Abstract
Background
Previous systematic reviews concluded that tricyclics antidepressants are superior to gabapentin for neuropathic pain, but were based on indirect comparisons from placebo-controlled trials.
Purpose
To evaluate gabapentin versus tricyclic antidepressants for diabetic neuropathy and post-herpetic neuralgia, using direct and indirect comparisons.
Data Sources
MEDLINE (1966 to March Week 4 2008), the Cochrane central register of controlled trials (1st quarter 2008), and reference lists.
Study Selection
We selected randomized trials directly comparing gabapentin versus tricyclic antidepressants or comparing either of these medications versus placebo.
Data Extraction
Studies were reviewed, abstracted, and quality-rated by two independent investigators using predefined criteria.
Data Synthesis
We performed a meta-analysis of head-to-head trials using a random effects model and compared the results to an adjusted indirect analysis of placebo-controlled trials.
Results
In three head-to-head trials, there was no difference between gabapentin and tricyclic antidepressants for achieving pain relief (RR 0.99, 95% CI 0.76 to 1.29). In adjusted indirect analyses, gabapentin was worse than tricyclic antidepressants for achieving pain relief (RR = 0.41, 95% CI 0.23 to 0.74). The discrepancy between direct and indirect analyses was statistically significant (p = 0.008). Placebo-controlled tricyclic trials were conducted earlier than the gabapentin trials, reported lower placebo response rates, had more methodological shortcomings, and were associated with funnel plot asymmetry.
Conclusions
Though direct evidence is limited, we found no difference in likelihood of achieving pain relief between gabapentin and tricyclic antidepressants for diabetic neuropathy and post-herpetic neuralgia. Indirect analyses that combine data from sets of trials conducted in different eras can be unreliable.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-008-0877-5) contains supplementary material, which is available to authorized users.
KEY WORDS: meta-analysis, neuropathic pain, gabapentin, tricyclic antidepressant
INTRODUCTION
A number of medications, including gabapentin and tricyclic antidepressants, have been suggested as first-line treatment options for neuropathic pain.1–3 Previously published systematic reviews concluded that tricyclics are more effective than newer medications such as gabapentin for neuropathic pain.3–5 These findings were not based on head-to-head trials directly comparing these drugs. Rather, the systematic reviews compared how gabapentin and tricyclics each performed versus placebo. Trials that evaluate different drugs versus a common comparator (such as placebo) can provide indirect evidence about comparative effectiveness, while preserving some of the benefits of randomization.6,7 However, although indirect comparisons usually agree with direct comparisons from head-to-head trials, in some cases large discrepancies have been reported between direct and indirect analyses.8,9 The validity of indirect comparisons depends on how well the trials meet the critical assumption of consistent treatment effects across all the trials.9 This assumption can be violated due to methodological shortcomings in the trials or differences in populations, interventions, measurement of outcomes, or other factors.7,9
Indirect comparisons in previously published systematic reviews were “informal,” or based on qualitative rank-ordering of pooled estimates for different drugs from placebo-controlled trials.3–5 Such qualitative comparisons may not be a reliable substitute for formal quantitative analyses.7 Disadvantages of informal indirect comparisons are that they do not provide overall summary estimates of effect and do not adjust for additional uncertainty that occurs when comparing evidence indirectly.7,9 Formal “adjusted” indirect methods, on the other hand, provide a summary combined estimate and incorporate additive variance from both sets of trials.6,7 Sensitivity and subgroup analyses can also be performed to evaluate effects of methodological shortcomings and differences in study design, population characteristics, and interventions on estimates and conclusions.
The purpose of this study is to evaluate comparative benefits and harms of gabapentin versus tricyclic antidepressants for diabetic neuropathy and post-herpetic neuralgia and to compare results of direct and formal adjusted indirect analyses. We chose gabapentin and tricyclics because they are commonly prescribed for neuropathic pain, and several head-to-head trials10–12 are available. We chose diabetic neuropathy and post-herpetic neuralgia because they are both peripheral neuropathies and the most common neuropathic pain conditions in clinical trials. Because most trials of tricyclics were undertaken before trials of gabapentin, we postulated that discrepancies between direct and indirect estimates are likely to occur because of differences that have occurred over time in patient characteristics, management of neuropathic pain, and design and conduct of randomized trials.
METHODS
Data Sources and Searches
To identify potentially relevant citations, we searched Ovid MEDLINE® (1966 to March Week 4 2008), the Cochrane Database of Systematic Reviews® (1st Quarter 2008), the Cochrane Central Register of Controlled Trials® (1st Quarter 2008), and the Database of Abstracts of Reviews of Effects (1st Quarter 2008), using terms for gabapentin and tricyclic antidepressants, neuropathic pain and specific neuropathic pain conditions, and randomized trials (the complete search strategy is shown in Appendix 1, available online). We also reviewed reference lists and solicited pharmaceutical companies for additional citations.
Study Selection
All citations were reviewed for inclusion using the criteria shown in Table 1. We applied no language restriction. Two reviewers (RC and SC) independently assessed titles and abstracts and full-text articles of potentially relevant citations for inclusion. Disagreements were resolved by consensus. Trials published only in abstract form (e.g., a conference proceeding) were not included.13
Table 1.
Study Inclusion Criteria
| Study meets all of the following criteria: |
|---|
| •Randomized controlled trial |
| •Enrolls adults with diabetic neuropathy and/or postherpetic neuralgia, or at least 75% of enrollees have either diabetic neuropathy or postherpetic neuralgia |
| •Evaluates gabapentin or a tricyclic antidepressant (versus each other or versus placebo) |
| •Reports at least one of the following outcomes: pain relief (the proportion of patients with >50% improvement in pain score or at least moderate pain relief or good overall response on a categorical scale), withdrawal due to adverse events, overall adverse events, serious adverse events, somnolence (including sedation, fatigue, tiredness, and lethargy), dizziness or vertigo, ataxia (including gait disturbance or incoordination), or dry mouth |
Data Extraction and Quality Assessment
Two independent reviewers (RC and SC) abstracted the following information from included trials: study design, population characteristics; eligibility and exclusion criteria, interventions (dose and duration), numbers lost to follow-up, method of outcome ascertainment, and results. We recorded intention-to-treat results when reported. For crossover trials, we abstracted results from both crossover periods.14 If this data were not available, we abstracted results from the first intervention period. Two independent reviewers (RC and SC) also assessed internal validity (quality) of controlled clinical trials using predefined criteria for randomization and allocation concealment, blinding of patients and outcomes assessors, and use of intention-to-treat analysis (Appendix 2, available online). Disagreements were resolved by consensus.
Data Synthesis and Analysis
Our primary outcome was the proportion of patients reporting significant pain relief. We defined significant pain relief as at least 50% improvement in pain score compared to baseline (preferred outcome) or the proportion reporting at least moderate or good improvement in pain or global efficacy on a categorical scale. A similar approach for defining pain relief was used in previously published systematic reviews.3,5,15,16 For adverse events, we evaluated withdrawals due to adverse events, serious adverse events, somnolence (including sedation, tiredness, fatigue, or lethargy), ataxia (including gait disturbance and incoordination), dizziness or vertigo, and dry mouth.
We estimated pooled relative risks and 95% confidence intervals using the DerSimonian-Laird method in a random effects model.17 We chose the random effects model because trials differed in patient populations, dosing of drugs, and other factors. For all pooled estimates, trials with no events in either group were excluded; trials with events only in one group were analyzed by adding 0.5 to all cells. Statistical heterogeneity was assessed by calculating the percent of the total variance due to between-study variability (I2 statistic18). Higher I2 values indicate greater between-study heterogeneity. Relative risks and confidence intervals were calculated using the meta package in R.19 Forest plots were generated using RevMan 4.2.8 (Review Manager 4.2 for Windows, The Nordic Cochrane Center, Copenhagen, Denmark). When data were available from at least six trials, we constructed L’Abbe plots to identify outlier trials and to assess whether treatment effects vary with differences in underlying risk.20 We assessed funnel plot asymmetry (which can be due to publication bias) with the Egger test.21
We performed adjusted indirect comparisons using the method described by Bucher et al.6
We calculated indirect relative risks (RRInd) for gabapentin versus tricyclic antidepressants for each outcome, adjusted by the results of their comparisons against placebo:
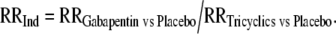 |
The variance was estimated as:
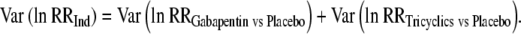 |
To test assumptions regarding similarity of treatment effects across trials, we compared mean placebo response rates in trials of gabapentin and tricyclics. We also performed subgroup and sensitivity analyses on study design factors (use of crossover versus parallel-group design, methodological quality criteria, and publication before or after 1997), intervention factors (evaluation of a dose of <2,400 mg/day of gabapentin, evaluation of a secondary versus tertiary amine tricyclic, and use of an active versus inert placebo), and population factors (exclusion of previous non-responders to gabapentin and evaluation of diabetic neuropathy or post-herpetic neuralgia). When funnel plot asymmetry was detected, we performed sensitivity analyses using the trim and fill method, which generates “missing” studies until the plot becomes symmetrical.22
We measured the discrepancy between direct and indirect estimates by calculating the difference in log relative risks. We deemed a p value of less than 0.05 statistically significant.9
RESULTS
Figure 1 shows the flow of studies from initial results of literature searches to final inclusion or exclusion. Literature searches identified 549 citations, and 55 of these appeared potentially relevant. After review of full-text articles, 18 trials met inclusion criteria: 3 head-to-head trials of gabapentin versus tricyclic antidepressants,10–12 6 trials of gabapentin versus placebo,23–28 and 9 trials of tricyclic antidepressants versus placebo.29–37 A list of excluded trials and reasons for exclusion is available from the authors.
Figure 1.
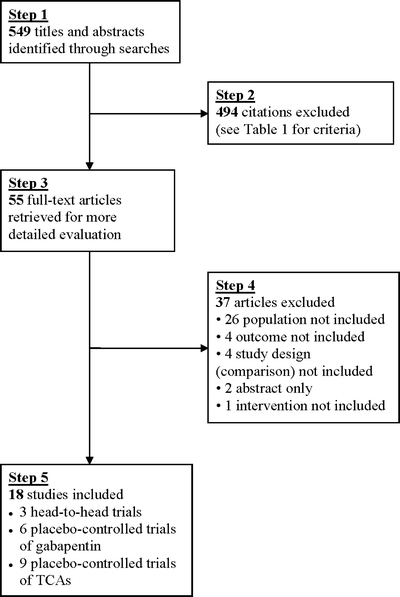
Literature search results.
Direct Meta-analysis
A total of 120 patients were evaluated in three head-to-head trials (n = 25 to 70) of gabapentin versus a tricyclic antidepressant for neuropathic pain (Table 2).10–12 Two trials compared gabapentin versus amitriptyline for diabetic neuropathy,11,12 and one trial compared gabapentin versus nortriptyline for post-herpetic neuralgia.10 The trials were published between 1999 and 2006. Dose of gabapentin varied, with titration up to 1,800 mg/day,12 2,400 mg/day,11 or 2,700 mg/day.10 Maximum doses of tricyclics ranged from 75 to 90 mg/day. One trial used a crossover design.12 Duration of therapy ranged from 4 to 6 weeks. None of the trials met all quality assessment criteria (Table 2). Two of the three trials did not describe funding source;11,12 the third10 reported funding from Pfizer India.
Table 2.
Characteristics of Included Trials and Main Results
| Author, year, design | Funding source | Drug, dose, duration (daily dose) | Type of neuropathic pain | Pain relief* | Quality items† |
|---|---|---|---|---|---|
| Gabapentin vs tricyclic antidepressanthead-to-head trials | |||||
| Chandra 200610 | Pfizer India Limited | A. Gabapentin up to 2,700 mg | Post-herpetic neuralgia | 16/34 (47.1%) vs 17/36 (47.2%) | 1)Y |
| 2)Y | |||||
| B. Nortriptyline up to 75 mg | 3)Y | ||||
| Parallel-group | 4)Y | ||||
| 8 weeks | 5)N | ||||
| Dalocchio 200011 | Not reported | A. Gabapentin mean 1,785 mg (max 2,400 mg) | Diabetic neuropathy | 11/13 (84.6%) | 1)NR |
| 2)NR | |||||
| B. Amitriptyline mean 53 mg (max 90 mg) | 9/12 (75.0%) | 3)N | |||
| Parallel-group | 4)N | ||||
| 4 weeks | 5)Y | ||||
| Morello 199912 | Not reported | A. Gabapentin 900-1,800 mg | Diabetic neuropathy | 11/21 (52.4%) | 1)NR |
| 2)NR | |||||
| B. Amitriptyline 25-75 mg | 14/21 (66.7%) | 3)Y | |||
| Crossover | 4)N | ||||
| 6 weeks | 5)N | ||||
| Gabapentin vs placebo | |||||
| Backonja 1998 23 | Parke-Davis Pharmaceuticals | Gabapentin up to 3,600 mg | Diabetic neuropathy | 47/79 (59.5%) vs 25/76 (32.9%) | 1)Y |
| 2)NR | |||||
| 3)Y | |||||
| Parallel-group | 8 weeks | 4)U | |||
| 5)Y | |||||
| Gilron 200524 | Canadian Institutes of Health Research | Gabapentin up to 3,200 mg (mean 2,207 mg) | Diabetic neuropathy or post-herpetic neuralgia | 27/44 (61.4%) vs 13/42 (31.0%) | 1)NR |
| 2)Y | |||||
| 3)Y | |||||
| Crossover | 5 weeks | 4)U | |||
| 5)U | |||||
| Gorson 199925 | Parke-Davis Pharma-ceuticals | Gabapentin 900 mg | Diabetic neuropathy | 17/19 (89.5%) vs 9/21 (42.9%) | 1)NR |
| 2)NR | |||||
| 3)U | |||||
| Crossover | 6 weeks | 4)U | |||
| 5)Y | |||||
| Rice 200126 | Pfizer Ltd. | Gabapentin 1,800 mg or 2,400 mg | Post-herpetic neuralgia | 74/223 (33.2%) vs 16/111 (14.4%) | 1)Y |
| 2)Y | |||||
| 3)Y | |||||
| Parallel-group | 7 weeks | 4)U | |||
| 5)N | |||||
| Rowbotham 199827 | Parke-Davis Pharmaceuticals | Gabapentin up to 3,600 mg | Post-herpetic neuralgia | 47/109 (43.1%) vs 14/116 (12.1%) | 1)Y |
| 2)NR | |||||
| 3)Y | |||||
| Parallel-group | 8 weeks | 4)U | |||
| 5)Y | |||||
| Simpson 200128 | Not reported | Gabapentin 900–2,700 mg | Diabetic neuropathy | 15/27 (55.6%) vs 7/27 (25.9%) | 1)NR |
| 2)NR | |||||
| 3)Y | |||||
| Parallel-group | 8 weeks | 4)U | |||
| 5)N | |||||
| Tricyclic antidepressant vs placebo | |||||
| Kishore-Kumar 199029 | Not reported | Desipramine 12.5–250 mg (mean 167 mg) | Post-herpetic neuralgia | 16/26 (61.5%) vs 3/26 (11.5%) | 1)NR |
| 2)NR | |||||
| 3)Y | |||||
| Crossover | 6 weeks | 4)U | |||
| 5)N | |||||
| Kvinesdal 198430 | Not reported | Imipramine 100 mg | Diabetic neuropathy | 7/12 (58.3%) vs 0/12 (0%) | 1)NR |
| 2)NR | |||||
| 3)Y | |||||
| Crossover | 5 weeks | 4)U | |||
| 5)N | |||||
| Max 198731 | Not reported | Amitriptyline 25–150 mg (mean 90 mg) | Diabetic neuropathy | 19/29 (65.5%) vs 1/29 (3.4%) | 1)NR |
| 2)NR | |||||
| 3)Y | |||||
| Crossover | 12 weeks | 4)Y | |||
| 5)N | |||||
| Max 198833 | Not reported | Amitriptyline 12.5–150 mg (mean 65 mg) | Post-herpetic neuralgia | 16/34 (47.1%) vs 4/25 (16.0%) | 1)NR |
| 2)NR | |||||
| 3)Y | |||||
| Crossover | 6 weeks | 4)U | |||
| 5)N | |||||
| Max 199132 | Not reported | Desipramine 12.5–250 mg (mean 201 mg) | Diabetic neuropathy | 13/24 (54.2%) vs 3/24 (12.5%) | 1)NR |
| 2)NR | |||||
| 3)Y | |||||
| Crossover | 6 weeks | 4)U | |||
| 5)N | |||||
| Raja 200234 | National Institutes of Health | Nortriptyline or desipramine 10–160 mg (mean 89 mg) | Post-herpetic neuralgia | NR | 1)Y |
| 2)Y | |||||
| 3)Y | |||||
| Crossover | 8 weeks | 4)U | |||
| 5)U | |||||
| Sindrup 198935 | Research Foundation of Vejle County, Denmark | Imipramine 125–225 mg (mean 178 mg) | Diabetic neuropathy | NR | 1)NR |
| 2)NR | |||||
| 3)Y | |||||
| Crossover | 3 weeks | 4)U | |||
| 5)N | |||||
| Sindrup 199036 | Danish Diabetes Association | A: Desipramine 50–200 mg | Diabetic neuropathy | NR | 1)NR |
| B: Clomipramine 50–75 mg | 2)NR | ||||
| C: Placebo | 3)Y | ||||
| Crossover | 2 weeks | 4)U | |||
| 5)N | |||||
| Watson 198237 | Not reported | Amitriptyline 25–125 mg (median 75 mg) | Post-herpetic neuralgia | 16/24 (66.7%) vs 1/24 (4.2%) | 1)NR |
| 2)NR | |||||
| 3)Y | |||||
| Crossover | 3 weeks | 4)U | |||
| 5)U | |||||
*≥50% improvement in pain or at least moderate pain relief; †1) Randomization method, 2) allocation concealment, 3) masked patients, 4) masked outcome assessors, 5) intention-to-treat analysis; NR = not reported; N = no; Y = yes; U = unclear; max = maximum
We found no difference between gabapentin and tricyclic antidepressants in likelihood of experiencing pain relief (Fig. 2) (RR = 0.99, 95% CI 0.76 to 1.29, I2 = 0%, three trials).10–12 Estimates were similar when trials were stratified according to whether they evaluated diabetic neuropathy (RR = 0.98, 95% CI 0.69 to 1.38, I2 = 26%, two trials11,12) or post-herpetic neuralgia (RR = 1.00, 95% CI 0.61 to 1.64, one trial10). Estimates were also similar after excluding the crossover trial12 and when trials were stratified by methodological quality indicators.
Figure 2.
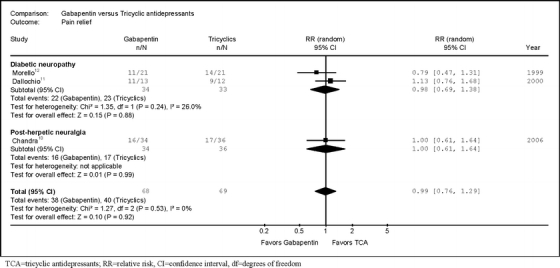
Relative risk for pain relief from head-to-head trials of gabapentin versus tricyclic antidepressants.
There was no difference between gabapentin versus tricyclics in rates of withdrawal due to adverse events (RR 0.27, 95% CI 0.03 to 2.34), but only three cases were reported in two trials.10,12 None of the trials reported serious adverse events. There was no significant difference between gabapentin and tricyclics in risk of somnolence (RR 1.22, 95% CI 0.59 to 2.52, two trials10,12), dry mouth (RR 0.16, 95% CI 0.01 to 2.66, two trials10,12), or dizziness (RR 3.65, 95% CI 0.85 to 15.78, one trial12), but all estimates were imprecise.
Indirect Meta-analysis
Analysis of Placebo-Controlled Trials
Sample sizes in six placebo-controlled trials of gabapentin23–28 ranged from 40 to 334 (median 112), and in nine placebo-controlled trials of tricyclics29–37 ranged from 12 to 76 (median 26). All of the gabapentin trials were published in or after 1997. All of the tricyclic trials that reported the primary outcome pain relief were published in or before 1991. Two gabapentin24,25 and all of the tricyclic trials used a crossover design. Target doses of gabapentin ranged from 900 to 3,600 mg/day (Table 2). Among the tricyclic trials, three evaluated a secondary amine (nortriptyline34 or desipramine29,32,34), five evaluated a tertiary amine (amitriptyline31,33,37 or imipramine30,35), and one evaluated both (clomipramine and desipramine36). Maximum doses of tricyclics ranged from 75 mg/day to 250 mg/day. Three gabapentin trials evaluated patients with diabetic neuropathy,23,25,28 two postherpetic neuralgia,26,27 and one included patients with both conditions.24 One gabapentin trial excluded previous non-responders to gabapentin.26 Five tricyclic antidepressant trials evaluated patients with diabetic neuropathy,30–32,35,36 and four evaluated patients with postherpetic neuralgia.29,33,34,37 Only one trial31 of either gabapentin or tricyclics evaluated a course of therapy longer than 8 weeks in duration. Five (83%) gabapentin trials23–27 and three (33%) tricyclic trials34–36 reported a funding source. Of trials reporting a funding source, four (80%) gabapentin trials23–27 were funded by pharmaceutical companies, and all tricyclic trials received government or foundation funding.
Gabapentin was superior to placebo for achieving pain relief (RR = 2.18, 95% CI 1.78 to 2.67, I2 = 0%, six trials,23–28 Fig. 3). Tricyclics were also superior to placebo for achieving pain relief (RR = 5.27, 95% CI 3.05 to 9.11, I2 = 10%, six trials,29–33,37 Fig. 4). Funnel plot asymmetry (p = 0.01 by Egger test, Fig. 5) was detected in the tricyclic, but not the gabapentin trials. The L’Abbe plot showed no outlier tricyclic trials (Fig. 6). Adjustment for funnel plot asymmetry using the trim-and-fill method slightly attenuated the estimate of pain relief (RR = 3.99, 95% CI 2.10 to 7.57, I2 = 30%).
Figure 3.

Relative risk for pain relief from placebo-controlled trials of gabapentin.
Figure 4.
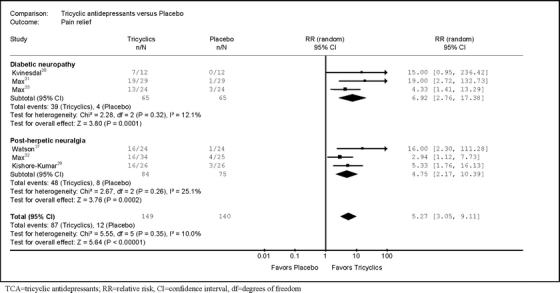
Relative risk for pain relief from placebo-controlled trials of tricyclic antidepressants.
Figure 5.
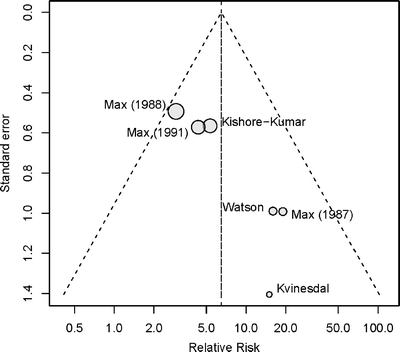
Funnel plot, placebo-controlled trials of tricyclic antidepressants, on relative risk for pain relief.
Figure 6.

L’Abbe plot, placebo-controlled trials of tricyclic antidepressants, on relative risk for pain relief.
There was no significant difference in stratified estimates between trials evaluating a secondary amine tricyclic versus a tertiary amine tricyclic (Table 3). For the gabapentin trials, excluding one trial evaluating a dose of 900 mg/day,25 excluding one trial that did not enroll previous non-responders to gabapentin,26 and stratifying trials by use of crossover versus parallel-group design had little effect on estimates. All of the other gabapentin trials titrated patients to a goal dose of at least 2,400 mg/day. For the gabapentin trials, stratifying trials according to whether they met various quality criteria showed no differences in estimates. For the tricyclic trials, we could not assess effects of methodological shortcomings. All tricyclic trials met the quality criterion for masking of patients, but only one small (n = 29) trial met even one other quality criterion.31 When stratified according to specific neuropathic pain condition, gabapentin and tricyclics (Table 3) were both superior to placebo for diabetic neuropathy.
Table 3.
Pain Relief in Placebo-Controlled Trials of Tricyclic Antidepressants for Neuropathic Pain
| Type of trial | Number of trials | Pooled relative risk (95% CI)* | Test for heterogeneity | p value† |
|---|---|---|---|---|
| All placebo-controlled trials of tricyclic antidepressants | 629–33,37 | 5.27 (3.05–9.11) | I2 = 10% | Not applicable |
| Stratified by evaluation of secondary or tertiary amine tricyclic | ||||
| Secondary amine tricyclic | 429–32 | 6.21 (3.07–12.58) | I2 = 0% | p = 0.65 |
| Tertiary amine tricyclic | 233,37 | 5.52 (1.11–27.46) | I2 = 62% | |
| Specific neuropathic pain condition | ||||
| Diabetic neuropathy | 330–32 | 6.92 (2.76–17.38) | I2 = 12% | p = 0.58 |
| Post-herpetic neuralgia | 329,33,37 | 4.75 (2.17–10.39) | I2 = 25% | |
| Adjusted for funnel plot asymmetry | ||||
| Trim and fill method | 629–33,37+ 3 trials ‘filled’ | 3.99 (2.10–7.57) | I2 = 30% | Not applicable |
*For ≥50% improvement in pain or at least moderate pain relief, tricyclic antidepressant versus placebo; †for difference in stratified estimates
“Serious” adverse events were reported by three gabapentin trials (range 0% to 1.8%).25–27 None defined the term “serious,” and two of the three trials reported zero events.25,27 There was no difference between gabapentin versus placebo for serious adverse events in the remaining trial,26 but the estimate was imprecise (RR = 1.99, 95% CI 0.23 to 17.60, I2 = 0%). Estimates were also imprecise for dry mouth and ataxia/gait disorder (Table 4; Appendix 3, available online). No trial of tricyclics reported serious adverse events or ataxia/gait disturbance. Gabapentin and tricyclics were both associated with a relative risk of about 1.70 for withdrawal due to adverse events compared to placebo, but the difference was only statistically significant for gabapentin. Gabapentin and tricyclics were both associated with greater risk of somnolence compared to placebo. Gabapentin was also associated with increased risk of dizziness and tricyclics with increased risk of dry mouth versus placebo. Stratification of tricyclic trials by evaluation of secondary versus tertiary amines or use of active versus inert placebo had little effect on estimates of harms (Appendix 4, available online). All trials of gabapentin used an inert placebo.
Table 4.
Direct and Adjusted Indirect Estimates from Placebo-Controlled Trials of Gabapentin and Tricyclic Antidepressants for Neuropathic Pain
| Comparison | Gabapentin versus placebo | Tricyclic antidepressants versus placebo | Gabapentin vs tricyclic antidepressant |
|---|---|---|---|
| RR (95% CI), test for heterogeneity [number of trials] | RR (95% CI), test for heterogeneity [number of trials] | RR (95% CI): Adjusted indirect estimate | |
| Pain relief* | 2.18 (1.78–2.67), I2 = 0% (623–28) | 5.27(3.05–9.11), I2 = 10% (629–33,37) | 0.41 (0.23–0.74) |
| Withdrawal due to adverse events | 1.69 (1.10–2.60), I2 = 0% (523,25–28) | 1.71 (0.68–4.31), I2 = 0% (529,30,32,35,36) | 0.99 (0.36–2.74) |
| Serious adverse events | 1.99 (0.23–17.60) (126) | No data [none] | No data |
| Somnolence, sedation, fatigue, or lethargy | 3.92 (2.45–6.27), I2 = 0% (426–28,38) | 1.50 (1.09–2.07), I2 = 17% (429,31–33) | 2.61 (1.48–4.62) |
| Dizziness or vertigo | 3.92 (2.55–6.02), I2 = 0% (423,26–28) | 1.24 (0.64–2.43), I2 = 36% (329,31,33) | 3.16 (1.43–6.99) |
| Ataxia, gait abnormality, or incoordination | 17.45 (1.02 to 299) (127) | No data [None] | No data |
| Dry mouth | 5.97 (0.79–45.35) (126) | 1.44 (1.13–1.83), I2 = 29% (629–33,35) | 4.15 (0.54–31.86) |
*≥50% improvement in pain score or at least moderate pain relief; CI = confidence interval; RR = relative risk
Adjusted Indirect Analyses
Pooled mean rates for at least moderate or >50% pain relief in patients randomized to placebo were four times higher in trials of gabapentin (24%, 95% CI 15% to 33%, six trials23–28) than in trials of tricyclics (6%, 95% CI 2% to 10%, six trials29–33,37). In adjusted indirect analyses, gabapentin was inferior to tricyclics for achieving pain relief (RR = 0.41, 95% CI 0.23 to 0.74, Table 4). The difference remained statistically significant when we restricted the analysis to diabetic neuropathy trials (RR = 0.28, 95% CI 0.11 to 0.73) or crossover trials (RR = 0.39, 95% CI 0.20 to 0.74) (Table 5). A similar trend was present when the analysis was restricted to post-herpetic neuralgia trials, though the difference was not statistically significant (RR 0.60, 95% CI 0.24 to 1.46).
Table 5.
Sensitivity Analyses on Adjusted Indirect Estimates for Achieving Pain Relief with Gabapentin Versus Tricyclic Antidepressants
| Analysis | Gabapentin versus placebo | Tricyclic antidepressants versus placebo | Gabapentin vs tricyclic anti-depressant: Indirect estimate |
|---|---|---|---|
| RR (95% CI),* test for heterogeneity [number of trials] | RR (95% CI),* test for heterogeneity [number of trials] | RR (95% CI)* | |
| All trials | 2.18 (1.78–2.67), I2 = 0% (623–28) | 5.27(3.05–9.11), I2 = 10% (629–33,37) | 0.41 (0.23–0.74) |
| Crossover trials only | 2.03 (1.41–2.92), I2 = 0% (224,25) | 5.27(3.05–9.11), I2 = 10% (629–33,37) | 0.39 (0.20 to 0.74) |
| Diabetic neuropathy | 1.93 (1.46–2.55), I2 = 0% (323,25,28) | 6.92 (2.76–17.38), I2 = 12% (330–32) | 0.28 (0.11–0.73) |
| Post-herpetic neuralgia only | 2.83 (1.84–4.35), I2 = 29% (226,27) | 4.75 (2.17–10.39), I2 = 25% (329,33,37) | 0.60 (0.24–1.46) |
| Adjusted for funnel plot asymmetry using the trim and fill method | 2.18 (1.78-2.67), I2 = 0% (623–28) | 3.99 (2.10-7.57), I2 = 30% (929–33,37) + 3 ‘filled’ trials | 0.55 (0.28-1.07) |
*For ≥50% improvement in pain score or at least moderate pain relief; CI = confidence interval; RR = relative risk
We found no statistically significant differences between gabapentin versus tricyclics in risk of withdrawal due to adverse events (Table 4). Gabapentin was associated with increased risk of somnolence/sedation (RR = 2.61, 95% CI 1.48 to 4.62) and dizziness (RR = 3.16, 95% CI 1.43 to 6.99) compared to tricyclics.
Discrepancies Between Direct and Indirect Estimates
When all trials were included in the analysis, the discrepancy between direct (RR = 0.99, 95% CI 0.76 to 1.29) and indirect estimates (RR = 0.41, 95% CI 0.23 to 0.74) for gabapentin versus tricyclics for achieving pain relief was statistically significant (p = 0.008, Table 6). The discrepancy was also statistically significant when the analysis was restricted to diabetic neuropathy trials (RR = 0.98, 95% CI 0.69 to 1.38 vs RR = 0.28, 95% CI 0.11 to 0.73, p = 0.016 for discrepancy). When the analysis was restricted to crossover trials, the discrepancy was not statistically significant (p = 0.09), but the direct estimate was based on only one head-to-head trial.12 There was no discrepancy between direct and indirect estimates for any adverse event, but data from head-to-head trials were sparse.
Table 6.
Discrepancies Between Direct and Indirect Analyses of Gabapentin Versus Tricyclic Antidepressants for Achieving Pain Relief
| Analysis | Direct analysis: Number of trials | Direct analysis: Relative risk for pain relief* (95% CI) | Adjusted indirect analysis: Number of trials | Indirect analysis: RR for pain relief* (95% CI) | Discrepancy between direct and indirect estimate: difference in log RR |
|---|---|---|---|---|---|
| All trials included | 310–12 | 0.99 (0.76–1.29) | 12 (6 gabapentin23–28 and 6 tricyclics29–33,37) | 0.41 (0.23-0.74) | Δ = 0.87, p = 0.008 |
| Crossover trials only | 112 | 0.79 (0.47 to 1.31) | 8 (2 gabapentin24,25 and 6 tricyclics29–33,37) | 0.39 (0.20 to 0.74) | Δ = 0.71, p = 0.09 |
| Adjusted for funnel plot asymmetry using trim-and-fill method | 310–12 | 0.99 (0.76 to 1.29) | 15 (6 gabapentin23–28 and 6 tricyclics29–33,37) + 3 ‘filled’ trials | 0.55 (0.28 to 1.07) | Δ = 0.59, p = 0.11 |
| Diabetic neuropathy | 211–12 | 0.98 (0.69 to 1.38) | 6 (3 gabapentin23,25,28 and 3 tricyclics30–32) | 0.28 (0.11 to 0.73) | Δ = 1.25, p = 0.016 |
| Post-herpetic neuralgia | 110 | 1.00 (0.61 to 1.64) | 5(2 gabapentin26,27 and 3 tricyclics29–33,37) | 0.60 (0.24 to 1.46) | Δ = 0.51, p = 0.32 |
*≥50% improvement in pain score or at least moderate pain relief; CI = confidence interval; RR = relative risk
DISCUSSION
In a direct meta-analysis of head-to-head trials, we showed no difference between gabapentin and tricyclic antidepressants for achieving pain relief for diabetic neuropathy or postherpetic neuralgia. Although this result is based on only three studies, the estimate is fairly precise and very close to a relative risk of 1.00. Even if statistical power were to be enhanced by the publication of new head-to-head trials, clinically relevant differences would only occur if future estimates of effects are substantially different than currently available evidence. Nonetheless, direct evidence is sparse, and more head-to-head trials are needed to confirm our findings. We found no difference between gabapentin and tricyclics for various adverse events, but analyses of harms are even more limited by sparse data than analyses of benefits.
Indirect meta-analysis of placebo-controlled trials yielded discordant conclusions compared with the direct analysis, showing gabapentin more than 50% less likely to achieve pain relief compared to tricyclics. The discrepancy was more pronounced when we restricted our analysis to diabetic neuropathy trials (gabapentin about one-fourth as likely as tricyclics to achieve pain relief). Our indirect analyses are consistent with previous systematic reviews, also based on indirect analyses, that concluded that tricyclics are superior to newer medications, including gabapentin, for diabetic neuropathy5 or peripheral neuropathic pain conditions in general.3 The discrepancy we found is in accordance with previous studies showing that direct and indirect analyses can be associated with discordant estimates of treatment effect.8,9 In this case, the discrepancy was both statistically and clinically significant (no difference between gabapentin and tricyclics in the direct meta-analysis versus gabapentin inferior to tricyclics in the indirect meta-analysis).
To our knowledge, this is the first study to compare direct meta-analyses to formal adjusted indirect analyses of medications for neuropathic pain. Previous systematic reviews3–5 comparing neuropathic pain drugs relied on informal indirect comparisons, or the qualitative rank-ordering of pooled estimates from placebo-controlled trials. Apparent differences between drugs in qualitative comparisons may not be statistically significant when formal adjusted indirect analysis is performed.6,7 In addition, for all indirect analysis, the validity of indirect comparisons depends on how well they meet the critical assumption of consistent treatment effects across all of the trials.6,7 This assumption can be violated by methodological shortcomings in the trials, differences in populations, interventions (e.g. dosing), assessment of outcomes, or other factors. It is critical to consider such factors when considering whether any indirect comparison—either formal or informal—is valid.
In our study, all placebo-controlled trials of gabapentin were published in or after 1998, while placebo-controlled tricyclic trials were generally published in or before 1991. Indirect analyses may be particularly problematic when trials from different eras are combined, because it is unlikely that patient characteristics, treatment regimens, assessment of outcomes, and design and conduct of randomized trials would remain similar enough to meet the assumption of consistent treatment effects across trials.8 Several findings from our study support this hypothesis. First, there was a four-fold difference in placebo response rates between the gabapentin (24%) and tricyclic (6%) trials. Second, the older tricyclic trials did not meet current standards for methodological quality. Only one small trial reporting pain relief met even one quality criterion other than masking of patients.31 Finally, all of the tricyclic trials reporting pain relief used a crossover design and evaluated small sample sizes (median 26). The gabapentin trials, on the other hand, mostly used a parallel group design and enrolled larger sample sizes (median 112). Although our results were similar when we restricted our analysis to crossover trials, small crossover trials tend to report higher estimates of effects compared to parallel-group trials.38
Our study has several potential limitations. First, there was clinical diversity in the trials evaluated, including the type of neuropathic pain evaluated and doses of drugs. We therefore used a random effects model. We also found little statistical heterogeneity and performed a number of subgroup and sensitivity analyses showing stable results. Second, as in several previously published systematic reviews,3,5,15,16 we analyzed a composite dichotomous measure for pain relief. An advantage of using such composite outcomes is that more trials can be entered into analyses. A disadvantage is that it is not certain how valid pooling of disparate methods for measuring pain outcomes is, particularly for poorly validated or described categorical scales.39 We found insufficient data to perform sensitivity analyses on different methods for classifying pain relief outcomes. Finally, we did not include pregabalin in our study, even though it is similar to gabapentin in structure and mechanism of action, because no trials of pregabalin versus tricyclics are available.
We identified several areas related to the conduct and reporting of randomized trials of medications for neuropathic pain that could be improved. First, no trial met all quality criteria. Second, all randomized trials included in our study are “efficacy” studies that applied numerous inclusion and exclusion criteria, were conducted in academic or specialty settings, and were relatively short-term.40 Third, assessment and reporting of outcomes were suboptimal. For example, one-third of placebo-controlled trials of tricyclics did not report usable data on the proportion of patients experiencing pain relief.34–36 Adverse events were reported even less consistently. Fourth, funnel plot asymmetry was present among tricyclic trials, which could be associated with publication bias.21 Finally, other factors, such as patient preferences, costs, or risk factors for serious adverse events, such as overdose or cardiac arrhythmias, may be relevant for making treatment choices, but are not well studied in randomized trials.2,41
Clinicians should be aware that conclusions of systematic reviews that compare different interventions are frequently based on indirect comparisons, even when they don’t use formal indirect methods, and that results based on such methods can be misleading. Our results are consistent with the hypothesis that assumptions underlying indirect comparisons are more likely to be violated when trials from different eras are combined. If direct evidence is sparse or unavailable, indirect comparisons should only be considered when critical underlying assumptions are met, formal adjusted indirect analysis should be performed if possible, and results should be verified against head-to-head evidence as it become available.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Search strategy for neuropathic pain drugs. (DOC 34.0 KB)
Quality assessment criteria for randomized controlled trials. (DOC 29.0 KB)
Adverse events, trials of gabapentin and tricyclic antidepressants for diabetic neuropathy or post-herpetic neuralgia. (DOC 66.5 KB)
Pooled results, adverse events in placebo-controlled trials of tricyclic antidepressants for neuropathic pain. (DOC 38.5 KB)
Acknowledgments
Conflict of Interest None disclosed.
Contributors All authors participated in the conception and design, analysis and interpretation of data, drafting the article, revising it critically for important intellectual content, and final approval of the version to be published. There were no other contributors to this manuscript. The guarantor of this manuscript is Roger Chou (corresponding author).
Funding This study is based on work funded by the Drug Effectiveness Review Project (http://www.ohsu.edu/drugeffectiveness/). The funder had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-008-0877-5) contains supplementary material, which is available to authorized users.
An erratum to this article can be found at http://dx.doi.org/10.1007/s11606-009-0960-6
References
- 1.Dworkin RH, O’Connor AB, Backonja M. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain. 2007;132:237–51. [DOI] [PubMed]
- 2.Gilron I, Watson CPN, Cahill CM, Moulin DE. Neuropathic pain: a practical guide for the clinician. CMAJ Can Med Assoc J. 2006;175:265–75. [DOI] [PMC free article] [PubMed]
- 3.Finnerup NB, Otto M, McQuay HJ, Jensen TS, Sindrup SH. Algorithm for neuropathic pain treatment: an evidence based proposal. Pain. 2005;118:289–305. [DOI] [PubMed]
- 4.Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83:389–400. [DOI] [PubMed]
- 5.Wong MC, Chung JW, Wong TK. Effects of treatments for symptoms of painful diabetic neuropathy: systematic review. BMJ. 2007;335:87. [DOI] [PMC free article] [PubMed]
- 6.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–91. [DOI] [PubMed]
- 7.Glenny AM, Altman DG, Song F, et al. Indirect comparisons of competing interventions. Health Technol Assess. 2005;9:1–134. [DOI] [PubMed]
- 8.Chou R, Fu R, Huffman LH, Korthuis PT. Initial highly-active antiretroviral therapy with a protease inhibitor versus a non-nucleoside reverse transcriptase inhibitor: discrepancies between direct and indirect analyses. Lancet. 2006;368:1503–15. [DOI] [PubMed]
- 9.Song F, Altman D, Glenny A-M, Deeks J. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326:472. [DOI] [PMC free article] [PubMed]
- 10.Chandra K, Shafiq N, Pandhi P, Gupta S, Malhotra S. Gabapentin versus nortriptyline in post-herpetic neuralgia patients: a randomized, double-blind clinical trial–the GONIP Trial. Int J Clin Pharmacol Therapeutics. 2006;44:358–63. [DOI] [PubMed]
- 11.Dallocchio C, Buffa C, Mazzarello P, Chiroli S. Gabapentin vs amitriptyline in painful diabetic neuropathy: an open-label pilot study. J Pain Symptom Manage. 2000;20:280–85. [DOI] [PubMed]
- 12.Morello CM, Leckband SG, Stoner CP, Moorhouse DF, Sahagian GA. Randomized double-blind study comparing the efficacy of gabapentin with amitriptyline on diabetic peripheral neuropathy pain. Arch Internal Med. 1999;159:1931–37. [DOI] [PubMed]
- 13.Toma M, McAlister FA, Bialy L, Adams D, Vandermeer B, Armstrong PW. Transition from meeting abstract to full-length journal article for randomized controlled trials. JAMA. 2006;295:1281–87. [DOI] [PubMed]
- 14.Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31:140–49. [DOI] [PubMed]
- 15.Hempenstall K, Nurmikko TJ, Johnson RW, A’Hern RP, Rice AS. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med/Public Library Sci. 2005;2:e164. [DOI] [PMC free article] [PubMed]
- 16.Wiffen PJ, McQuay, Edward JE, Moore RA. Gabapentin for acute and chronic pain. Cochrane Database Syst Rev. 2005;3:CD005452. [DOI] [PubMed]
- 17.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for Meta-Analysis in Medical Research. West Sussex: John Wiley & Sons; 2000.
- 18.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;2:1539–58. Jun. [DOI] [PubMed]
- 19.Team RDC. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2006.
- 20.Song F. Exploring heterogeneity in meta-analysis: Is the L’Abbe plot useful. J Clin Epidemiol. 1999;52:725–30. [DOI] [PubMed]
- 21.Egger M, Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. [DOI] [PMC free article] [PubMed]
- 22.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320:1574–77. [DOI] [PMC free article] [PubMed]
- 23.Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280:1831–36. [DOI] [PubMed]
- 24.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. New England J Med. 2005;352:1324–34. [DOI] [PubMed]
- 25.Gorson KC, Schott C, Herman R, Ropper AH, Rand WM. Gabapentin in the treatment of painful diabetic neuropathy: a placebo controlled, double blind, crossover trial. J Neurol, Neurosurgery & Psychiatry. 1999;66:251–52. [DOI] [PMC free article] [PubMed]
- 26.Rice AS, Maton S, Postherpetic Neuralgia Study G. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94:215–24. [DOI] [PubMed]
- 27.Rowbotham M, Harden N, Stacey B, Bernstein P, Magnus-Miller L. Gabapentin for the treatment of postherpetic neuralgia: a randomized controlled trial. JAMA. 1998;280:1837–42. [DOI] [PubMed]
- 28.Simpson DA. Gabapentin and venlafaxine for the treatment of painful diabetic neuropathy. J Clin Neuromuscular Dis. 2001;3:53–62. [DOI] [PubMed]
- 29.Kishore-Kumar R, Max MB, Schafer SC, et al. Desipramine relieves postherpetic neuralgia. Clin Pharmacol Therapeutics. 1990;47(3):305–12. Mar. [DOI] [PubMed]
- 30.Kvinesdal B, Molin J, Froland A, Gram LF. Imipramine treatment of painful diabetic neuropathy. JAMA. 1984;251:1727–30. [DOI] [PubMed]
- 31.Max MB, Culnane M, Schafer SC, et al. Amitriptyline relieves diabetic neuropathy pain in patients with normal or depressed mood. Neurology. 1987;37:589–96. [DOI] [PubMed]
- 32.Max MB, Kishore-Kumar R, Schafer SC, et al. Efficacy of desipramine in painful diabetic neuropathy: a placebo-controlled trial. Pain. 1991;45:3–9. [DOI] [PubMed]
- 33.Max MB, Schafer SC, Culnane M, Smoller B, Dubner R, Gracely RH. Amitriptyline, but not lorazepam, relieves postherpetic neuralgia. Neurology. 1988;38:1427–32. [DOI] [PubMed]
- 34.Raja SN, Haythornthwaite JA, Pappagallo M, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2002;59:1015–21. [DOI] [PubMed]
- 35.Sindrup SH, Ejlertsen B, Froland A, Sindrup EH, Brosen K, Gram LF. Imipramine treatment in diabetic neuropathy: relief of subjective symptoms without changes in peripheral and autonomic nerve function. Eur J Clin Pharmacol. 1989;37(2):151–3. [DOI] [PubMed]
- 36.Sindrup SH, Gram LF, Skjold T, Grodum E, Brosen K, Beck-Nielsen H. Clomipramine vs desipramine vs placebo in the treatment of diabetic neuropathy symptoms. A double-blind cross-over study. British J Clin Pharmacol. 1990;30(5):683–91. [DOI] [PMC free article] [PubMed]
- 37.Watson CP, Evans RJ, Reed K, Merskey H, Goldsmith L, Warsh J. Amitriptyline versus placebo in postherpetic neuralgia. Neurology. 1982;32:671–73. [DOI] [PubMed]
- 38.Lathyris D, Trikalinos T, Ioannidis JP. Evidence from crossover trials: empirical evaluation and comparison against parallel arm trials. Int J Epidemiol. 2007;36:422–30. [DOI] [PubMed]
- 39.Ferreira-Gonzalez I, Permanyer-Miralda G, Busse JW, et al. Methodologic discussions for using and interpreting composite endpoints are limited, but still identify major concerns. J Clin Epidemiol. 2007;60:651–7. [DOI] [PubMed]
- 40.Gartlehner G, Hansen RA, Nissman D, Lohr KN, Carey TS. A simple and valid tool distinguished efficacy from effectiveness studies. J Clin Epidemiol. 2006;59:1040–48. [DOI] [PubMed]
- 41.Dworkin R, O’Connor A, Backonja M, et al. Pharmacologic management of neuropathic pain: Evidence-based recommendations. Pain. 2007;132(3):237–51. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Search strategy for neuropathic pain drugs. (DOC 34.0 KB)
Quality assessment criteria for randomized controlled trials. (DOC 29.0 KB)
Adverse events, trials of gabapentin and tricyclic antidepressants for diabetic neuropathy or post-herpetic neuralgia. (DOC 66.5 KB)
Pooled results, adverse events in placebo-controlled trials of tricyclic antidepressants for neuropathic pain. (DOC 38.5 KB)


