Abstract
A recent study by our group demonstrates pharmacologically that the transient receptor potential vanilloid-1 (TRPV1) is activated by intradermal injection of capsaicin to initiate neurogenic inflammation by the release of neuropeptides in the periphery. In this study, expression of TRPV1, phosphorylated protein kinase C (p-PKC) and calcitonin gene-related peptide (CGRP) in dorsal root ganglion (DRG) neurons were visualized using immunofluorescence, real-time PCR and Western blots to examine whether increases in TRPV1 mRNA and protein levels evoked by capsaicin injection are subject to modulation by the activation of PKC and to analyze the role of this process in the pathogenesis of neurogenic inflammation. Capsaicin injection into the hindpaw skin of anesthetized rats evoked increases in the expression of TRPV1, CGRP and p-PKC in mRNA and/or protein levels and in the number of single labeled TRPV1, p-PKC and CGRP neurons in ipsilateral L4–5 DRGs. Co-expressions of TRPV1 with p-PKC and/or CGRP in DRG neurons were also significantly increased after CAP injection. These evoked expressions both at molecular and cellular levels were significantly inhibited after TRPV1 receptors were blocked by 5′-iodoresiniferatoxin (5 μg) or PKC was inhibited by chelerythrine chloride (5 μg). Taken together, these results provide evidence that up-regulation of TRPV1 mRNA and protein levels under inflammatory conditions evoked by capsaicin injection is subject to modulation by the PKC cascade in which increased CGRP level in DRG neurons may be related to the initiation of neurogenic inflammation. Thus, up-regulation of TRPV1 receptors in DRG neurons seems critical for initiating acute neurogenic inflammation.
Keywords: TRPV1, protein kinase C, primary afferent neuron, real-time PCR, phosphorylation
The transient receptor potential vanilloid-1 (TRPV1) is a non-selective ligand-gated cationic channel that mediates responses to a number of pain-inducing stimuli, including heat, protons and chemical irritants, such as capsaicin (CAP), a potent pain-causing principal of hot chili peppers (Caterina et al., 1997; Szallazi and Blumberg, 1999). Since TRPV1 is predominantly expressed in primary afferent nociceptive neurons and their terminals, activation of TRPV1 can selectively sensitize these neurons (Caterina et al., 1997; Ma, 2001; Carlton and Coggeshall, 2001; Carlton and Hargett, 2002). Thus, sensitization of primary afferent nociceptive terminals due to activation of TRPV1 contributes critically to primary (and may trigger secondary) hyperalgesia and allodynia (Simone et al., 1989; Baumann et al., 1991; Willis, 1992; Walker et al., 2003).
Since many primary afferent nociceptive neurons and their axons are peptidergic with the capability to release inflammatory peptides, such as calcitonin gene-related peptide (CGRP) and substance P (SP) (Gibbins et al., 1985; Alvarez et al., 1988; Kruger et al., 1989), sensitization of these neurons due to activation of TRPV1 should lead to the local release of these inflammatory mediators to initiate neurogenic inflammation and resulting pain (Szolcsanyi, 1993,1996; Lynn, 1990; Holzer, 1991; Kilo et al., 1997; Kessler et al., 1999). On the other hand, the released inflammatory mediators may activate several signaling pathways in the peripheral nervous system, leading to the modulation of TRPV1 (Cesare et al., 1999; Chuang et al., 2001; Linhart et al., 2003; Zhang et al., 2005). Numerous studies demonstrate that modulation of TRPV1 involves the triggering of signal transduction cascades (Khasar et al., 1999; Aley et al., 2001; Olah et al., 2002; Cortright and Szallasi, 2004; Jung et al., 2004). We propose that this should be a positive feedback mechanism that would help up-regulate the activity of TRPV1 either by increasing protein expression or sensitizing the receptors to integrate the pathophysiological process of neurogenic inflammation. However, how the triggering of signaling transduction pathways targets TRPV1, which causes an up-regulation of the receptors, still remains obscure. Also, there is a lack of evidence that this process is associated with neurogenic inflammation.
The present study investigates the effect of activated protein kinase C (PKC) on regulation of TRPV1 and analyzes a possible role of this process in initiation of neurogenic inflammation by examining the molecular and cellular changes that occur in the expression of TRPV1, phosphorylated PKC (p-PKC) and CGRP in dorsal root ganglion (DRG) neurons following CAP injection. Activation of PKC is one of the earliest events in a cascade that involves a variety of cellular sequential responses (Nishizuka, 1984; Keranen et al., 1995). p-PKC serves as a marker of its activation status (Ferri et al., 2006; Langham et al., 2008). PKCα and PKCε are two major PKC isozymes in the plasma membrane of DRG neurons and involved in modulation of TRPV1 (Cesare et al., 1999; Numazaki et al., 2002; Olah et al., 2002). Thus, changes in TRPV1 and CGRP mRNA due to CAP injection were quantified using real-time PCR, and changes in TRPV1and p-PKC proteins were analyzed using Western blots. Single, double and triple staining of expression of these three molecules and their co-expression in DRG nociceptive neurons were visualized before and after CAP injection using immunofluorescence to analyze the possible role of PKC in neurogenic inflammation. The analysis was also combined with pharmacological manipulations in which the effects of blockade of TRPV1 receptors or inhibition of PKC were tested, so that anatomical co-localization of TRPV1 and PKC would be linked functionally. Acute neurogenic inflammation was induced by intradermal injection of CAP. Preliminary data have been presented in abstract form (Zou et al., 2004a; Lin et al., 2006).
MATERIALS AND METHODS
Adult male Sprague-Dawley rats weighing 250–350 g were used. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and was consistent with the ethical guidelines of the National Institutes of Health and of the International Association for the Study of Pain. Efforts were made to minimize the number of animals used and their suffering.
Inflammatory pain model
Rats were anesthetized with sodium pentobarbital (50 mg kg−1; i.p.). 3% CAP (30 μl, prepared in a solution of 7% Tween 80, 20% ethanol and 73% saline) was injected intradermally into the glabrous skin of the hindpaw on one side (Zou et al., 2004b). The level of anesthesia was monitored by examination of pupillary reflexes and/or the eye-blink reflex to air-puffs to assure that the tissue sampling was done under anesthetized conditions. Extra anesthetic was given when necessary. For control purposes, vehicle (7% Tween 80, 20% ethanol and 73% saline) was injected intradermally.
Immunohistochemistry analysis
DRG tissue sampling, fixation and sectioning were performed after anesthetized rats were perfused using the procedure described previously (Zou et al., 2004b). DRG tissue at L4–5 on the side ipsilateral to the CAP injection was sampled at 30, 60 and 90 min after intradermal injection of CAP or vehicle, because the glabrous skin of the hindpaw is innervated mainly by these two segments (Takahashi et al., 1994). Triple labeling immunofluorescence staining for anti-TRPV1 (1:1,000, guinea-pig polyclonal, Chemicon Inc), anti-CGRP (1:1,000, mouse monoclonal, Chemicon Inc), and anti-p-PKCα or anti-p-PKCε (1:500, Ser 657 or Ser 729, rabbit polyclonal, Santa Cruz Biotechnology, Inc) was performed. Sections were incubated with a mixture of three primary antibodies for 24 h at 4°C. Then the sections were transferred to a secondary antibody solution containing Alexa Fluor® 405 goat anti-mouse IgG (1:200), Alexa Fluor® 488 goat anti-guinea-pig IgG (1:200) and Alexa Fluor® 568 goat anti-rabbit IgG (1:200) for 1 h respectively at room temperature. Controls included: 1) omission of primary antibody resulted in no detectable labeling; 2) incubation with a single primary antibody followed by the appropriate secondary antibody, to ensure that the labeling pattern for each substance in the triple-stained sections was in agreement with that observed in the single-labeled section; 3) incubation with a single primary antibody followed by a mixture of 2 or 3 secondary antibodies, in order to test the species specificity of the secondary antibodies used; 4) incubation with 3 primary antibodies followed by only one appropriate secondary antibody to check cross-reactivity between secondary antibodies and primary antibodies.
Labeled sections were examined and processed by confocal laser scanning microscopy (Nikon EZ-C1) for staining of TRPV1-, CGRP- and p-PKCα- or p-PKCε-immunofluorescence. Three fluorescence filters (Fluor 405 filter for blue, Fluor 488 for green and Fluor 568 for red) were used to separate individual wave lengths for staining. A sequential scanning method was used to avoid bleed-through. Thus, digitized images were obtained of three different colors for TRPV1, CGRP and p-PKCα (or p-PKCε), respectively. Analysis of co-staining was done by matching of corresponding regions. A neuronal profile was considered as positively double or triple labeled if it showed two or three color codes overlapping in space (see Figs. 1 and 4). For quantification, the average number of single-, double-, or triple-labeled neuronal profiles with different labeling per section from each DRG in 10 sections (at least 50 μm between consecutive sections) per animal was calculated and averaged.
Fig. 1.
Confocal immunofluorescence images showing single labeling of TRPV1, p-PKCα and CGRP, and double or triple labeling of these three molecules in L4 DRG on the side ipsilateral to CAP injection at 30 min after intradermal (i.d.) injection of vehicle (A–D) or CAP (E–H). Scale bar=100 μm.
Fig. 4.
Confocal immunofluorescence images showing single labeling of TRPV1, p-PKCα and CGRP, and double or triple labeling of these three molecules in L4 DRG on the side ipsilateral to CAP injection at 30 min after CAP injection in vehicle-, 5′-iodoresiniferatoxin- and chelerythrine chloride-pretreated rats. Scale bar=100 μm.
The following counts were made in DRG tissue: 1) the numbers of TRPV1, CGRP and p-PKC positive neuronal profiles; 2) the total number of TRPV1 positive profiles with p-PKC (α or ε subunits) staining (double staining); 3) total number of TRPV1 positive profiles with CGRP staining (double staining); 4) total number of TRPV1/p-PKC (α or ε subunits) positive profiles with CGRP staining (triple staining). The percentages of TRPV1 positive neurons doubly labeled with p-PKC or CGRP and TRPV1/p-PKC positive neurons triple labeled with CGRP were determined, respectively.
Quantification of mRNA for TRPV1 and CGRP from DRG
Total RNA extraction and cDNA synthesis
DRG tissue at L4–5 on the side ipsilateral to CAP injection was dissected at the same time points as done for Immunohistochemistry experiments. Tissue was also dissected in rats without any treatments to serve as baseline. Total RNA from DRG tissue was extracted using an RNAqueous® Kit provided by Ambion®, RNA Company. RNA extraction was quantified by measurement of its absorbance at 260 and 280 nm (Eppendorf Biophotometer). cDNAs of TRPV1 and CGRP were obtained by RT-PCR from total RNA. Reverse transcription of RNA was performed in a final volume of 20 μl containing 0.8 μl of deoxynucleotide triphosphate, 2 μl of 1×PCR buffer, 3.2 μl of DEPC treated DI water, 1 μl of RNase inhibitor, 2 μl of random hexamer, 1 μl of MuLV Reverse Transcriptase (High Capacity cDNA Reverse Transcription Kit, Applied Biosystems) and 10 μl (2 μg) of total RNA. The programmatic sequence was 25°C for 10 min and 37°C for 120 min and 85°C for 5 sec, cooling to 4°C (GeneAmp® PCR System 9700).
Real-time PCR assay
Real-time PCR was performed with Taqman reagents (TaqMan® Fast Universal PCR Master Mix, Applied Biosystems). All primers and probes with high efficiency were obtained from Applied Biosystems (Rn01460297 for rat TRPV1, Rn00569199 for rat CGRP, and 4352931E for rat ACTB). The Step One™ Real-time system (version 1.0) and the StepOne™ software were used for real-time PCR amplification. β-actin gene transcript was measured as an endogenous control by means of the comparative CT (ΔΔCT) method. Measurements of target genes were normalized by comparing them to the endogenous control. The software determined the relative quantity of target genes in each sample by comparing normalized target quantity in each sample to normalized target quantity in the reference sample. Relative quantification of gene expression was calculated according the method of 2 −ΔΔCT (RQ).
Western blot analysis
Western blotting was performed as previously reported (Zou et al., 2004b) to determine the relative expression levels of TRPV1 receptors, p-PKCα and p-PKCε in ipsilateral L4–5 DRG tissue after intradermal injection of CAP or vehicle. DRG tissue was sampled at the same time points as for Immunohistochemistry experiments. Each sample was collected from 3 rats and homogenized in ice-cold homogenization buffer containing phosphatase inhibitors. The concentration of protein in the homogenate was measured using a bicinchoninic acid (BCA) kit on a microplate reader. After assuring linearity of band density, equivalent amounts of protein (20 μg) for each sample were fractionated by 7.5% SDS-polyacrylamide gels (Fisher Scientific) and transferred onto a polyvinylidene difluoride (PVDF) membrane (Invitrogen). The PVDF membranes were blocked in 5% nonfat dry milk for 1 h in Tris-buffered saline containing Tween 20 and then incubated respectively with primary antibodies to TRPV1 (1:2,000), p-PKCα (1:200) or p-PKCε (1:200) overnight at 4°C, followed by incubation with horseradish peroxidase linked goat anti-rabbit IgG (1:2,000) for 2 h. The membranes were washed with buffer three times for 30 min and enhanced with a chemiluminescence Western blotting detection reagent. The blots were exposed to autoradiographic film and the intensity of immunoreactive bands of interest was quantified using densitometric-scanning analyses. β-Actin immunoreactivity was used as a loading control. A single band for TRPV1, p-PKCα or p-PKCε in Western blots was expressed relative to the values for β-actin.
Administration of TRPV1 antagonist and PKC inhibitor
Close-by intra-arterial injection (Ren et al., 2005) was used for local administration of 5′-iodoresiniferatoxin (I-RTX, from Tocris), a potent TRPV1 antagonist, in the hindpaw (Wahl et al., 2001). I-RTX was dissolved in a vehicle of ethanol/saline (1:10) and administered intra-arterially at the dose of 5 μg in a volume of 25 μl 10 min before CAP injection. In vitro study of isolated neurons has shown that the CAP effect was selectively antagonized by the similar dose of I-RTX (Marinelli et al., 2002). Chelerythrine chloride (C.C., from Tocris), a PKC inhibitor (Herbert et al., 1990), was dissolved in a vehicle of dimethyl sulfoxide (DMSO)/saline (1:20) and topically applied to the DRG. After laminectomy to expose L4–5 DRG (Lyu et al., 2001), A small piece of absorbable gelatin sponge (Gelfoam) that was filled with C.C. solution (5 μg in 5 μl saline) was put on the surface of each DRG for 20 min before CAP injection. The dose was chosen based on the results of our previous study in which the PKC-mediated phosphorylation of NMDA receptor 1 subunits in spinal dorsal horn neurons could be selectively inhibited dose-dependently (Zou et al., 2004b). For control purposes, vehicle used for making the drug solution was given using the same procedure as for the drug.
Statistical analysis
Five animals were included in each group for each type of experiment. All data were expressed as means±SEM, evaluated using SigmaStat and plotted with SigmaPlot. The numbers of immunoreactive positive neuronal profiles and percentages of double or triple staining were counted using Metamorph offline software. mRNA levels for TRPV1and CGRP were calculated using relative quantification (RQ). Densitometric analysis of TRPV1 receptors or p-PKCα or p-PKCε and β-actin of Western blot immunoreactivity results was conducted using Metamorph offline. β-actin immunoreactivity was used as a loading control and immunoreactivity of TRPV1 and p-PKC was normalized to β-actin. Statistical differences between groups of intradermal vehicle and CAP injections and groups of CAP injections at different time points were determined by one-way ANOVA followed by Dunnett’s post hoc test. The Student’s t test was used to determine statistical differences between groups having vehicle and drug pre-treatments. In all tests, p<0.05 was considered significant.
RESULTS
1. Immunofluorescence
Immunofluorescence staining of TRPV1, p-PKC and CGRP in DRG neurons in response to CAP injection
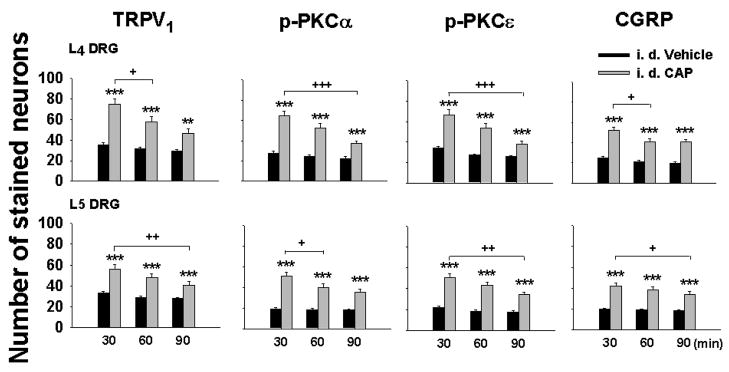
Changes in numbers of TRPV1, p-PKC (α and ε subunits) and CGRP immunofluorescence stained neuronal profiles in L4–5 DRGs due to unilateral intradermal injection of CAP were examined at 30, 60 and 90 min after CAP injection, respectively. The 1st to 3rd columns in Fig. 1 show examples of staining of these three molecules in L4 DRG neurons 30 min after intradermal injection of vehicle and CAP, respectively. Neurons stained for these three molecules were mostly of small size. There were substantial increases in the numbers of all three molecule-positive neurons seen at 30 min after CAP injection compared to that after intradermal vehicle injection.
Fig. 2 summarizes the changes observed in grouped data of the numbers of three kinds of molecule stained neurons in L4–5 DRG 30, 60 and 90 min after CAP injection. The largest increase in staining for these time points was seen at 30 min after CAP injection for all three molecules. For TRPV1 staining, the number of positive neurons in the CAP group at 30 min was 74.7±5.2 and 56.3±3.9, respectively, which were significantly more than those in 30 min vehicle groups (35.4±2.0 and 33.1±1.3, P<0.001 and P<0.001). For p-PKCα staining, the number of positive neurons in the CAP group at 30 min was 64.5±4.7 and 51.0±3.7, respectively, which were significantly more than those in 30 min vehicle groups (27.8±2.1 and 19.0±1.1, P<0.001 and P<0.001). Changes in p-PKCε after CAP injection were similar to those for p-PKCα described above. For CGRP staining, the number of positive neurons in the CAP group at 30 min were 51.9±3.7 and 42.0±3.7, respectively, which were significantly more than those in 30 min vehicle groups (24.9±1.2 and 20.2±0.9, P<0.001 and P<0.001). The increased staining for all three molecules was still statistically evident in the groups at 60 and 90 min after CAP injection, but showed gradual recovery.
Fig. 2.
Grouped data summarizing changes in the numbers of TRPV1, p-PKC (α and ε subunits), and CGRP positive neurons in L4–5 DRGs after CAP injection. The Y-axis shows the average number of stained neurons per section. DRG tissue was sampled at 30, 60 and 90 min after vehicle or CAP injection. Statistical analysis (**, P<0.01; ***, P<0.001) was made of changes in the numbers of these three molecules due to CAP injection vs. those due to vehicle injection at the same time point, and of the CAP-induced effects between the groups at different time points (+, P<0.05; ++, P<0.01; +++, P<0.001).
Percent TRPV1 immunoreactivity with p-PKC in DRG neurons in response to CAP Injection
Double labeling showed that p-PKC immunoreactivity was co-localized to a great extent with TRPV1 immunoreactivity in DRG neurons after CAP injection as compared with vehicle injection (Fig. 1D vs. H). Fig. 3A,B summarizes the percent TRPV1 positive neurons with p-PKCα (A) or p-PKCε (B) double staining at 30, 60 and 90 min after vehicle or CAP injection in L4–5 DRGs. The percentage of TRPV1 positive neurons with p-PKC labeling was most obvious at 30 min after CAP injection. An average of 67.4±1.9% (L4) and 60.6±2.6% (L5) of TRPV1 positive neurons were doubly labeled for p-PKCα, significantly higher than the percentages in vehicle groups at the same time points (P<0.001 and P<0.001), suggesting that CAP injection produced increased TRPV1 expression and more co-expression with p-PKC. At 60 min after CAP injection, 60.1±3.8% (L4) and 50.8±3.4% (L5) of TRPV1 positive neurons were doubly labeled for p-PKCα, still significantly higher than the percentages in vehicle groups at the same time points. A recovery of enhanced double labeling was seen at 90 min after CAP injection. Results of double staining for TRPV1 and p-PKCε were similar to those for TRPV1 and p-PKCα described above (Fig. 3B).
Fig. 3.
Grouped data summarizing changes in percent TRPV1 positive neurons with p-PKC double staining (A and B), percent TRPV1 positive neurons with CGRP double staining (C), and percent TRPV1-p-PKC positive neurons with CGRP triple staining (D and E) after CAP injection. Statistical analysis (*,P<0.05; **, P<0.01; ***, P<0.001) was made of percent changes due to CAP injection vs. those due to vehicle injection at the same time point, and of the CAP-induced effects between the groups at different time points (+, P<0.05; ++, P<0.01; +++, P<0.001).
Percent TRPV1 immunoreactivity with CGRP in DRG neurons in response to CAP injection
Co-localization of TRPV1 and CGRP in DRG neurons was also analyzed by counting the double labeling of these two molecules in the same neurons after intradermal injection of vehicle or CAP (Fig. 1D,H). Fig. 3C shows that 23.5–27.9% of TRPV1 positive neurons in L4–5 DRGs were stained for CGRP with vehicle injection. In CAP injection groups, the proportion of TRPV1 positive neurons with staining for CGRP was increased. This increase was not due to a decrease in the number of TRPV1 staining (see results of Fig. 1E). At 30 min after CAP, 59.4±2.5% (L4) and 42.6±2.2% (L5) of TRPV1-containing neurons were labeled for CGRP, which were significantly higher than percentages in vehicle groups at the same time points (P<0.001 and P<0.001). A recovery of double staining was seen at 60 and 90 min after CAP injection.
Percent TRPV1-p-PKC immunoreactivity with CGRP in DRG neurons in response to CAP injection
Triple immunostaining of TRPV1, p-PKC and CGRP shows that 20.0–23.7% of TRPV1/p-PKCα positive neurons were stained for CGRP after vehicle injection. CAP injection produced a significant increase in staining for CGRP in a proportion of TRPV1/p-PKCα-contaning neurons (Figs. 1H and 3D). This increase was not due to a decrease in the number of TRPV1 or p-PKCα staining neurons (see results of Fig. 1E,F). Percentage of TRPV1/p-PKCα positive neurons with CGRP labeling was most obvious at 30 min after CAP injection. 42.5±1.5% (L4), and 36.9±1.4% (L5) of TRPV1/p-PKCα-containing neurons were labeled for CGRP, which was significantly higher than percentages in vehicle groups at the same time points (P<0.001 and P<0.001). A recovery of enhanced triple staining was seen at 60 and 90 min after CAP injection (Fig. 3D). Results of triple staining for TRPV1, p-PKCε and CGRP (Fig. 3E) were similar to those for TRPV1, p-PKCα and CGRP described above.
The effects of TRPV1 blockade or PKC inhibition
Tests started with the effects of 5′-iodoresiniferatoxin (I-RTX), a TRPV1 antagonist, or chelerythrine chloride (C.C.), a PKC inhibitor, given in the absence of CAP injection on the expression of these molecules. Table 1 shows that I-RTX or C.C. given alone produced slight decreases without statistical significance in expression of these molecules compared with those in naïve rats. The effects of TRPV1 blockade or PKC inhibition on the CAP-evoked changes in numbers of stained neurons in L4–5 DRGs 30 min after CAP injection were then tested and summarized in Table 2 (see confocal fluorescence images also in Fig. 4). Control groups were set by vehicle pretreatments. In the group having I-RTX pretreatment (I-RTX+CAP), the CAP-evoked changes in numbers of TRPV1, p-PKC and CGRP stained neurons were all significantly reduced, and the reduction in TRPV1 expression was more obvious (Table 2). Accordingly, a dramatic reduction in double and triple staining was obtained mostly due to a greater reduction in TRPV1 staining. A similar effect on the CAP-evoked changes in staining was seen in another group with C.C. pretreatment (C.C.+CAP), but inhibition of PKC appeared to cause a greater reduction in the CAP-evoked p-PKC expression (Table 2). Accordingly, the reduction in double and triple labeling was more obvious.
TABLE 1.
Effects of administration of a TRPV1 antagonist or PKC inhibitor on the expressions of TRPV1, p-PKC and CGRP in DRG neurons
| Number of stained neuronsa | Naïve | TRPV1 antagonist
(I-RTX)b |
PKC inhibitor
(C.C)c |
|
|---|---|---|---|---|
| L4 DRG | TRPV1 | 33.4±5.7 | 31.1±5.2 | 32.9±5.5 |
| p-PKCα | 25.2±5.2 | 24.6±5.3 | 23.7±4.9 | |
| p-PKCε | 31.7±5.7 | 31.3±4.8 | 28.9±4.5 | |
| CGRP | 24.6±3.3 | 23.1±3.6 | 22.7±3.6 | |
|
| ||||
| L5 DRG | TRPV1 | 30.6±5.0 | 28.9±5.2 | 29.9±4.9 |
| p-PKCα | 18.4±2.8 | 18.0±2.8 | 16.6±3.0 | |
| p-PKCε | 20.7±3.6 | 20.7±3.6 | 18.1±3.5 | |
| CGRP | 18.5±2.8 | 18.1±2.8 | 17.9±2.7 | |
I-RTX: 5′-iodoresiniferatoxin; C.C.: chelerythrine chloride;
: Average number of single stained neurons;
: Effect of local application of a TRPV1 antagonist (I-RTX);
: Effect of local application of a PKC inhibitor (C.C.). The effect of drug was compared with the naïve group. After either I-RTX or C.C. was given alone, there were no statistically significant differences in any molecules between naïve and drug groups.
TABLE 2.
Effects of TRPV1 blockade or PKC inhibition on the CAP-evoked expressions of TRPV1, p-PKC, CGRP and their co-expressions in DRG neurons
| Number of stained neuronsa | TRPV1 antagonist (I-RTX)b |
PKC inhibitor (C.C.)c |
|||||
|---|---|---|---|---|---|---|---|
| Veh.+CAP | I-RTX+CAP | % decrease | Veh.+CAP | C.C.+CAP | % decrease | ||
| L4 DRG | TRPV1 | 72.3±4.5 | 49.7±3.1*** | 31.2±1.4 | 76.1±5.3 | 40.6±3.1*** | 48.2±1.6 |
| p-PKCα | 63.2±4.2 | 48.3±3.7* | 26.6±1.9 | 65.2±4.8 | 35.0±2.6*** | 46.4±1.2 | |
| p-PKCε | 65.7±5.0 | 47.7±3.6* | 25.7±1.9 | 67.4±5.4 | 33.4±2.6*** | 48.6±1.0 | |
| CGRP | 49.9±3.5 | 33.4±2.2*** | 30.9±1.6 | 52.4±3.7 | 38.2±2.7*** | 26.9±0.3 | |
| TRPV1-p-PKCα | 46.8±3.6 | 18.7±1.8*** | 61.8±1.0 | 49.8±3.8 | 15.0±1.1*** | 70.1±2.2 | |
| TRPV1-p-PKCε | 44.0±3.4 | 20.0±1.8*** | 56.8±1.8 | 44.9±3.4 | 16.0±1.2*** | 64.3±1.8 | |
| TRPV1-p-PKCα-CGRP | 30.3±2.3 | 16.6±1.6*** | 61.5±1.9 | 31.5±2.4 | 11.7±0.8*** | 62.0±1.7 | |
| TRPV1-p-PKCε-CGRP | 26.7±2.1 | 15.6±1.6*** | 46.8±3.0 | 27.4±2.1 | 11.9±0.9*** | 57.3±1.6 | |
|
| |||||||
| L5 DRG | TRPV1 | 58.1±3.7 | 41.8±2.9*** | 28.9±1.4 | 57.0±3.9 | 32.6±2.5*** | 44.3±1.6 |
| p-PKCα | 50.0±3.6 | 40.1±3.3* | 24.6±2.0 | 51.6±3.8 | 29.8±2.2*** | 42.1±1.1 | |
| p-PKCε | 52.1±3.9 | 39.6±3.1* | 23.4±1.3 | 49.8±3.9 | 31.0±2.3*** | 40.6±1.4 | |
| CGRP | 42.9±2.9 | 31.6±2.1** | 26.1±1.4 | 42.4±2.9 | 29.4±2.0*** | 30.5±1.0 | |
| TRPV1-p-PKCα | 31.9±2.5 | 14.9±1.4*** | 55.6±1.6 | 32.9±2.6 | 10.3±0.8*** | 68.5±2.0 | |
| TRPV1-p-PKCε | 28.4±2.2 | 15.6±1.4*** | 47.8±2.4 | 28.4±2.2 | 11.0±0.8*** | 61.2±1.4 | |
| TRPV1-p-PKCα-CGRP | 22.5±2.1 | 13.5±1.3** | 42.7±2.4 | 21.9±2.0 | 7.2±0.7*** | 65.5±1.1 | |
| TRPV1-p-PKCε-CGRP | 20.2±1.8 | 12.4±1.3*** | 40.0±2.5 | 19.9±1.8 | 7.6±0.7*** | 62.6±1.5 | |
I-RTX: 5′-iodoresiniferatoxin; C.C.: chelerythrine chloride;
: Average number of single, double or triple stained neurons;
: Effect of TRPV1 antagonist (I-RTX) pretreatment;
, Effect of PKC inhibitor (C.C.) pretreatment; Veh.+CAP: vehicle pretreatment with CAP injection; I-RTX+CAP: 5′-iodoresiniferatoxin pretreatment with CAP injection; C.C.+CAP: chelerythrine chloride pretreatment with CAP injection. % decrease: percent decrease compared with the vehicle pretreatment group.
P<0.05,
P<0.01 and
P<0.001, compared with the vehicle pretreatment groups.
2. Expressions of TRPV1 and CGRP mRNA in DRG
CAP evoked expressions of TRPV1 and CGRP mRNA
The effects of CAP injection on TRPV1 and CGRP levels in DRG were also assessed by measurements of their mRNA expressions using real-time PCR. As shown in Fig. 5A,B, CAP injection evoked significant increases both in TRPV1 and CGRP mRNA expressions as compared to the vehicle injection groups. The mRNA levels peaked at 30 min, and then declined gradually toward the baseline level. No statistically significant changes were observed among the vehicle injection and baseline groups.
Fig. 5.
CAP-evoked expressions of TRPV1 and CGRP mRNA (A and B) and the effects of a TPRV1 antagonist and a PKC inhibitor (C and D). Relative quantification (RQ) of TRPV1 and CGRP mRNA level was done by TaqMan® Fast Universal PCR Master Mix and calculated by the ΔΔCT method after normalization to internal controls. RQ=2 −ΔΔCT. A and B: Changes in TRPV1 and CGRP mRNA levels at 30, 60 and 90 min after CAP injection were analyzed by comparing with those in the vehicle injection group at the same time point (*, ** and ***: P<0.05, P<0.01 and P<0.001). C and D: The effects of 5′-iodoresiniferatoxin (I-RTX) or chelerythrine chloride (C.C) pretreatment were analyzed by comparisons of the evoked expressions of TRPV1 and CGRP at 30 min after CAP injection between groups having been pretreated with vehicle and 5′-iodoresiniferatoxin or chelerythrine chloride. * and **: P<0.05 and P<0.01, compared with vehicle pretreated group.
The effects of TRPV1 blockade or PKC inhibition
Since the enhanced expressions of TRPV1 and CGRP mRNA were seen to be maximal at 30 min after CAP injection, changes in mRNA when TRPV1 receptors were blocked or PKC was inhibited were examined in groups of animals at 30 min after CAP injection. I-RTX pretreatment significantly inhibited the CAP-evoked enhancement of expressions of TRPV1 and CGRP mRNA (Fig. 5C, vehicle+CAP, 2.07±0.16 vs. I-RTX+CAP, 1.27±0.04 for TRPV1 expression; vehicle+CAP, 1.90±0.18 vs. I-RTX+CAP, 1.26±0.05 for CGRP expression). When PKC was inhibited by pretreatment with C.C., the CAP-evoked enhancement of expressions of TRPV1 and CGRP mRNA was also significantly inhibited (Fig. 5D, vehicle+CAP, 1.97±0.14 vs. C.C.+CAP, 1.42±0.03 for TRPV1 expression; vehicle+CAP, 1.95±0.20 vs. C.C.+CAP 1.27±0.04 for CGRP expression).
3. Western blot
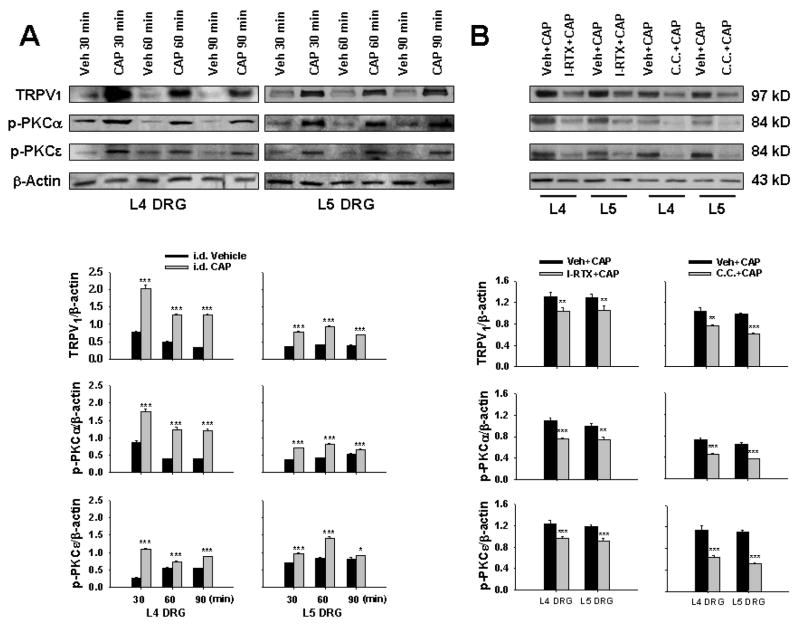
Figure 6A shows the relative density of immunoblots of TRPV1, p-PKCα and p-PKCε proteins from L4–5 DRG tissue after vehicle or CAP injection. The relative density of all three molecules was significantly increased at 30 min after CAP injection as compared with those with vehicle injection. The enhanced expressions remained at a high level at 30 to 60 min and lasted over 90 min after CAP injection.
Fig. 6.
Western blot analysis of the relative density of TRPV1, p-PKCα and p-PKCε in L4–5 DRG tissue evoked by CAP injection. A: Changes the relative density of TRPV1, p-PKCα and p-PKCε in DRG tissue ipsilateral to CAP injection at 30 (CAP 30 min), 60 (CAP 60 min) and 90 min (CAP 90 min) after unilateral intradermal injection of CAP. Immunoreactivity of TRPV1, p-PKCα and p-PKCε was normalized to β-actin. * and ***: P<0.05 and P<0.001, compared with intradermal vehicle injection (Veh 30min, Veh 60 min and Veh 90 min). B: Effects of TPRV1 antagonist and PKC inhibitor. Tests were performed in the groups at 30 min after CAP injection with vehicle (Veh+CAP) or drug (I-RTX+CAP or C.C.+CAP) pretreatment. *, ** and ***: P<0.05, P<0.01 and P<0.001, compared with vehicle pretreatment (Veh+CAP).
Changes in the CAP-evoked immunoblots of TRPV1 and p-PKC when TRPV1 antagonist or PKC inhibitor was given were examined in the groups of 30 min after CAP injection. In Fig. 6B, the relative density of immunoblots of TRPV1 and p-PKC after CAP injection with I-RTX or C.C. pretreatment (I-RTX+CAP or C.C.+CAP) was compared with those after CAP injection with vehicle pretreatment (Veh.+CAP). Results show that either I-RTX or chelerythrine chloride pretreatment inhibited significantly the CAP-evoked expressions of TRPV1 and p-PKC immunoblots in L4 and L5 DRGs (Fig. 6B).
DISCUSSION
Using rats receiving an intradermal injection of CAP, our and other groups have provided evidence that CAP injection to activate TRPV1 receptors plays a critical role in sensitization of primary afferent nociceptors and in induction of acute neurogenic inflammation (Lin et al., 1999; Garcia-Nicas et al., 2001; Ren et al., 2005; Valencia De Ita et al., 2006). During these pathological processes, the TRPV1 seems to be a key target subject to modulation. In the present study, we used p-PKC as a marker of enzyme activation (Ferri et al., 2006; Langham et al., 2008) and CGRP as an indicator related to neurogenic inflammation (Galeazza et al., 1995; Traub et al., 1999, Ambalavanar et al., 2006a) in the DRG neurons in vivo to analyze the role of PKC activation in up-regulation of TRPV1 both at cellular and molecular levels, which would participate critically in neurogenic inflammation in the same model. We found that 1) an enhanced expression of TRPV1, CGRP and p-PKC in mRNA and/or protein levels and increased number of single labeled neurons in L4-5 DRG were evoked by CAP injection; 2) Co-expression of TRPV1 with p-PKC and/or CGRP (double and triple labeling) in DRG neurons was also increased after CAP injection. Observations made at 30 to 90 min after CAP injection show that evoked increases in mRNA and/or protein were most obvious at 30 min after injection. So far there is little evidence that acute changes in mRNA and protein levels of TRPV1 or CGRP were evoked by noxious stimulation as early as 30 min, even though there is a report that CGRP mRNA level peaked at 1 h after adjuvant injection (Bulling et al., 2001). This rapid up-regulation after noxious stimulation was proposed to be afferent activity dependent but remains to be further investigated (Bulling et al., 2001; Puehler et al., 2004). In addition, we assumed that a detectable evoked change in mRNA should be seen earlier than change in protein expression if more time points had been sampled. However, we propose that there might be an overlap of peaks of mRNA and protein in the acute stage of inflammation since our previous report showed that the enhanced expression of receptor protein reached its peak as early as 5 min after CAP injection (Fang et al., 2003). Finally, a TRPV1 antagonist or PKC inhibitor given alone did not alter baseline expression of these molecules. However, the CAP evoked expressions were significantly inhibited by either blockade of TRPV1 receptors or inhibition of PKC.
TRPV1 is one of the key nociceptive molecules and well known to play a critical role in pain sensation by activating or sensitizing primary afferent nociceptors because it is highly expressed in small diameter nociceptive primary neurons and their afferent terminals (Tominaga et al., 1998; Davis et al., 2000; Caterina and Julius, 2001). The expression of TRPV1 at cellular and molecular levels has been previously reported in DRG neurons (Price, 1985; Guo et al., 1999; Michael and Priestley, 1999; Carlton and Hargett, 2002; Carlton et al., 2004). The data in the present study show that the neurons labeled for TRPV1 were mostly of small size, in general agreement with previous reports. CAP application in cultured DRG neurons evokes a membrane current gated by cation-permeable ion channels that can be blocked by a TRPV1 antagonist (Oh et al., 1996; Jung et al., 1999; Xu et al., 2007). In the present study, CAP, a potent TRPV1 ligand, was used to evoke the expression of TRPV1 cellularly and molecularly, and this evoked expression was then further verified pharmacologically by a TRPV1 antagonist. Quantitative analysis of immunofluorescence, real-time PCR and Western blots indicate that intradermal CAP evoked a remarkable increase in the number of TRPV1 labeled neurons and expression of TRPV1 mRNA and protein in DRG, and this evoked response could be inhibited by a TRPV1 antagonist. TRPV1 expression after peripheral inflammation increases both in small and medium-sized DRG neurons (Amaya et al., 2003), and this increased expression enhances the transport of this receptor, which subsequently increases TRPV1 density in the nerve terminals in inflamed tissue (Carlton and Coggeshall, 2001; Yiangou et al., 2001). Thus, this ligand-receptor response shown accounts for one of the major mechanisms by which primary afferent nociceptors are sensitized due to CAP injection (Ren et al., 2005).
It is well established that activation of TRPV1 also has an efferent function by initiating neurogenic inflammation. Anatomically, TRPV1 frequently co-localizes with neuropeptides, such as CGRP and SP, in primary afferent neurons (Guo et al., 1999; Aoki et al., 2005; Price and Flores, 2007). This has been confirmed by our present study. Functionally, the opening of TRPV1 ion channels is likely to trigger the Ca2+ dependent release of tachykinins and CGRP from the terminals of peptidergic nociceptors (Holzer, 1988; Szolcsanyi, 1996; Kessler et al., 1999). Using immunofluorescent co-staining, we wanted to determine further whether activation of TRPV1 by the ligand (CAP) is closely associated with a change in neuropeptide content in the nociceptive neurons that would indicate a neurogenic mechanism by which inflammation is induced. To do this, the content of CGRP in DRG cell bodies was evaluated using fluorescence staining and double labeling for TRPV1. Following CAP injection, significant increases both in the number of CGRP staining and the proportion of TRPV1-positive neurons with CGRP staining were observed and this increase lasted over 60 min after CAP injection. This change in peptide content of DRG neurons was further supported by real-time PCR data showing that an up-regulation of CGRP mRNA was evident and by a pharmacological experiment demonstrating that blockade of TRPV1 inhibited significantly the CAP-evoked increases in the CGRP expression (mRNA and positive neurons) and the proportion of TRPV1-positive neurons with CGRP staining, indicating that an increased production of CGRP in neurons is triggered due to activation of TRPV1 receptors. In the same model of acute neurogenic inflammation as used in the present study, we have recently demonstrated pharmacologically that vasodilation and edema of the hindpaw skin induced by CAP injection involve the release of CGRP and SP from primary afferent terminals (Lin et al. 2007). Therefore, we presume that acute neurogenic inflammation evoked by CAP injection should include a pathophysiological process in which primary afferent nociceptive neurons are sensitized by activation of TRPV1 receptors, which would cause an enhanced production of neuropeptides likely to be released in the periphery. The correlation between the changes in CGRP content in the DRG neurons and peripheral inflammation has been also seen in other pain models (Galeazza et al. 1995; Traub et al., 1999; Ambalavanar et al. 2006a,b). Combined with our recent study (Lin et al., 2007), we suggest that acute neurogenic inflammation induced by CAPinjection is associated with increases in CGRP mRNA expression and content in DRG neurons.
PKC in primary sensory neurons plays a prominent role in the modulation of hypersensitivity to thermal, mechanical and inflammatory stimuli after tissue injury in which TRPV1 is thought to be a substrate of PKC. Electrophysiological studies in cultured DRG neurons have shown that PKC potentiates heat, proton, CAP and pro-inflammatory substance responses (Cesare et al., 1999; Premkumar and Ahern, 2000; Vellani et al., 2001; Zhou et al., 2001). PKCα and PKCε are two major isozymes in the plasma membrane of DRG neurons and regulate pain sensation by modulating TRPV1(Cesare et al., 1999; Numazaki et al., 2002; Olah et al., 2002). Inhibition of PKCε reduces inflammatory mediator-induced hyperalgesia, and diminishes PKC-mediated enhancement of heat currents in sensory neurons (Cesare et al., 1999; Khasar et al., 1999; Aley et al., 2000). PKCα expression is suggested to correlate with the ability of phorbol esters to activate TRPV1 directly (Olah et al., 2002). In these processes, protein phosphorylation is suggested to be a major way by which TRPV1 is up-regulated (Ahern and Premkumar, 2002; Numazaki et al., 2002; Bhave et al., 2003). On the other hand, CAP or released inflammatory mediators can activate TRPV1, causing Ca2+ influx and triggering of the PKC cascade (Harvey et al 1995; Zhang et al., 2005). In the present study, a co-expression of TRPV1, CGRP and p-PKC in DRG neurons was immunofluorescently visualized to indicate a modulatory effect of PKC on TRPV1 mediating neurogenic inflammation. Data show that CAP injection resulted in an enhanced expression of both p-PKCα and p-PKCε and an increase in number of TRPV1 positive neurons that co-expressed p-PKC. This is consistent with observations on other pain models (Zhou et al., 2003). Triple staining reveals that the numbers of TRPV1-p-PKC positive neurons with CGRP labeling were accordingly increased. The data from Western blot analysis of p-PKC have further supported the results of immunofluorescence studies. Furthermore, a pharmacological experiment by topical administration of a PKC inhibitor on DRG has shown that inhibition of PKC not only inhibited the enhanced expressions of p-PKC but also reduced TRPV1 and CGRP as well as their co-expressions evoked by CAP injection. Thus, it is anatomically and functionally suggested that activation of TRPV1 by CAP involves a triggering of the PKC cascade in DRG neurons, which would up-regulate TRPV1 receptors either by increasingprotein expression or phosphorylation.
In summary, up-regulation of TRPV1 receptors in DRG neurons is modulated by activation of PKC under inflammatory conditions induced by CAP injection. In this process, increase in CGRP level in DRG neurons is related to the initiation of neurogenic inflammation.
Acknowledgments
We are very grateful to Dr. W.D. Willis for his valuable suggestions and criticism of this study. This work was supported by NIH/NINDS R01 Grant NS40723 to Q. Lin, NIH/NINDS P01 Grant NS011255 and NIH/NIDCR R03 Grant DE14814 to L. Fang.
References
- Ahern GP, Premkumar LS. Voltage-dependent priming of rat vanilloid receptor: effects of agonist and protein kinase C activation. J Physiol. 2002;545:441–451. doi: 10.1113/jphysiol.2002.029561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Messing RO, Mochly-Rosen D, Levine JD. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO. Nociceptor sensitization by extracellular signal-regulated kinase. J Neurosci. 2001;21:6933–6939. doi: 10.1523/JNEUROSCI.21-17-06933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez FJ, Cervantes C, Blasco I, Villalba R, Martinez-Murillo R, Polak JM, Rodrigo J. Presence of calcitonin gene-related peptide (CGRP) and substance P (SP) immunoreactivity in intraepidermal free nerve endings of cat skin. Brain Res. 1988;442:391–395. doi: 10.1016/0006-8993(88)91532-6. [DOI] [PubMed] [Google Scholar]
- Amaya F, Oh-hashi K, Naruse Y, Iijima N, Ueda M, Shimosato G, Tominaga M, Tanaka Y, Tanaka M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroup of DRG neurons. Brain Res. 2003;963:190–196. doi: 10.1016/s0006-8993(02)03972-0. [DOI] [PubMed] [Google Scholar]
- Ambalavanar R, Moritani M, Moutanni A, Gangula P, Yallampalli C, Dessem D. Deep tissue inflammation upregulates neuropeptides and evokes nociceptive behaviors which are modulated by a neuropeptide antagonist. Pain. 2006a;120:53–68. doi: 10.1016/j.pain.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Ambalavanar R, Dessem D, Moutanni A, Yallampalli C, Yallampalli U, Gangula P, Bai G. Muscle inflammation induces a rapid increase in calcitonin gene-related peptide (CGRP) mRNA that temporally relates to CGRP immunoreactivity and nociceptive behavior. Neuroscience. 2006b;143:875–884. doi: 10.1016/j.neuroscience.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Aoki Y, Ohtori S, Takahashi K, Ino H, Douya H, Ozawa T, Saito T, Moriya H. Expression and Co-expression of VR1, CGRP, and IB-4-binding glycoprotein in dorsal root ganglion neurons in rats: differences between the disc afferents and the cutaneous afferents. Spine. 2005;30:1496–1500. doi: 10.1097/01.brs.0000167532.96540.31. [DOI] [PubMed] [Google Scholar]
- Baumann TK, Simone DA, Shain CN, LaMotte RH. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulling DGS, Kelly D, Bond S, McQueen DS, Seckl JR. Adjuvant-induced joint inflammation causes very rapid transcription of β-preprotachykinin and a-CGRP genes in innervating sensory ganglia. J Neurochem. 2001;77:373–382. doi: 10.1046/j.1471-4159.2001.00175.x. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Coggeshall RE. Peripheral capsaicin receptors increase in the inflamed rat hindpaw: a possible mechanism for peripheral sensitization. Neurosci Lett. 2001;310:53–56. doi: 10.1016/s0304-3940(01)02093-6. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL. Stereological analysis of Ca2+/calmodulin-dependent protein kinase IIα-containing dorsal root ganglion neurons in the rat: colocalization with isolectin Griffonia simplicifolia, calcitonin gene-related peptide, or vanilloid receptor 1. J Comp Neurol. 2002;448:102–110. doi: 10.1002/cne.10250. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Zhou S, Du J, Hargett GL, Ji G, Coggeshall RE. Somatostatin modulates the transient receptor potential vanilloid 1 (TRPV1) ion channel. Pain. 2004;110:616–627. doi: 10.1016/j.pain.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKCε in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- Chuang H, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Cortright DN, Szallasi A. Biochemical pharmacology of the vanilloid receptor TRPV1. Eur J Biochem. 2004;271:1814–1819. doi: 10.1111/j.1432-1033.2004.04082.x. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Zhang X, Lin Q, Willis WD. Increased phosphorylation of the GluR1 subunit of spinal cord α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor in rats following intradermal injection of capsaicin. Neuroscience. 2003;122:237–245. doi: 10.1016/s0306-4522(03)00526-8. [DOI] [PubMed] [Google Scholar]
- Ferri P, Cecchini T, Ambrogini P, Betti M, Cuppini R, Del Grande P, Ciaroni S. a-Tocopherol affects neuronal plasticity in adult rat dentate gyrus: the possible role of PKCδ. J Neurobiol. 2006;66:793–810. doi: 10.1002/neu.20255. [DOI] [PubMed] [Google Scholar]
- Garcia-Nicas E, Laird JM, Cervero F. Vasodilatation in hyperalgesic rat skin evoked by stimulation of afferent Aβ-fibers: further evidence for a role of dorsal root reflexes in allodynia. Pain. 2001;94:283–291. doi: 10.1016/S0304-3959(01)00365-7. [DOI] [PubMed] [Google Scholar]
- Galeazza MT, Garry MG, Yost HJ, Strait KA, Hargreaves KM, Seybold VS. Plasticity in the synthesis and storage of substance P and calcitonin gene-related peptide in primary afferent neurons during peripheral inflammation. Neuroscience. 1995;66:443–458. doi: 10.1016/0306-4522(94)00545-g. [DOI] [PubMed] [Google Scholar]
- Gibbins IL, Furness JB, Costa M, MacIntyre I, Hillyard CJ, Girgis S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pig. Neurosci Lett. 1985;57:125–130. doi: 10.1016/0304-3940(85)90050-3. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Harvey JS, Davis C, James IF, Burgess GM. Activation of protein kinase C by the capsaicin analogue resiniferatoxin in sensory neurons. J Neurochem. 1995;65:1309–1317. doi: 10.1046/j.1471-4159.1995.65031309.x. [DOI] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- Jung J, Hwang SW, Kwak J, Lee SY, Kang CJ, Kim WB, Kim D, Oh U. Capsaicin binds to the intracellular domain of the capsaicin-activated ion channel. J Neurosci. 1999;19:529–538. doi: 10.1523/JNEUROSCI.19-02-00529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- Keranen LM, Dutil EM, Newton AC. Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr Biol. 1995;5:1394–1403. doi: 10.1016/s0960-9822(95)00277-6. [DOI] [PubMed] [Google Scholar]
- Kessler F, Habelt C, Averbeck B, Reeh PW, Kress M. Heat-induced release of CGRP from isolated rat skin and effects of bradykinin and the protein kinase C activator PMA. Pain. 1999;83:289–295. doi: 10.1016/s0304-3959(99)00108-6. [DOI] [PubMed] [Google Scholar]
- Khasar SG, Lin YH, Martin A, Dadgar J, McMahon T, Wang D, Hundle B, Aley KO, Isenberg W, McCarter G, Green PG, Hodge CW, Levine JD, Messing RO. A novel nociceptors signaling pathway revealed in protein kinase Cε zmutant mice. Neuron. 1999;24:253–260. doi: 10.1016/s0896-6273(00)80837-5. [DOI] [PubMed] [Google Scholar]
- Kilo S, Harding-Rose C, Hargreaves KM, Flores CM. Peripheral CGRP release as a marker for neurogenic inflammation: a model system for the study of neuropeptide secretion in rat paw skin. Pain. 1997;73:201–207. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- Kruger L, Silverman JD, Mantyh PW, Sternini C, Brecha NC. Peripheral patterns of calcitonin-gene-related peptide general somatic sensory innervation: cutaneous and deep terminations. J Comp Neurol. 1989;280:291–302. doi: 10.1002/cne.902800210. [DOI] [PubMed] [Google Scholar]
- Langham RG, Kelly DJ, Gow RM, Zhang Y, Cox AJ, Qi W, Thai K, Pollock CA, Christensen PK, Parving HH, Gilbert RE. Increased renal gene transcription of protein kinase C-β in human diabetic nephropathy: relationship to long-term glycaemic control. Diabetologia. 2008;51:668–674. doi: 10.1007/s00125-008-0927-x. [DOI] [PubMed] [Google Scholar]
- Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation following intradermal injection of capsaicin in rats. J Neurophysiol. 1999;82:2602–2611. doi: 10.1152/jn.1999.82.5.2602. [DOI] [PubMed] [Google Scholar]
- Lin Q, Zou X, Fang L. Sympathetic effect on neurogenic inflammation by modulation of TRPV1 receptors via the protein kinase C cascade. Society for Neurosci Program. 2006 No. 51.8/L14 (Abstract) [Google Scholar]
- Lin Q, Li D, Xu X, Zou X, Fang L. Roles of TRPV1 and neuropeptidergic receptors in dorsal root reflex-mediated neurogenic inflammation induced by intradermal injection of capsaicin. Mol Pain. 2007;3:30. doi: 10.1186/1744-8069-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhart O, Obreja O, Kress M. The inflammatory mediators serotonin, prostaglandin E2 and bradykinin evoke calcium influx in rat sensory neurons. Neuroscience. 2003;118:69–74. doi: 10.1016/s0306-4522(02)00960-0. [DOI] [PubMed] [Google Scholar]
- Lynn B. Capsaicin: actions on nociceptive C-fibres and therapeutic potential. Pain. 1990;41:61–69. doi: 10.1016/0304-3959(90)91110-5. [DOI] [PubMed] [Google Scholar]
- Lyu YS, Park SK, Chung K, Chung JM. Low dose of tetrodotoxinreduces neuropathic pain behaviors in an animal model. Brain Res. 2000;871:98–103. doi: 10.1016/s0006-8993(00)02451-3. [DOI] [PubMed] [Google Scholar]
- Ma QP. Vanilloid receptor homologue, VR1, is expressed by both A- and C-fiber sensory neurons. NeuroReport. 2001;12:3693–3695. doi: 10.1097/00001756-200112040-00018. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Vaughan CW, Christie MJ, Connor M. Capsaicin activation of glutamatergic synaptic transmission in the rat locus coeruleus in vitro. J Physiol. 2002;543:531–540. doi: 10.1113/jphysiol.2002.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984;308:693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cε and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- Oh U, Hwang SW, Kim D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J Neurosci. 1996;16:1659–1667. doi: 10.1523/JNEUROSCI.16-05-01659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah Z, Karai L, Iadarola J. Protein kinase Cα zis required for vanilloid receptor 1 activation-Evidence for multiple signaling pathways. J Biol Chem. 2002;277:35752–35759. doi: 10.1074/jbc.M201551200. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Price J. An immunohistochemical and quantitative examination of dorsal root ganglion neuronal subpopulations. J Neurosci. 1985;5:2051–2059. doi: 10.1523/JNEUROSCI.05-08-02051.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price T, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8:263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puehler W, Zollner C, Brack A, Shaqura MA, Krause H, Schafer M, Stein C. Rapid upregulation of μ opioid receptor mRNA in dorsal root ganglia in reponse to peripheral inflammation depends on neuronal conduction. Neuroscience. 2004;129:473–479. doi: 10.1016/j.neuroscience.2004.06.086. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zou X, Fang L, Lin Q. Sympathetic modulation of activity in Aδ- and C-primary nociceptive afferents after intradermal injection of capsaicin in rats. J Neurophysiol. 2005;93:365–377. doi: 10.1152/jn.00804.2004. [DOI] [PubMed] [Google Scholar]
- Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- Szolcsanyi J. Actions of capsaicin on sensory receptors. In: Wood JN, editor. Capsaicin in the Study of Pain. London: Academic Press; 1993. pp. 1–26. [Google Scholar]
- Szolcsanyi J. Neurogenic inflammation: reevaluation of axon reflex theory. In: Geppetti P, Holzer P, editors. Neurogenic inflammation. New York: CRC Press; 1996. pp. 33–42. [Google Scholar]
- Takahashi Y, Nakajima Y, Sakamoto T. Dermatome mapping in the rat hindlimb by electrical stimulation of the spinal nerves. Neurosci Lett. 1994;168:85–88. doi: 10.1016/0304-3940(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Hutchcroft K, Gebhart GF. The peptide content of colonic afferents decreases following colonic inflammation. Peptide. 1999;20:267–273. doi: 10.1016/s0196-9781(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Valencia De Ita S, Lawand NB, Lin Q, Castaneda-Hernandez G, Willis WD. The role of the Na+-K+-2Cl− cotransporter in the development of capsaicin-induced neurogenic inflammation. J Neurophysiol. 2006;95:3553–3561. doi: 10.1152/jn.01091.2005. [DOI] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl P, Foged C, Tullin S, Thomsen C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, McIntyre P. The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain. J Pharmacol Exp Ther. 2003;304:56–62. doi: 10.1124/jpet.102.042010. [DOI] [PubMed] [Google Scholar]
- Willis WD. Hyperalgesia and Allodynia. 2. Bristol-Myers Squibb Symposium on Pain Research; Raven Press, New York: 1992. [Google Scholar]
- Xu GY, Winston JH, Shenoy M, Yin H, Pendyala S, Pasricha PJ. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology. 2007;133:1282–1292. doi: 10.1053/j.gastro.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Dyer NH, Chan CL, Knowles C, Williams NS, Anand P. Vanilloid receptor 1 immunoreactivity in flamed human bowel. Lancet. 2001;357:1338–1339. doi: 10.1016/s0140-6736(00)04503-7. [DOI] [PubMed] [Google Scholar]
- Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, Oppenheim JJ. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci. 2005;102:4536–4541. doi: 10.1073/pnas.0406030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhou ZS, Zhao Z. PKC regulates capsaicin-induced currents of dorsal root ganglion neurons in rats. Neuropharmacol. 2001;41:601–608. doi: 10.1016/s0028-3908(01)00106-x. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Li G, Zhao Z. State-dependent phosphorylation of ε-isozyme of protein kinase C in adult rat dorsal root ganglia after inflammation and nerve injury. J Neurochem. 2003;85:571–580. doi: 10.1046/j.1471-4159.2003.01675.x. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Image analysis of phosphorylation of ε-isozyme of protein kinase C in transient receptor potential vanilloid-1 (TRPV1)-containing neurons in dorsal root ganglia following intradermal injection of capsaicin; Society for Neurosci; 2004a. Program No. 407.19. (Abstract) [Google Scholar]
- Zou X, Lin Q, Willis WD. Effect of protein kinase C blockade on phosphorylation of NR1 in dorsal horn and spinothalamic tract cells caused by intradermal capsaicin injection in rats. Brain Res. 2004b;1020:95–105. doi: 10.1016/j.brainres.2004.06.017. [DOI] [PubMed] [Google Scholar]