CNS inflammatory response. For theses studies, we used an apoE peptide (EP) that consisted of a tandem repeat of receptor binding domain; this peptide binds apoE receptors and activates signaling processes in neurons (Hoe et al. 2005; Hoe et al. 2006). We studied mitogen-activated protein kinase (MAPK) family members, a group implicated in microglial activation (Bhat et al. 1998; Han et al. 2002; Pyo et al. 1998; Xie et al. 2004) and neuronal apoE signaling (Hoe et al. 2005; Hoe et al. 2006). Microglial activation was monitored by LPS-induced accumulation of nitric oxide (NO) and reduced apoE expression. Inhibition of the MAPK family member c-Jun N-terminal kinase (JNK) by EP proved to be essential to overcome LPS-induced microglial inflammatory responses.
METHODS
BV2 Microglial Cell Culture
BV2 microglia were provided by Dr. Richard Banati (Max-Planck-Institute of Psychiatry, Martinsried/Munich, Germany) (Blasi et al. 1990). Cells were maintained in Opti-MEM (Invitrogen, Carlsbad, CA) with 5% fetal bovine serum (FBS, Invitrogen) in a 5% CO2 incubator. For experiments, cells were plated in 24-well plates at 7.5 × 104 cells / well. Plated cells were grown in Opti-MEM with 5% FBS overnight and then the medium was changed to serum-free Opti-MEM for 1 hr. After 1 hr, the medium was replaced with serum-free Opti-MEM containing either control PBS or experimental agents.
Primary Microglial Cell Culture
Primary mouse microglial cell cultures were prepared from postnatal day 1 Swiss-Webster mouse pups using a slightly modified method from that of (Su et al. 2007). Mixed glial cultures were grown to confluency and microglia were harvested by shaking the flasks at 100 rpm for 1 hr at 37°C. Microglial were resuspended in minimum essential media (MEM, Invitrogen) supplemented with 10 % FBS, 1% L-glutamine (Invitrogen), 1% sodium pyruvate (Invitrogen), 1% Pen/Strep (Invitrogen), and 1% Fungizone (Invitrogen) and plated at a density of 1 × 105 cells/ml on glass coverslips. Microglial cell purity was confirmed to be >95% using immunoflourescence for OX42 (microglial marker, Serotec, Raleigh, NC) and DAPI (Vector Laboratories, Burlingame, CA) nuclear counterstain. For nitrite assay experiments, cells were plated in 96-well plates at 2.5 × 104 cells / well. For Western blot analysis experiments, cells were plated in 24-well plates at 12.5 × 104 cells / well. Plated cells were grown in supplemented media overnight and then the medium was replaced with serum-free Opti-MEM containing either control PBS or experimental agents.
Antibodies
Phosphorylation site-specific antibodies were used against phospho-p38 (Thr180/Tyr182), phospho- ERK (Thr202/Tyr204), and phospho-JNK (Thr183/Tyr185) (Cell Signaling Technologies, Beverly, MA). Phospho-ERK antibody detected levels of both p42 (Erk2) and p44 (Erk1) MAP Kinases when phosphorylated. Phospho-JNK antibody detected levels of p46 (JNK1) and p54 (JNK2) kinases when phosphorylated. Total ERK and JNK antibodies (Cell Signaling Technologies) detected total levels of ERK and JNK proteins, including both ERK proteins (p42 and p44) and both JNK proteins (p46 and p54). ApoE was detected by rabbit polyclonal antibody against rodent apoE (Abcam, Cambridge, MA). Rabbit polyclonal antibody was used to detect iNOS (BD Biosciences Pharmigen, San Diego, CA). From the same blots, β-actin (Abcam) was detected by monoclonal antibody to ensure equal protein levels in each lane. To detect LDL receptors, we used rabbit polyclonal antibodies against LDLR (Irene) and LRP (RRR) provided by Dr. Guojun Bu (Washington University, St. Louis, MO). VLDLR was detected by mouse monoclonal antibody (5F3), provided by Dr. Dudley Strickland (University of Maryland, Baltimore, MD). OX42 (Serotec, Raleigh, NC) was used to stain primary microglia.
Chemicals
The apoE-derived peptide(141-149) (EP), consisting of a duplicated sequence of apoE amino acids 141 through 149, was synthesized by Johns Hopkins University of Medicine (Biosynthesis and Sequencing Facility, Baltimore, MD) (Hoe et al. 2005). Recombinant RAP was a generous gift from Dr. Dudley Strickland (University of Maryland) and Dr. Guojun Bu (Washington University). Wortmannin (Sigma) inhibited PI3K/AKT, PD98059 (Sigma) inhibited ERK, and SP600125 (Invitrogen) inhibited JNK. Phosphatase inhibitor cocktails (Sigma) and protease inhibitor (Sigma) were used in cell lysis buffer. LPS was purchased from Calbiochem (San Diego, CA). Human recombinant α2M (Athens Research, Athens, GA) was activated in 100mM methylamine (Sigma) at 1mg/ml as stock solution. FITC-α2M* was made with EZ-Label™ FITC Protein Labeling Kit (Pierce Biotechnology, Rockford, IL). VECTASHIELD Mounting Medium with DAPI (Vector Laboratories, Burlingame, CA) was used to fluorescently label nuclei.
Western blot analyses
For analysis of secreted molecules, conditioned media was collected from cultured cells. Cell associated proteins were harvested in ice-cold lysis buffer containing phosphatase inhibitor cocktails and protease inhibitor. Proteins were separated under denatured and reduced conditions using Tris-glycine SDS-polyacrylamide gel electrophoresis (Biorad, Hercules, CA). Separated proteins were detected on poly(vinyldifuoride) membranes incubated with primary antibodies. Immunoreactivity was detected using horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA) visualized by enhanced chemiluminescence detection film. Band density was determined using Quantity One 1-D analysis software (Biorad).
Nitrite quantification
The production of NO was assessed as the accumulation of nitrite from the spontaneous oxidation of NO in serum-free cell conditioned media after 24 hrs and 48 hrs. Accumulation of nitrite was quantified using a colorimetric reaction with Griess reagent (Invitrogen). Absorbance was measured at 570nm by spectrophotometry.
Statistical analysis
All experiments were repeated a minimum of three times. The data were analyzed using ANOVA with Graphpad Prism 4 software, using Newman-Keuls Multiple Comparison Test for posthoc analysis with significance determined at a P value of <0.05.
RESULTS
Effects of microglial cell stimulation on nitric oxide production and apoE production
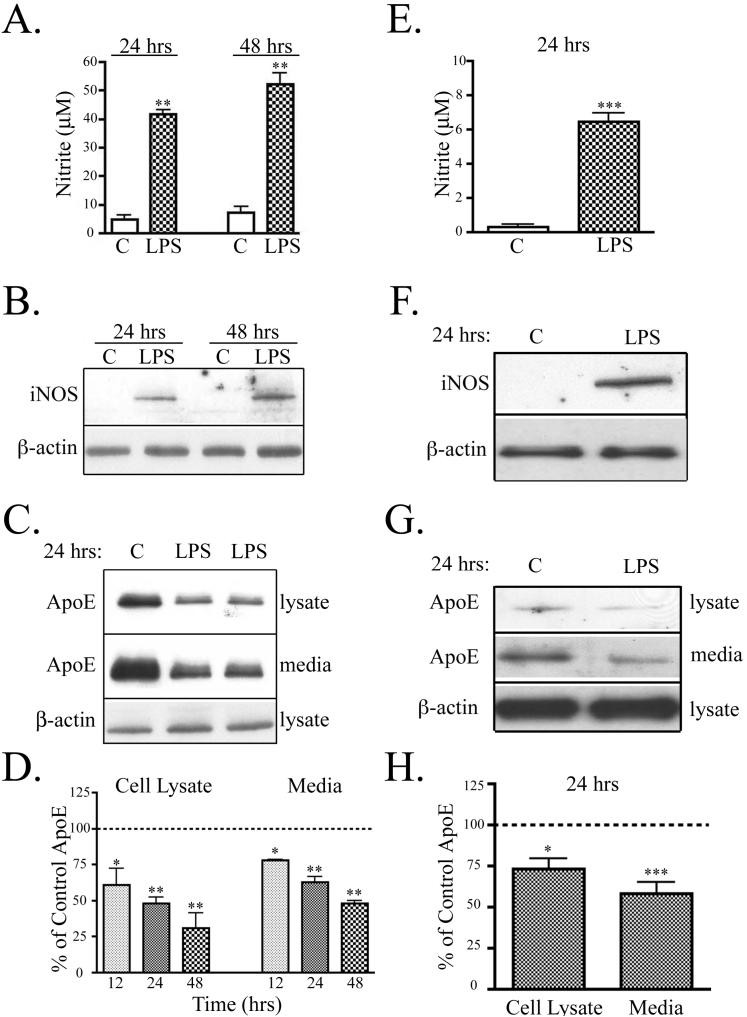
The Toll-like receptor 4 (TLR4) is traditionally considered the primary receptor for LPS (Lien et al. 2000). Upon receptor activation, LPS induces intracellular signaling cascades, downstream synthesis of inducible nitric oxide synthase (iNOS) and release of NO in microglia (Corradin et al. 1993). For our experiments, mouse microglial BV2 cells and primary mouse microglia were treated with 100 ng/ml LPS in serum-free media. LPS treatment caused a significant increase of NO from BV2 cells at 24 hrs and 48 hrs (Fig. 1A). BV2 cells treated with LPS accumulated 42 ± 2 μM nitrite at 24 hrs and 52 ± 4 μM nitrite at 48 hrs, compared to 4 ± 1 μM and 7 ± 2 μM nitrite from untreated cells. Expression of iNOS was readily detected by Western blot analysis of BV2 cells treated with LPS for 24 and 48 hrs, but undetected in untreated cells (Fig. 1B). Similarly, in primary microglia, LPS caused a significant increase in NO at 24 hrs (Fig. 1E). Microglia treated with LPS accumulated 6 ± 1 μM nitrite compared to 0.3 ± 0.1 μM nitrite from untreated cells. Expression of iNOS was detected by Western blot analysis in microglia treated with LPS for 24 hrs, but undetected in control cultures Fig. 1F).
Figure 1. Activation of microglia by LPS increased NO and decreased apoE.
BV2 microglia and primary microglia were treated with 100 ng/ml LPS. A. In BV2 cells, LPS promoted nitrite accumulation in the conditioned media at 24 hrs and 48 hrs (mean ± SEM; **P<0.01; n = 6). B. BV2 cell lysates were analyzed by Western blotting with antibodies to iNOS and β-actin (as a control). A representative blot shows that LPS increased iNOS levels (n = 4). C. BV2 cell lysates were analyzed for apoE and β-actin and conditioned media was analyzed for apoE. A representative blot shows that treatment of cells with LPS for 24 hrs reduced apoE in both cell lysates and media. D. Quantification of Western blots showed that apoE in cell lysate and media of BV2 cells was significantly decreased at 12 hrs, 24 hrs, and 48 hrs. The data were quantified as percent of control (mean ± SEM; *P<0.05; **P<0.01 compared with corresponding control cultures; n = 6). E. Primary microglia treated with LPS for 24 hrs accumulated a significant amount of nitrite in the conditioned media (mean ± SEM; ***P<0.001; n=4). F. Cell lysates from primary microglia were analyzed by Western blotting with antibodies to iNOS and β-actin (as a control). A representative blot shows that LPS increased iNOS levels (n=3). G. Representative Western blots of primary microglia cell lysate analyzed for apoE and β-actin and conditioned media analyzed for apoE is shown. Microglia treated with LPS for 24 hrs showed reduced apoE in both cell lystes and media. H. Quantification of Western blot (Panel G) showed that apoE in cell lysate and media of primary microglia was significantly decreased at 24 hrs (mean ± SEM; *P<0.05; ***P<0.001 compared with untreated cultures; n = 4).
LPS also reduced endogenous apoE in cell lysates and conditioned media of BV2 cells (Fig. 1C) and primary microglia (Fig. 1G). Total protein levels of β-actin remained unchanged. Quantification of Western blot analysis showed that apoE levels in the BV2 cell lysate and conditioned media significantly decreased with LPS stimulation at 12 hrs, 24 hrs, and 48 hrs when compared to apoE expression in unstimulated cells at the same times (Fig. 1D). LPS significantly reduced cellular apoE levels by 39% at 12 hrs, 52% at 24 hrs, and 69% at 48 hrs, compared to control cells. ApoE levels in the conditioned media were reduced similarly (22% at 12 hrs, 37% at 24 hrs, and 52% at 48 hrs) (Fig. 1D). Western blot analysis of primary microglia was quantified showing that apoE levels in the cell lysate and conditioned media were also significantly decreased with LPS stimulation at 24 hrs when compared to apoE expression in unstimulated cells (Fig. 1H). Cellular apoE levels in primary microglia were decreased 27% with LPS treatment for 24 hrs. ApoE levels in the conditioned media were decreased 42% with LPS treatment (Fig. 1H). Thus, activation of both BV2 cells and primary microglia by LPS increased iNOS levels and production of NO, and decreased levels of apoE.
ApoE Peptide Attenuates LPS Induced Inflammatory Responses
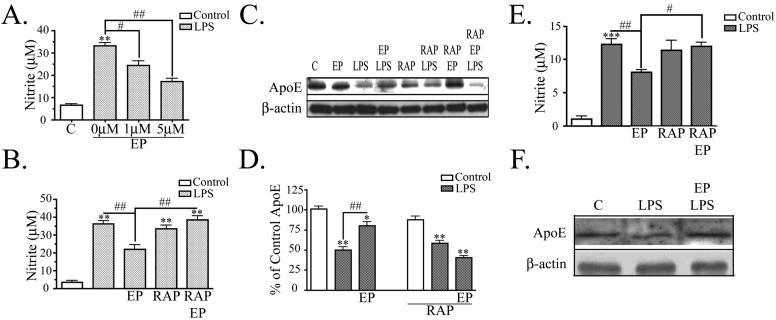
Various apoE peptides, formed from the receptor-binding region of apoE, have anti-inflammatory properties (Laskowitz et al. 2001). In this study, we used the apoE mimetic peptide, EP, consisting of a tandem repeat of the nine amino acid receptor-binding domain of apoE. We hypothesized that EP could attenuate the LPS induced nitrite accumulation and reduction of apoE expression. Preliminary studies showed that pretreatment of cells with EP was not necessary for its effects (data not shown); therefore EP treatment was performed at the same time as LPS stimulation. BV2 microglia were treated with various doses of EP, ranging from 100 nM to 5 μM. EP modulated LPS-induced nitrite production optimally at doses of 1 μM or higher. BV2 cells stimulated with LPS accumulated 33 ± 2 μM nitrite at 24 hrs. Cultures treated with 1 μM EP and LPS accumulated 24 ± 2 μM nitrite, a significant 27% decrease compared to cells treated with LPS alone. Cultures treated with 5 μM EP and LPS accumulated 17 ± 1 μM nitrite, a significant 48% decrease compared to cells treated with LPS alone (Fig. 2A). Thus, EP counteracts the effects of LPS on nitrite production.
Figure 2. ApoE peptide attenuates LPS induced NO increase and apoE decrease.
A. BV2 cells were treated with 100 ng/ml LPS and nitrite was measured in the conditioned media at 24 hrs. LPS induced nitrite accumulation and EP showed dose-dependent attenuation of LPS-induced nitrite production (n = 6). B. Cells were treated as in Panel A with 5 μM EP and LPS. EP attenuated LPS-induced nitrite production and 50 nM RAP prevented the effect of EP, while RAP alone had no effect (mean ± SEM; **P<0.01 compared with corresponding control cultures; #P<0.05; ##P<0.01 compared with indicated cultures; n = 6). C. BV2 cell lysates were analyzed by Western blot analysis for apoE and β-actin. A representative blot is shown. LPS decreased apoE levels and 1 μM EP prevented that decrease, while EP alone had no effect on apoE levels. 50 nM RAP alone had no effect, but RAP prevented EP from reversing the effects of LPS. β-actin levels were the same across treatment conditions. D. Western blot data were quantified as percent of control (mean ± SEM; *P<0.05; **P<0.01; ***P<0.001 compared with control cultures; ## P<0.01 compared with indicated cultures; n = 3). E. Primary microglia were treated with 100 ng/ml LPS and nitrite was measured in the conditioned media at 24 hrs. LPS induced nitrite accumulation and 5 μM EP showed attenuation of LPS-induced nitrite production. 50 nM RAP prevented the effect of EP, while RAP alone had no effect (mean ± SEM; ***P<0.001 compared with corresponding control cultures; #P<0.05; ##P<0.01 compared with indicated cultures; n = 3). F. Cells lysates from primary microglia were analyzed by Western blot analysis for apoE and β-actin. LPS decreased apoE levels and 1 μM EP prevented that decrease. β-actin levels were the same across treatment conditions (n = 3).
To test whether BV2 cells have functional receptors of the LDL receptor family, FITC-labeled activated alpha-2-macroglobulin (α2M*), a ligand for several members of this family, was applied and detected intracellularly by fluorescence microscopy (data not shown). Receptor associated protein (RAP) was used as a tool to block ligand binding to the receptors. Cells treated with RAP were unable to endocytose FITC-labeled α2M*, proving that RAP was active (data not shown). We used RAP to block EP from binding the receptors and to test whether EP was reducing nitrite accumulation through interactions with the LDL receptor family. BV2 cells treated with 50 nM RAP, 5 μM EP, and LPS accumulated significantly higher levels of nitrite compared to cells treated with 5 μM EP and LPS alone (39 ± 2 μM compared to 22 ± 2 μM) (Fig. 2B). Cells were treated with RAP and LPS alone as a control and those cells accumulated approximately the same amount of nitrite as LPS stimulated cells (34 ± 2 μM nitrite). Thus, LDL receptor family mediated the inhibitory effect of EP on nitrite production.
Similar results were observed in primary microglia. LPS increased nitrite levels, and this effect was partially blocked by EP (8 ± 0.3 μM compared to 12 ± 1 μM) (Fig. 2E). Microglia treated with 50 nM RAP, 5 μM EP, and LPS accumulated significantly higher amounts of nitrite compared to cells treated with 5 μM EP and LPS alone (12 ± 1 μM compared to 8 ± 0.3 μM). As a control, microglia were treated with RAP and LPS alone and those cultures accumulated similar amounts of nitrite as LPS stimulated cells (11 ± 2 μM). The data from both primary microglia and BV2 cells support the idea that LDL receptors mediate inhibitory effects of EP on nitrite production.
We also tested whether EP altered the effect of LPS on apoE levels. These experiments were done in both BV2 cells and primary microglia. For these experiments, apoE expression in the cell lysate was analyzed by Western blot (Fig. 2C for BV2 cells; 2F for primary microglia) and quantified (Fig. 2D for BV2 cells; quantification not shown for primary microglia). Cells treated with 1 μM EP alone did not have a significant change in endogenous apoE expression compared to controls. Cells stimulated with LPS had significantly less apoE than control (50 ± 4% of control in BV2 cells; 66 ± 5% of control in primary microglia), consistent with Fig. 1. Cells treated with 1 μM EP and LPS had significantly more apoE than cells treated with LPS alone (80 ± 5% of control in BV2 cells; 85 ± 6% in primary microglia). Thus, EP treatment attenuated the reduction of endogenous apoE caused by LPS.
To examine whether members of the LDL receptor family mediated the effect of EP, we again used the receptor blocker RAP. When BV2 cells were treated with RAP alone, there was no significant change in apoE levels (88 ± 4% of control); LPS also still reduced apoE levels in the presence of RAP (58 ± 3% of control). However, in the presence of RAP, EP no longer reversed the effect of LPS (40 ± 2% of control). These data indicate that EP attenuates LPS induced reduction in apoE expression via the LDL receptor family.
ApoE Peptide Inhibits JNK Activation
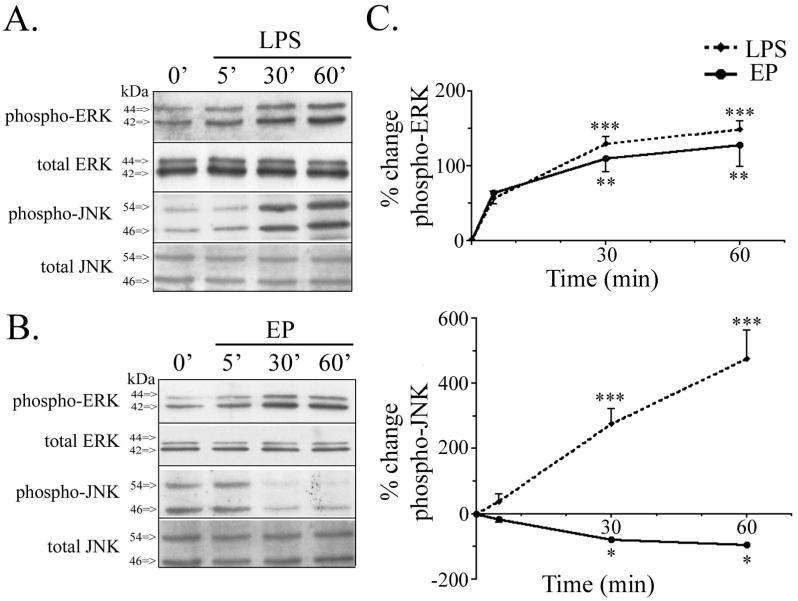
We have shown that EP attenuates nitrite production and changes in endogenous apoE, caused by LPS in BV2 cells and primary microglia. To examine the signaling pathways that may mediate these responses, we continued our research in BV2 microglia and studied the activation of kinases JNK and ERK, both implicated in LPS-induced signaling cascades (Bhat et al. 1998; Han et al. 2002; Pyo et al. 1998; Schumann et al. 1998). Activation of JNK and ERK was initially studied from 5 min to 60 min and cells were treated with either 100 ng/ml LPS or 1 μM EP. LPS caused an increase in phospho-ERK levels, while levels of total ERK remained unchanged (Fig. 3A). LPS also caused a dramatic increase in phospho-JNK levels and levels of total JNK remained unchanged (Fig. 3A). Quantification of the Western blot analysis showed that LPS induced significant time-dependent increases in both phospho-ERK and phospho-JNK levels (Fig. 3C). Levels of phospho-ERK and phospho-JNK activation were also analyzed for cultures stimulated with EP from 5 min to 60 min. EP caused an increase in phospho-ERK levels, while total levels of ERK remained unchanged (Fig. 3B). In contrast, EP induced a decrease in phospho-JNK, while levels of total JNK remained unchanged (Fig. 3B). Together, these data show that EP caused a significant time-dependent increase in phospho-ERK and a time-dependent decrease in phospho-JNK (Fig. 3C). Thus, LPS and EP have qualitatively similar effects on ERK activation, but strikingly different effects on JNK activation (Fig. 3C).
Figure 3. ApoE peptide increased ERK activation and reduced JNK activation.
BV2 microglia were treated with 1 μM EP or 100 ng/ml LPS from 5 to 60 min. Cell lysates were analyzed by Western blotting with antibodies to phospho-ERK, total ERK, phospho-JNK, and total JNK. A. Cells treated with LPS showed a time-dependent increase in both phospho-ERK and phospho-JNK. Total levels of ERK and JNK protein remain unchanged. B. Cells treated with EP showed a time-dependent increase in phospho-ERK and decrease in phospho-JNK. Total levels of ERK and JNK protein remain unchanged. C. Western blot data were quantified as percent change of phospho-ERK (upper graph) and percent change of phospho-JNK (lower graph) comparing EP and LPS treatment. LPS significantly increased phospho-ERK and phospho-JNK by 30 min. EP significantly increased phospho-ERK and decreased phospho-JNK by 30 min (mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001; n = 3).
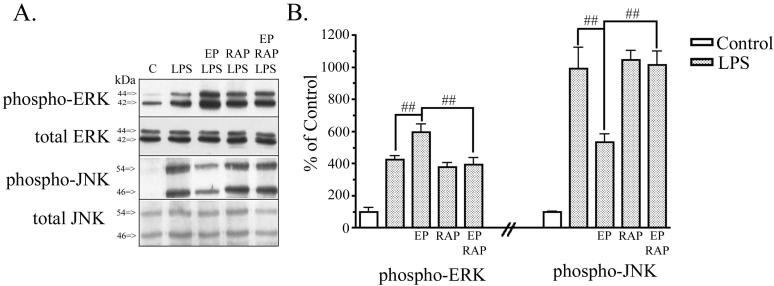
We asked how these signaling pathways were affected with simultaneous EP and LPS treatment. ERK and JNK phosphorylation was examined 1 hr after treatments because the most pronounced signaling effects were seen at 1 hr (Fig. 3). As before, treatment of BV2 cells with LPS for 1 hr increased phospho-ERK and phospho-JNK expression, while levels of total ERK and JNK remained unchanged (Fig. 4A). LPS-treated cells had 425 ± 24% of control levels in phospho-ERK and 990 ± 130% of control levels of phospho-JNK. Simultaneous treatment of BV2 cells with EP and LPS for 1 hr induced an even greater increase in phospho-ERK (596 ± 5% of control) and a significant decrease in phospho-JNK (532 ± 53% of control) levels when compared to cultures treated with LPS alone (Fig. 4B). To test whether apoE receptors were involved in the effects of EP on signaling, we again used the inhibitor RAP. Treatment of cultures with RAP, EP and LPS showed an increase in phospho-ERK levels (393 ± 44% of control) and an increase in phospho-JNK levels (1012 ± 89% of control) when compared to control cultures. RAP treatment prevented EP from potentiating LPS induced phospho-ERK activation and prevented EP from attenuating LPS induced phospho-JNK activation (Fig. 4B). Thus, EP acts via the LDL receptor family to induce a potentiation of LPS-induced ERK activation and an attenuation of LPS-induced JNK activation.
Figure 4. ApoE peptide signaling effects are LDL receptor family mediated.
BV2 microglia were treated with various combinations of PBS (control), 1 μM EP, 100 ng/ml LPS, and 50 nM RAP for 1 hr. Cell lysates were analyzed by Western blotting with antibodies to phospho-ERK, total ERK, phospho-JNK, and total JNK. A. Cells treated with EP and LPS showed increased phospho-ERK and reduced phospho-JNK compared to cells treated with LPS alone. Treatment of BV2 cells with RAP prevented EP-induced potentiation of phospho-ERK and EP-induced attenuation of phospho-JNK. Total levels of ERK and JNK protein were unchanged. B. Western blot data were quantified as percent of control for phospho-ERK and phospho-JNK (mean ± SEM; ##P < 0.01 compared with indicated cultures; n = 4).
Inhibition of JNK Prevents LPS Induced Inflammatory Response
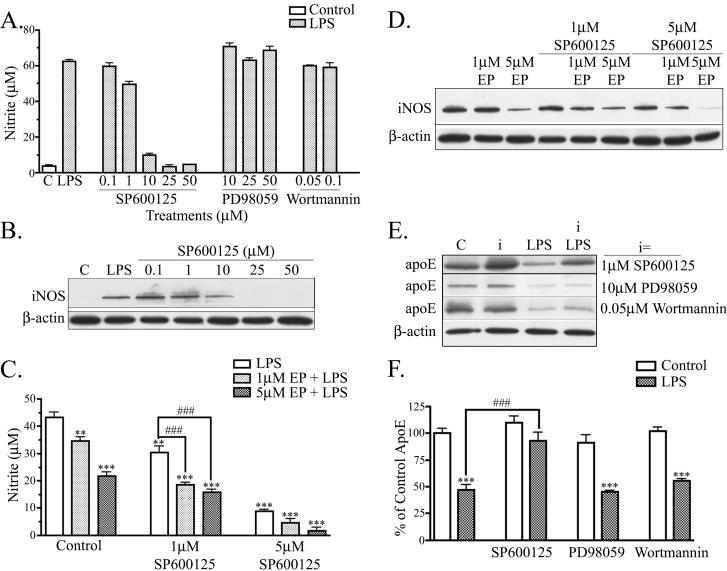
The data in Figs. 3 and 4 show that activation of JNK and ERK can be modulated by EP in BV2 microglia. We next asked whether various kinases were involved in LPS-induced nitrite accumulation and apoE reduction using various inhibitors. We used inhibitors to the MAPK pathway family members, JNK and ERK, and an inhibitor to the phosphoinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway, implicated in regulating cellular inflammatory responses (Cantley 2002). Cells were treated with doses of JNK inhibitor (SP600125), ERK inhibitor (PD98059), and PI3K/Akt inhibitor (Wortmannin). The lowest dose used for each inhibitor blocked phosphorylation of its respective kinase at 1 hr (data not shown). Previous reports implicated JNK activation in iNOS expression and NO accumulation (Moon et al. 2007; Pawate and Bhat 2006) and our findings confirmed these results. Cells treated with LPS and SP600125 accumulated significantly less nitrite in a dose dependent manner when compared to cultures treated with LPS alone (Fig. 5A). Cells treated with PD98050 or Wortmannin showed no attenuation of the LPS induced nitrite response (Fig. 5A). Further, SP600125 blocked the increase in iNOS accumulation caused by LPS (Fig. 5B). In cells treated with low doses of SP600125 (0.1 μM and 1 μM) and LPS, iNOS levels remained equal to control cells treated with LPS alone. Cells treated with 10 μM SP600125 and LPS treatment showed a 20 % reduction in iNOS expression compared to cells treated with LPS alone. At higher doses of SP600125 (25 μM and 50 μM), iNOS expression was reduced by nearly 100 % compared to cells treated with LPS alone. The reduction in iNOS levels and nitrite levels occurred with the same doses of SP600125 (between 1 μM and 10 μM SP600125). These data show that JNK activation, but not ERK or PI3K/AKT activation, is important for LPS induced NO accumulation in BV2 cells.
Figure 5. JNK modulates NO production and apoE levels.
A. BV2 cells were treated with indicated doses of a JNK inhibitor (SP600125), ERK inhibitor (PD98059), or PI3K/AKT inhibitor (Wortmannin), and stimulated with 100 ng/ml LPS for 24 hrs. SP600125 treatment reduced nitrite production in a dose-dependent manner. PD98059 and Wortmannin did not effect LPS induced nitrite production (n = 3). B. Cell lysates were analyzed by Western blotting with antibodies to iNOS and β-actin. A representative blot shows that SP600125 reduced iNOS levels in a dose-dependant manner (n = 3). C. BV2 cells were treated with suboptimal doses of EP and SP600125. Each alone showed attenuation of LPS-induced nitrite accumulation; in combination, they showed significant additive effects (***P < 0.001; **P < 0.01 compared with control cultures. ###P<0.001 compared with indicated cultures; n = 6). D. Cell lysates of BV2 cells treated with suboptimal doses of EP and SP600125 were analyzed by Western blotting antibodies to iNOS and β-actin. Combined EP and SP600125 treatment reduced of LPS-induced iNOS accumulation (n = 3). E. BV2 cells were treated with LPS and/or kinase inhibitors (i) and cell lysates were analyzed for apoE and β-actin expression. A representative blot shows that SP600125 increased apoE levels, but neither other inhibitor had any effects. F. Western blot data were quantified as percent of control for apoE in the cell lysate. Cells treated with 1 μM SP600125 and LPS showed an increase in lysate apoE compared to cells treated with LPS alone, whereas cells treated with 10 μM PD98059 or 0.05 μM Wortmannin and LPS did not show an increase in lysate apoE compared to cells treated with LPS alone (mean ± SEM; ***P < 0.001 compared with corresponding control cultures; ###P < 0.001 compared with indicated cultures; n = 3).
We next asked whether the effects of EP were also mediated by the JNK pathway. We used suboptimal doses of both SP600125 (1μM and 5μM) and EP (1μM or 5μM) to test whether they would act synergistically on JNK activation and LPS-induced nitrite accumulation. As before, BV2 cells stimulated with LPS and treated with 1μM EP or 5μM EP had a significantly lower amount of nitrite accumulation than cells stimulated with LPS alone (Fig. 5C). Cells stimulated with 1μM SP600125 accumulated 30% (30 ± 2 μM) less nitrite than cells treated with LPS alone; cells stimulated with 1μM EP alone accumulated 21% (34 ± 2 μM nitrite) less nitrite than cells treated with LPS alone. Cells treated with 1μM SP600125 and 1μM EP alongside LPS stimulation accumulated 58% (18 ± 1 μM) less nitrite than cells treated with LPS alone and significantly less than cells treated with either drug alone. Further, cells treated with a higher dose of EP (5 μM) and 1 μM SP600125 also accumulated significantly less nitrite compared to cells treated with LPS alone (65% less; 15 ± 1 μM). Thus, EP and SP600125 can additively suppress LPS-induced nitrite accumulation. Western blot analysis indicated that iNOS levels were similarly decreased with a combinational treatment of EP and SP600125 (Fig. 5D).
We tested whether these mechanisms defined in BV2 cells also occurred in primary microglia. Similar to Fig. 2E, primary microglia stimulated with LPS and treated with 1 μM EP had a significant 25 % reduction in nitrite accumulation (6 ± 0.1 μM) than cells stimulated with LPS alone (8 ± 0.3 μM) (data not shown). Primary microglia stimulated with 1 μM SP600125 accumulated 75 % (2 ± 0.1 μM nitrite) less nitrite than cells treated with LPS alone. Cells treated with 1 μM SP600125 and 1 μM EP alongside LPS stimulation accumulated 89 % (1 ± 0.03 μM nitrite) less nitrite than cells treated with LPS alone, which was significantly less nitrite compared to cells treated with either drug alone. These effects of EP on nitrite levels in primary microglia were paralleled by effects on decreasing iNOS levels (data not shown).
Finally, to determine whether JNK was also involved in the down-regulation of apoE by LPS, we tested the effects of SP600125 on apoE levels after stimulation with LPS. For these experiments, apoE levels in BV2 cell lysates were analyzed by Western blot (Fig. 5E) and quantified (Fig. 5F). As before, LPS decreased apoE levels to 46 ± 5% of control (Fig. 5E & 5F). Cells treated with LPS and SP600125 showed a significant attenuation of the decrease in apoE (93 ± 7% of control). SP600125 alone increased apoE levels, suggesting that there is also regulation of apoE by JNK under normal conditions in cell cultures. Cells treated with the other kinase inhibitors, PD98059 and Wortmannin, did not show an attenuation of LPS induced decrease in apoE expression. Thus, as with NO production, the effect of LPS on reducing apoE levels depended on the JNK signaling pathway.
DISCUSSION
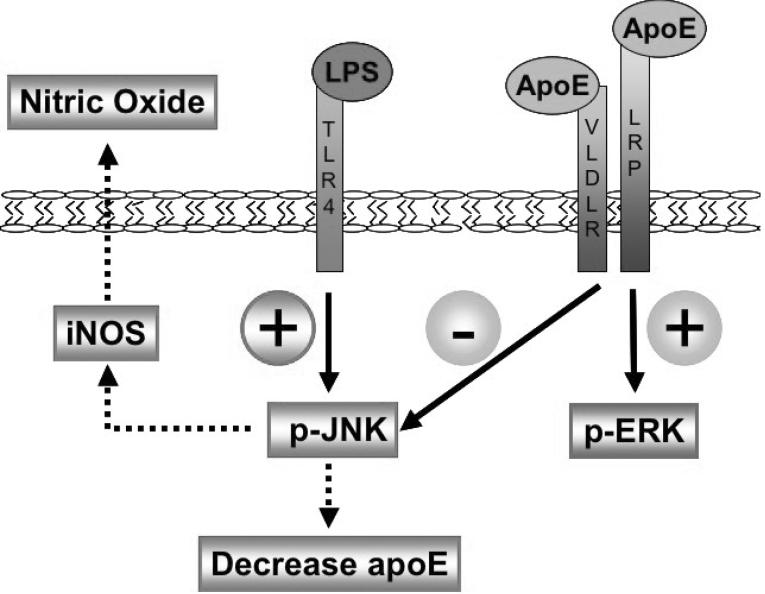
Our current study demonstrates that apoE-induced anti-inflammatory properties involve MAPK signaling pathways that can be promoted through the activation of LDL receptors. This work is consistent with reports that apoE has anti-inflammatory properties in the CNS (Laskowitz et al. 1997; Laskowitz et al. 2000; Laskowitz et al. 1998; Laskowitz et al. 2001; Lynch et al. 2001; Lynch et al. 2003; Mace et al. 2007). Our data show that an apoE mimetic peptide, EP, attenuated LPS-induced inflammatory responses by modulating JNK activation. In microglia LPS activation of JNK increased NO and decreased apoE, the two inflammatory responses that we studied. We showed that EP treatment decreased JNK through the LDL receptor family and counteracted LPS-induced inflammatory responses through this modulation. Taken together, we developed a model of apoE receptor immunomodulatory signaling (Fig. 6), suggesting that the LDL receptor family regulates the microglial inflammatory response by suppressing JNK activation.
Figure 6. LDL receptor immunomodulatory signaling in microglia.
LPS binds TLR4 receptors on the cell surface and signals an increase in phospho-JNK, leading to an increase in iNOS synthesis, an accumulation of NO extracellularly, and an independent decrease of intracellular apoE. ApoE binds LDL receptors on the cell surface and signals an increase in ERK activation and decrease in JNK activation. The decrease in JNK activation suppresses iNOS synthesis, reduces NO accumulation extracellularly, and suppresses a reduction of intracellular apoE.
Several studies have implicated MAPK activation in microglia treated with various stimuli. LPS induces activation of all three major classes of MAPKs: p38, JNK, and ERK (Bhat et al. 1998; Han et al. 2002; Pyo et al. 1998; Xie et al. 2004). Our study confirmed these previous reports showing LPS-induced activation of p38 (data not shown), ERK and JNK (Fig. 3) in BV2 microglia. Our previous work in neurons showed EP-induced an increase in ERK activation and a decrease in JNK activation (Hoe et al. 2005). In microglia, we found that EP affects ERK and JNK activation, but does not alter p38 activation (data not shown). One potential mechanism through which MAPKs might contribute to microglial activation is through effects on NO generation (Chao et al. 1992; Saha and Pahan 2006; Simmons and Murphy 1992). Previous work showed that JNK is the major regulator of iNOS expression in primary astrocytes (Pawate and Bhat 2006). Our research focused primarily on the effects on JNK activation because our data show that specific JNK inhibitor (SP600125) inhibited iNOS and nitrite accumulation in a dose-dependent manner, implicating JNK as an important mediator of LPS-induced NO production, a finding that is in line with a recent study in BV2 cells (Moon et al. 2007). These findings support the idea that apoE protects against inflammatory signaling and NO production via inhibition of the JNK pathways.
LPS also decreased endogenous apoE expression in BV2 microglia and primary microglia. There are reports of LPS-induced down regulation of apoE gene and protein expression in macrophages (Dory 1993; Gafencu et al. 2007; Menju et al. 1989; Mouchel et al. 1995; Saura et al. 2003; Zuckerman and O'Neal 1994) or in mixed glial cultures and microglia (Saura et al. 2003). However, the mechanism by which LPS down regulates apoE expression in microglia has not been elucidated. Our work suggests that apoE regulation depends on JNK signaling. Treatment of microglia with EP prevented the LPS-induced decrease of apoE. Further, we found that inhibition of JNK by specific inhibitor (SP600125) prevented LPS-induced reduction of endogenous apoE. Treatment of BV2 cells with ERK inhibitor (PD98059) or PI3K/Akt inhibitor (Wortmannin) did not inhibit NO production. These findings support the idea that apoE protects against inflammatory signaling and NO production via inhibition of the JNK pathways.
LPS-induced JNK activation leads to c-Jun phosphorylation and activation of AP-1 transcription factors (Hidding et al. 2002). A recent study suggests LPS treatment induces both AP-1 and NFκB to bind the apoE promoter and interfere with promoter activity resulting in repression of apoE gene expression (Gafencu et al. 2007). However, in another study, LPS-induced down regulation of apoE was not attenuated with inhibitors of NFκB (Saura et al. 2003), suggesting that factors besides NFκB regulate apoE expression. Our findings that JNK inhibition is important to overcome LPS-induced down regulation of apoE support a role for JNK in regulating apoE expression, perhaps by regulating AP-1 activation.
We showed that EP significantly inhibited JNK in a time-dependent manner and attenuated the LPS-induced inflammatory response in microglia. The effects of EP were found to be mediated by members of the LDL receptor family. This family has previously been implicated in modulating the glial inflammatory response (LaDu et al. 2000; LaDu et al. 2001; Laskowitz et al. 2001), but this is the first study to define specific LDL receptor family signaling pathways in microglial cells. These receptors interact with various cytoplasmic adaptor proteins that mediate receptor signaling (Gotthardt et al. 2000; Trommsdorff et al. 1998). The cytoplasmic tails contain NPxY sequences, which are recognized by various adaptor proteins that mediate intracellular signaling cascades. Of particular relevance to this study, the cytoplasmic domain of LRP1 binds JNK-interacting proteins (JIP-1 and JIP-2) (Stockinger et al. 2000). JIPs have been identified as both inhibitors and activators of the JNK signal transduction pathway (Dickens et al. 1997; Mooney and Whitmarsh 2004; Whitmarsh et al. 2001; Willoughby et al. 2003). In neurons, we found that the decrease in JNK activation by apoE receptors depends on release of the cytoplasmic domain from the cell membrane by γ-secretase (Hoe et al. 2005). Therefore, we propose that a similar mechanism may be employed in microglia. Both LRP1 and VLDLR have been found in microglia (Christie et al. 1996; Moestrup et al. 1992; Rebeck et al. 1993; Swanson et al. 1988) and we have identified them in BV2 cells by Western blot analysis (data not shown). Because a low concentration of RAP was used for these studies, we speculate that either LRP1 or VLDLR mediated the signaling effects of EP rather than the LDLR receptor, which is inhibited only by a high concentration of RAP (LaDu et al. 2000). However, exactly which of these receptors affect which kinase pathways is currently unknown.
In this work, we demonstrated that the LDL receptor family has immunomodulatory signaling properties based on studies of EP overcoming the LPS-induced inflammatory response in BV2 cells and primary microglia. We found that EP decreased JNK activation and thereby suppressed the LPS-induced JNK activation. Inhibition of JNK proved to be essential to overcome LPS-induced increase in NO and decrease in apoE production. EP also induced an increase in ERK activation, but inhibition of ERK did not overcome the microglial inflammatory response. These studies provide new insight into the molecular mechanisms of LDL receptor family signaling in microglia and changes in apoE expression with microglial inflammation.
ACKNOWLEDGEMENTS
The authors wish to thank Dr. Dudley Strickland and Dr. Guojun Bu for generously providing RAP and antibodies to study LDL receptor expression. This work is supported by National Institute of Health (RO1-AG14473).
REFERENCES
- Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406(6797):782–7. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- Beffert U, Stolt PC, Herz J. Functions of lipoprotein receptors in neurons. J Lipid Res. 2004;45(3):403–9. doi: 10.1194/jlr.R300017-JLR200. [DOI] [PubMed] [Google Scholar]
- Bhat NR, Zhang P, Lee JC, Hogan EL. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18(5):1633–41. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27(2-3):229–37. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76(4):1501–13. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–7. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149(8):2736–41. [PubMed] [Google Scholar]
- Christie RH, Chung H, Rebeck GW, Strickland D, Hyman BT. Expression of the very low-density lipoprotein receptor (VLDL-r), an apolipoprotein-E receptor, in the central nervous system and in Alzheimer's disease. J Neuropathol Exp Neurol. 1996;55(4):491–8. doi: 10.1097/00005072-199604000-00012. [DOI] [PubMed] [Google Scholar]
- Corradin SB, Mauel J, Donini SD, Quattrocchi E, Ricciardi-Castagnoli P. Inducible nitric oxide synthase activity of cloned murine microglial cells. Glia. 1993;7(3):255–62. doi: 10.1002/glia.440070309. [DOI] [PubMed] [Google Scholar]
- Dickens M, Rogers JS, Cavanagh J, Raitano A, Xia Z, Halpern JR, Greenberg ME, Sawyers CL, Davis RJ. A cytoplasmic inhibitor of the JNK signal transduction pathway. Science. 1997;277(5326):693–6. doi: 10.1126/science.277.5326.693. [DOI] [PubMed] [Google Scholar]
- Dory L. Post-transcriptional regulation of apolipoprotein E expression in mouse macrophages by phorbol ester. Biochem J. 1993;292(Pt 1):105–11. doi: 10.1042/bj2920105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafencu AV, Robciuc MR, Fuior E, Zannis VI, Kardassis D, Simionescu M. Inflammatory signaling pathways regulating ApoE gene expression in macrophages. J Biol Chem. 2007;282(30):21776–85. doi: 10.1074/jbc.M611422200. [DOI] [PubMed] [Google Scholar]
- Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000;275(33):25616–24. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- Han IO, Kim KW, Ryu JH, Kim WK. p38 mitogen-activated protein kinase mediates lipopolysaccharide, not interferon-gamma, -induced inducible nitric oxide synthase expression in mouse BV2 microglial cells. Neurosci Lett. 2002;325(1):9–12. doi: 10.1016/s0304-3940(02)00218-5. [DOI] [PubMed] [Google Scholar]
- Hidding U, Mielke K, Waetzig V, Brecht S, Hanisch U, Behrens A, Wagner E, Herdegen T. The c-Jun N-terminal kinases in cerebral microglia: immunological functions in the brain. Biochem Pharmacol. 2002;64(5-6):781–8. doi: 10.1016/s0006-2952(02)01139-5. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Harris DC, Rebeck GW. Multiple pathways of apolipoprotein E signaling in primary neurons. J Neurochem. 2005;93(1):145–55. doi: 10.1111/j.1471-4159.2004.03007.x. [DOI] [PubMed] [Google Scholar]
- Hoe HS, Pocivavsek A, Dai H, Chakraborty G, Harris DC, Rebeck GW. Effects of apoE on neuronal signaling and APP processing in rodent brain. Brain Res. 2006;1112(1):70–9. doi: 10.1016/j.brainres.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Kim DH, Iijima H, Goto K, Sakai J, Ishii H, Kim HJ, Suzuki H, Kondo H, Saeki S, Yamamoto T. Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J Biol Chem. 1996;271(14):8373–80. doi: 10.1074/jbc.271.14.8373. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Shah JA, Reardon CA, Getz GS, Bu G, Hu J, Guo L, van Eldik LJ. Apolipoprotein E receptors mediate the effects of beta-amyloid on astrocyte cultures. J Biol Chem. 2000;275(43):33974–80. doi: 10.1074/jbc.M000602200. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Shah JA, Reardon CA, Getz GS, Bu G, Hu J, Guo L, Van Eldik LJ. Apolipoprotein E and apolipoprotein E receptors modulate A beta-induced glial neuroinflammatory responses. Neurochem Int. 2001;39(5-6):427–34. doi: 10.1016/s0197-0186(01)00050-x. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Goel S, Bennett ER, Matthew WD. Apolipoprotein E suppresses glial cell secretion of TNF alpha. J Neuroimmunol. 1997;76(1-2):70–4. doi: 10.1016/s0165-5728(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Lee DM, Schmechel D, Staats HF. Altered immune responses in apolipoprotein E-deficient mice. J Lipid Res. 2000;41(4):613–20. [PubMed] [Google Scholar]
- Laskowitz DT, Matthew WD, Bennett ER, Schmechel D, Herbstreith MH, Goel S, McMillian MK. Endogenous apolipoprotein E suppresses LPS-stimulated microglial nitric oxide production. Neuroreport. 1998;9(4):615–8. doi: 10.1097/00001756-199803090-00010. [DOI] [PubMed] [Google Scholar]
- Laskowitz DT, Thekdi AD, Thekdi SD, Han SK, Myers JK, Pizzo SV, Bennett ER. Downregulation of microglial activation by apolipoprotein E and apoE-mimetic peptides. Exp Neurol. 2001;167(1):74–85. doi: 10.1006/exnr.2001.7541. [DOI] [PubMed] [Google Scholar]
- Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton MJ, Oikawa M, Qureshi N, Monks B. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105(4):497–504. doi: 10.1172/JCI8541. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JR, Morgan D, Mance J, Matthew WD, Laskowitz DT. Apolipoprotein E modulates glial activation and the endogenous central nervous system inflammatory response. J Neuroimmunol. 2001;114(1-2):107–13. doi: 10.1016/s0165-5728(00)00459-8. [DOI] [PubMed] [Google Scholar]
- Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278(49):48529–33. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- Mace BE, Wang H, Lynch JR, Moss J, Sullivan P, Colton H, Morgan K, Renauld JC, Laskowitz DT. Apolipoprotein E modifies the CNS response to injury via a histamine-mediated pathway. Neurol Res. 2007;29(3):243–50. doi: 10.1179/016164107X158974. [DOI] [PubMed] [Google Scholar]
- Menju M, Tajima S, Yamamoto A. Expression of the apolipoprotein E gene in a human macrophage-like cell line, THP-1. J Biochem. 1989;106(3):505–10. doi: 10.1093/oxfordjournals.jbchem.a122882. [DOI] [PubMed] [Google Scholar]
- Moestrup SK, Gliemann J, Pallesen G. Distribution of the alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein in human tissues. Cell Tissue Res. 1992;269(3):375–82. doi: 10.1007/BF00353892. [DOI] [PubMed] [Google Scholar]
- Moon DO, Park SY, Lee KJ, Heo MS, Kim KC, Kim MO, Lee JD, Choi YH, Kim GY. Bee venom and melittin reduce proinflammatory mediators in lipopolysaccharide-stimulated BV2 microglia. Int Immunopharmacol. 2007;7(8):1092–101. doi: 10.1016/j.intimp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Mooney LM, Whitmarsh AJ. Docking interactions in the c-Jun N-terminal kinase pathway. J Biol Chem. 2004;279(12):11843–52. doi: 10.1074/jbc.M311841200. [DOI] [PubMed] [Google Scholar]
- Mouchel Y, Lefrancois T, Fages C, Tardy M. Apolipoprotein E gene expression in astrocytes: developmental pattern and regulation. Neuroreport. 1995;7(1):205–8. [PubMed] [Google Scholar]
- Murakami M, Ushio Y, Morino Y, Ohta T, Matsukado Y. Immunohistochemical localization of apolipoprotein E in human glial neoplasms. J Clin Invest. 1988;82(1):177–88. doi: 10.1172/JCI113568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophir G, Amariglio N, Jacob-Hirsch J, Elkon R, Rechavi G, Michaelson DM. Apolipoprotein E4 enhances brain inflammation by modulation of the NF-kappaB signaling cascade. Neurobiol Dis. 2005;20(3):709–18. doi: 10.1016/j.nbd.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Pawate S, Bhat NR. C-Jun N-terminal kinase (JNK) regulation of iNOS expression in glial cells: predominant role of JNK1 isoform. Antioxid Redox Signal. 2006;8(5-6):903–9. doi: 10.1089/ars.2006.8.903. [DOI] [PubMed] [Google Scholar]
- Poirier J, Hess M, May PC, Finch CE. Astrocytic apolipoprotein E mRNA and GFAP mRNA in hippocampus after entorhinal cortex lesioning. Brain Res Mol Brain Res. 1991;11(2):97–106. doi: 10.1016/0169-328x(91)90111-a. [DOI] [PubMed] [Google Scholar]
- Pyo H, Jou I, Jung S, Hong S, Joe EH. Mitogen-activated protein kinases activated by lipopolysaccharide and beta-amyloid in cultured rat microglia. Neuroreport. 1998;9(5):871–4. doi: 10.1097/00001756-199803300-00020. [DOI] [PubMed] [Google Scholar]
- Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron. 1993;11(4):575–80. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- Saha RN, Pahan K. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid Redox Signal. 2006;8(5-6):929–47. doi: 10.1089/ars.2006.8.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura J, Petegnief V, Wu X, Liang Y, Paul SM. Microglial apolipoprotein E and astroglial apolipoprotein J expression in vitro: opposite effects of lipopolysaccharide. J Neurochem. 2003;85(6):1455–67. doi: 10.1046/j.1471-4159.2003.01788.x. [DOI] [PubMed] [Google Scholar]
- Schumann RR, Pfeil D, Freyer D, Buerger W, Lamping N, Kirschning CJ, Goebel UB, Weber JR. Lipopolysaccharide and pneumococcal cell wall components activate the mitogen activated protein kinases (MAPK) erk-1, erk-2, and p38 in astrocytes. Glia. 1998;22(3):295–305. doi: 10.1002/(sici)1098-1136(199803)22:3<295::aid-glia8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Simmons ML, Murphy S. Induction of nitric oxide synthase in glial cells. J Neurochem. 1992;59(3):897–905. doi: 10.1111/j.1471-4159.1992.tb08328.x. [DOI] [PubMed] [Google Scholar]
- Stockinger W, Brandes C, Fasching D, Hermann M, Gotthardt M, Herz J, Schneider WJ, Nimpf J. The reelin receptor ApoER2 recruits JNK-interacting proteins-1 and -2. J Biol Chem. 2000;275(33):25625–32. doi: 10.1074/jbc.M004119200. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(5):1977–81. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson's disease. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Simmons DM, Hofmann SL, Goldstein JL, Brown MS. Localization of mRNA for low density lipoprotein receptor and a cholesterol synthetic enzyme in rabbit nervous system by in situ hybridization. Proc Natl Acad Sci U S A. 1988;85(24):9821–5. doi: 10.1073/pnas.85.24.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trommsdorff M, Borg JP, Margolis B, Herz J. Interaction of cytosolic adaptor proteins with neuronal apolipoprotein E receptors and the amyloid precursor protein. J Biol Chem. 1998;273(50):33556–60. doi: 10.1074/jbc.273.50.33556. [DOI] [PubMed] [Google Scholar]
- Vitek MP, Brown CM, Colton CA. APOE genotype-specific differences in the innate immune response. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgraber KH. Apolipoprotein E: structure-function relationships. Adv Protein Chem. 1994;45:249–302. doi: 10.1016/s0065-3233(08)60642-7. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Kuan CY, Kennedy NJ, Kelkar N, Haydar TF, Mordes JP, Appel M, Rossini AA, Jones SN, Flavell RA. Requirement of the JIP1 scaffold protein for stress-induced JNK activation. Genes Dev. 2001;15(18):2421–32. doi: 10.1101/gad.922801. and others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby EA, Perkins GR, Collins MK, Whitmarsh AJ. The JNK-interacting protein-1 scaffold protein targets MAPK phosphatase-7 to dephosphorylate JNK. J Biol Chem. 2003;278(12):10731–6. doi: 10.1074/jbc.M207324200. [DOI] [PubMed] [Google Scholar]
- Xie Z, Smith CJ, Van Eldik LJ. Activated glia induce neuron death via MAP kinase signaling pathways involving JNK and p38. Glia. 2004;45(2):170–9. doi: 10.1002/glia.10314. [DOI] [PubMed] [Google Scholar]
- Zuckerman SH, O'Neal L. Endotoxin and GM-CSF-mediated down-regulation of macrophage apo E secretion is inhibited by a TNF-specific monoclonal antibody. J Leukoc Biol. 1994;55(6):743–8. doi: 10.1002/jlb.55.6.743. [DOI] [PubMed] [Google Scholar]