Abstract
Penetrating limb injuries are common and usually heal without long-lasting effects, even when nerves are cut. However, rare nerve-injury patients develop prolonged and disabling chronic pain (neuralgia). When pain severity is disproportionate to severity of the inciting injury, physicians and insurers may suspect exaggeration and limit care or benefits, although the nature of the relationship between lesion-size and the development and persistence of neuralgia remains largely unknown. We compared cellular changes in the spinal dorsal-horn (the initial CNS pain-processing area) after partial or total tibial-nerve axotomies in male Sprague–Dawley rats to determine if these changes are proportional to the numbers of peripheral axons cut. Unoperated rats provided controls. Plantar hind-paw responses to touch, pin, and cold were quantitated bilaterally to identify hyperalgesic rats. We also compared data from nerve-injured rats with or without hyperalgesic responses to mechanical hind-paw stimulation to evaluate concordance between pain behaviors and dorsal-horn cellular changes. Hyperalgesia was no less prevalent or severe after partial than after total axotomy. L5 spinal-cord sections from rats killed 7 days postoperatively were labeled for markers of primary afferents (substance P calcitonin gene-related peptide isolectin B4, gamma aminobutyric acid, and glial fibrillary acidic protein), then labeled cells were stereologically quantitated in somatotopically defined dorsal-horn regions. Total axotomy reduced markers of primary afferents more than partial axotomy. In contrast, GABA-immunoreactive profiles were similarly reduced after both lesions, and in rats with sensory loss versus hyperalgesia. Numbers of GFAP-immunoreactive astrocytes increased independently of lesion size and pain status. Small nerve injuries can thus have magnified and disproportionate effects on dorsal-horn neurons and glia, perhaps providing a biological correlate for the disproportionate pain of post-traumatic neuralgias (including complex regional pain syndrome-I) that follow seemingly minor nerve injuries. However, the presence of similar dorsal-horn changes in rats without pain behaviors suggests that not all transcellular responses to axotomy are pain-specific.
Keywords: GABA, astrocyte, chronic pain, rat, axotomy, spinal cord
Peripheral-nerve axons are protected from blunt trauma by the tough, collagenous epineurium and perineurium, and their zigzag course protects them from stretch, but axons are vulnerable to transection from penetrating injuries. The usual outcome is hypoalgesia (sensory loss, numbness) that resolves when adjacent axons sprout in, but rare patients develop spontaneous pain and/or hyperalgesia (an exaggerated painful response to stimuli), even after minor injuries. Mechanical allodynia (pain from light touch or contact) is the most common and disabling stimulus-evoked symptom of neuralgia.
The biological determinants of who will or will not develop mechanical allodynia and other pain symptoms after nerve injury remain unclear. Risk for post-herpetic neuralgia after shingles correlates highly with the severity of loss of unmyelinated cutaneous axons (Oaklander, 2001), but a different relationship has been identified in post-traumatic neuralgias such as complex regional pain syndrome-I (CRPS-I/reflex sympathetic dystrophy) where neuralgic pain can persist in patients with only subtle axonal losses (van der Laan et al., 1998; Albrecht et al., 2006; Oaklander et al., 2006). In a model of CRPS created by injuring the tibial nerve with different diameter needles, the presence and severity of evoked pain behaviors were also independent of the number of axons cut (Siegel et al., 2007). These findings direct attention toward the central changes evoked by peripheral axotomies. We here examine the question of whether or not the disproportion between the size of nerve lesions and their ability to cause chronic pain arises in the spinal-cord dorsal horn; the first nociceptive synapse.
Many rodent studies have characterized the profound effects of peripheral axotomy on the dorsal horn. Incoming nociceptive axons synapse somatotopically in distinct zones (Devor and Claman, 1980) and are influenced by surrounding cells (Roberts et al., 1986; Garrison et al., 1991). GABAergic and glycinergic interneurons tonically inhibit activation of 2nd order nociceptive neurons to focus incoming pain signals in space and time (Todd and Sullivan, 1990). Reduction in dorsal-horn inhibition that facilitates firing of ascending pain neurons has been associated with evoked pain behaviors in animal models. For instance, rats with hyperalgesic responses after axotomy have reduced mRNA for GABA synthetic enzymes (Moore et al., 2002) and transporters (Miletic et al., 2003) in their dorsal horns, as well as fewer GABA-immunoreactive (IR) cells (Eaton et al., 1998). Administering GABA antagonists worsens hyperalgesic responses (Yamamoto and Yaksh, 1993).
Glial cells also help to initiate and maintain neuralgia (Garrison et al., 1991; Watkins et al., 1997). Dorsal-horn microglia (Colburn et al., 1997), followed by astrocytes (Hajos et al., 1990; Murray et al., 1990), increase in number and size. Their cytokine secretions sensitize nociceptive primary afferents (Sommer and Schafers, 1998) and second-order projection neurons (DeLeo et al., 2000).
Most of these dorsal-horn studies used large and/or proximal nerve injuries, but these are rare in patients. Small distal nerve injuries are far more common but their effects on the dorsal horn are inadequately studied. Moreover, all or most lesioned rats develop hyperalgesia in most rat models of neuralgia (Bennett and Xie, 1988; Kim and Chung, 1992; Decosterd and Woolf, 2000; Hama and Borsook, 2005), leaving it unclear how many of the identified dorsal-horn changes are related to pain pathogenesis. For these reasons, we compared the effect of partial- and total-nerve injury on three cellular components of the spinal-cord dorsal horn; central axons of primary afferents, inhibitory interneurons, and astroglia. We also compared these changes in nerve-injured rats with and without mechanical allodynia plus hyperalgesia.
Experimental Procedures
Animal care and distal nerve injury (DNI) surgery
Male Sprague–Dawley rats (200–230 g, Charles River Laboratories, Wilmington, MA, USA) were housed in smooth-bottomed cages with free access to food and water. Rats were randomly assigned to undergo partial-DNI (n=32), total-DNI (n=28), or to serve as unoperated controls (n=8). Rats were rested for at least 48 h after arrival and then underwent 3 days of behavioral testing, the median of which defined the baseline value. All procedures were approved by the Institutional Animal Care and Use Committee and conformed to ethical guidelines (Zimmermann, 1983). DNI rats were anesthetized with sodium pentobarbital (50 mg/kg i.p.; Abbott Laboratories, North Chicago, IL, USA) and their left tibial nerves were tightly ligated in two locations three mm apart with 8.0 silk suture (Surgical Specialties Corporation, Reading, PA, USA). Either the entire tibial nerve or a portion comprising approximately one-third of its diameter was ligated. The 1–2 mm segment of interposed tissue was removed with iris scissors (FST, Foster City, CA, USA). The sural and peroneal nerves remained untouched. The muscles were approximated with 4.0 silk suture (Ethicon Inc., Somerville, NJ, USA), and the skin was closed with 9 mm wound clips (Becton Dickinson, Sparks, MD, USA). No medications were administered.
Sensory testing for evoked-pain behaviors
Sensory testing was performed in random order in a quiet room by a single person who was unaware of treatment group. On each testing day (baseline and days 1, 3, 7, 14, 21 post-operatively), rats were weighed and habituated to the testing apparatus for 15–30 min. Data were acquired from four sites on the left (ipsilesional) and right (contralesional) hind paws. Two sites were studied on each hind paw. The tibial-innervated site was central and proximal to the tori, and the sural-innervated area was on the lateral glabrous skin (Bajrovic and Sketelj, 1998). Thresholds for hind-paw withdrawal from static, punctuate, mechanical stimuli were measured using balance-calibrated Semmes-Weinstein monofilaments (Stoelting Co., Wood Dale, IL, USA). Rats were placed in plastic boxes on an elevated wire grid and their plantar hind paws were stimulated from below (Chaplan et al., 1994; Tal and Bennett, 1994). The threshold for hind-paw withdrawal was defined as the lowest force that evoked hind-paw withdrawal in two out of five consecutive trials (Anseloni et al., 2002) for two consecutive monofilaments. Punctate mechanical hyperalgesia was measured by applying a single safety-pin stimulus to rats' plantar hind paws from below. The duration of hind-paw withdrawal was timed with a digital chronometer. Normal withdrawals were very brief and scored as 0.5 s. Mechanical hyperalgesia was defined as withdrawals lasting 2.0 s or longer (cutoff 20 s).
Cold sensation was measured by applying acetone (∼100 μl) onto the central plantar skin of the hind paw using a syringe that did not touch the skin (Choi et al., 1994). Duration of hind-paw withdrawal was timed by digital chronometer. Withdrawals lasting 2.0 s or longer defined cold allodynia (cutoff 20 s). In preliminary experiments heat sensation was evaluated by radiant heating through a transparent plastic floor, but abnormal hind-paw postures precluded full hind-paw contact so these measurements were discontinued.
Seventeen partial-DNI and 21 total-DNI rats were killed for pathological studies 7 days after DNI, the nadir of behavioral changes. Rats to be killed were selected randomly without knowledge of their sensory testing results. The remaining rats continued behavioral testing until day 21 to characterize the long-term behavioral effects of these lesions. Tissues from specific rats were then selected for pathological study on the basis of their behavioral testing results at day 7 postoperatively. To be categorized as free of pain behaviors, a rat had to have no postoperative decrease in threshold for hind-paw withdrawal from monofilaments and to have maintained normal hind-paw withdrawals from pin and cold. To be characterized as having post-DNI evoked pain behavior, a rat had to have more than a 75% reduction in the threshold for withdrawal from monofilaments, plus a hyperalgesic withdrawal from pin. Each sequentially thinner filament applies about 50% less force, so our criteria for mechanical allodynia correspond to a reduction of at least two monofilaments. Since fewer DNI rats developed prolonged withdrawal from cold (see Results) than developed mechanical allodynia or hyperalgesia, abnormal thermal response was not included in the criteria used to select hyperalgesic rats for morphometric analysis. Had it been included, fewer rats would have qualified for analysis and statistical power would have dropped.
Tissue acquisition and processing
After completing sensory testing, rats were anesthetized with sodium pentobarbital overdose and perfused transcardially with 100 units of heparin (Elkins-Sinn, Inc., Cherry Hill, NJ, USA), then 400 ml cold 0.9% saline followed by 400 ml cold fixative (4% paraformaldehyde in 0.1 M phosphate buffer with 0.13% picric acid, pH 7.4). Spinal cords were immediately removed and post-fixed overnight at 4 °C in fresh 4% paraformaldehyde, then rinsed, cryoprotected in 30% sucrose-phosphate buffer solution, and stored at 4 °C.
The lumbar enlargements of spinal cords (L3-5) were cut into 30 μm sections using a freezing microtome (Microm, Waldorf, Germany). Sections were incubated with primary antibodies against neural and glial antigens using the manufacturers' recommended methods. Antibodies comprised rat anti-substance P (SP) (1:200; Santa Cruz Biotech, Santa Cruz, CA, USA), goat anti–calcitonin gene-related peptide (CGRP) (1:200), rabbit anti-caspase 3 (1:200), mouse anti–glial fibrillary acidic protein (GFAP) (1:200; Chemicon, Temecula, CA, USA), rabbit anti-GAD65/67 (1:200), and guinea-pig anti-GABA (1:200; Protos Biotech, New York, NY, USA). Biotinylated Isolectin B4 (1:50; Vector Laboratories, Burlingame, CA, USA) was used to label non-peptidergic unmyelinated primary afferents. Primary antibodies were diluted in 5% normal serum-0.4% TX-100/PB, incubated overnight, washed 3× for 10 min each in PB, and incubated for 2 h with secondary antibodies in 5% normal serum-0.4% TX-100/PB (Alexa Fluor 488 or 564; Molecular Probes, Eugene, OR, USA). Omission of primary antibody caused failure of labeling. Sections were mounted on gel-coated slides, coverslipped with anti-fade mounting media (Vectashield, Vector Laboratories, Burlingame, CA, USA) and visualized using a Zeiss Axioplan fluorescent microscope. Sections incubated with biotinylated-IB4 solution were processed using standard ABC histology methods and visualized with 3′ 3′-diamino benzidine (DAB, Vector Laboratories, Burlingame, CA, USA). These sections were mounted on gel-coated slides, dehydrated, cleared in xylene and coverslipped with Permount (Fisher Sci., Fair Lawn, NJ, USA).
Stereological quantification of labeled cells
Accurate measurement of cell numbers can be compromised by measuring cell density if the reference space shrinks due to cell death or atrophy. To avoid this, we made stereological estimates of total numbers of GABA-IR and GFAP-IR cells present in histologically defined regions of L5 spinal cord cross-sections. Stereo-Investigator 6.0 software (MBF Bioscience, Williston, VT, USA) was used for all measurements. Four transverse L5 spinal serial sections (180 μm interval between sections) were used for systematic random sampling of GABA-IR and GFAP-IR cells. Three reference zones were identified from each half of the L5 dorsal horn (Fig. 1A). These represented the termination zone for the tibial nerve (medial aspect of laminae I, II, III), the sural nerve (central aspect of laminae I, II, III), and the common peroneal nerve (lateral aspect of laminae I, II, III) (Devor and Claman, 1980; Swett and Woolf, 1985; Shields et al., 2003). For GABA-IR cells, a 75×75 μm counting frame, a 75×75 μm sampling grid, and a dissector height of 20 μm was used. For counting GFAP-IR cells, a 95×95 μm counting frame and a 150×150 μm sampling grid was used for the tibial, sural, and common peroneal zones. For the neck of the dorsal horn, a 95×95 μm counting frame and a 250×250 μm sampling grid with a dissector height of 20 μm was used. Cells were counted at a final magnification of 600× (GABA), and 400× (GFAP).
Fig. 1.
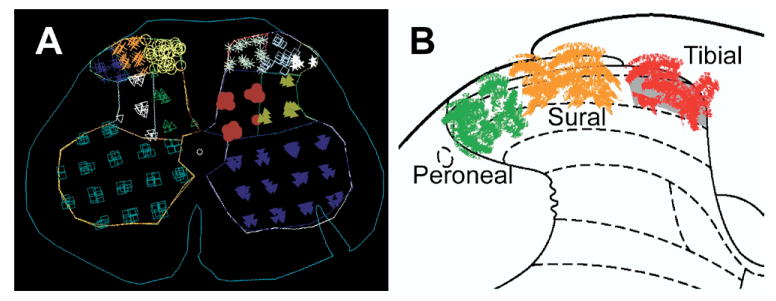
A screen-capture image from the Stereo Investigator software showing the demarcation of the six spinal gray-matter zones used for stereological morphometric analysis (A). The superficial dorsal horn was divided into three zones representing termination of specific peripheral nerves (B; red: tibial zone, orange: sural zone, green: peroneal zone). A notch, placed at the time of spinal-cord removal, identifies the right (contralesional) side. For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.
Central axons of primary afferents cannot be individuated or counted at light microscopic resolution, so we compared relative luminosity (at 520 nm wavelength) of SP-IR fibers from the tibial, sural, peroneal, and the neck of dorsal horn (laminae III to VI) of ipsilesional and contralesional gray matter using fluorescent microscopy. Luminosity intensity was measured (StereoInvestigator 6.0 software) by drawing a boundary around the area of interest with the marquee tool. Luminosity was calculated as the average of three intensity measurements (pixel brightness/mm2). To control for background luminosity, value from neck of dorsal horn was subtracted from luminosity values from tibial, sural, and peroneal sites. This method is standard for comparison of axon numbers in the spinal dorsal horn and in the skin (Gonzalez et al., 2005; Forrest and Keast, 2008).
Data analysis
Means±standard deviations summarize data from groups. Analysis of variance (Super ANOVA, Abacus Concepts, Berkeley, CA, USA) was used to assess the effects of DNI. Fisher-protected post hoc testing evaluated between-group differences. Chi square tests were used to analyze between-group comparisons of dichotomous outcomes such as the presence or absence of abnormal hind-paw posture at a specific time point. P<0.05 defined statistical significance.
Results
DNI rats appeared to ambulate and explore as much control rats, and their feeding was judged unimpaired because weight gain in both groups of DNI rats was similar to that of unoperated rats (P≥0.49). Neither autotomy nor tremors were detected in any rats. Some DNI rats developed sustained (tonic) abnormal postures of their ipsilesional hind paws, specifically lateral hind-paw-margin elevation and paw eversion, or plantar-flexion of all digits with ambulation on the volar surface of the digit. These never developed in unoperated rats, or in hind paws contralateral to DNI (contralesional hind paws). One hundred percent of total-DNI rats had abnormal hind-paw postures on postoperative days 1, 7, 14, and 21. Among partial-DNI rats, the prevalence of abnormal hind-paw postures was 100% on postoperative day 1, 50% on day 7, and 27% on days 14 and 21 postoperatively.
Effects of DNI on evoked-pain behaviors
Unoperated rats never developed mechanical allodynia. Fig. 2 illustrates that roughly half of partial-DNI rats (Fig. 2A) and a slightly smaller proportion of total-DNI rats (Fig. 2B) developed mechanical allodynia. Hence, development of allodynia was not proportionate to lesion size. Allodynia did not consistently develop in the hind paw contralateral to DNI. We performed a secondary analysis, group means of hind-paw withdrawal from monofilaments (Fig. 3A) to permit comparison of our data to similar data collected from other animal models of neuralgia. This confirmed findings that total-tibial axotomy has greater effects in the zone mostly innervated by the sural nerve (Bajrovic and Sketelj, 1998) than in the tibial-innervated zone. However, the relatively unchanged mean tibial-zone withdrawal thresholds mask a more complex outcome since withdrawal thresholds went up in some DNI rats (sensory loss), down in others (allodynia), and stayed unchanged in other rats. For instance, the mean tibial-site withdrawal threshold at day 7 postoperatively among “no pain” total-DNI rats increased to 272±93% (n=11), whereas among “painful” total-DNI rats it was reduced to 16±2% (n=6) of baseline values (P<0.01). In contrast, partial-DNI did not leave any rats with sensory loss, so mean thresholds were reduced in the tibial zone (P=0.0004) as well as in the sural-innervated extraterritorial zone (P=0.004). These same variable consequences of nerve injuries on sensory function are observed in nerve-injury patients.
Fig. 2.
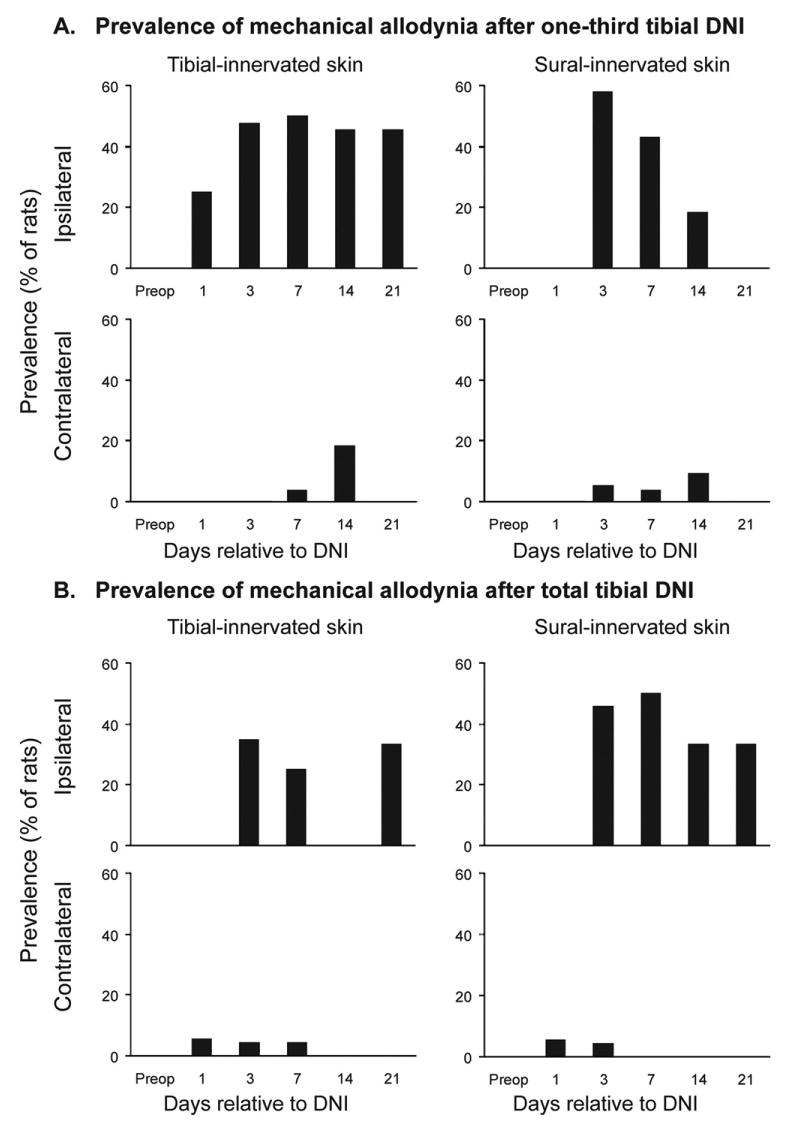
The prevalence of mechanical allodynia in rat plantar hind paws after partial (one-third) (A) or total (B) tibial-nerve axotomy. Mechanical allodynia was defined by a >75% reduction in hind-paw withdrawal threshold from preoperative values.
Fig. 3.
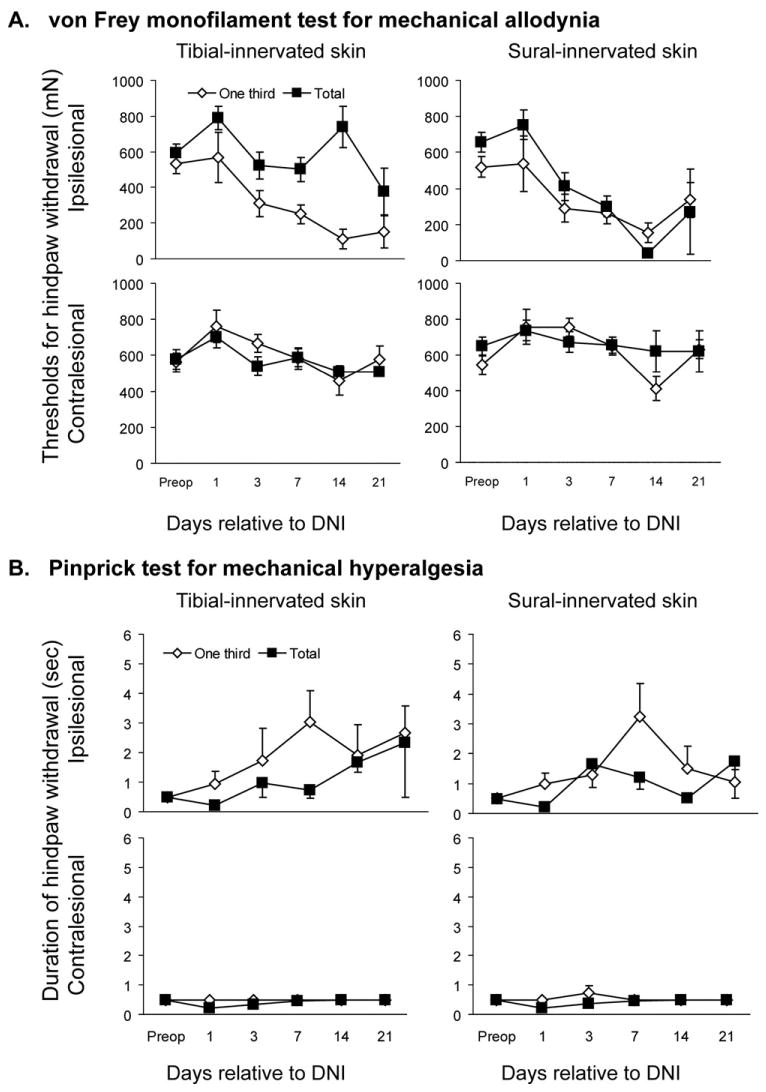
(A) Group means of withdrawal threshold from Semmes-Weinstein monofilaments, (B) group means of hind-paw withdrawal from pin (B) after partial-DNI and total-DNI. This analysis combines data from rats with hypoesthesia and hyperalgesia after DNI.
Unoperated rats never had pin hyperalgesia, and this was less prevalent in DNI rats than mechanical allodynia. Prolonged withdrawal from pin was present at day 7 postoperatively in 25% of partial-DNI rats and in 4% of total-DNI rats (P=0.09). With such low prevalence, group means of duration of withdrawal from pin in both groups of DNI rats (Fig. 3B) were not significantly different from baseline values (P≥0.11).
Unoperated rats never had prolonged hind-paw withdrawal from acetone. Prolonged withdrawal from acetone was present at day 7 postoperatively in 19% of partial-DNI rats and 29% of total-DNI rats; values that were similar (P=0.87). Mean duration of withdrawal in the six partial-DNI rats with prolonged withdrawal was 14.2±2.7 s and in the eight total-DNI rats with prolonged withdrawal it was 8.9±2.9 s (P≥0.69).
Effects on central axons of primary afferent fibers
In the ipsilesional spinal cord, DNI caused somatotopically restricted reductions in mean pixel intensity for all markers studied (Fig. 4A). After total-DNI, there were very few remaining SP-, CGRP-, and IB-4-positive fibers visible in the tibial zone of the lumbar dorsal horn, whereas after partial-DNI there was partial loss of labeling. Pixel intensity in SP-IR images was significantly reduced from values in unoperated rats (to 69% after partial-DNI, and to 40% after total-DNI vs. control) in the ipsilesional tibial zone of the dorsal horn (Fig. 4B; P=0.022). There were no significant changes in the sural (middle) or peroneal (outer) nerve-termination zones. Analyses of reference spaces from contralateral dorsal horn showed elevated pixel intensities of SP-IR fibers in the sural and peroneal zones as compared with values from unoperated rats.
Fig. 4.
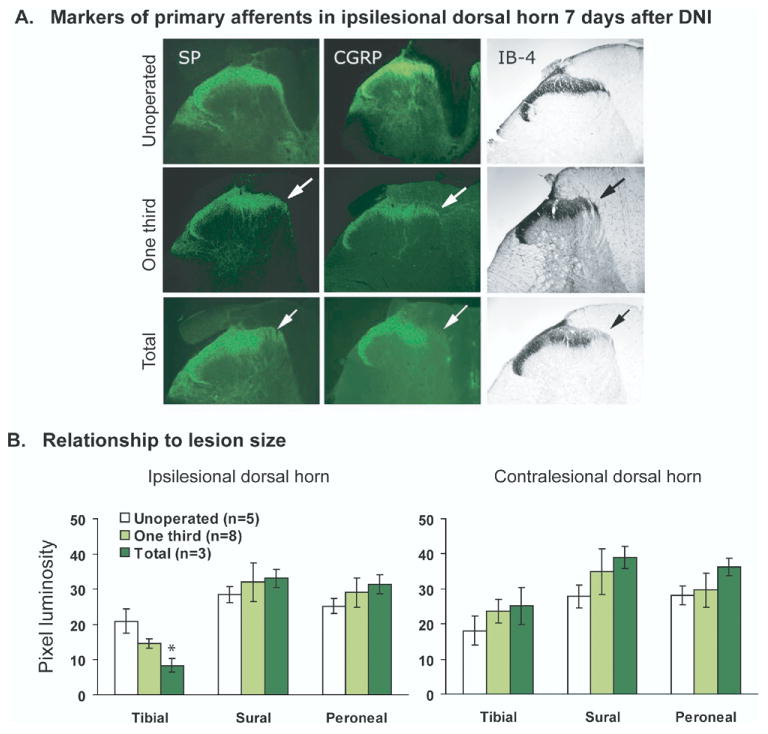
Changes in markers of incoming primary afferent fibers at the L5 level of the spinal cord 7 days after DNI. (A) Labeling for SP, CGRP, and IB-4-positive fibers from representative individual rats. The top row illustrates dense fiber staining in Rexed's laminae I and II of unoperated rats. The middle row shows partial loss in the expected somatotopic termination zone in partial-DNI rats. The bottom row shows severe reduction in total-DNI rats. Images were obtained at 10×. (B). SP-IR pixels are reduced in the ipsilateral tibial zone of the dorsal horn of total-DNI rats as compared with unoperated controls.
Effects of DNI on inhibitory interneurons in spinal dorsal horn
Unoperated control rats had 451.8±70.7 (tibial zone), 441±98.1 (sural zone), and 311.4±34.7 (peroneal zone) GABA-IR cells within these ipsilateral dorsal horn reference spaces. At day 7 after DNI, experimental rats had fewer GABA-IR cells than unoperated rats in the ipsilateral tibial (P<0.01) and peroneal zones, (P<0.03; Fig. 5B). After partial-DNI, numbers of labeled cell profiles were significantly reduced from control values in the tibial (by 51%) and peroneal (by 64%) zones (Fig. 5A). Contralesionally, reductions of at least 35% were observed in all zones. When DNI rats were stratified according to whether or not they had evoked mechanical pain behaviors (algesic responses to both monofilament and pin, Fig. 5C), there were no significant differences between the two groups of DNI rats (P=0.18). Additional spinal sections were double-labeled for GABA and caspase-3 to look for evidence of apoptosis of inhibitory interneurons. At 7D post-lesion, DNI rats had many caspase 3-IR cells present throughout the spinal gray matter, but GABA- and caspase 3-IR did not co-localize (data not shown).
Fig. 5.
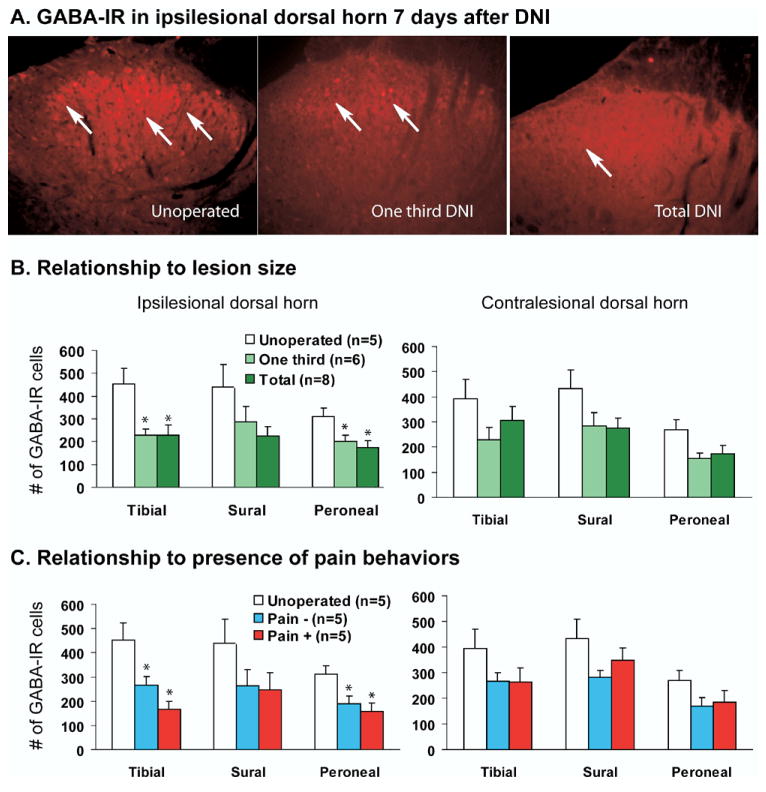
Effects of DNI on numbers of GABA-IR cell in the dorsal spinal cord 7 days after lesioning. (A) Representative image of GABA-IR cells present in laminae I and II of superficial dorsal horns of an unoperated, partial-DNI, and total-DNI rats (20× magnification). (B) Numbers of GABA-IR cells after partial (1/3) or total-DNI, and (C) comparison of numbers of GABA-IR cells in rats with pain behaviors versus those without. One-way ANOVA, * vs. unoperated, P<0.05.
Effects of DNI on numbers of GFAP-IR astrocytes
On average, unoperated control rats had 1817.5±258.7 (tibial zone), 1667.8±178.7 (sural zone), and 1039.6±113.2 (peroneal zone) GFAP-IR astrocytes in their ipsilesional dorsal horns. At day 7 after total-DNI, experimental rats had more GFAP-IR astrocytes in their ipsilesional tibial and sural zones than unoperated control rats (Fig. 6B; P<0.02). After partial-DNI, labeled cell profiles were significantly increased from control values in the ipsilesional tibial (164%) and sural (180%) zones (Fig. 6B). When DNI rats were stratified according to whether or not they had evoked-pain behaviors (Fig. 6C), increases were similar in both groups (P<0.009).
Fig. 6.
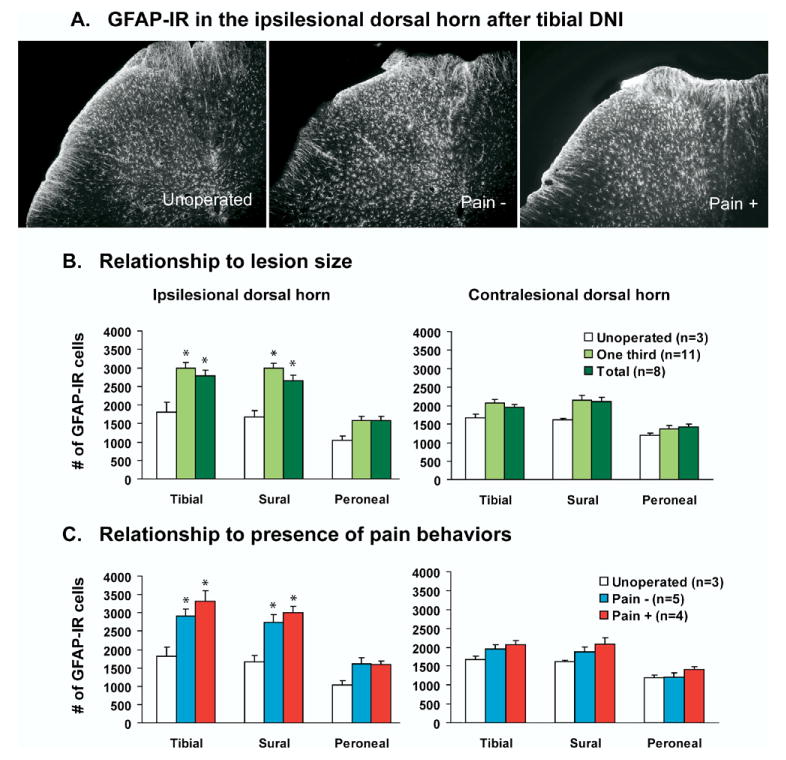
Effects of DNI on numbers of astrocytes in the dorsal spinal cord of unoperated, partial-DNI, and total-DNI rats 7 days after tibial DNI (10× magnification). (A) DNI rats had increased number of GFAP-IR cells in the spinal gray matter compared with unoperated control rats. (B) Numbers of GFAP-IR cells after partial-DNI or total-DNI, and (C) comparison of numbers of GFAP-IR cells in rats with pain behaviors versus those without. One-way ANOVA, * vs. unoperated, P<0.05.
We performed a secondary analysis on the astrocytes identified as activated or hypertrophic (defined by diameter greater than 2.0 μm). Activated astrocytes were never present in samples from unoperated rats. About 50% of GFAP-IR cells from the dorsal horn (tibial+sural+peroneal) of DNI rats were hypertrophic. Numbers of activated astrocytes were not significantly different in partial-DNI versus total-DNI rats, or in DNI rats with or without pain behaviors.
Discussion
We generated a nerve injury that models the epidemiological characteristic of neuralgia, namely, that it does not always follow nerve injury. Most extant rodent models of neuralgia produce pain-behaviors in all or most lesioned animals. While efficient for preclinical testing, this precludes exploration of which post-lesioning changes are specifically associated with pain development. Seven days after our tibial-nerve lesions, 63% of rats had mechanical allodynia; 29% had prolonged withdrawal from pin, and 19% had prolonged withdrawal from cold. Comparing partial and total lesions revealed that evoked pain-behaviors were more prevalent (Fig. 2) and more severe (Fig. 3) after partial than after total axotomy. This models the situation in patients, where neuralgia is far more common after partial than total nerve transections.
Symptom prevalence data, which are unusual in animal-model pain research, demonstrated three outcomes from lesioning—reduced thresholds (allodynia), unchanged thresholds, or increased thresholds (hypoesthesia). These multiple possible outcomes would have been obscured had we merely reported group means of thresholds as is customary. The smaller, partial-DNI lesion was disproportionately likely to induce pain-behaviors when compared with the larger lesion, confirming earlier studies on the efficiency of small DNI in causing neuralgia (Lee et al., 2000; Hama and Borsook, 2005). This parallels medical experience, where lesions as small as a single needle-stick or injury to a small terminal nerve branch can cause neuralgia or CRPS (Horowitz, 1994).
We analyzed spinal-cord sections from these behaviorally characterized rats to investigate effects of lesion size on cellular changes identified in other rat models of neuralgia such as chronic constriction injury (Colburn et al., 1997; Ibuki et al., 1997; Somers and Clemente, 2002) and spared nerve injury (Decosterd and Woolf, 2000). Comparison of numbers or density of 1) incoming central axons of peptidergic and non-peptidergic afferents, 2) inhibitory GABAergic interneurons, 3) all GFAP-IR astrocytes, and 4) activated GFAP-IR astrocytes did not reveal specific associations with the presence of pain-behaviors. This suggests that not all dorsal-horn abnormalities caused by experimental nerve injury are tightly linked to stimulus-evoked pain behaviors.
Finding somatotopically appropriate and proportional pattern of loss of markers of primary afferent axons confirmed that our immunolabeling accurately represented the central axons of the specific neurons that had been cut, and that our nerve-termination zones were correctly mapped. Although such labeling cannot distinguish between transient changes in gene expression and frank degeneration, ultrastructural examination confirms that sciatic axotomy causes degeneration of central axon terminals (Kapadia and LaMotte, 1987), and that subsequent return of afferent markers represents axonal sprouting (Villar et al., 1989), so we interpret our data as more likely reflecting axonal degeneration rather than mere depletion of markers.
Concordance between dorsal-horn changes and severity and location of distal axotomy vanished when we examined cells other than the axotomized primary afferents. Reductions in GABAergic interneurons and increases in GFAP-IR astrocytes spread to involve the sural and peroneal zones. Changes in numbers of GABA-IR and GFAP-IR profiles were similar after partial and total-DNI. These transcellular changes illustrate spread of axotomy-induced changes beyond the somatotopic area of the injured axons. They appeared to involve the contralateral dorsal horn (Figs. 4–6) and even the ventral horn (data not shown). Similar bilateral reductions of GABAergic cells have been reported after unilateral CCI (Ibuki et al., 1997; Eaton et al., 1998). It is possible that these are a cellular substrate for extra-territorial spread of pain behavior after nerve injury.
Pharmacological and electrophysiological evidence identifies loss of dorsal-horn GABA, the chief spinal inhibitory neurotransmitter, as an important correlate of neuropathic pain (Ibuki et al., 1997; Jones and Sorkin, 1998; Moore et al., 2002). It was thus unexpected that DNI rats without pain behaviors also had fewer GABA-IR cells (Fig. 5C). Although it is possible that DNI rats without pain behaviors had spontaneous pain, there was no evidence of this. Some investigators interpret vocalizations, autotomy, or specific movements as indices of spontaneous pain, but the validity of these interpretations is impossible to confirm (Vierck et al., 2008) and has been disputed (Dey et al., 2005). Exploration, feeding, weight gain, and behavior were indistinguishable in DNI rats without evoked-pain behaviors and unoperated rats, and DNI rats did not develop excess vocalizations, paw licking, or autotomy, so nothing suggested the presence of spontaneous pain. Moreover, other studies also note discordance between the presence of hind-paw allodynia (after CCI) and numbers of spinal dorsal horn GABAergic cells (Polgár et al., 2003). Because there was a trend toward fewer GABA-IR cells in the ipsilateral tibial zone of DNI rats with pain-behaviors as compared with those without them (Fig. 5C) we conducted a post hoc analysis to clarify these results. Power analysis (α=0.05; power=80%) suggested that analyzing tissues from 13 DNI rats with pain behaviors and 13 without pain behaviors would identify statistically significantly greater loss of GABA-IR profiles in DNI rats with pain-behaviors. The stringency of the behavioral criteria used to define rats as with or without pain-behaviors also influences the outcome of statistical analysis. When we used less-stringent criteria to differentiate between the two groups; algesic responses to monofilaments only, no differences were apparent (P=0.89). In aggregate, these results suggest that any association between numbers of GABA-IR cells at day 7 postoperatively and the presence of mechanical allodynia is modest at best. Since allodynia is already well-established by then, an earlier time point might have yielded a more robust association.
In contrast, we found complete dissociation between the severity and location of DNI and numbers of GFAP-IR astrocytes' response in the dorsal horn (Fig. 6). Astrocyte hypertrophy, present by 3–4 days after nerve injury, is also implicated in the pathogenesis of neuralgia (Garrison et al., 1991), perhaps by sensitizing primary afferents and 2nd order spinal neurons via secretion of proinflammatory cytokines (DeLeo et al., 2000; Zelenka et al., 2005). Our findings contrast with several earlier studies that found no increase in numbers of dorsal-horn astrocytes after sciatic-nerve axotomy (Murray et al., 1990; Liu et al., 2000). We found bilateral increases in astrocyte numbers after one-sided axotomies, and so believe that some of these earlier results may have been compromised by using the contralesional dorsal horn as a control site (Garrison et al., 1991). Several groups have now implicated activated dorsal-horn glia in creating and maintaining neuropathic pain after nerve injuries, particularly by release of proinflammatory products such as cytokines that increase neuronal excitability. Various data show that inhibiting glial effects can attenuate mechanical allodynia after nerve injuries (Ledeboer et al., 2006). Although increases in cell numbers are one common marker of cellular activation, our data suggest that increased numbers of dorsal-horn astrocytes 7 days after nerve injury do not correlate with the presence of mechanical allodynia (Fig. 6C). Perhaps an earlier time point might have captured such an association, or perhaps the contribution of astrocytes is better measured by expression profiling.
Conclusion
In summary, this study demonstrates that partial axotomies of one distal nerve can be as effective as or more effective than total axotomies at producing long-lasting intraterritorial and extraterritorial hind-paw mechanical and thermal pain behaviors. A smaller, partial lesion was as effective as total axotomy at causing trans-synaptic and widespread effects on GABAergic interneurons and astrocytes in the dorsal-horn. This cellular “magnification” appears to develop within the dorsal horn rather than in the nerve because changes in central axons of primary axons remained proportional to the severity and location of the DNI. Changes in numbers of GABAergic interneurons and astrocytes were not restricted to rats with neuropathic pain behaviors, although there was a trend toward greater loss of GABA-IR cells in lesioned rats with pain behaviors. Studying rats that do not develop chronic pain behaviors after nerve injury in parallel with rats that do, may help clarify which responses to nerve injury are specifically pain-associated, and thus are attractive targets for development of new treatments for neuropathic pain.
Acknowledgments
The technical assistance of Li Zheng, Ralph Gott, YangFeng Li, and statistical support of Dr. Yuchiao Chang are gratefully acknowledged. Supported in part by the Public Health Service (R01NS42866, K24NS059892, P30 EY 12196), the Beatrice and Roy Backus Foundation, and the RSDSA Rachel Tobias Young Investigator Award. Presented to the Society for Neuroscience in abstract form.
Abbreviations
- CGRP
calcitonin gene-related peptide
- CRPS
complex regional pain syndrome
- DNI
distal nerve injury
- GABA
gamma aminobutyric acid
- GFAP
glial fibrillary acidic protein
- IB-4
isolectin
- IR
immunoreactive
- SP
substance P
References
- Albrecht PJ, Hines S, Eisenberg E, Pud D, Finlay DR, Connolly MK, Pare M, Davar G, Rice FL. Pathologic alterations of cutaneous innervation and vasculature in affected limbs from patients with complex regional pain syndrome. Pain. 2006;120:244–266. doi: 10.1016/j.pain.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Anseloni VC, Weng HR, Terayama R, Letizia D, Davis BJ, Ren K, Dubner R, Ennis M. Age-dependency of analgesia elicited by intraoral sucrose in acute and persistent pain models. Pain. 2002;97:93–103. doi: 10.1016/s0304-3959(02)00010-6. [DOI] [PubMed] [Google Scholar]
- Bajrovic F, Sketelj J. Extent of nociceptive dermatomes in adult rats is not primarily maintained by axonal competition. Exp Neurol. 1998;150:115–121. doi: 10.1006/exnr.1997.6734. [DOI] [PubMed] [Google Scholar]
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- Colburn RW, DeLeo JA, Rickman AJ, Yeager MP, Kwon P, Hickey WF. Dissociation of microglial activation and neuropathic pain behaviors following peripheral nerve injury in the rat. J Neuroimmunol. 1997;79:163–175. doi: 10.1016/s0165-5728(97)00119-7. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Rutkowski MD, Stalder AK, Campbell IL. Transgenic expression of TNF by astrocytes increases mechanical allodynia in a mouse neuropathy model. Neuroreport. 2000;11:599–602. doi: 10.1097/00001756-200002280-00033. [DOI] [PubMed] [Google Scholar]
- Devor M, Claman D. Mapping and plasticity of acid phosphatase afferents in the rat dorsal horn. Brain Res. 1980;190:17–28. doi: 10.1016/0006-8993(80)91156-7. [DOI] [PubMed] [Google Scholar]
- Dey DD, Landrum O, Oaklander AL. Central neuropathic itch from spinal-cord cavernous hemangioma: a human case, a possible animal model, and hypotheses about pathogenesis. Pain. 2005;113:233–237. doi: 10.1016/j.pain.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD- and GABA-immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat. 1998;16:57–72. doi: 10.1016/s0891-0618(98)00062-3. [DOI] [PubMed] [Google Scholar]
- Forrest SL, Keast JR. Expression of receptors for glial cell line-derived neurotrophic factor family ligands in sacral spinal cord reveals separate targets of pelvic afferent fibers. J Comp Neurol. 2008;506:989–1002. doi: 10.1002/cne.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison CJ, Dougherty PM, Kajander KC, Carlton SM. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 1991;565:1–7. doi: 10.1016/0006-8993(91)91729-k. [DOI] [PubMed] [Google Scholar]
- Gonzalez HL, Carmichael N, Dostrovsky JO, Charlton MP. Evaluation of the time course of plasma extravasation in the skin by digital image analysis. J Pain. 2005;6:681–688. doi: 10.1016/j.jpain.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Hajos F, Csillik B, Knyihar-Csillik E. Alterations in glial fibrillary acidic protein immunoreactivity in the upper dorsal horn of the rat spinal cord in the course of transganglionic degenerative atrophy and regenerative proliferation. Neurosci Lett. 1990;117:8–13. doi: 10.1016/0304-3940(90)90111-l. [DOI] [PubMed] [Google Scholar]
- Hama AT, Borsook D. Behavioral and pharmacological characterization of a distal peripheral nerve injury in the rat. Pharmacol Biochem Behav. 2005;81:170–181. doi: 10.1016/j.pbb.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Horowitz SH. Peripheral nerve injury and causalgia secondary to routine venipuncture. Neurology. 1994;44:962–964. doi: 10.1212/wnl.44.5.962. [DOI] [PubMed] [Google Scholar]
- Ibuki T, Hama AT, Wang XT, Pappas GD, Sagen J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–858. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- Jones DL, Sorkin LS. Systemic gabapentin and S(+)-3-isobutyl-gamma-aminobutyric acid block secondary hyperalgesia. Brain Res. 1998;810:93–99. doi: 10.1016/s0006-8993(98)00890-7. [DOI] [PubMed] [Google Scholar]
- Kapadia SE, LaMotte CC. Deafferentation-induced alterations in the rat dorsal horn: I. Comparison of peripheral nerve injury vs. rhizotomy effects on presynaptic, postsynaptic, and glial processes. J Comp Neurol. 1987;266:183–197. doi: 10.1002/cne.902660205. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Ledeboer A, Liu T, Shumilla JA, Mahoney JH, Vijay S, Gross MI, Vargas JA, Sultzbaugh L, Claypool MD, Sanftner LM, Watkins LR, Johnson KW. The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron Glia Biol. 2006;2:279–291. doi: 10.1017/S1740925X0700035X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Won R, Baik EJ, Lee SH, Moon CH. An animal model of neuropathic pain employing injury to the sciatic nerve branches. Neuroreport. 2000;11:657–661. doi: 10.1097/00001756-200003200-00002. [DOI] [PubMed] [Google Scholar]
- Liu L, Rudin M, Kozlova EN. Glial cell proliferation in the spinal cord after dorsal rhizotomy or sciatic nerve transection in the adult rat. Exp Brain Res. 2000;131:64–73. doi: 10.1007/s002219900273. [DOI] [PubMed] [Google Scholar]
- Miletic G, Draganic P, Pankratz MT, Miletic V. Muscimol prevents long-lasting potentiation of dorsal horn field potentials in rats with chronic constriction injury exhibiting decreased levels of the GABA transporter GAT-1. Pain. 2003;105:347–353. doi: 10.1016/s0304-3959(03)00250-1. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M, Wang SD, Goldberger ME, Levitt P. Modification of astrocytes in the spinal cord following dorsal root or peripheral nerve lesions. Exp Neurol. 1990;110:248–257. doi: 10.1016/0014-4886(90)90036-r. [DOI] [PubMed] [Google Scholar]
- Oaklander AL. The density of remaining nerve endings in human skin with and without postherpetic neuralgia after shingles. Pain. 2001;92:139–145. doi: 10.1016/s0304-3959(00)00481-4. [DOI] [PubMed] [Google Scholar]
- Oaklander AL, Rissmiller JG, Gelman LB, Zheng L, Chang Y, Gott R. Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome-I (reflex sympathetic dystrophy) Pain. 2006;120:235–243. doi: 10.1016/j.pain.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Polgár E, Hughes DI, Riddell JS, Maxwell DJ, Puskar Z, Todd AJ. Selective loss of spinal GABAergic or glycinergic neurons is not necessary for development of thermal hyperalgesia in the chronic constriction injury model of neuropathic pain. Pain. 2003;104:229–239. doi: 10.1016/s0304-3959(03)00011-3. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Beyer C, Komisaruk BR. Nociceptive responses to altered GABAergic activity at the spinal cord. Life Sci. 1986;39:1667–1674. doi: 10.1016/0024-3205(86)90164-5. [DOI] [PubMed] [Google Scholar]
- Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- Siegel SM, Lee JW, Oaklander AL. Needlestick distal nerve injury in rats models symptoms of complex regional pain syndrome. Anesth Analg. 2007;105:1820–1829. doi: 10.1213/01.ane.0000295234.21892.bc. [DOI] [PubMed] [Google Scholar]
- Somers DL, Clemente FR. Dorsal horn synaptosomal content of aspartate, glutamate, glycine and GABA are differentially altered following chronic constriction injury to the rat sciatic nerve. Neurosci Lett. 2002;323:171–174. doi: 10.1016/s0304-3940(02)00157-x. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schafers M. Painful mononeuropathy in C57BL/Wld mice with delayed wallerian degeneration: differential effects of cytokine production and nerve regeneration on thermal and mechanical hypersensitivity. Brain Res. 1998;784:154–162. doi: 10.1016/s0006-8993(97)01327-9. [DOI] [PubMed] [Google Scholar]
- Swett JE, Woolf CJ. The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neurol. 1985;231:66–77. doi: 10.1002/cne.902310106. [DOI] [PubMed] [Google Scholar]
- Tal M, Bennett GJ. Extra-territorial pain in rats with a peripheral mononeuropathy: mechano-hyperalgesia and mechano-allodynia in the territory of an uninjured nerve. Pain. 1994;57:375–382. doi: 10.1016/0304-3959(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Sullivan AC. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol. 1990;296:496–505. doi: 10.1002/cne.902960312. [DOI] [PubMed] [Google Scholar]
- van der Laan L, ter Laak HJ, Gabreels-Festen A, Gabreels F, Goris RJ. Complex regional pain syndrome type I (RSD): pathology of skeletal muscle and peripheral nerve. Neurology. 1998;51:20–25. doi: 10.1212/wnl.51.1.20. [DOI] [PubMed] [Google Scholar]
- Vierck CJ, Hansson PT, Yezierski RP. Clinical and pre-clinical pain assessment: are we measuring the same thing? Pain. 2008;135:7–10. doi: 10.1016/j.pain.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Villar MJ, Cortes R, Theodorsson E, Wiesenfeld-Hallin Z, Schalling M, Fahrenkrug J, Emson PC, Hokfelt T. Neuropeptide expression in rat dorsal root ganglion cells and spinal cord after peripheral nerve injury with special reference to galanin. Neuroscience. 1989;33:587–604. doi: 10.1016/0306-4522(89)90411-9. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Martin D, Ulrich P, Tracey KJ, Maier SF. Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain. 1997;71:225–235. doi: 10.1016/s0304-3959(97)03369-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Yaksh TL. Effects of intrathecal strychnine and bicuculline on nerve compression-induced thermal hyperalgesia and selective antagonism by MK-801. Pain. 1993;54:79–84. doi: 10.1016/0304-3959(93)90102-U. [DOI] [PubMed] [Google Scholar]
- Zelenka M, Schafers M, Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005;111:257–263. doi: 10.1016/j.pain.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


