Abstract
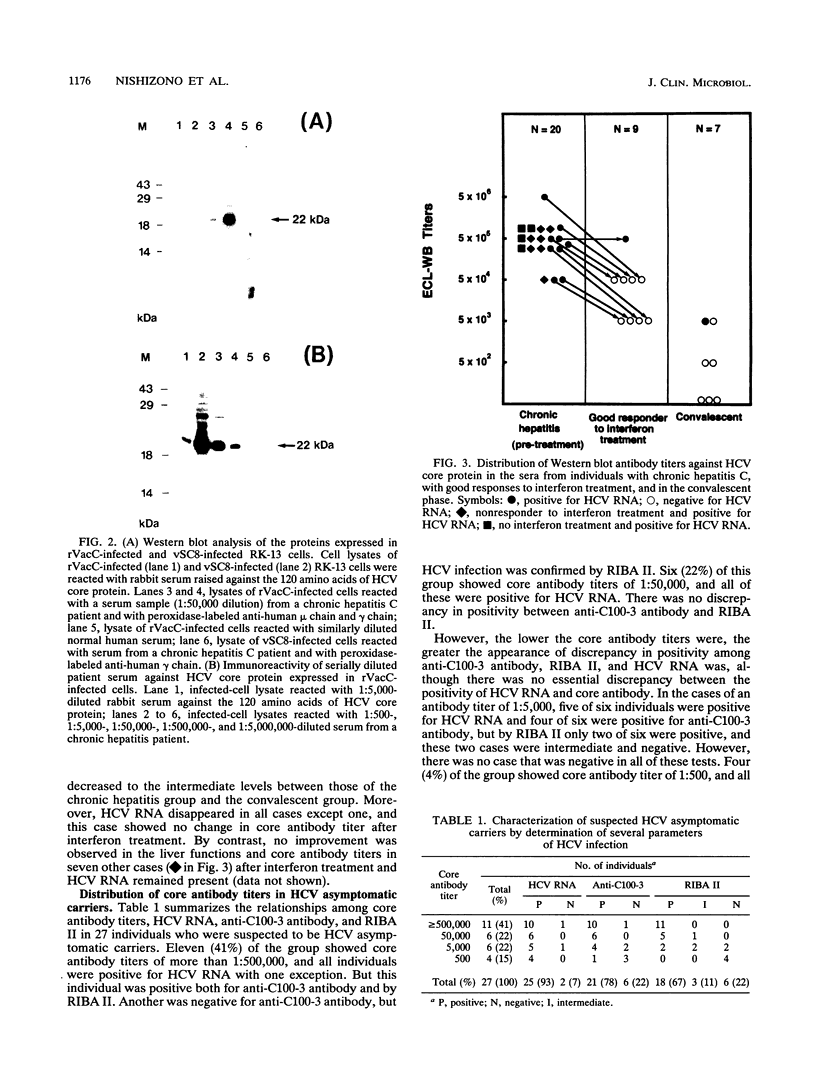
In order to study the relationships among the clinical features of hepatitis C patients, the presence of hepatitis C virus (HCV) RNA in their blood, and their serum antibody titers against the core protein of virus and to study the antibody levels in asymptomatic HCV carriers, a recombinant vaccinia virus containing a core protein gene was constructed. The recombinant virus expressed a protein with a molecular mass of 22 kDa in RK-13 cells as determined by Western blot (immunoblot) analysis. By using the cell lysate of virus-infected cells and serially diluted serum samples, core antibody titers in the groups of patients in the chronic hepatitis phase and in the convalescent phase as well as in asymptomatic carriers were determined by enhanced chemiluminescence Western blot analysis. Almost all patients in the chronic phase were shown to have high antibody titers of more than 1:500,000 and with no exception had of HCV RNA in their sera. On the other hand, patients who had recovered naturally and were in the convalescent phase were shown to have significantly lower antibody titers, and the antibody was not detected in the lowest serum dilution of 1:500 in 43% of these patients (three of seven total patients). Antibody levels of patients who showed a good response to interferon treatment decreased to intermediate levels between those of patients in the chronic phase and those of patients in convalescent phase. The antibody titers in asymptomatic carriers varied considerably from 1:500,000 to 1:500, and 41% (11 of 27 total individuals) of these carriers showed a high titer equivalent to that of those in the chronic phase. Core antibody was detected consistently in the individuals in whom HCV RNA was detected. This system for core antibody might be useful for identifying the stage of an apparent HCV infection.
Full text
PDF




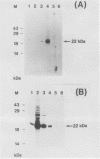
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley D. W., Maynard J. E., Popper H., Cook E. H., Ebert J. W., McCaustland K. A., Schable C. A., Fields H. A. Posttransfusion non-A, non-B hepatitis: physicochemical properties of two distinct agents. J Infect Dis. 1983 Aug;148(2):254–265. doi: 10.1093/infdis/148.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. W., McCaustland K. A., Cook E. H., Schable C. A., Ebert J. W., Maynard J. E. Posttransfusion non-A, non-B hepatitis in chimpanzees. Physicochemical evidence that the tubule-forming agent is a small, enveloped virus. Gastroenterology. 1985 Mar;88(3):773–779. [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba J., Ohba H., Matsuura Y., Watanabe Y., Katayama T., Kikuchi S., Saito I., Miyamura T. Serodiagnosis of hepatitis C virus (HCV) infection with an HCV core protein molecularly expressed by a recombinant baculovirus. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4641–4645. doi: 10.1073/pnas.88.11.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garson J. A., Tedder R. S., Briggs M., Tuke P., Glazebrook J. A., Trute A., Parker D., Barbara J. A., Contreras M., Aloysius S. Detection of hepatitis C viral sequences in blood donations by "nested" polymerase chain reaction and prediction of infectivity. Lancet. 1990 Jun 16;335(8703):1419–1422. doi: 10.1016/0140-6736(90)91446-h. [DOI] [PubMed] [Google Scholar]
- Han J. H., Shyamala V., Richman K. H., Brauer M. J., Irvine B., Urdea M. S., Tekamp-Olson P., Kuo G., Choo Q. L., Houghton M. Characterization of the terminal regions of hepatitis C viral RNA: identification of conserved sequences in the 5' untranslated region and poly(A) tails at the 3' end. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1711–1715. doi: 10.1073/pnas.88.5.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S., Watanabe Y., Takeuchi K., Suzuki T., Katayama T., Takebe Y., Saito I., Miyamura T. Expression of processed core protein of hepatitis C virus in mammalian cells. J Virol. 1991 Jun;65(6):3015–3021. doi: 10.1128/jvi.65.6.3015-3021.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosein B., Fang C. T., Popovsky M. A., Ye J., Zhang M., Wang C. Y. Improved serodiagnosis of hepatitis C virus infection with synthetic peptide antigen from capsid protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3647–3651. doi: 10.1073/pnas.88.9.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda K., Omata M., Yokosuka O., Kato N., Ohto M. Non-A, non-B chronic hepatitis is chronic hepatitis C: a sensitive assay for detection of hepatitis C virus RNA in the liver. Hepatology. 1992 May;15(5):777–781. doi: 10.1002/hep.1840150506. [DOI] [PubMed] [Google Scholar]
- Kato N., Hijikata M., Ootsuyama Y., Nakagawa M., Ohkoshi S., Sugimura T., Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimms L., Vallari D., Ducharme L., Holland P., Kuramoto I. K., Zeldis J. Specificity of anti-HCV ELISA assessed by reactivity to three immunodominant HCV regions. Lancet. 1990 Dec 22;336(8730):1590–1591. doi: 10.1016/0140-6736(90)93377-2. [DOI] [PubMed] [Google Scholar]
- Nasoff M. S., Zebedee S. L., Inchauspé G., Prince A. M. Identification of an immunodominant epitope within the capsid protein of hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5462–5466. doi: 10.1073/pnas.88.12.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Kurai K., Iizuka H., Machida A., Miyakawa Y., Mayumi M. Nucleotide sequence of the genomic RNA of hepatitis C virus isolated from a human carrier: comparison with reported isolates for conserved and divergent regions. J Gen Virol. 1991 Nov;72(Pt 11):2697–2704. doi: 10.1099/0022-1317-72-11-2697. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Yotsumoto S., Tanaka T., Yoshizawa H., Tsuda F., Miyakawa Y., Mayumi M. The 5'-terminal sequence of the hepatitis C virus genome. Jpn J Exp Med. 1990 Jun;60(3):167–177. [PubMed] [Google Scholar]
- Scheuer P. J., Ashrafzadeh P., Sherlock S., Brown D., Dusheiko G. M. The pathology of hepatitis C. Hepatology. 1992 Apr;15(4):567–571. doi: 10.1002/hep.1840150402. [DOI] [PubMed] [Google Scholar]
- Sugimoto M., Yasuda A., Miki K., Morita M., Suzuki K., Uchida N., Hashizume S. Gene structures of low-neurovirulent vaccinia virus LC16m0, LC16m8, and their Lister original (LO) strains. Microbiol Immunol. 1985;29(5):421–428. doi: 10.1111/j.1348-0421.1985.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Okamoto H., Kishimoto S., Munekata E., Tachibana K., Akahane Y., Yoshizawa H., Mishiro S. Demonstration of a hepatitis C virus-specific antigen predicted from the putative core gene in the circulation of infected hosts. J Gen Virol. 1992 Mar;73(Pt 3):667–672. doi: 10.1099/0022-1317-73-3-667. [DOI] [PubMed] [Google Scholar]
- Takamizawa A., Mori C., Fuke I., Manabe S., Murakami S., Fujita J., Onishi E., Andoh T., Yoshida I., Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991 Mar;65(3):1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Poel C. L., Cuypers H. T., Reesink H. W., Weiner A. J., Quan S., Di Nello R., Van Boven J. J., Winkel I., Mulder-Folkerts D., Exel-Oehlers P. J. Confirmation of hepatitis C virus infection by new four-antigen recombinant immunoblot assay. Lancet. 1991 Feb 9;337(8737):317–319. doi: 10.1016/0140-6736(91)90942-i. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Kuo G., Bradley D. W., Bonino F., Saracco G., Lee C., Rosenblatt J., Choo Q. L., Houghton M. Detection of hepatitis C viral sequences in non-A, non-B hepatitis. Lancet. 1990 Jan 6;335(8680):1–3. doi: 10.1016/0140-6736(90)90134-q. [DOI] [PubMed] [Google Scholar]
- Yoshizawa H., Itoh Y., Iwakiri S., Kitajima K., Tanaka A., Tachibana T., Nakamura T., Miyakawa Y., Mayumi M. Non-A, non-B (type 1) hepatitis agent capable of inducing tubular ultrastructures in the hepatocyte cytoplasm of chimpanzees: inactivation by formalin and heat. Gastroenterology. 1982 Mar;82(3):502–506. [PubMed] [Google Scholar]