ABSTRACT
BACKGROUND
Diabetes increases the risk of breast and colorectal cancers and has an undetermined relationship to cervical cancer. Improved screenings for these cancers are effective in reducing cancer mortality.
OBJECTIVES
To examine the prevalence of receiving recommended screenings for these cancers and to assess the trends in the screening rates over time among US women with diagnosed diabetes in comparison with women without diabetes.
DESIGN
Cross-sectional.
PARTICIPANTS
A total of 63,650 to 182,168 adult women participated in the 1996−2006 (biennially) Behavioral Risk Factor Surveillance System.
METHODS
The prevalence of receiving cancer screenings was age-standardized to the 2000 US population. The adjusted prevalence and adjusted odds ratios (AORs) with 95% confidence intervals (CIs) were estimated using logistic regression analyses. The linear trends in the screening rates were tested using orthogonal polynomial contrasts.
RESULTS
In 2006, women with diabetes had a lower adjusted prevalence (74% versus 79%, P < 0.05) and the AOR (0.73, 95% CI: 0.66−0.81) for receiving cervical cancer screenings, but had a higher adjusted prevalence (63% versus 60%, P < 0.05) and the AOR (1.14, 95% CI: 1.04−1.24) for receiving colorectal cancer screenings compared to those without. In both women with diabetes and those without, the screening rate for colorectal cancer increased linearly during 2002−2006, whereas the screening rates for breast and cervical cancers changed little during 1996−2006.
CONCLUSION
Women with diabetes were equally likely to be screened for breast cancer, less likely to be screened for cervical cancer, but more likely to be screened for colorectal cancer compared to those without. Overall, the screening rates in both groups remain below the recommended levels.
KEY WORDS: diabetes mellitus, mammogram, Papanicolaou test, fecal occult blood test, sigmoidoscopy/colonoscopy
INTRODUCTION
Breast, colorectal and cervical cancers are the leading causes of cancer death among US women1,2. Screening tests for these cancers have been effective in early detection, and improved screening modalities are largely responsible for decreased cancer mortality3–5. The US Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS) recommend that all women aged ≥40 years should have a mammogram every 1-2 years and that all women should have a regular Papanicolaou (Pap) test every year starting approximately 3 years after they become sexually active, but no later than their 21st birthday [except for those over the age of 65 (by the USPSTF) or 70 (by the ACS) who have had three or more consecutive normal Pap test results within the previous 10 years]2,6. Screening for colorectal cancer should be done every year with a fecal occult blood test (FOBT), or every 5 years with a sigmoidoscopy, or every 10 years with a colonoscopy beginning at age 50 for both men and women2,6. Presently, the precise age at which to discontinue these cancer screenings is uncertain2,6. People at increased risk for cancers should get screened earlier and more often, or may have additional tests 2.
Diabetes is associated with an increased risk for breast and colorectal cancers7,8. In addition, diabetes adversely alters the presentation of breast cancer and is associated with negative prognostic factors9. Major risk factors for diabetes, such as obesity, hyperglycemia or impaired glucose tolerance and the metabolic syndrome, are all associated with an increased risk for colorectal cancer10–12, and hyperinsulinemia may be involved in carcinogenesis13. The relationship between diabetes and risk of cervical cancer remains to be evaluated.
In 1999−2000, the prevalence of diabetes in US women aged ≥20 years was 8.2%14, and it continues to increase15. Although the cancer screenings have been recommended for all women in certain age groups, patients with diabetes may have a greater need for cancer screenings than those without given the increases risk for common cancers in these patients. However, adherence to the cancer screening guidelines is not well known among US women with diabetes at the national level. By analyzing data from large nationally representative samples, we aimed to (1) examine the prevalence of receiving breast, cervical and colorectal cancer screenings within the recommended intervals among US women with diagnosed diabetes, (2) examine the trends in these screening rates over time and (3) compare these measures with those seen in women without diabetes.
METHODS
Data for our analyses came from the Behavioral Risk Factor Surveillance System (BRFSS), a population-based telephone survey of health-related behaviors regarding the leading causes of death among noninstitutionalized US adults aged ≥18 years. We analyzed the data collected biennially from female survey participants in all 50 states and the District of Columbia during 1996−2006. The median cooperation rate (the percentage of eligible persons contacted who completed the interview) ranged from 68.2% to 74.5%. The survey was reviewed by the Human Research Protection Office at the Centers for Disease Control and Prevention and determined to be exempt from human subject guidelines. Further information on BRFSS is available at http://www.cdc.gov/brfss/.
Respondents’ diabetes status was assessed by asking them whether they had ever been told by a doctor, nurse or other health professional that they had diabetes. Those who answered that they had not been so told or had been told so only during pregnancy or had borderline diabetes were not considered to have diagnosed diabetes. Breast and cervical cancer screenings were assessed by asking respondents whether they had received a mammogram (assessed only in women aged ≥40 years) or Pap test (assessed in women aged 18−<70 years) within the previous 2 years. Colorectal cancer screenings were assessed only among survey participants aged ≥50 years during the year of 2002, 2004 and 2006, who were asked whether they had had a FOBT within the previous year or a sigmoidoscopy/colonoscopy within the previous 10 years (an answer of “yes” to either question was defined as having colorectal screenings). The screening intervals for these cancers were based on the USPSTF and the ACS recommendations described above2,6, which were used in a recent study16.
The demographic variables in our analyses included respondents’ age, body mass index (BMI, self-reported weight in kilograms divided by the square of height in meters), race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and others), marital status (married, divorced, never married, and others) and education (< high school diploma, high school graduate and ≥ some college or college graduate). We also included in our analyses respondents’ status on smoking, health insurance and a routine checkup visit. Current smokers were those participants who had smoked ≥100 cigarettes during their lifetime and were still smoking. Current non-smokers were those who either had smoked <100 cigarettes during their lifetime or had smoked ≥100 cigarettes in their entire life but had stopped. Health insurance status was assessed by asking respondents whether they had any kind of health-care coverage including health insurance, prepaid plans (i.e., HMOs) or governmental plans (i.e., Medicare). A routine checkup visit was assessed by asking respondents whether they had visited a doctor for a routine checkup within the previous 2 years. BRFSS data have consistently been found to provide valid and reliable estimates of the population prevalence of certain chronic conditions and of routine screening examinations when compared to other national household surveys in the US17,18.
After excluding from the analyses participants who responded “don’t know/not sure,” refused to answer or had missing responses for any of the questions described previously, a total of 38,111 (in 1996) to 140,936 (in 2006) women (aged ≥40 years) were included in our analyses to assess the trends in the prevalence of receiving breast cancer screenings, a total of 54,181 (in 1996) to 150,634 (in 2006) women (aged 18−<70 years) were included to assess the trends in the prevalence of receiving cervical cancer screenings, and a total of 58,312 (in 2002) to 103,601 (in 2006) women (aged ≥50 years) were included to assess the trends in the prevalence of receiving colorectal cancer screenings. The prevalence estimates for receiving cancer screenings within the recommended intervals by diabetes status were age-standardized to the 2000 US population. Logistic regression analyses were conducted on the data of 2006 to estimate the adjusted prevalence and the adjusted odds ratios (AORs) with 95% confidence intervals (CIs) for receiving these screenings in women with diabetes (women without diabetes used as the referent). The linear trends in the screening rates were tested using orthogonal polynomial contrasts with SUDAAN software (release 9.0; Research Triangle Institute, Research Triangle Park, NC), which was used to account for the multi-stage, disproportionate stratified sampling design.
RESULTS
Among the participants included in our analyses, the number of women with diagnosed diabetes aged ≥40 years ranged from 2,736 in 1996 to 16,256 in 2006, the number of women with diagnosed diabetes aged 18−<70 years ranged from 2,017 in 1996 to 11,665 in 2006, and the number of women with diagnosed diabetes aged ≥50 years ranged from 6,940 in 2002 to 14,183 in 2006.
Univariate and Multivariate Analyses of Receipt of Cancer Screenings by Diabetes Status During 2006
In 2006, among both those with diabetes and those without, the percentages of women who were screened for breast and colorectal cancers generally increased with age, while the percentages of women who were screened for cervical cancer generally decreased with age (P < 0.05 for linear trends, Tables 1 and 2). In addition, the prevalence of receiving screenings for all three cancers increased with increases in educational levels and was significantly higher among women who had health insurance coverage or had had a routine checkup within the previous 2 years than among those who did not (P < 0.05 for all).
Table 1.
Age-Standardized Percentages of US Women with or Without Diabetes Who Had Received Screenings for Breast and Cervical Cancers Within the Previous 2 Years by Selected Characteristics, BRFSS 2006
| Breast cancer | Cervical cancer | |||
|---|---|---|---|---|
| Diabetes (n = 16,256) | No diabetes (n = 124,680) | Diabetes (n = 11,665) | No diabetes (n = 138,969) | |
| Total | 76.6 (0.8) | 76.7 (0.2) | 75.1 (1.8)† | 79.3 (0.3) |
| Age (years) | ||||
| 18–29 | — * | — | 73.7 (6.1) | 77.4 (0.8) |
| 30–39 | — | — | 83.1 (3.5) | 85.6 (0.4) |
| 40–49 | 71.0 (1.9) | 69.6 (0.5) | 75.6 (1.9)‡ | 81.4 (0.4) |
| 50–59 | 80.6 (1.2) | 80.6 (0.4) | 72.1 (1.4)‡ | 77.1 (0.5) |
| 60–69 | 82.9 (1.1) | 83.3 (0.4) | 65.0 (1.4)‡ | 69.7 (0.6) |
| ≥70 | 75.9 (1.3)§ | 78.5 (0.5) | — | — |
| Race | ||||
| Non-Hispanic white | 74.9 (1.0)† | 77.0 (0.2) | 75.6 (2.0)† | 79.6 (0.3) |
| Non-Hispanic black | 81.0 (1.2)† | 77.7 (0.9) | 85.8 (1.8) | 83.0 (0.7) |
| Hispanic | 75.7 (3.0) | 76.0 (1.2) | 67.4 (5.0)† | 77.9 (0.9) |
| Other | 78.4 (3.0) | 71.3 (1.5) | 74.0 (4.3) | 72.7 (1.2) |
| Marital status | ||||
| Married | 79.2 (1.1) | 79.9 (0.3) | 78.0 (2.6) | 84.0 (0.3) |
| Divorced | 74.6 (1.7) | 72.6 (0.7) | 77.7 (1.8) | 78.8 (0.8) |
| Never married | 75.6 (2.8) | 71.6 (1.0) | 74.3 (3.8) | 73.5 (0.7) |
| Other | 70.4 (2.5) | 70.0 (0.9) | 74.0 (5.0) | 76.6 (0.8) |
| Education | ||||
| < High school diploma | 70.2 (2.3)† | 64.9 (1.2) | 68.5 (4.0) | 69.7 (1.1) |
| High school graduate | 77.5 (1.3)‡ | 73.7 (0.4) | 72.6 (3.1) | 75.1 (0.5) |
| ≥ Some college or college graduate | 78.3 (1.2) | 79.8 (0.3) | 79.9 (1.7) | 82.3 (0.3) |
| BMI (kg/m2) | ||||
| <25.0 | 74.2 (2.3) | 76.5 (0.4) | 75.8 (2.9) | 79.9 (0.4) |
| 25.0-<30.0 | 78.9 (1.6) | 77.5 (0.4) | 75.5 (3.5) | 81.1 (0.4) |
| ≥30.0 | 76.3 (1.0) | 75.9 (0.5) | 74.8 (2.7) | 77.2 (0.5) |
| Smoking | ||||
| Smoker | 68.3 (1.9)† | 64.2 (0.7) | 72.6 (3.2) | 72.5 (0.5) |
| Non-smoker | 78.6 (0.9) | 79.3 (0.3) | 75.7 (2.1)† | 80.9 (0.3) |
| Health insurance | ||||
| Yes | 79.2 (0.8) | 79.3 (0.2) | 77.8 (2.2)† | 82.2 (0.3) |
| No | 64.6 (2.8)‡ | 53.1 (1.3) | 63.6 (3.1) | 62.0 (0.8) |
| Routine checkup within the past 2 years | ||||
| Yes | 79.1 (0.8)‡ | 82.4 (0.2) | 78.0 (1.9)‡ | 85.2 (0.3) |
| No | 46.3 (3.6)† | 37.0 (0.8) | 46.1 (4.6) | 47.5 (0.7) |
Data expressed as percentages with standard errors in parentheses. *Not recommended for cancer screenings at those age groups; †P < 0.05, ‡P < 0.01 and §P < 0.1 for comparisons between women with diabetes and those without; BRFSS: Behavioral Risk Factor Surveillance System
Table 2.
Age-Standardized Percentages of US Women with or Without Diabetes Who Had Received Screenings* for Colorectal Cancer by Selected Characteristics, BRFSS 2006
| Colorectal cancer | ||
|---|---|---|
| Diabetes (n = 14,183) | No diabetes (n = 89,418) | |
| Total | 63.1 (0.9)† | 60.8 (0.3) |
| Age (years) | ||
| 50–59 | 55.4 (1.6)† | 51.5 (0.5) |
| 60–69 | 66.1 (1.7) | 66.4 (0.6) |
| ≥70 | 68.3 (1.2) | 67.6 (0.6) |
| Race | ||
| Non-Hispanic white | 62.7 (0.9) | 62.0 (0.3) |
| Non-Hispanic black | 65.2 (2.1)‡ | 57.1 (1.4) |
| Hispanic | 53.6 (4.2) | 50.4 (2.2) |
| Other | 66.6 (4.0) | 59.7 (2.1) |
| Marital status | ||
| Married | 65.8 (1.3) | 64.3 (0.4) |
| Divorced | 59.6 (2.0) | 57.6 (0.9) |
| Never married | 53.6 (3.7) | 56.0 (1.4) |
| Other | 60.6 (2.1) | 54.6 (1.5) |
| Education | ||
| < High school diploma | 54.5 (2.6)† | 47.6 (1.2) |
| High school graduate | 62.2 (1.4)‡ | 57.3 (0.5) |
| ≥ Some college or college graduate | 67.1 (1.2) | 65.2 (0.4) |
| BMI (kg/m2) | ||
| <25.0 | 63.4 (3.1) | 60.7 (0.5) |
| 25.0-<30.0 | 61.8 (1.6) | 61.5 (0.5) |
| ≥30.0 | 63.0 (1.2)† | 60.2 (0.7) |
| Smoking | ||
| Smoker | 56.7 (2.4)‡ | 48.5 (0.9) |
| Non-smoker | 63.2 (1.0) | 62.9 (0.4) |
| Health insurance | ||
| Yes | 64.0 (0.9) | 62.7 (0.3) |
| No | 49.2 (4.3)‡ | 36.8 (1.8) |
| Routine checkup within the past 2 years | ||
| Yes | 63.6 (0.9) | 64.3 (0.3) |
| No | 46.0 (3.7)‡ | 31.0 (0.9) |
Data expressed as percentages with standard errors in parentheses. *A composite measure of colorectal cancer screenings by a fecal occult blood test within the previous year and/or a sigmoidoscopy/colonoscopy within the previous 10 years. †P < 0.05 and ‡P < 0.01 for comparisons between women with diabetes and those without; BRFSS: Behavioral Risk Factor Surveillance System
Overall, compared to women without diabetes, women with diabetes had a similar screening rate for breast cancer, had a significantly lower screening rate for cervical cancer (P < 0.05, Table 1) and had a significantly higher screening rate for colorectal cancer (P < 0.05, Table 2). Among non-Hispanic white women, significantly lower percentages of women with diabetes were screened for breast or cervical cancers than those without diabetes (P < 0.05 for both); however, among non-Hispanic black women, significantly higher percentages of women with diabetes were screened for breast or colorectal cancers than those without diabetes (P < 0.05 or P < 0.01). Hispanic women with diabetes had a significantly lower screening rate for cervical cancer than Hispanic women without diabetes (P < 0.05). In addition, among those who attained educational levels of ≤ high school diploma, were smokers, had no health insurance coverage or had no routine checkup within the past 2 years, women with diabetes had significantly higher screening rates for breast and colorectal cancers than those without diabetes (P < 0.05 for all). In contrast, among those who were non-smokers, had health insurance coverage or had had routine checkup within the past 2 years, women with diabetes had a significantly lower screening rate for cervical cancer than those without diabetes (P < 0.05 for all).
For specific colorectal cancer screening tests, women with diabetes were significantly more likely to be screened by a FOBT than those without diabetes (18.3% versus 15.6%, P < 0.05). However, the percentages of women who were screened by a sigmoidoscopy/colonoscopy test did not differ by diabetes status (56.9% versus 56.2%).
After adjustment for age, race/ethnicity, BMI, education, marital status, current smoking, health insurance and a routine checkup visit, women with diabetes had a significantly lower adjusted prevalence (74% versus 79%, P < 0.05) and the AOR (0.73, 95% CI: 0.66−0.81) for receiving cervical cancer screenings, but had a significantly higher adjusted prevalence (63% versus 60%, P < 0.05) and the AOR (1.14, 95% CI: 1.04−1.24) for receiving colorectal cancer screenings compared to those without diabetes (Table 3). No significant difference was observed between women with diabetes and those without in receiving breast cancer screenings.
Table 3.
Adjusted Prevalence of Receiving Screenings for Breast, Cervical and Colorectal Cancers Within the Recommended Intervals in Women with and Without Diabetes, and the Odds Ratios for Receiving These Screenings in Women with Diabetes Using Women Without Diabetes as the Referent, BRFSS 2006
| Cancer screening | Age group | Adjusted* prevalence (SE) | Odd ratios (95% CI) | ||
|---|---|---|---|---|---|
| Diabetes | No diabetes | Unadjusted | Adjusted* | ||
| Breast cancer | ≥40 years | 76.4 (0.7) | 77.1 (0.2) | 1.09 (1.00-1.18) | 0.96 (0.87-1.05) |
| Cervical cancer | 18-<70 years | 74.2 (0.8)† | 79.0 (0.2) | 0.65 (0.60-0.71) | 0.73 (0.66-0.81) |
| Colorectal cancer | ≥50 years | 63.2 (0.9)‡ | 60.4 (0.3) | 1.15 (1.06-1.25) | 1.14 (1.04-1.24) |
| Fecal occult blood test | ≥50 years | 18.3 (0.6)† | 15.5 (0.2) | 1.27 (1.15-1.40) | 1.24 (1.12-1.36) |
| Sigmoidoscopy/ colonoscopy | ≥50 years | 57.0 (0.9) | 55.8 (0.3) | 1.06 (0.98-1.15) | 1.05 (0.97-1.14) |
*Adjusted for age, body mass index, race/ethnicity, education, marital status, current smoking, health insurance and a routine checkup visit. †P < 0.001 and ‡P < 0.01 for comparisons between women with diabetes and those without. BRFSS: Behavioral Risk Factor Surveillance System; CI: confidence interval; SE: standard error
Trends in the Prevalence of Receiving Cancer Screenings by Diabetes Status
During the past 10 years, among women aged ≥40 years, the percentages of women with diabetes who were screened for breast cancer increased slightly from 74.4% (95% CI: 71.3-77.3%) in 1996 to 76.6% (95% CI: 74.9-78.1%) in 2006, similar to the percentages observed in women without diabetes (Fig. 1a).
Figure 1.
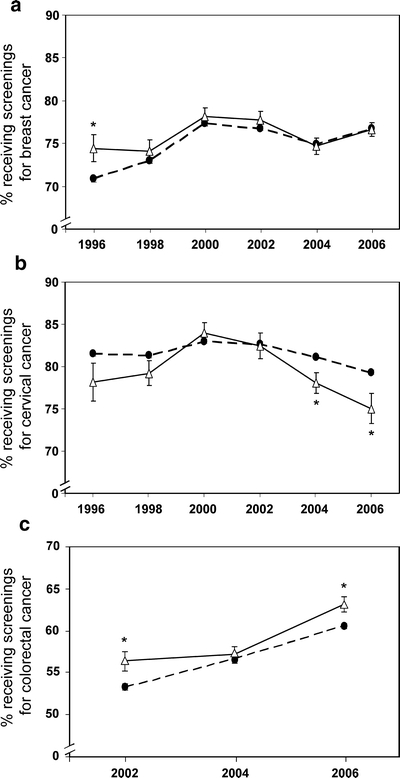
Age-standardized percentages of US women with diagnosed diabetes (solid lines) and those without (dashed lines) who received screenings for (A) breast cancer (among those aged ≥40 years) within the previous 2 years during the period of 1996-2006, (B) cervical cancer (among those aged 18−<70 years) within the previous 2 years during the period of 1996-2006 and (C) colorectal cancer (among those aged ≥50 years) within the recommended intervals during the period of 2002-2006. *P < 0.05 for comparisons between women with diabetes and those without in a year.
Among women aged 18−<70 years, the percentages of women with diabetes who were screened for cervical cancer decreased slightly from 78.2% (95% CI: 73.5-82.2%) in 1996 to 75.1% (95% CI: 71.4-78.3%) in 2006. In 2004 and 2006, the screening rate for cervical cancer was significantly lower in women with diabetes than in those without diabetes (P < 0.05 for both, Fig. 1b).
Among women aged ≥50 years, the percentages of women with diabetes who were screened for colorectal cancer increased significantly from 56.4% (95% CI: 54.1-58.7%) in 2002 to 63.1% (95% CI: 61.3-64.9%) in 2006 (P < 0.05 for a linear trend, Fig. 1c); the similar trend was also observed in women without diabetes. However, in 2002 and 2006, women with diabetes were significantly more likely to be screened for colorectal cancer than women without diabetes (P < 0.05 for both). For specific colorectal cancer screening tests, we found that women with diabetes who were screened by a FOBT decreased significantly from 22.0% (95% CI: 20.2-23.9%) in 2002 to 18.3% (95% CI: 17.0-19.7%) in 2006 (P < 0.01 for a linear trend), whereas women with diabetes who were screened by a sigmoidoscopy/colonoscopy increased significantly from 46.6% (95% CI: 44.4-48.9%) in 2002 to 56.9% (95% CI: 55.0-58.7%) in 2006 (P < 0.01 for a linear trend).
DISCUSSION
Since the prevalence of diabetes in the US continues to increase14,15 and women with diabetes are at a higher risk for common cancers showing increased incidence and mortality of cancers7,8,19, it is of paramount importance that women with diabetes receive the recommended screenings for various cancers. However, our results, at the national level, demonstrate that the screening rates for breast, cervical and colorectal cancers among US women with diabetes are below the Healthy People 2010 Objectives on cancer screenings for all US women20.
To our knowledge, this was the first large study to examine screening rates over time for highly prevalent cancers by diabetes status. Our findings showed that, overall, the screening rates for breast and cervical cancers among US women with diabetes did not change much from 1996 to 2006. Although we did find that the screening rate for colorectal cancer increased from 2002 to 2006, even by 2006, only 63% of US women with diabetes were screened for colorectal cancer. Importantly, we found that the rate of having a FOBT for colorectal cancer screenings among women with diabetes actually decreased from 2002 to 2006. Thus, a wide gap exists between increased risk of cancers and the receipt of cancer screenings in this population.
Two early and one recent case-control studies showed that the rate of having a mammography screening were significantly lower among women with diabetes than among those without diabetes21–23; however, these studies were limited either by small sample sizes or the inclusion of women aged ≥50 years. In addition, a Canadian study of women aged 50−67 years also showed a lower rate of receiving a mammography screening among those with diabetes than those without 24. In contrast, our results showing that, among women aged 40−69 years, those with diabetes had a similar screening rate for breast cancer to those without diabetes are apparently opposite to the findings of the previous studies. However, our results partially agree with the previous findings that, among women aged ≥70 years, the screening rate for breast cancer tended to be lower among those with diabetes than among those without (76% versus 79% in 2006, P = 0.069). Thus, the apparent differences between our results and those of others may partially result from the different age compositions of the study populations.
For cervical cancer screenings, our results demonstrate that women with diabetes were less likely to be screened for cervical cancer than women without diabetes and that the screening rate for cervical cancer was in a declining trend in 2004 and 2006. Whether or not the provision of diabetes-related services during a health-care visit competes for resources and time availability with preventive services in patients with diabetes remains controversial25. Nonetheless, attention needs to be directed to the impact that a decline in the screening rate for cervical cancer may have on the health of women with diabetes, and the similar concern exists among women without diabetes as well.
Previous studies of colorectal cancer screenings among women with diabetes have produced mixed results. Bell et al. reported that people with diabetes were as likely or more likely than those without diabetes to report having been screened for colorectal cancer26; however, a recent study showed that elderly women (≥67 years old) with diabetes were less likely to be screened for colorectal cancer than those without diabetes23, though the type of colorectal cancer screenings was not analyzed separately in this study23. Our results demonstrated that women with diabetes were more likely than those without to be screened for colorectal cancer (being screened either by the composite measure of a FOBT and/or a sigmoidoscopy/colonoscopy, or by a FOBT only). In addition, although we have demonstrated that the overall screening rate for colorectal cancer among women with diabetes increased in a linear manner from 2002 to 2006, the screening rate by a FOBT actually decreased during this period; thus, the goal of universal screenings for colorectal cancer in the US remains a distant target.
Our study has several limitations. First, self-reported measures of diabetes status and the receipt of cancer screenings were used, and are thus subject to recall bias. Second, we were unable to exclude women who had already had cancers or had conditions to be diagnosed; therefore, we were not sure whether a test was for screening only or for a diagnostic purpose. Also, we were unable to exclude women for whom screenings for cancers were no longer needed (e.g., women who had had a normal Pap test for many years). Third, for diabetes status, we were unable to distinguish types of diabetes (type 1 versus type 2), which may be differentially associated with an increased risk of cancers. It has been reported that type 1 diabetes accounts for 7% of all diabetes in the BRFSS participants27. Moreover, detailed information about whether women with diabetes were taking insulin or other diabetes medications was not collected in the present study. Results of a recent study showed that diabetes patients receiving medications were more likely to undergo low endoscopy than those treated by diet control alone28. In addition, duration of diabetes may also influence the adherence to the cancer screening guidelines among diabetes patients, which could not be evaluated in the present study. Future studies may assess the cancer screening rates by the type, duration and medications of diabetes.
In conclusion, in the US, the proportion of women with diagnosed diabetes who were screened for breast, cervical and colorectal cancers remains below the recommended levels. Women with diabetes were equally likely to be screened for breast cancer, less likely to be screened for cervical cancer, but more likely to be screened for colorectal cancer compared to those without diabetes. Given the important role of physicians, especially obstetrician/gynecologists23, in promoting preventive screening practices, our results call for more efforts from health-care professionals to educate diabetes patients about the health benefits of cancer screenings and to advise them to adopt good preventive health behaviors.
Acknowledgements
We thank Dr. Mary White from the Division of Cancer Prevention and Control, Centers for Disease Control and Prevention, for reviewing the manuscript and providing useful comments.
The abstract was presented in the 68th American Diabetes Association (ADA) Scientific Session, June 6-10, 2008 in San Francisco, CA.
Conflict of Interest None disclosed.
Disclaimer The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–52. [DOI] [PubMed]
- 2.American Cancer Society. Cancer Statistics 2007. Available at URL:http://www.cancer.org/downloads/STT/Cancer_Statistics_Combined_2007.ppt#256,1,Cancer Statistics. Accessed Nov. 12, 2008.
- 3.Tabar L, Yen MF, Vitak B, Chen HH, Smith RA, Duffy SW. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet. 2003;361:1405–10. [DOI] [PubMed]
- 4.Gustafsson L, Ponten J, Zack M, Adami HO. International incidence rates of invasive cervical cancer after introduction of cytological screening. Cancer Causes Control. 1997;8:755–63. [DOI] [PubMed]
- 5.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota colon cancer control study. N Engl J Med. 1993;328:1365–71. [DOI] [PubMed]
- 6.U.S. Department of Health and Human Services. The guide to clinical preventive services, 2007: Recommendations of the U.S. Preventive Services Task Force. Available at URL: http://www.ahrq.gov/clinic/pocketgd07/pocketgd07.pdf. Accessed Nov. 12, 2008.
- 7.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–87. [DOI] [PubMed]
- 8.Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–62. [DOI] [PubMed]
- 9.Wolf I, Sadetzki S, Gluck I, et al. Association between diabetes mellitus and adverse characteristics of breast cancer at presentation. Eur J Cancer. 2006;42:1077–82. [DOI] [PubMed]
- 10.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. 2006;55:285–91. [DOI] [PMC free article] [PubMed]
- 11.Marugame T, Lee K, Eguchi H, Oda T, Shinchi K, Kono S. Relation of impaired glucose tolerance and diabetes mellitus to colorectal adenomas in Japan. Cancer Causes Control. 2002;13:917–21. [DOI] [PubMed]
- 12.Morita T, Tabata S, Mineshita M, Mizoue T, Moore MA, Kono S. The metabolic syndrome is associated with increased risk of colorectal adenoma development: the Self-Defense Forces health study. Asian Pac J Cancer Prev. 2005;6:485–9. [PubMed]
- 13.Yoshida I, Suzuki A, Vallee M, et al. Serum insulin levels and the prevalence of adenomatous and hyperplastic polyps in the proximal colon. Clin Gastroenterol Hepatol. 2006;4:1225–31. [DOI] [PubMed]
- 14.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: national health and nutrition examination survey 1999-2002. Diabetes Care. 2006;29:1263–8. [DOI] [PubMed]
- 15.Centers for Disease Control and Prevention. Diabetes Data & Trends. Available at URL: http://www.cdc.gov/diabetes/statistics/prev/national/figbysex.htm. Accessed Nov. 12, 2008.
- 16.Ahluwalia IB, Bolen J, Garvin B. Health insurance coverage and use of selected preventive services by working-age women, BRFSS, 2006. J Womens Health. 2007;16:935–40. [DOI] [PubMed]
- 17.Nelson DE, Holtzman D, Bolen J, Stanwyck CA, Mack KA. Reliability and validity of measures from the Behavioral Risk Factor Surveillance System (BRFSS). Soc Prev Med. 2001;46(suppl 1):S3-S42. [PubMed]
- 18.Martin LM, Leff M, Calonge N, Garrett C, Nelson DE. Validation of self-reported chronic conditions and health services in a managed care population. Am J Prev Med. 2000;18:215–8. [DOI] [PubMed]
- 19.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol. 2004;159:1160–7. [DOI] [PubMed]
- 20.U.S. Department of Health and Human Services. Healthy People 2010. Available at URL: http://www.healthypeople.gov/Search/objectives.htm. Accessed Nov. 12, 2008.
- 21.Beckman TJ, Cuddihy RM, Scheitel SM, Naessens JM, Killian JM, Pankratz VS. Screening mammogram utilization in women with diabetes. Diabetes Care. 2001;24:2049–53. [DOI] [PubMed]
- 22.Fontana SA, Baumann LC, Helberg C, Love RR. The delivery of preventive services in primary care practices according to chronic disease status. Am J Public Health. 1997;87:1190–6. [DOI] [PMC free article] [PubMed]
- 23.McBean AM, Yu X. The underuse of screening services among elderly women with diabetes. Diabetes Care. 2007;30:1466–72. [DOI] [PubMed]
- 24.Lipscombe LL, Hux JE, Booth GL. Reduced screening mammography among women with diabetes. Arch Intern Med. 2005;165:2090–5. [DOI] [PubMed]
- 25.Tabaei BP, Herman WH, Jabarin AF, Kim C. Does diabetes care compete with the provision of women's preventive care services? Diabetes Care. 2005;28:2644–9. [DOI] [PubMed]
- 26.Bell RA, Shelton BJ, Paskett ED. Colorectal cancer screening in North Carolina: associations with diabetes mellitus and demographic and health characteristics. Prev Med. 2001;32:163–7. [DOI] [PubMed]
- 27.Beckles GL, Engelgau MM, Narayan KM, Herman WH, Aubert RE, Williamson DF. Population-based assessment of the level of care among adults with diabetes in the U.S. diabetes care. 1998;21:1432–8. [DOI] [PubMed]
- 28.Lewis JD, Capra AM, Achacoso NS, et al.. Medical therapy for diabetes is associated with increased use of lower endoscopy. Pharmacoepidemiol Drug Saf. 2007;16:1195–202. [DOI] [PubMed]


