Abstract
Objective:
To present a unique case of a young pubertal female athlete who was prospectively monitored for previously identified anterior cruciate ligament (ACL) injury risk factors for 3 years before sustaining an ACL injury.
Background:
In prospective studies, previous investigators have examined cross-sectional measures of anatomic, hormonal, and biomechanical risk factors for ACL injury in young female athletes. In this report, we offer a longitudinal example of measured risk factors as the participant matured.
Differential Diagnosis:
Partial or complete tear of the ACL.
Measurements:
The participant was identified from a cohort monitored from 2002 until 2007. No injury prevention training or intervention was included during this time in the study cohort.
Findings:
The injury occurred in the year after the third assessment during the athlete's club basketball season. Knee examination, magnetic resonance imaging findings, and arthroscopic evaluation confirmed a complete ACL rupture. The athlete was early pubertal in year 1 of the study and pubertal during the next 2 years; menarche occurred at age 12 years. At the time of injury, she was 14.25 years old and postpubertal, with closing femoral and tibial physes. For each of the 3 years before injury, she demonstrated incremental increases in height, body mass index, and anterior knee laxity. She also displayed decreased hip abduction and knee flexor strength, concomitant with increased knee abduction loads, after each year of growth.
Conclusions:
During puberty, the participant increased body mass and height of the center of mass without matching increases in hip and knee strength. The lack of strength and neuromuscular adaptation to match the increased demands of her pubertal stature may underlie the increased knee abduction loads measured at each annual visit and may have predisposed her to increased risk of ACL injury.
Keywords: knee injuries, neuromuscular adaptations, lower extremity injuries, anterior cruciate ligament injury mechanisms, high-risk athletes, female athlete triad, neuromuscular spurt
Adolescent and adult female athletes are 4 to 6 times more likely to sustain a sport-related noncontact anterior cruciate ligament (ACL) injury than are male athletes.1–3 Although strong evidence indicates a sex difference in ACL injury rates in postpubertal athletes, no similar trend is present in prepubescent athletes.4–8 However, knee injuries in preadolescent athletes constitute up to two-thirds of the sport-related injuries in children reported as joint sprains, with most sprains occurring at the knee.7 Sprains to the ACL are rare in preadolescent children, and sex differences do not appear to be present in children before their growth spurt.4–6 However, after their growth spurt, female athletes have higher rates of knee sprains, a trend that continues into maturity.9
Currently, few investigators have prospectively quantified risk factors for ACL injury in young female athletes. LaPrade and Burnett10 performed the first prospective investigation to identify factors related to increased ACL injury risk in collegiate athletes. No sex differences were reported, but intercondylar notch stenosis was related to an increased risk of injury.10 Uhorchak et al11 determined that female cadets with increased joint laxity, smaller femoral notch width, and greater body mass index were more likely to sustain ACL injuries during 4 years of military training. Hewett et al12 prospectively measured biomechanics in high school volleyball, soccer, and basketball players. Measures of lower extremity valgus, which included knee abduction torque, during vertical jump task predicted ACL injury risk. More recently, Myer et al13 used a case-control study design from a large prospective data set to evaluate joint laxity measures and their relationship to ACL injury risk. Greater joint laxity, especially at the knee, also may contribute to increased risk of knee injury in high school and collegiate female athletes. Previous authors provided snapshots of injury risk factors in adult and late-adolescent athletes; however, to our knowledge, no prospective longitudinal reports have quantified risk factors in early pubertal athletes, nor is there evidence of changes in these risk factors as females mature. Our purpose was to present the case of a young, pubertal female athlete who was prospectively monitored for ACL injury risk factors for 3 years before sustaining an ACL injury. Although the injury mechanism and surgical procedure were not unique, the inclusion of 3 consecutive years of data provides information related to injury risk factors during growth and development.
Case Report
The 14-year-old female athlete was injured when she “took a charge” while playing Amateur Athletic Union basketball. She felt an immediate “pop” in her left knee, fell awkwardly, and could not stand or walk without assistance. She had no previous history of participation-limiting knee pain or injury. Her examination was remarkable for difficulty fully extending the knee, and she had a low-grade effusion. Both her Lachman and anterior drawer tests were positive, and she had lateral joint line pain with the McMurray test. Plain radiographs of the left knee indicated closing physes (both tibia and femur) with no specific abnormalities. Magnetic resonance imaging (MRI) indicated a complete disruption of the ACL, a tear in the anterior horn of the lateral meniscus, and contusions to the lateral femoral condyle and posterolateral tibial plateau in a pattern characteristic of ACL failure. The athlete and her parents opted for arthroscopically assisted ACL reconstruction with ipsilateral hamstrings tendon autograft.
The novelty of this case report is that at the time of her injury, the athlete was a research participant enrolled in a prospective biomechanical-epidemiologic study to determine the relationship of maturational development to risk factors for ACL injury in female athletes.14 The participant was first evaluated at age 11 years and then reevaluated annually before each fall basketball season to monitor changes in maturation status and potential risk factors for ACL injury.
Measurements
Demographic and Anthropometric Data
The participant was identified from a cohort that was screened prospectively before participating in sports from 2002 until 2007. Data were collected annually, just before the start of the athlete's competitive basketball season, for 3 years prior to the ACL injury. Informed written consent was obtained from her guardian, and the investigation was approved by the institutional review board before screening began.
The participant's height and mass were recorded. She was tested for generalized joint laxity, using tests previously described by Pasque and Hewett15 (visual approximation and goniometric measures). Anterior-posterior tibiofemoral translation was quantified using the CompuKT knee arthrometer (Medmetric Corp, San Diego, CA) to measure total anterior-posterior displacement of the tibia relative to the secured femur. During the measurement, each leg was placed on the adjustable thigh support with the knee stabilized at 20° to 35° of knee flexion. The arthrometer was secured to the lower leg such that the patella sensor pad rested on the patella, with the knee joint line reference mark on the CompuKT aligned with the athlete's joint line.13 The ankle and foot were stabilized to limit leg rotation. The tester provided posterior and anterior pressure (±134 N) on an axis perpendicular to the tibia. Total displacement (mm) was plotted on the computer and recorded. The CompuKT measurements of anterior knee laxity demonstrated high reliability,13 with intraclass correlation coefficients between testers ranging from 0.81 to 0.86 and within-testers correlations ranging from 0.92 to 0.95. All laxity testing for this participant was performed by 1 investigator, who demonstrated moderate to high intrarater reliability, with coefficients ranging from 0.891 to 0.972 for the CompuKT and joint laxity scores, respectively, during pilot testing. In addition, general joint laxity classifications similar to those used in the current investigation also demonstrate good to excellent interrater (R = 0.75) and intrarater (R = 0.81) reliability.13
Notch width index was determined from MRI after the injury as the ratio of the width of the intercondylar notch to the width of the distal femur at the level of the popliteal groove. The intercondylar notch was measured at the narrowest point of the notch at the level parallel to the popliteal groove.16,17
Maturational Status
The athlete was classified into 1 of 4 pubertal categories (prepubertal, early pubertal, pubertal, or postpubertal) using the modified Pubertal Maturational Observational Scale18 at each visit. The scale uses parental questionnaires and investigator observations to classify participants into the pubertal categories: prepubertal (equivalent to Tanner stage 1), early pubertal (Tanner stage 2), pubertal (Tanner stages 3 and 4), or postpubertal (Tanner stage 5).19 The scale has shown high reliability and can be used to differentiate between pubertal stages based on indicators of adolescent growth, breast development, menstruation status, axillary and leg hair growth, muscular development, presence of acne, and evidence of sweating during physical activities.19–22
Menstrual History
Included with the consent form was a detailed medical history and previous sport participation questionnaire. The forms, which included redundant questions for cross-checking the reliability of answers, yielded an annual objective documentation from the parents regarding the athlete's current menstrual status and date of menarche.21 In addition, the participant was interviewed after the injury to determine her menstrual status at the time of injury.
Biomechanics
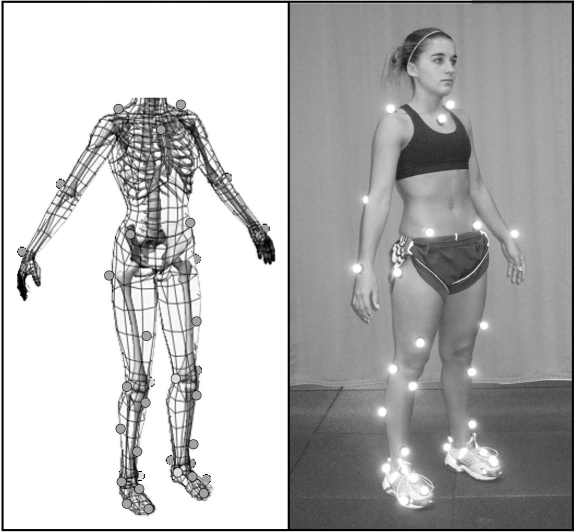
The biomechanical methods we employed have been detailed previously23 but will be explained briefly here. Each year, the participant had 37 retroreflective markers placed by the same investigator at defined anatomic locations (Figure 1). The athlete's neutral (zero) alignment and subsequent measures were referenced to a static measurement taken each year. Once the static trial was completed, the participant was positioned at the top of a 31-cm box with her feet aligned 35 cm apart (distance measured between toe markers)24 for the drop vertical jump. She was instructed to drop directly down off the box and immediately perform a maximal-effort vertical jump. Knee abduction moment recorded during a drop vertical jump has been identified as an important risk factor and provides moderate-to-high within-sessions and between-sessions reliability over extended time intervals.12,23 The first landing on the force platforms (ie, the drop from the box) was used for analysis; 3 successful trials were recorded and averaged. Trials were collected with a motion analysis system (EVaRT version 4; Motion Analysis Corp, Santa Rosa, CA) consisting of 8 cameras (sampling at 240 Hz) and 2 force platforms (sampling at 1200 Hz) (model OR6; AMTI, Watertown, MA).
Figure 1. Biomechanical marker set and static position used during motion analysis testing. Reprinted with permission from the National Athletic Trainers' Association.27.
Biomechanical data were imported into Visual3D (version 3.65; C-Motion, Inc, Germantown, MD) and MATLAB (version 7.0; The MathWorks, Natick, MA) for data reduction and analysis. The vertical ground reaction force (VGRF) data were used to calculate initial contact with the ground immediately after the athlete dropped from the box. Initial contact was defined as occurring when VGRF first exceeded 10 N. Toe-off was subsequently calculated after initial contact when the VGRF fell below 10 N. To minimize possible peak impact errors induced by different force and video cutoff frequencies, the force plate data and marker trajectories were filtered through a low-pass, fourth-order Butterworth filter at the same cutoff frequency of 12 Hz before joint moment calculations.25 We used these data to calculate joint moments using inverse dynamics.26 The net external knee abduction moment integral over time (impulse) (N·m·s) represented external load on the joint during the entire stance phase. Center of mass was estimated for the entire body from each segment within Visual3D.27
Dynamic Strength
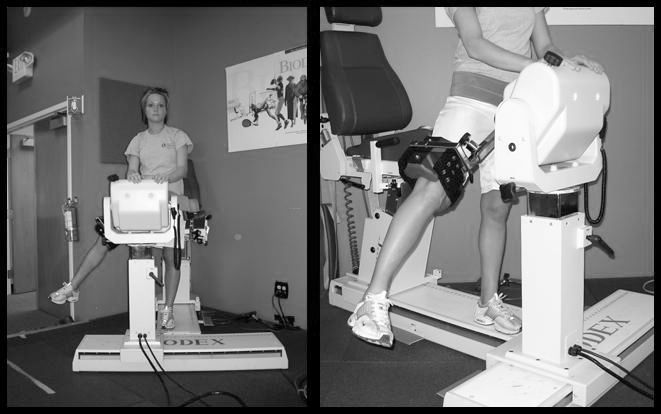
Isokinetic knee extension-flexion (concentric-concentric muscle action) strength was measured with the participant seated on the dynamometer and the trunk perpendicular to the floor, the hip flexed to 90°, and the knee flexed to 90°. Before each data collection set, a warm-up set, which consisted of 5 submaximal knee flexion-extension movements for each leg at 300°/s, was performed. The test session consisted of 10 knee flexion-extension repetitions for each leg at 300°/s. Peak flexion and extension torques were recorded. Concentric hip abduction strength was assessed with the athlete standing erect and fully supported, with a stabilization strap around the pelvis and her hands gripping a stable hand rest (Figure 2). The test leg was positioned lateral to the opposite leg at 0° of hip and knee flexion. The axis of abduction-adduction of the hip was aligned with the axis of rotation of the dynamometer. The resistance pad was affixed to the participant's leg just proximal to the knee joint. She was provided instructions and allowed to execute 5 submaximal warm-up hip abduction-adduction movements at the test speed. The warm-up was immediately followed by the test session, consisting of 10 repetitions of maximal-effort hip abduction with passive adduction at 120°/s. The protocol was repeated on the opposite limb. Peak hip abduction torques were recorded.
Figure 2. Standing hip abduction test measurement technique.
Results
Demographic and Anthropometric Data
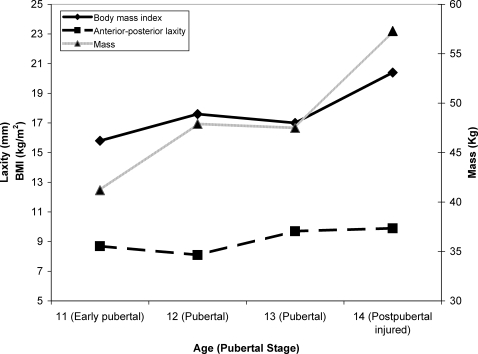
At the time of injury, the athlete had increased in total height (4%; Figure 3) and body mass index (29%), with small incremental increases in anterior-posterior tibiofemoral knee laxity (14%) relative to the first biomechanical testing 3 years earlier (Figure 4). The calculated height of the center of mass also incrementally increased with each annual visit (Figure 3). At the time of injury, the 22.8 ratio of notch width relative to knee width was within the normal ranges (23.1 ± 4.4; Figure 5) and did not indicate narrowing, which can be related to increased injury risk.17
Figure 3. The athlete demonstrated incremental increases in center of mass height at each testing visit.
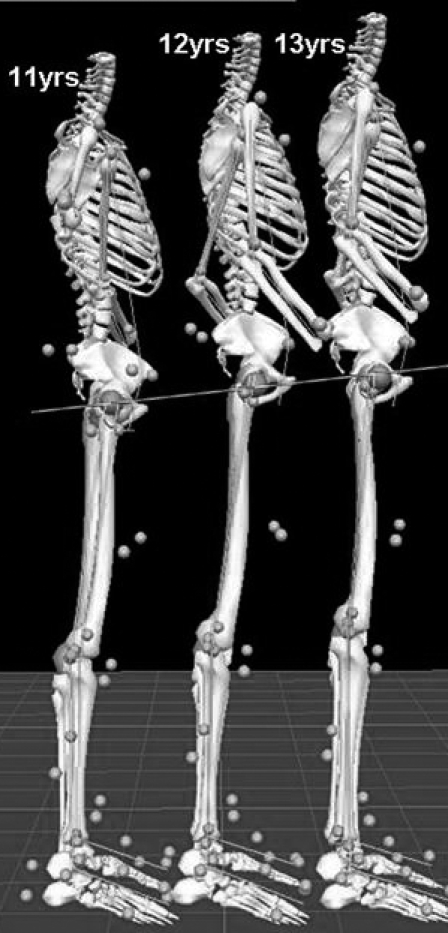
Figure 4. The measured anthropometric changes associated with each year of growth and development. Laxity and body mass index (BMI) are shown on the same scale.
Figure 5. Notch width index was measured on magnetic resonance imaging after the injury. Notch width index was calculated as the ratio of the width of the intercondylar notch to the width of the distal femur at the level of the popliteal groove and the narrowest point of the notch at the level parallel to the popliteal groove.
Maturational Status and Menstrual History
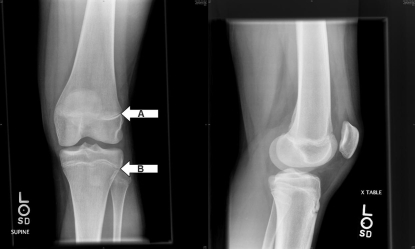
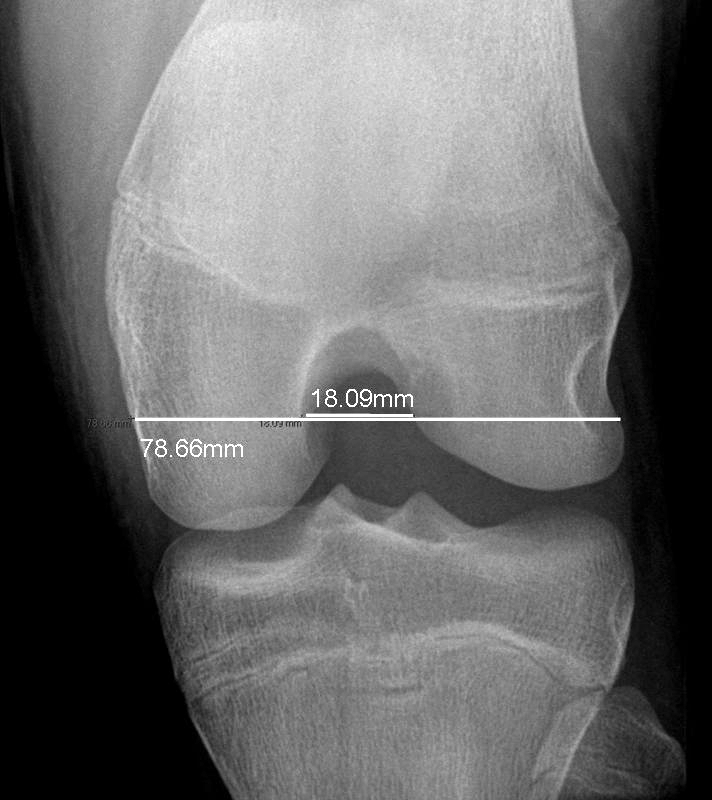
The participant was determined to be in early puberty in year 1, pubertal in years 2 and 3, and postpubertal at the time of injury (6 months later). At the time of injury, her age was 14 years 3 months (171 months). Postinjury radiographs indicated closing physes of the femur and tibia, with no remarkable abnormalities (Figure 6); bone age was estimated (via the Greulich and Pyle method)28 at 14 years (168 months). The athlete reported that menarche occurred at age 12, between the measurements for years 2 and 3. At the interview, she indicated that she had missed 3 successive menstrual cycles at the time of injury, with more than 3 months since the last menses.
Figure 6. Radiographs indicating closing A, femoral and B, tibial physes with no remarkable abnormalities in the athlete at the time of injury.
Biomechanics and Strength
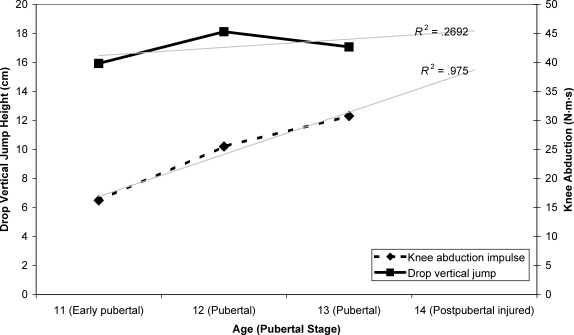
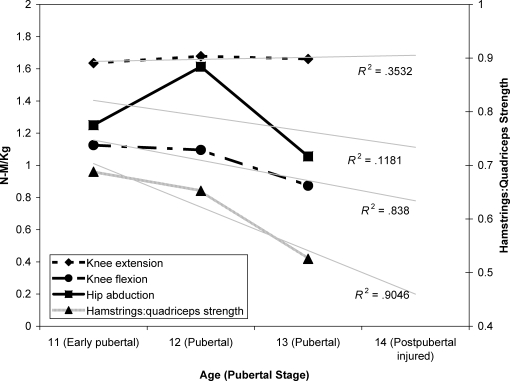
During each annual visit, the participant displayed an increased knee abduction impulse during the drop vertical jump, with limited change in vertical jump performance height during maturation (Figure 7). She also demonstrated limited quadriceps muscle strength increases, with decreased hip abduction and relative knee flexor-to-extensor strength during maturation (Figure 8).
Figure 7. Measured biomechanics changes associated with growth and development.
Figure 8. Measured isokinetic strength changes demonstrated at each visit.
Mechanism of Injury
The athlete and parent reported on the mechanism of injury. The former described that she was “attempting to take a charge at the baseline when [the] knee gave out.” The MRI indicated bone contusions on the left lateral femoral condyle and left posterolateral tibia, reflective of a medial knee collapse (dynamic valgus) injury mechanism (Figure 9).
Figure 9. Magnetic resonance imaging shows A, disrupted anterior cruciate ligament, B, bone bruises on the left lateral femoral condyle, and C, left posterolateral tibia are related to a valgus mechanism at time of injury.
Outcome
The athlete was offered treatment options for the ACL rupture to the left knee, including hamstrings tendon autograft, patellar tendon autograft, and nonsurgical treatment. She and her parents consented to arthroscopy and ACL reconstruction with hamstrings tendon autograft. She pursued return-to-sport phased rehabilitation at The Cincinnati Children's Sports Medicine Biodynamics Center. However, before achieving any of the functional milestones outlined in phase 4, which are indicated before progressing back into sport, the participant began reintegrating into team activities.29,30 At 6.8 months after surgical reconstruction, the athlete reported that while playing defense at basketball practice, she tripped, heard a “pop,” and felt pain in her left knee. Both MRI and arthroscopic evaluation confirmed a complete ACL disruption of her previously reconstructed knee ligament.
Discussion
The mechanism underlying the sex disparity in ACL injury risk is likely multifactorial in nature. Several theories have been proposed to identify the mechanisms that underlie ACL injury risk in female athletes. These theories include related extrinsic (physical and visual perturbations) and intrinsic (anatomic, hormonal, neuromuscular, and biomechanical) mechanisms.31 The participant reported that she was attempting to defend the baseline drive to the basket, and her knee gave out just before she had contact with her opponent. In 1990, McNair et al32 reported that 70% of ACL injuries were noncontact and 30% were contact injuries. In 2004, Olsen et al33 performed a videographic examination of ACL injury mechanisms in team handball and concluded that the ACL injury mechanism in women was a forceful valgus collapse with the knee close to full extension, combined with tibial rotation. Although the exact biomechanical mechanism cannot be determined directly, these data in combination with the MRI images reflective of bone contusions on the left lateral femoral condyle and left posterolateral tibia may indicate a medial knee collapse (dynamic valgus) injury mechanism.
The athlete was determined to be early pubertal in year 1, pubertal in years 2 and 3, and postpubertal at the time of injury (6 months later). Previous authors12,21 have indicated that biomechanical risk factors often peak once a female athlete reaches the postpubertal stage of development. The participant reported menarche had occurred at age 12, between the year 2 and year 3 measurements. During the interview, she indicated she had missed 3 successive menstrual cycles at the time of injury, with more than 3 months passing since last menses. A systematic review of the literature indicates that the menstrual cycle may have a significant effect on ACL injury risk.34 In the time period between menses and ovulation, surrounding the preovulatory (first half) of the menstrual cycle, the risk of injury appears to be increased.34 The irregularity of the athlete's menstrual cycle at the time of injury makes an accurate determination of the cycle phase difficult. Hormonal influences on neuromuscular control of the joints and dynamic knee abduction loads could be an underlying mechanism for ACL injury risk in female athletes. In 1996, Sarwar et al35 reported quadriceps strength increases and a slowing of muscle relaxation during the ovulatory phase of the menstrual cycle.31 Serum estrogen concentrations fluctuate radically throughout the cycle, and estrogen has measurable effects on muscle function and tendon and ligament strength.31,35 In 1994, Lebrun36 reported differences in isokinetic strength, anaerobic and aerobic capacity, and high-intensity endurance in females during different phases of the menstrual cycle.31 In 1987, Posthuma et al37 demonstrated a decrease in motor skills in the premenstrual phase.31 These data indicate that estrogen may have had effects on neuromuscular function and the potential for increased dynamic knee abduction motion and load in the participant as she matured.21 Conversely, reports on postpubertal females suggest that serum hormone levels are not related to biomechanical performance changes.38,39 Although hormones and the menstrual cycle may have contributed to the athlete's injury risk as she matured, these relationships would be speculative in the current report. Future research to determine potential relationships between the menstrual cycle and knee ligament injury in female athletes is warranted.
The participant demonstrated significant musculoskeletal changes in addition to the likely hormonal changes experienced during the study period. She was 14 years old at the time of her injury and demonstrated incremental increases in her body mass index and mass at each annual visit. It is possible that the 17% increase in body mass demonstrated from the time of her last testing visit to the injury may have influenced the mechanism of injury. A longitudinal study of youth soccer players 5 to 12 years of age demonstrated that age greater than 11 years was a risk factor for knee injury in girls. For female players older than 8 years of age, body mass index was also a risk factor for increased knee injury risk.5,31 Uhorchak et al11 reported that women with body weight or body mass index greater than 1 SD above the mean had a 3.2 or 3.5 times greater risk, respectively, of ACL injury than those with lower body weight or body mass index.31 Increased ACL injury incidence increases incrementally on a yearly basis, reaching its peak at age 16 years in females.8 Cross-sectional data also indicate that female athletes demonstrate incremental increases in knee abduction load as they increase in chronological age.40 Interestingly, the knee abduction loads that are related to increased knee injury risk also peak at age 16, concomitant with peak ACL injury incidence in female athletes.8,40 Adding to the loss of lower extremity control, maturing females also demonstrate decreased passive joint stability as they mature, compared with males.18 The current athlete also increased her anterior tibiofemoral laxity as she matured. The greater joint laxity, especially at the knee, also may contribute to the increased tendency for dynamic valgus laxity that can predispose female athletes to increased dynamic valgus loads and ACL injury risk as prepubescent females mature.12,13,21,40 Cumulatively, these data may indicate a relationship between maturational development and the tendency for high-risk female athletes to demonstrate an increased coronal-plane load strategy, as opposed to a sagittal-plane load absorption strategy, during dynamic sport-related activities. Loading in the coronal plane, combined with increased passive laxity, may destabilize the knee, load the ACL, and increase knee injury risk in postpubertal athletes12,24,41–48
During peak growth (height and mass) velocity in pubertal athletes, the tibia and femur grow at rapid rates in both sexes.49 Rapid growth of the 2 longest lever arms in the human body initiates height increases concomitant with increasing height of the center of mass, as was demonstrated in the participant (Figure 3). Beynnon et al50 reported that increased thigh length was an ACL injury risk factor in female skiers.31 Lower extremity bone lengths may underlie the increased risk of ACL injuries; however, anatomic measures often do not correlate with dynamic injury mechanisms.31,51 In the current report, the apparent increase in vertical height of the athlete's center of mass as she matured (Figure 3) may have placed greater demands on her core and trunk control. Yet she did not demonstrate matching increases in relative hip abduction or hamstring strength to help meet these demands (Figure 8). As she reached maturity, in the absence of adaptations in core power and control to match her whole-body increases in inertial load, increased knee abduction loads may have been influenced.14,21,52–57
During maturation, male athletes naturally demonstrate a “neuromuscular spurt” (increased strength and power during maturational growth and development) to match the increased demands of growth and development.20,21,53,58,59 The participant did not demonstrate any similar neuromuscular strength adaptations to match the increased demands created by structural and inertial changes from her pubertal development. This lack of adaptation is common in female athletes.20,21,53,58,59 One might hypothesize that after the onset of puberty (concomitant with or just after peak height velocity), the increased tibia and femur lever length, combined with increased body mass and height of the center of mass, in the absence of increases in strength and recruitment of the musculature at the hip and torso, may have led to the athlete's decreased control of lower extremity alignment and knee loads during dynamic tasks.14,21,60
Evidence that neuromuscular training reduces the levels of potential biomechanical risk factors for ACL injury, increases measures related to the “neuromuscular spurt,” and decreases general knee and ACL injury incidence in female athletes exists.21,43,48,61–65 However, reevaluation of ACL injury rates in female athletes indicates that this important health issue has yet to be resolved in society, because increased knowledge and application of injury prevention techniques have not provided dramatic reductions in ACL injury incidence in female athletes.66 A recent investigation by Grindstaff et al67 indicated that standard, nontargeted neuromuscular training programs require application to 89 female athletes to prevent 1 ACL injury. Theoretically, by identifying female athletes at greater risk for ACL injury, the numbers needed to treat to prevent an ACL injury could be reduced substantially. Pilot work testing this theory suggests that female athletes categorized as high risk for ACL injury based on excessive dynamic knee valgus loads are more responsive to neuromuscular training aimed at reducing this risk factor.12,48 Due to the near-100% risk of osteoarthritis in the ACL-injured population,68 with or without surgical reconstruction, prevention is currently the only effective intervention for these life-altering injuries. The greatly increased risk of knee osteoarthritis suggests the need to develop more specific injury prevention protocols targeted to high-risk mechanisms. In addition, determining the timing of these interventions for the greatest effectiveness is salient. Through both longitudinal and prospective cohort study designs used to determine the contributing mechanisms of increased knee loads associated with maturational development, progress toward characterizing risk factors for ACL injury continues.
Conclusions
The anatomic and neuromuscular changes associated with puberty may underlie biomechanical changes, which may lead to an increased risk of ACL injury in female athletes.21,69 As with all case reports, the interpretation and generalization of the reported data are limited by sample size; however, we present interesting comparisons of ACL risk factors across pubertal developmental stages in a young female athlete. The current case indicates that after the onset of puberty, the participant increased body mass and height of the center of mass without matched increases in hip and knee strength. For this athlete, the absence of adaptive increases in lower extremity control may have predisposed her to sustain an ACL injury during competition. Although multiple factors were likely to have contributed to her increased risk of ACL injury, neuromuscular control and lower extremity strength are perhaps the most modifiable factors. A focus on research into modifiable factors, so that effective interventions can be instituted, is imperative. Similarly, investigating innate and potentially nonmodifiable variables that contribute to ACL injury in female athletes will promote a better understanding of developmental patterns and how they might relate and contribute to identified modifiable risk factors. This research is especially important in the pubescent athlete, because the individual in the current case demonstrated significant developmental changes, both anatomically and hormonally (the latter inferred). The musculoskeletal changes that occur during puberty may alter both passive joint laxity and dynamic joint stability and potentially lead to higher injury rates in this population. If all risk factors (modifiable and nonmodifiable) are identified, clinical algorithms can be developed to accurately identify those at high risk. These algorithms can then be used to target high-risk athletes with the most effective intervention instituted in the right time frame. Prospective longitudinal designs are needed to scientifically test hypotheses related to development of increased risk for ACL injury in the female athlete.
Acknowledgments
We acknowledge funding support from National Institutes of Health/NIAMS Grant R01-AR049735. We thank the Boone County School District in Kentucky, and especially School Superintendent Dr Brian Blavatt, for participation in this study. We also thank Kim Foss and the Sports Medicine Biodynamics Center team for their work on the project and her help with the development of the manuscript.
Footnotes
Gregory D. Myer, MS, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting, critical revision, and final approval of the article. Kevin R. Ford, MS, contributed to acquisition and analysis and interpretation of the data and critical revision and final approval of the article. Jon G. Divine, MD, and Eric J. Wall, MD, contributed to analysis and interpretation of the data and critical revision and final approval of the article. Leamor Kahanov, EdD, ATC, and Timothy E. Hewett, PhD, contributed to conception and design, analysis and interpretation of the data, and critical revision and final approval of the article.
References
- 1.Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer. NCAA data and review of literature. Am J Sports Med. 1995;23(6):694–701. doi: 10.1177/036354659502300611. [DOI] [PubMed] [Google Scholar]
- 2.Malone T.R, Hardaker W.T, Garrett W.E, Feagin J.A, Bassett F.H. Relationship of gender to anterior cruciate ligament injuries in intercollegiate basketball players. J South Orthop Assoc. 1993;2(1):36–39. [Google Scholar]
- 3.Myklebust G, Maehlum S, Holm I, Bahr R. A prospective cohort study of anterior cruciate ligament injuries in elite Norwegian team handball. Scand J Med Sci Sports. 1998;8(3):149–153. doi: 10.1111/j.1600-0838.1998.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 4.Andrish J.T. Anterior cruciate ligament injuries in the skeletally immature patient. Am J Orthop. 2001;30(2):103–110. [PubMed] [Google Scholar]
- 5.Buehler-Yund C. A Longitudinal Study of Injury Rates and Risk Factors in 5- to 12-Year-Old Soccer Players [dissertation] Cincinnati, OH: University of Cincinnati; 1999. [Google Scholar]
- 6.Clanton T.O, DeLee J.C, Sanders B, Neidre A. Knee ligament injuries in children. J Bone Joint Surg Am. 1979;61(8):1195–1201. [PubMed] [Google Scholar]
- 7.Gallagher S.S, Finison K, Guyer B, Goodenough S. The incidence of injuries among 87,000 Massachusetts children and adolescents: results of the 1980–81 Statewide Childhood Injury Prevention Program Surveillance System. Am J Public Health. 1984;74(12):1340–1347. doi: 10.2105/ajph.74.12.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shea K.G, Pfeiffer R, Wang J.H, Curtin M, Apel P.J. Anterior cruciate ligament injury in pediatric and adolescent soccer players: an analysis of insurance data. J Pediatr Orthop. 2004;24(6):623–628. doi: 10.1097/00004694-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Tursz A, Crost M. Sports-related injuries in children. A study of their characteristics, frequency, and severity, with comparison to other types of accidental injuries. Am J Sports Med. 1986;14(4):294–299. doi: 10.1177/036354658601400409. [DOI] [PubMed] [Google Scholar]
- 10.LaPrade R.F, Burnett QM I.I. Femoral intercondylar notch stenosis and correlation to anterior cruciate ligament injuries: a prospective study. Am J Sports Med. 1994;22(2):198–203. doi: 10.1177/036354659402200208. [DOI] [PubMed] [Google Scholar]
- 11.Uhorchak J.M, Scoville C.R, Williams G.N, Arciero R.A, St Pierre P, Taylor D.C. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31(6):831–842. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- 12.Hewett T.E, Myer G.D, Ford K.R, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 13.Myer G.D, Ford K.R, Paterno M.V, Nick T.G, Hewett T.E. The effects of generalized joint laxity on risk of anterior cruciate ligament injury in young female athletes. Am J Sports Med. 2008;36(6):1073–1080. doi: 10.1177/0363546507313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewett T.E, Biro F.M, McLean S.G, Van den Bogert A.J, editors. Identifying Female Athletes at High Risk for ACL Injury. Cincinnati, OH: Cincinnati Children's Hospital and National Institutes of Health; 2003. [Google Scholar]
- 15.Pasque C.B, Hewett T.E. A prospective study of high school wrestling injuries. Am J Sports Med. 2000;28(4):509–515. doi: 10.1177/03635465000280041101. [DOI] [PubMed] [Google Scholar]
- 16.Souryal T.O, Moore H.A, Evans J.P. Bilaterality in anterior cruciate ligament injuries: associated intercondylar notch stenosis. Am J Sports Med. 1988;16(5):449–454. doi: 10.1177/036354658801600504. [DOI] [PubMed] [Google Scholar]
- 17.Souryal T.O, Freeman T.R. Intercondylar notch size and anterior cruciate ligament injuries in athletes: a prospective study. Am J Sports Med. 1993;21(4):535–539. doi: 10.1177/036354659302100410. [DOI] [PubMed] [Google Scholar]
- 18.Quatman C.E, Ford K.R, Myer G.D, Paterno M.V, Hewett T.E. The effects of gender and pubertal status on generalized joint laxity in young athletes. J Sci Med Sport. 2008;11(3):257–263. doi: 10.1016/j.jsams.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies P.L, Rose J.D. Motor skills of typically developing adolescents: awkwardness or improvement. Phys Occup Ther Pediatr. 2000;20(1):19–42. [PubMed] [Google Scholar]
- 20.Quatman C.E, Ford K.R, Myer G.D, Hewett T.E. Maturation leads to gender differences in landing force and vertical jump performance: a longitudinal study. Am J Sports Med. 2006;34(5):806–813. doi: 10.1177/0363546505281916. [DOI] [PubMed] [Google Scholar]
- 21.Hewett T.E, Myer G.D, Ford K.R. Decrease in neuromuscular control about the knee with maturation in female athletes. J Bone Joint Surg Am. 2004;86(8):1601–1608. doi: 10.2106/00004623-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Davies P.S. Assessment of Cognitive Development in Adolescents by Means of Neuropsychological Tasks [dissertation] Laramie: University of Wyoming; 1995. [Google Scholar]
- 23.Ford K.R, Myer G.D, Hewett T.E. Reliability of landing 3D motion analysis techniques: implications for longitudinal analyses. Med Sci Sports Exerc. 2007;39(11):2021–2028. doi: 10.1249/mss.0b013e318149332d. [DOI] [PubMed] [Google Scholar]
- 24.Ford K.R, Myer G.D, Hewett T.E. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003;35(10):1745–1750. doi: 10.1249/01.MSS.0000089346.85744.D9. [DOI] [PubMed] [Google Scholar]
- 25.Bisseling R.W, Hof A.L. Handling of impact forces in inverse dynamics. J Biomech. 2006;39(13):2438–2444. doi: 10.1016/j.jbiomech.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Winter D.A. Biomechanics and Motor Control of Human Movement. New York, NY: John Wiley & Sons; 1990. pp. 91–95. [Google Scholar]
- 27.Smith R, Ford K.R, Myer G.D, Holleran A, Treadway E, Hewett T.E. Biomechanical and performance differences between female soccer athletes in National Collegiate Athletic Association Divisions I and III. J Athl Train. 2007;42(4):470–476. [PMC free article] [PubMed] [Google Scholar]
- 28.Christoforidis A, Badouraki M, Katzos G, Athanassiou-Metaxa M. Bone age estimation and prediction of final height in patients with beta-thalassaemia major: a comparison between the two most common methods. Pediatr Radiol. 2007;37(12):1241–1246. doi: 10.1007/s00247-007-0656-1. [DOI] [PubMed] [Google Scholar]
- 29.Myer G.D, Paterno M.V, Ford K.R, Hewett T.E. Neuromuscular training techniques to target deficits before return to sport after anterior cruciate ligament reconstruction. J Strength Cond Res. 2008;22(3):987–1014. doi: 10.1519/JSC.0b013e31816a86cd. [DOI] [PubMed] [Google Scholar]
- 30.Myer G.D, Paterno M.V, Ford K.R, Quatman C.E, Hewett T.E. Rehabilitation after anterior cruciate ligament reconstruction: criteria based progression through the return-to-sport phase. J Orthop Sports Phys Ther. 2006;36(6):385–402. doi: 10.2519/jospt.2006.2222. [DOI] [PubMed] [Google Scholar]
- 31.Hewett T.E, Myer G.D, Ford K.R. Anterior cruciate ligament injuries in female athletes: part 1: mechanisms and risk factors. Am J Sports Med. 2006;34(2):299–311. doi: 10.1177/0363546505284183. [DOI] [PubMed] [Google Scholar]
- 32.McNair P.J, Marshall R.N, Matheson J.A. Important features associated with acute anterior cruciate ligament injury. N Z Med J. 1990;103(901):537–539. [PubMed] [Google Scholar]
- 33.Olsen O.E, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 34.Hewett T.E, Zazulak B.T, Myer G.D. Effects of the menstrual cycle on anterior cruciate ligament injury risk: a systematic review. Am J Sports Med. 2007;35(4):659–668. doi: 10.1177/0363546506295699. [DOI] [PubMed] [Google Scholar]
- 35.Sarwar R, Niclos B, Rutherford O.M. Changes in muscle strength, relaxation rate and fatiguability during the human menstrual cycle. J Physiol. 1996;493(pt 1):267–272. doi: 10.1113/jphysiol.1996.sp021381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebrun C.M. The effect of the phase of the menstrual cycle and the birth control pill on athletic performance. Clin Sports Med. 1994;13(2):419–441. [PubMed] [Google Scholar]
- 37.Posthuma B.W, Bass M.J, Bull S.B, Nisker A.J. Detecting changes in functional ability in women with premenstrual syndrome. Am J Obstet Gynecol. 1987;156(2):275–278. doi: 10.1016/0002-9378(87)90267-5. [DOI] [PubMed] [Google Scholar]
- 38.Abt J.P, Sell T.C, Laudner K.G, et al. Neuromuscular and biomechanical characteristics do not vary across the menstrual cycle. Knee Surg Sports Traumatol Arthrosc. 2007;15(7):901–907. doi: 10.1007/s00167-007-0302-3. [DOI] [PubMed] [Google Scholar]
- 39.Chaudhari A.M, Lindenfeld T.N, Andriacchi T.P, et al. Knee and hip loading patterns at different phases in the menstrual cycle: implications for the gender difference in anterior cruciate ligament injury rates. Am J Sports Med. 2007;35(5):793–800. doi: 10.1177/0363546506297537. [DOI] [PubMed] [Google Scholar]
- 40.Ford K.R, Myer G.D, Divine J.G, Hewett T.E. Landing differences in high school female soccer players grouped by age [abstract] Med Sci Sports Exerc. 2004;36(5):S293. [Google Scholar]
- 41.McLean S.G, Lipfert S.W, van den Bogert A.J. Effect of gender and defensive opponent on the biomechanics of sidestep cutting. Med Sci Sports Exerc. 2004;36(6):1008–1016. doi: 10.1249/01.mss.0000128180.51443.83. [DOI] [PubMed] [Google Scholar]
- 42.McLean S.G, Neal R.J, Myers P.T, Walters M.R. Knee joint kinematics during the sidestep cutting maneuver: potential for injury in women. Med Sci Sports Exerc. 1999;31(7):959–968. doi: 10.1097/00005768-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 43.Hewett T.E, Stroupe A.L, Nance T.A, Noyes F.R. Plyometric training in female athletes. decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765–773. doi: 10.1177/036354659602400611. [DOI] [PubMed] [Google Scholar]
- 44.Ford K.R, Myer G.D, Toms H.E, Hewett T.E. Gender differences in the kinematics of unanticipated cutting in young athletes. Med Sci Sports Exerc. 2005;37(1):124–129. [PubMed] [Google Scholar]
- 45.Ford K.R, Myer G.D, Smith R.L, Vianello R.M, Seiwert S.L, Hewett T.E. A comparison of dynamic coronal plane excursion between matched male and female athletes when performing single leg landings. Clin Biomech (Bristol, Avon) 2006;21(1):33–40. doi: 10.1016/j.clinbiomech.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 46.Kernozek T.W, Torry M.R, H V.H, Cowley H, Tanner S. Gender differences in frontal and sagittal plane biomechanics during drop landings. Med Sci Sports Exerc. 2005;37(6):1003–1013. [PubMed] [Google Scholar]
- 47.Lloyd D.G, Buchanan T.S, Besier T.F. Neuromuscular biomechanical modeling to understand knee ligament loading. Med Sci Sports Exerc. 2005;37(11):1939–1947. doi: 10.1249/01.mss.0000176676.49584.ba. [DOI] [PubMed] [Google Scholar]
- 48.Myer G.D, Ford K.R, Brent J.L, Hewett T.E. Differential neuromuscular training effects on ACL injury risk factors in “high-risk” versus “low-risk” athletes. BMC Musculoskelet Disord. 2007;8(39):1–7. doi: 10.1186/1471-2474-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tanner J.M, Davies P.S. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107(3):317–329. doi: 10.1016/s0022-3476(85)80501-1. [DOI] [PubMed] [Google Scholar]
- 50.Beynnon B, Slauterbeck J, Padua D, Hewett T.E. Proceedings of the National Athletic Trainers' Association 52nd Annual Meeting and Clinical Symposia. Champaign, IL: Human Kinetics; 2001. Update on ACL risk factors and prevention strategies in the female athlete; pp. 15–18. [Google Scholar]
- 51.Myer G.D, Ford K.R, Hewett T.E. The effects of gender on quadriceps muscle activation strategies during a maneuver that mimics a high ACL injury risk position. J Electromyogr Kinesiol. 2005;15(2):181–189. doi: 10.1016/j.jelekin.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Willson J.D, Dougherty C.P, Ireland M.L, Davis I.M. Core stability and its relationship to lower extremity function and injury. J Am Acad Orthop Surg. 2005;13(5):316–325. doi: 10.5435/00124635-200509000-00005. [DOI] [PubMed] [Google Scholar]
- 53.Hewett T.E, Myer G.D, Ford K.R, Slauterbeck J.R. Preparticipation physical exam using a box drop vertical jump test in young athletes: the effects of puberty and sex. Clin J Sport Med. 2006;16(4):298–304. doi: 10.1097/00042752-200607000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Hodges P.W, Richardson C.A. Contractions of the abdominal muscles associated with movement of the lower limb. Phys Ther. 1997;77(2):132–144. doi: 10.1093/ptj/77.2.132. [DOI] [PubMed] [Google Scholar]
- 55.Hodges P.W, Richardson C.A. Feedforward contraction of transversus abdominis is not influenced by the direction of arm movement. Exp Brain Res. 1997;114(2):362–370. doi: 10.1007/pl00005644. [DOI] [PubMed] [Google Scholar]
- 56.Brent J.L, Myer G.D, Ford K.R, Hewett T.E. The effect of gender and age on isokinetic hip abduction [abstract] Med Sci Sports Exerc. 2006;38(suppl 5):S290–S291. [Google Scholar]
- 57.Padua D.A, Marshall S.W, Beutler A.I, et al. Predictors of knee valgus angle during a jump-landing task [abstract] Med Sci Sports Exerc. 2005;37(suppl 5):S398. [Google Scholar]
- 58.Kellis E, Tsitskaris G.K, Nikopoulou M.D, Moiusikou K.C. The evaluation of jumping ability of male and female basketball players according to their chronological age and major leagues. J Strength Cond Res. 1999;13(1):40–46. [Google Scholar]
- 59.Malina R.M, Bouchard C. Growth, Maturation, and Physical Activity. Champaign, IL: Human Kinetics; 1991. p. 501. [Google Scholar]
- 60.Ford K.R, Myer G.D, Hewett T.E. Increased trunk motion in female athletes compared to males during single leg landing [abstract] Med Sci Sports Exerc. 2007;39(suppl 5):S70. [Google Scholar]
- 61.Myer G.D, Ford K.R, Divine J.G, Hewett T.E. Specialized dynamic neuromuscular training can be utilized to induce neuromuscular spurt in female athletes. Med Sci Sports Exerc. 2004;36(suppl 5):343–344. [Google Scholar]
- 62.Hewett T.E, Ford K.R, Myer G.D. Anterior cruciate ligament injuries in female athletes, part 2: a meta-analysis of neuromuscular interventions aimed at injury prevention. Am J Sports Med. 2006;34(3):490–498. doi: 10.1177/0363546505282619. [DOI] [PubMed] [Google Scholar]
- 63.Myer G.D, Ford K.R, Brent J.L, Hewett T.E. The effects of plyometric versus dynamic balance training on power, balance, and landing force in female athletes. J Strength Cond Res. 2006;20(2):345–353. doi: 10.1519/R-17955.1. [DOI] [PubMed] [Google Scholar]
- 64.Myer G.D, Ford K.R, McLean S.G, Hewett T.E. The effects of plyometric versus dynamic stabilization and balance training on lower extremity biomechanics. Am J Sports Med. 2006;34(3):490–498. doi: 10.1177/0363546505281241. [DOI] [PubMed] [Google Scholar]
- 65.Myer G.D, Ford K.R, Palumbo J.P, Hewett T.E. Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. J Strength Cond Res. 2005;19(1):51–60. doi: 10.1519/13643.1. [DOI] [PubMed] [Google Scholar]
- 66.Agel J, Arendt E.A, Bershadsky B. Anterior cruciate ligament injury in National Collegiate Athletic Association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524–530. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- 67.Grindstaff T.L, Hammill R.R, Tuzson A.E, Hertel J. Neuromuscular control training programs and noncontact anterior cruciate ligament injury rates in female athletes: a numbers-needed-to-treat analysis. J Athl Train. 2006;41(4):450–456. [PMC free article] [PubMed] [Google Scholar]
- 68.Myklebust G, Bahr R. Return to play guidelines after anterior cruciate ligament surgery. Br J Sports Med. 2005;39(3):127–131. doi: 10.1136/bjsm.2004.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Croce R.V, Russell P.J, Swartz E.E, Decoster L.C. Knee muscular response strategies differ by developmental level but not gender during jump landing. Electromyogr Clin Neurophysiol. 2004;44(6):339–348. [PubMed] [Google Scholar]