Abstract
Objective
Although non-specific, cerebral atrophy and white matter hyperintensities (WMH) are features of the neurodegeneration associated with Alzheimer’s disease (AD). The purpose of the current study was to determine if baseline measurements of cerebral atrophy and WMH predict the rate of future cognitive decline in AD.
Design
Data were drawn from the Predictors Study, a longitudinal study that enrolls mild AD patients and re-asseses them every six months with the Columbia modified Mini Mental State Examination (mMMS; 0–57). MR images were analyzed to determine the severity of WMH (Scheltens Scale) and the degree of atrophy (bicaudate ratio). Generalized estimating equations (GEE) were used to determine whether severity of baseline MRI measurements and their interaction predicted the rate of mMMS decline at subsequent visits.
Setting
Three university-based AD centers in the United States (Predictors Study).
Participants
Eighty-four AD patients from the Predictors Study received structural MRI at baseline and were selected for analysis. They had an average of 6 follow-up evaluations.
Main outcome measure
Cognitive (Columbia modified Mini-Mental State Examination).
Results
Generalized estimating equation models demonstrated that degree of baseline atrophy (β = −0.316, p = 0.036), severity of WMH (β = −0.173, p = 0.028), and their interaction (β = − 6.061, p = 0.018) predicted rate of decline in mMMS scores.
Conclusions
Both degree of cerebral atrophy and severity of WMH are associated with the rapidity of cognitive decline in AD. Atrophy and WMH may interact to have a synergistic effect on future decline, such that AD patients with a high degree of both have a particularly precipitous cognitive course. These findings lend further support to the hypothesis that cerebrovascular pathology contributes to the clinical syndrome of Alzheimer’s disease.
Search Terms: Alzheimer’s disease, MRI, neuropsychological assessment
In the absence of definitive diagnostic instruments for Alzheimer’s disease (AD), structural magnetic resonance imaging (MRI) has emerged as an important tool for the characterization of morphological changes associated with the disease (for review, see1). Increased brain atrophy is a consistent structural neuroimaging findings that has emerged as a hallmark of AD2, 3. Longitudinal analyses revealed greater rates of brain atrophy among AD patients than among healthy adults4–6 and a correlation between the severity of atrophic change and severity of cognitive deficits2, 7. These findings suggest that measures of cerebral atrophy provide a reasonable marker for disease staging in AD.
Areas of increased intensity on T2-weighted, including FLAIR, MRI sequences, termed “white matter hyperintensities” (WMH) are thought to reflect small vessel cerebrovascular disease8–12 and may contribute to age-associated cognitive decline13. Similar to degree of cerebral atrophy, WMH are prevalent in AD14 and the absence neuroradiologic markers of cerebrovascular disease among individuals with dementia is rare13.
Whether evaluation of neuroimaging data at one point in time has prognostic value for determination of future cognitive decline remains an important question. Data from the Cardiovascular Health Study showed that baseline ventricular volumes were larger among individuals who progressed from neurological health to dementia over a four year period15. In one study16, ventricular volume and severity of WMH both predicted rate of future decline among healthy older adults, but only ventricular volume predicted rate of decline among those with MCI and AD. However, only 39 AD patients were included and the investigators did not examine the interaction between ventricular volume and WMH severity. Examining the two phenomena together may help determine the relative importance of cerebrovascular disease in the course of AD and whether it synergistically interacts with disease state.
In the current study we used data from the Predictors cohort17–19 to examine the prognostic utility of baseline measurements of atrophy and cerebrovascular disease on rates of cognitive decline among individuals with early AD. An overarching goal of the Predictors Study is to elucidate the determinants of the cognitive and functional course of AD. A subset of participants received standard MRI studies as part of their diagnostic workup. We used these data and rated the severity of cerebral atrophy and WMH to predict future decline in cognitive abilities. We predicted that both baseline atrophy WMH ratings would predict future decline in cognition and that the two measures would interact.
Methods
Sample characteristics
The sample was drawn from the Predictors cohort and included individuals with AD20, 21. Full inclusion criteria and details of the study are described elsewhere17, 18. Briefly, participants met diagnostic criteria for dementia of the Alzheimer’s type21 and probable AD20. Exclusion criteria included parkinsonism, stroke, alcoholism, schizophrenia, schizoaffective disorder, and history of electroconvulsive treatments. Participants were recruited from three sites (Columbia University, Johns Hopkins School of Medicine, and Massachusetts General Hospital) between April 1989 and September 2005. The study was approved by local ethics committees. Neuroimaging was acquired for clinical diagnostic purposes and was available for 84 individuals in the study cohort. These subjects comprise the current study sample. The mean age at the time of the neuroimaging study was 73.24 (SD=8.02), 47.6% (n=40) was male, and 90.5% (n=76) was Caucasian. Mean number of years of education was 14.77 (SD=3.74). Participants were relatively mildly impaired at the time of their neuroimaging studies, as indicated by a mean mMMS of 41.29 (SD=7.11), which is comparable to a Mini Mental State Examination (MMSE)22 score of about 22. The average number of follow-up visits, at approximate 6 month intervals was 5.76 (SD=2.99). Fifty percent (n=31, data available for 62 subjects) had the APOE-4 allele. At baseline, 9.5% (n=8) had self-reported history of diabetes, 16.7% (n=14) had dyslipidemia, 16.7% (n=14) had coronary artery disease (CAD), and 35.7% had hypertension (n=30). Compared to Predictors Study participants without available neuroimaging (n=456), the current study participants were similar in age, sex, number of evaluations, and proportion with the APOE-4 allele, diabetes, dyslipidemia, CAD, and hypertension. Those without neuroimaging had fewer years of education (13.43±3.52 vs. 14.77±3.740, t (537)=3.192,p=0.001).
Neuroimaging
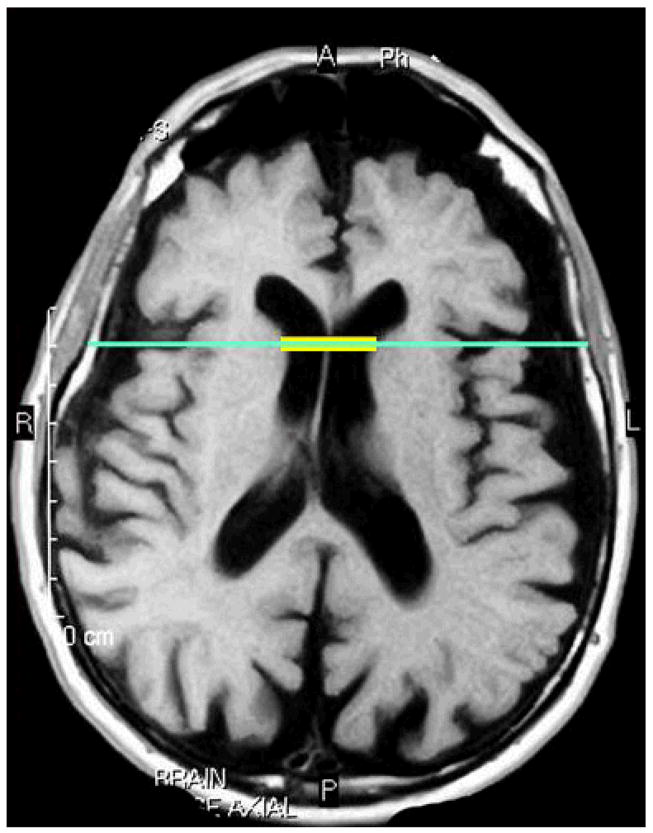
Estimates of total brain atrophy were computed primarily from T1-weighted images, which were available for 53 (68%) of the 84 participants with neuroimaging data; atrophy measurements were computed for the remainder of participants on FLAIR-, proton-, or T2-weighted images. Findings did not change when we included image sequence as a dummy-coded covariate. Bicaudate ratios were derived on axially-acquired images as an estimate of total brain atrophy following established protocols23–25. To derive the bicaudate ratio, the axial slice on which the caudate nuclei produced the greatest amount of indentation on the lateral ventricles was identified and the distance in millimeters between the two caudate apices was measured. This value was divided by the maximum width of the skull at the same level of the caudate measurement (Figure 1). Using this approach, enlarged ventricles increase the distance between the two caudate nuclei resulting in a higher bicaudate ratio, so a larger value indicates a greater degree of atrophy. Bicaudate ratio measurements were made by two experienced raters (AMB, LH). Interrater reliability, computed on 12 images, was good for the bicaudate distance (intraclass correlation coefficient [ICC]=0.861), skull width (ICC=0.991), and bicaudate ratio (ICC=0.740). Further, in an independent sample of 17 dementia patients with digital T1-weighted MR images, we calculated bicaudate ratios and compared them to manually derived full relative brain volumes and found a strong relationship between the two measures (Spearman’s Rho=−0.814,p<0.001). These findings suggest that the bicaudate ratio is a reliable and valid index of cerebral atrophy.
Figure 1.
Bicaudate ratio. The yellow represents the distance between the two apices of the caudate nuclei. The inner skull dimension is shown in turquoise. The bicaudate ratio is derived by dividing the ventricular dimension (yellow) by the inner skull dimension (turquoise). A higher ratio represents greater atrophy.
White matter hyperintensity severity ratings were made on FLAIR- and T2-weighted images using the Scheltens Scale26. The Scheltens Scale is a visual rating scale that includes anchored, 7-point severity ratings in periventricular (i.e., frontal horn, occipital horn, lateral bands), cortical (i.e., frontal lobe, temporal lobe, parietal lobe, occipital lobe), subcortical (i.e., caudate, putamen, globus pallidus, internal capsule, thalamus), and infratentorial (i.e., mesencephalon, pons, medulla, cerebellum) regions. The WMH severity measure for the current study was the summation of ratings in each of the four regions. All WMH ratings were performed by one rater (LSH). Intrarater reliability, computed on 12 images, was high (ICC=0.912).
Clinical Evaluation
Performance on the mMMS27 was the primary outcome measure. The mMMS is an expanded version of the MMSE22 and consists of attention/calculation, general knowledge, language, and construction tasks. The scale has a maximum score of 57. All Predictors Study participants were required to score 30 or higher for inclusion, which is equivalent to a score of about 16 on the MMSE. The mMMS was administered at each semi-annual visit and thus served as the indicator of cognitive change.
Additional risk factor clinical data included presence or absence of the following variables: APOE-e4 allele28, diabetes, dyslipidemia, CAD, and hypertension, which were coded as present based on reported history of treatment or diagnosis.
Statistical approach
Baseline associations between MRI-derived measurements and cognition were evaluated with multiple linear regression analyses. First, baseline bicaudate ratio, age, sex, education, and risk factor variables were entered as independent variables and total score on the mMMS was the dependent variable. The model was re-run without the risk factors. Next, the same two regression analyses were run substituting Scheltens Scale WMH severity ratings for the bicaudate ratio. Finally, the same two regression analyses were run with WMH ratings, atrophy ratings, and their interaction term.
A similar approach was taken to evaluate the impact of baseline atrophy and WMH severity on longitudinal change in cognition. A series of generalized estimating equations (GEE)29 models tested associations between baseline bicaudate ratio or WMH severity on change in mMMS score with and without risk factor covariates. This approach takes into account multiple visits per subject and the likelihood that characteristics of the same individuals over time are correlated. To establish the rate of cognitive decline, the first GEE model was run with mMMS score as the dependent variable and time (in years from baseline) as the independent variable. In all subsequent GEE models, mMMS score was the dependent variable and the MRI measurement (i.e., Scheltens score or bicaudate ratio), time, and an MRI measurement by time interaction term were included as predictors. All models included age, sex, and education as additional covariates. In a final GEE model, we included WMH severity ratings and bicaudate ratios, their interaction terms with time, and their 3-way interaction (i.e., bicaudate ratio by Scheltens score by time) to evaluate their combined effects on decline. The three models were run with and without risk factor variables as additional covariates. Significant main effects of MRI measurements would indicate a difference in cognitive performance for each unit of measurement. A significant time effect would indicate a change in test scores over time. A significant interaction term with time would indicate differential rates of change in cognition over time as a function of the MRI measurement. Finally, a significant three-way interaction would suggest an interaction of the two MRI measurements with time.
Results
Baseline associations with cognition
Mean bicaudate ratio and WMH ratings were 0.1602 (SD=0.018) and 3.79 (SD=4.55), respectively. The overall multiple regression model testing the baseline association of atrophy with cognition was significant (F (9, 55)=2.357, p=0.028), although only increased number of years of education entered into the model as a predictor of higher mMMS scores (β=1.161, SE=0.307, p<0.001).
The regression model testing the association of WMH severity on baseline cognition was not significant with (F (9, 53)=1.981,p=0.065) or without (F (4, 76)=2.302, p=0.067)risk factor variables included. Similarly, when the two MRI measurements and their interaction terms were included, the model was not significant with (F (11, 53)=1.898, p=0.067) or without (F(6, 76)=1.960, p=0.083) risk factor variables.
In a separate bivariate correlational analysis, severity of WMH was not significantly associated with atrophy ratings, controlling for age (r (74)=0.103, p=0.376).
Longitudinal analysis
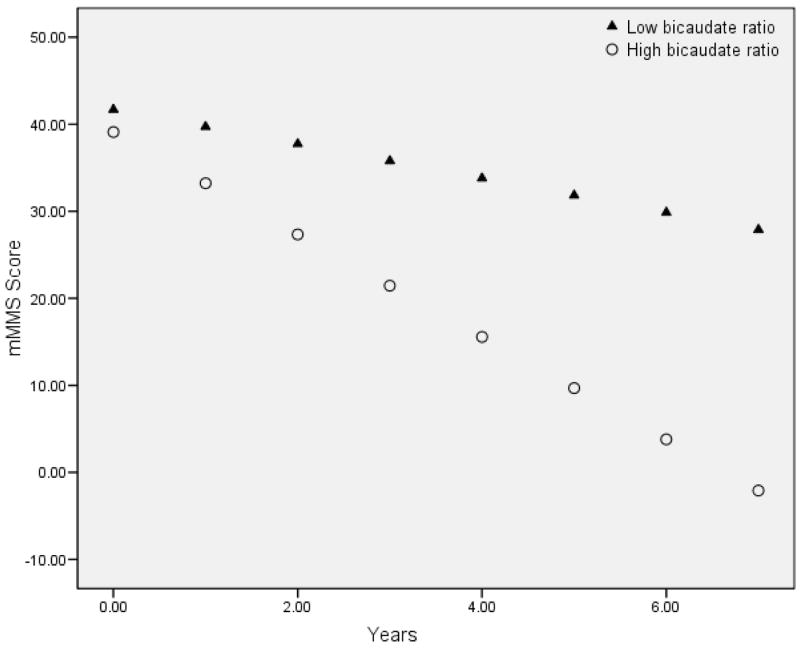
Modified Mini Mental State Examination scores declined an average of 3.5 points per year (estimated β= −3.455, p<0.001). Table 1 displays the primary results of the three GEE analyses. For every 1% difference (i.e., increase) in baseline bicaudate ratio, there was an additional associated 0.316 point decrease in mMMS score per year (significant time by bicaudate interaction). Increased age (β=0.452, p=0.037), being male (β=5.263, p=0.041), and lower education (β=1.239, p<0.001) were associated with poorer mMMS scores. The effect of bicaudate ratio on mMMS decline was similar when the vascular risk factors were excluded. Figure 2 displays the estimated rate of decline in mMMS scores in participants with low and high bicaudate ratio values, which was defined on the basis of a median split (i.e.,.1567).
Table 1.
Results from the three GEE analyses testing the associations between baseline measures of Atrophy, WMH, and their combined effects on rate of cognitive decline. Each analysis was run with (Model 1) and without (Model 2) vascular risk factor variables. The main effect of “Time” shows the rate of mMMS change per 1 year interval. Interactions involving Time show the annualized rate of change on mMMS as a function of each unit of the independent variable. For example, the “Time X Bicaudate Ratio” interaction shows the impact of baseline atrophy measures on rate of annualized mMMS change (i.e., for every 1% increase in baseline bicaudate ratio, there is an associated 0.316 point decrease in mMMS score per year).
| GEE Analysis | Variable of Interest | Model 1* | Model 2¥ | ||
|---|---|---|---|---|---|
| β | P | β | P | ||
| Atrophy | Time | 1.619 | 0.485 | 2.964 | 0.116 |
| Bicaudate Ratio | −0.786 | 0.023 | −0.497 | 0.092 | |
| Time X Bicaudate ratio | −0.316 | 0.036 | −0.415 | <0.001 | |
| WMH | Time | −2.706 | <0.001 | −3.160 | <0.001 |
| Scheltens Score | −0.082 | 0.683 | −0.106 | 0.558 | |
| Time X Scheltens Score | −0.173 | 0.028 | −0.122 | 0.71 | |
| Atrophy + WMH | Time | −1.988 | 0.295 | 0.977 | 0.504 |
| Bicaudate Ratio | −0.445 | 0.148 | −0.427 | 0.121 | |
| Scheltens Score | −0.224 | 0.245 | −0.145 | 0.360 | |
| Time X Bicaudate Ratio | −6.277 | 0.620 | −0.278 | 0.003 | |
| Time X Scheltens Score | 0.968 | 0.049 | 0.512 | 0.094 | |
| Time X Bicaudate Ratio X Scheltens Score | −6.061 | 0.018 | −3.250 | 0.050 | |
Model adjusted for Age, Sex, Education, APOE4, Diabetes, Dyslipidemia, CAD, and Hypertension
Model adjusted for Age, Sex, and Education
Figure 2.
Predicted rates of cognitive change based on baseline characterization of bicaudate ratio. For graphical presentation, baseline bicaudate ratio is presented as a dichotomous variable based on the median split of the entire sample (median=0.1567). Note that higher bicaudate ratio indicates greater amounts of atrophy. The results from the GEE analysis suggest that the greater the amount of atrophy at baseline, the greater the rate of cognitive decline.
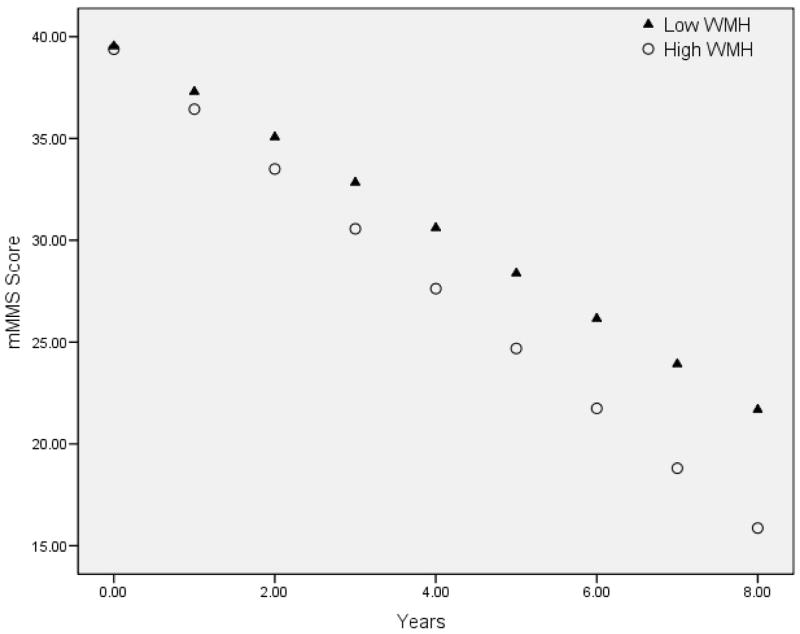
A similar pattern emerged when examining the impact of baseline WMH severity on future decline. For each Scheltens Scale point (i.e., increase in WMH severity), there was an additional 0.173 point loss in mMMS score per visit (significant WMH by time interaction; see Figure 3). Being male (β=4.801, p=0.039) and lower education (β=1.018, p=0.002) was associated with lower mMMS scores. None of the risk factor variables reached significance for this model. When they were removed from the analysis, the findings remained essentially unchanged, although the significance of the time by WMH interaction was reduced to a trend level effect.
Figure 3.
Predicted rates of cognitive change based on baseline characterization of WMH severity. For graphical presentation, the baseline Scheltens Scale score is presented as a dichotomous variable based on the median split of the entire sample (median = 3.0). Note that higher “High WMH” refers to more severe WMH. The results from the GEE analysis suggest that the greater the more severe the baseline WMH, the greater the rate of cognitive decline.
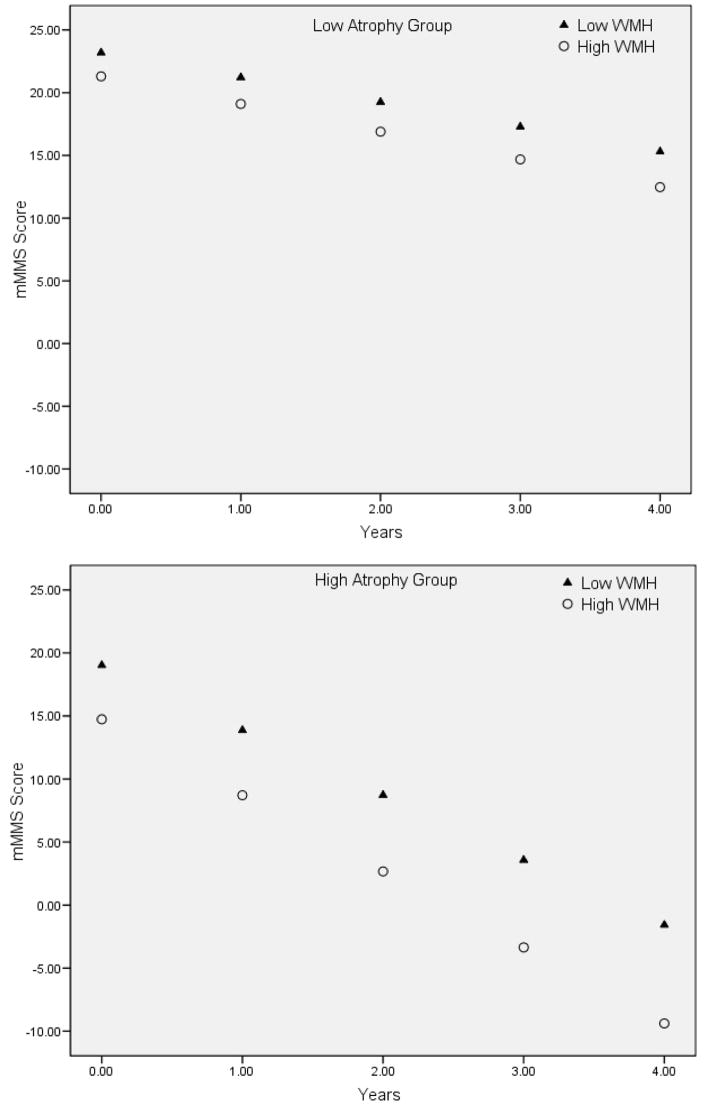
When we examined the combined impact of baseline atrophy and WMH severity on future decline, a significant three-way interaction indicated that individuals with the highest severity of WMH and greatest atrophy had the most precipitous rate of cognitive decline (see Table 1, Figure 4). Excluding the vascular risk factors, the three-way interaction remained statistically significant and baseline atrophy ratings significantly predicted rate of decline.
Figure 4.
Graphical representation of the significant Time by baseline WMH by baseline atrophy interaction. For the purpose of graphical presentation, WMH severity (circles and triangles) and degree of atrophy (upper and lower panel) are displayed in high and low groups based on the median severity. Note that the predicted mMMS scores decline most precipitously among individuals with greater level of atrophy and the greater baseline WMH burden (lower panel, circles).
Comment
The current study sought to determine whether measures of cerebral atrophy and WMH were associated with cognitive function and the rate of future cognitive decline in AD. Several important findings emerged. First, when examined cross-sectionally, increased atrophy was modestly associated with poorer cognitive performance, but the relationship between severity of WMH and cognition was unremarkable. When examined longitudinally, both severity of baseline atrophy and severity of baseline WMH were associated with a faster rate of future cognitive decline. When both severity measures were considered in the same statistical model, they interacted and had an effect on future decline. Taken together, the findings suggest a synergistic interaction of AD pathology and small vessel vascular disease on the course of cognitive symptoms in AD.
The results add to a growing corpus of work examining the associations among measures of AD pathology, vascular disease, and their clinical expression. In most studies, atrophy is more prominent among AD patients than controls1–3, 30 and is associated with more severe cognitive symptoms31, 32. Others have reported that increased atrophy is associated with future risk of developing AD15, 33, 34 and that the rate of regional atrophy predicts decline in patients with mild cognitive impairment and AD35, 36. The current study extends these findings. Although cross-sectional associations with cognitive abilities were weak, global atrophy was robustly associated with a more precipitous cognitive decline. The findings suggest that among individuals with mild AD, the severity of pathology-associated atrophy at one point in time has prognostic utility. Whole brain atrophy is not specific to the diagnosis of AD; however, within a sample of well-defined AD patients, it is a good marker of disease severity. Thus, the current study supports previous cognitive studies showing an accelerated rate of future decline among more severely affected patients with AD37.
White matter hyperintensity burden has been reported to be more severe among patients with AD than nondemented elderly3, 14, 38, although not all studies have shown this association39, 40. Further, some studies have shown that increased burden is associated with poorer cognitive abilities in AD41, 42, while others have not40, 43, 44, but the relationship between severity of WMH and cognitive abilities among older adults without dementia, has been more consistently demonstrated (45–47 c.f. 42). It is possible that small vessel vascular disease, reflected in the severity of WMH, significantly impacts cognitive abilities among individuals with no or very little AD pathology. Among those with more severe disease, AD pathology may play a more salient role in cognitive abilities, effectively concealing the impact of small vessel vascular disease. Our results partially support this idea: on a cross-sectional basis, the severity of WMH was not associated with severity of cognitive impairment.
Our findings are consistent with the idea that cerebrovascular pathology interacts with AD pathology. While both severity of baseline atrophy and severity of baseline WMH were associated with rate of cognitive decline, when the two measures were included in the same model, the interaction effect was significant and the individual main effects were not. The observation is reminiscent of autopsy studies that have shown that individuals with vascular disease and AD pathology were more likely to have clinical dementia compared to those without vascular disease48 or findings that less AD pathology is required to produce the same degree of cognitive impairment when vascular disease is present49. Our findings are most consistent with a report50 that demonstrated a significant interaction of WMH and medial temporal lobe atrophy in the classification of older adults as AD or normal; the risk associated with having both a high degree of atrophy and a high degree of WMH volume was greater than the risk incurred by the product of the two factors. Although we did not observe an interaction between degree of atrophy and WMH cross-sectionally, the significant effect on future decline suggests a synergistic interaction of the two pathologies on the course of AD.
From a mechanistic perspective, the interaction of cerebrovascular and AD pathology may be a reflection of β-amyloid (Aβ) peptide deposition, which comprises both the senile plaques characteristic of AD pathology (in the Aβ42 species) and cerebrovascular amyloid (in its Aβ40 form). Indeed plasma concentrations of Aβ40 peptide were associated with increased WMH volume among patients with AD and cerebral amyloid angiopathy in a clinic-based sample51 and among participants in the population-based Rotterdam Study52. In the current study, WMH severity predicted rate of decline in AD only after statistical adjustment for common vascular risk factors (see Table 1), suggesting that the variance in WMH related to cognitive course in AD is not due to these risk factors. Rather, it is possible that WMH associated cerebrovascular Aβ deposition specifically accounts for this association. Future work should examine the association among concentrations of plasma Aβ, WMH, and longitudinal changes in cognition.
While inconsistencies exist in the literature regarding the exact relationship among AD pathology, vascular pathology, and cognition, findings do converge in demonstrating that increased cerebrovascular disease burden is not beneficial and is most likely harmful. At present, there are no available disease-modifying treatments for AD. However, there are a number of potentially modifiable risk factors and behaviors for cerebrovascular disease53–55. The reduction of these conditions could have therapeutic effects for the treatment of the cognitive symptoms associated with AD and further study of the determinants of WMH among patients with AD may highlight newer treatment targets.
This study has several limitations. First, neuroimaging was available for only a subset of participants in the Predictors Study, although participants with and without neuroimaging were similar to each other. Second, analyses were conducted on ratings of clinical MRI scans, as opposed to digital scans that were acquired uniformly and analyzed automatively. Because clinical scans were acquired in the axial orientation, our analyses focused on global atrophy as opposed to atrophy in areas more specific to AD, such as the medial temporal lobe, which would require scans oriented in the coronal plane. Newer protocols exist that are able to quantify regional atrophy and WMH signal with higher precision and accuracy. It should be noted, however, that rater reliability for both the bicaudate ratio and Scheltens scale was high and that estimates of atrophy from the bicaudate ratio were highly correlated with whole-brain atrophy measures derived in an independent sample. Third, while the study comprised well characterized longitudinal cognitive data, neuroimaging data were only available at one point in time, thus limiting inference about the emergence and evolution of the imaging markers themselves. Future studies should incorporate prospectively collected longitudinal clinical and neuroradiologic data.
This study also has a number of particular strengths. This is among the largest studies that have examined prognostic utility of baseline neuroimaging characteristics on future cognitive decline in AD. Patients were carefully diagnosed at academic medical centers with specific expertise in aging and dementia and diagnoses were based on uniform application of widely accepted criteria via consensus diagnosis procedures. Diagnoses from the Predictors Study are accurate. For example, 93% of patients that came to autopsy had pathologically confirmed AD at postmortem evaluation 56. Patients were evaluated prospectively and relatively frequently (i.e., biannually). As participants were generally mildly demented at their baseline evaluation, we were able to capture a large range of progression over time.
Acknowledgments
This work was supported in part by National Institutes of Health grants AG07370, RR000645, and AG029949 and a grant from the Alzheimer’s Association (NIRG 05-14586). A portion of this work was presented at the annual meeting of the American Neurological Association (October 2006, Chicago, IL).
Footnotes
Disclosures: The authors report no conflicts of interest.
Author contributions
Study concept and design: Drs. Brickman, Honig, Scarmeas, and Stern. Acquisition of data: Drs. Brickman, Honig, Scarmeas, Albert, Brandt, Blacker, and Stern and Ms. Tatarina and Sanders. Analysis and interpretation of the data: Drs. Brickman, Honig, Scarmeas, and Stern. Drafting of the manuscript: Drs. Brickman and Scarmeas. Critical revision of the manuscript for important intellectual content: Drs. Honig, Scarmeas, Brandt, Blacker, and Stern. Statistical analysis: Drs. Brickman and Scarmeas. Obtained funding: Drs. Brickman and Stern. Administrative, technical, and material support: Drs. Honig, Brandt, Blacker, and Stern. Ms.Tatarina and Sanders. Study supervision: Drs. Scarmeas, Albert, Brandt, Blacker, and Stern.
References
- 1.Brickman AM, Buchsbaum MS. Alzheimer’s disease and normal aging: Neurostructures. In: Byrne J, editor. Learning and Memory: A Comprehensive Review. New York: Elsevier; in press. [Google Scholar]
- 2.Murphy DG, DeCarli CD, Daly E, et al. Volumetric magnetic resonance imaging in men with dementia of the Alzheimer type: correlations with disease severity. Biol Psychiatry. 1993 Nov 1;34(9):612–621. doi: 10.1016/0006-3223(93)90153-5. [DOI] [PubMed] [Google Scholar]
- 3.Tanabe JL, Amend D, Schuff N, et al. Tissue segmentation of the brain in Alzheimer disease. AJNR Am J Neuroradiol. 1997 Jan;18(1):115–123. [PMC free article] [PubMed] [Google Scholar]
- 4.Fox NC, Freeborough PA, Rossor MN. Visualisation and quantification of rates of atrophy in Alzheimer’s disease. Lancet. 1996 Jul 13;348(9020):94–97. doi: 10.1016/s0140-6736(96)05228-2. [DOI] [PubMed] [Google Scholar]
- 5.Chan D, Fox NC, Jenkins R, Scahill RI, Crum WR, Rossor MN. Rates of global and regional cerebral atrophy in AD and frontotemporal dementia. Neurology. 2001 Nov 27;57(10):1756–1763. doi: 10.1212/wnl.57.10.1756. [DOI] [PubMed] [Google Scholar]
- 6.de Leon MJ, George AE, Reisberg B, et al. Alzheimer’s disease: longitudinal CT studies of ventricular change. AJR Am J Roentgenol. 1989 Jun;152(6):1257–1262. doi: 10.2214/ajr.152.6.1257. [DOI] [PubMed] [Google Scholar]
- 7.Fox NC, Scahill RI, Crum WR, Rossor MN. Correlation between rates of brain atrophy and cognitive decline in AD. Neurology. 1999 May 12;52(8):1687–1689. doi: 10.1212/wnl.52.8.1687. [DOI] [PubMed] [Google Scholar]
- 8.Englund E. Neuropathology of white matter changes in Alzheimer’s disease and vascular dementia. Dement Geriatr Cogn Disord. 1998 Jul;9(Suppl 1):6–12. doi: 10.1159/000051183. [DOI] [PubMed] [Google Scholar]
- 9.Fazekas F, Kleinert R, Offenbacher H, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993 Sep;43(9):1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 10.Thomas AJ, O’Brien JT, Davis S, et al. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002 Sep;59(9):785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- 11.Udaka F, Sawada H, Kameyama M. White matter lesions and dementia: MRI-pathological correlation. Ann N Y Acad Sci. 2002 Nov;977:411–415. doi: 10.1111/j.1749-6632.2002.tb04845.x. [DOI] [PubMed] [Google Scholar]
- 12.Jagust WJ, Zheng L, Harvey DJ, et al. Neuropathological basis of magnetic resonance images in aging and dementia. Ann Neurol. 2008 Jan;63(1):72–80. doi: 10.1002/ana.21296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Flier WM, Barkhof F, Scheltens P. Shifting paradigms in dementia: toward stratification of diagnosis and treatment using MRI. Ann N Y Acad Sci. 2007 Feb;1097:215–224. doi: 10.1196/annals.1379.013. [DOI] [PubMed] [Google Scholar]
- 14.Scheltens P, Barkhof F, Valk J, et al. White matter lesions on magnetic resonance imaging in clinically diagnosed Alzheimer’s disease. Evidence for heterogeneity. Brain. 1992 Jun;115( Pt 3):735–748. doi: 10.1093/brain/115.3.735. [DOI] [PubMed] [Google Scholar]
- 15.Carmichael OT, Kuller LH, Lopez OL, et al. Ventricular volume and dementia progression in the Cardiovascular Health Study. Neurobiol Aging. 2007 Mar;28(3):389–397. doi: 10.1016/j.neurobiolaging.2006.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adak S, Illouz K, Gorman W, et al. Predicting the rate of cognitive decline in aging and early Alzheimer disease. Neurology. 2004 Jul 13;63(1):108–114. doi: 10.1212/01.wnl.0000132520.69612.ab. [DOI] [PubMed] [Google Scholar]
- 17.Stern Y, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the “predictors study”). I. Study design, cohort description, and intersite comparisons. Alzheimer Dis Assoc Disord. 1993 Spring;7(1):3–21. doi: 10.1097/00002093-199307010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Richards M, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the “predictors study”). II. Neurological, psychiatric, and demographic influences on baseline measures of disease severity. Alzheimer Dis Assoc Disord. 1993 Spring;7(1):22–32. doi: 10.1097/00002093-199307010-00003. [DOI] [PubMed] [Google Scholar]
- 19.Scarmeas N, Albert M, Brandt J, et al. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005 May 24;64(10):1696–1703. doi: 10.1212/01.WNL.0000162054.15428.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Doraiswamy PM, Patterson L, Na C, et al. Bicaudate index on magnetic resonance imaging: effects of normal aging. J Geriatr Psychiatry Neurol. 1994 Jan-Mar;7(1):13–17. [PubMed] [Google Scholar]
- 24.van Zagten M, Kessels F, Boiten J, Lodder J. Interobserver agreement in the assessment of cerebral atrophy on CT using bicaudate and sylvian-fissure ratios. Neuroradiology. 1999 Apr;41(4):261–264. doi: 10.1007/s002340050743. [DOI] [PubMed] [Google Scholar]
- 25.Barr AN, Heinze WJ, Dobben GD, Valvassori GE, Sugar O. Bicaudate index in computerized tomography of Huntington disease and cerebral atrophy. Neurology. 1978 Nov;28(11):1196–1200. doi: 10.1212/wnl.28.11.1196. [DOI] [PubMed] [Google Scholar]
- 26.Scheltens P, Barkhof F, Leys D, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993 Jan;114(1):7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- 27.Stern Y, Sano M, Paulson J, Mayeux R. Modified Mini-mental State Examination: Validity and reliability. Neurology. 1987;37(suppl):179. [Google Scholar]
- 28.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990 Mar;31(3):545–548. [PubMed] [Google Scholar]
- 29.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrica. 1986;73:13–22. [Google Scholar]
- 30.Lehericy S, Baulac M, Chiras J, et al. Amygdalohippocampal MR volume measurements in the early stages of Alzheimer disease. AJNR Am J Neuroradiol. 1994 May;15(5):929–937. [PMC free article] [PubMed] [Google Scholar]
- 31.Duarte A, Hayasaka S, Du A, et al. Volumetric correlates of memory and executive function in normal elderly, mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2006 Oct 2;406(1–2):60–65. doi: 10.1016/j.neulet.2006.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du AT, Schuff N, Amend D, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001 Oct;71(4):441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoub TR, Bulgakova M, Leurgans S, et al. MRI predictors of risk of incident Alzheimer disease: a longitudinal study. Neurology. 2005 May 10;64(9):1520–1524. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- 34.Convit A, de Asis J, de Leon MJ, Tarshish CY, De Santi S, Rusinek H. Atrophy of the medial occipitotemporal, inferior, and middle temporal gyri in non-demented elderly predict decline to Alzheimer’s disease. Neurobiol Aging. 2000 Jan-Feb;21(1):19–26. doi: 10.1016/s0197-4580(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 35.Jack CR, Jr, Petersen RC, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000 Aug 22;55(4):484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jack CR, Jr, Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004 Feb 24;62(4):591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stern Y, Liu X, Albert M, et al. Application of a growth curve approach to modeling the progression of Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 1996 Jul;51(4):M179–184. doi: 10.1093/gerona/51a.4.m179. [DOI] [PubMed] [Google Scholar]
- 38.Capizzano AA, Acion L, Bekinschtein T, et al. White matter hyperintensities are significantly associated with cortical atrophy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004 Jun;75(6):822–827. doi: 10.1136/jnnp.2003.019273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erkinjuntti T, Gao F, Lee DH, Eliasziw M, Merskey H, Hachinski VC. Lack of difference in brain hyperintensities between patients with early Alzheimer’s disease and control subjects. Arch Neurol. 1994 Mar;51(3):260–268. doi: 10.1001/archneur.1994.00540150054016. [DOI] [PubMed] [Google Scholar]
- 40.Leys D, Soetaert G, Petit H, Fauquette A, Pruvo JP, Steinling M. Periventricular and white matter magnetic resonance imaging hyperintensities do not differ between Alzheimer’s disease and normal aging. Arch Neurol. 1990 May;47(5):524–527. doi: 10.1001/archneur.1990.00530050040010. [DOI] [PubMed] [Google Scholar]
- 41.Bracco L, Piccini C, Moretti M, et al. Alzheimer’s disease: role of size and location of white matter changes in determining cognitive deficits. Dement Geriatr Cogn Disord. 2005;20(6):358–366. doi: 10.1159/000088562. [DOI] [PubMed] [Google Scholar]
- 42.Burns JM, Church JA, Johnson DK, et al. White matter lesions are prevalent but differentially related with cognition in aging and early Alzheimer disease. Arch Neurol. 2005 Dec;62(12):1870–1876. doi: 10.1001/archneur.62.12.1870. [DOI] [PubMed] [Google Scholar]
- 43.Hirono N, Kitagaki H, Kazui H, Hashimoto M, Mori E. Impact of white matter changes on clinical manifestation of Alzheimer’s disease: A quantitative study. Stroke. 2000 Sep;31(9):2182–2188. doi: 10.1161/01.str.31.9.2182. [DOI] [PubMed] [Google Scholar]
- 44.Kono I, Mori S, Nakajima K, et al. Do white matter changes have clinical significance in Alzheimer’s disease? Gerontology. 2004 Jul-Aug;50(4):242–246. doi: 10.1159/000078353. [DOI] [PubMed] [Google Scholar]
- 45.Au R, Massaro JM, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006 Feb;63(2):246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 46.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000 Apr;14(2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- 47.Cook IA, Leuchter AF, Morgan ML, et al. Cognitive and physiologic correlates of subclinical structural brain disease in elderly healthy control subjects. Arch Neurol. 2002 Oct;59(10):1612–1620. doi: 10.1001/archneur.59.10.1612. [DOI] [PubMed] [Google Scholar]
- 48.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997 Mar 12;277(10):813–817. [PubMed] [Google Scholar]
- 49.Zekry D, Duyckaerts C, Moulias R, et al. Degenerative and vascular lesions of the brain have synergistic effects in dementia of the elderly. Acta Neuropathol (Berl) 2002 May;103(5):481–487. doi: 10.1007/s00401-001-0493-5. [DOI] [PubMed] [Google Scholar]
- 50.van der Flier WM, Middelkoop HA, Weverling-Rijnsburger AW, et al. Interaction of medial temporal lobe atrophy and white matter hyperintensities in AD. Neurology. 2004 May 25;62(10):1862–1864. doi: 10.1212/01.wnl.0000125337.65553.8a. [DOI] [PubMed] [Google Scholar]
- 51.Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006 Jan 10;66(1):23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 52.van Dijk EJ, Prins ND, Vermeer SE, et al. Plasma amyloid beta, apolipoprotein E, lacunar infarcts, and white matter lesions. Ann Neurol. 2004 Apr;55(4):570–575. doi: 10.1002/ana.20050. [DOI] [PubMed] [Google Scholar]
- 53.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001 Jan;70(1):9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DeCarli C, Miller BL, Swan GE, et al. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999 Mar;30(3):529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 55.Liao D, Cooper L, Cai J, et al. The prevalence and severity of white matter lesions, their relationship with age, ethnicity, gender, and cardiovascular disease risk factors: the ARIC Study. Neuroepidemiology. 1997;16(3):149–162. doi: 10.1159/000368814. [DOI] [PubMed] [Google Scholar]
- 56.Scarmeas N, Hadjigeorgiou GM, Papadimitriou A, et al. Motor signs during the course of Alzheimer disease. Neurology. 2004 September 28, 2004;63(6):975–982. doi: 10.1212/01.wnl.0000138440.39918.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]