Abstract
Objective:
To assess existing original research addressing the efficiency of whole-body cooling modalities in the treatment of exertional hyperthermia.
Data Sources:
During April 2007, we searched MEDLINE, EMBASE, Scopus, SportDiscus, CINAHL, and Cochrane Reviews databases as well as ProQuest for theses and dissertations to identify research studies evaluating whole-body cooling treatments without limits. Key words were cooling, cryotherapy, water immersion, cold-water immersion, ice-water immersion, icing, fanning, bath, baths, cooling modality, heat illness, heat illnesses, exertional heatstroke, exertional heat stroke, heat exhaustion, hyperthermia, hyperthermic, hyperpyrexia, exercise, exertion, running, football, military, runners, marathoner, physical activity, marathoning, soccer, and tennis.
Data Synthesis:
Two independent reviewers graded each study on the Physiotherapy Evidence Database (PEDro) scale. Seven of 89 research articles met all inclusion criteria and a minimum score of 4 out of 10 on the PEDro scale.
Conclusions:
After an extensive and critical review of the available research on whole-body cooling for the treatment of exertional hyperthermia, we concluded that ice-water immersion provides the most efficient cooling. Further research comparing whole-body cooling modalities is needed to identify other acceptable means. When ice-water immersion is not possible, continual dousing with water combined with fanning the patient is an alternative method until more advanced cooling means can be used. Until future investigators identify other acceptable whole-body cooling modalities for exercise-induced hyperthermia, ice-water immersion and cold-water immersion are the methods proven to have the fastest cooling rates.
Keywords: exertional heat illness, evidence-based practice, cryotherapy, modalities
Certified athletic trainers need to assess and appropriately care for individuals with exertional heat illnesses to prevent possible serious consequences or fatalities.1–4 The most serious of these illnesses is exertional heat stroke (EHS), a condition marked by an elevated core body temperature (greater than 40°C–41°C) and central nervous system dysfunction.1–6 Exertional heat exhaustion is diagnosed with a moderately elevated core body temperature (generally less than 40°C) and the inability to continue exercising; exertional heat stroke can develop if heat exhaustion is managed improperly, but heat exhaustion need not precipitate EHS.1,5
The National Athletic Trainers' Association2 and American College of Sports Medicine3 have published guidelines for the treatment of this condition. Because the most critical predictor of outcome after EHS is the amount of time that core body temperature remains above a critical threshold, certified athletic trainers should, whenever possible, implement the most effective cooling modality as supported by evidence.1,5–9
Authors of reviews in this area have not critically appraised the research and have combined treatment data for both classic heat stroke and EHS, which can be distinguished by noteworthy differences.10–12 Classic heat stroke involves passive thermal exposure, normally affecting the elderly in a non–air-conditioned environment or youths abandoned in vehicles in the summer months. The body's response to cooling modalities differs with age, possible heart complications, and existing unhealthy conditions before the illness.6 Instead of critical assessments of the literature, conclusions from these reviews may reflect author bias. Because author bias exists in the medical literature, attention recently has been directed toward evidence-based medicine,13 defined as the practice of medicine based on a complete appraisal of methodologically sound research. Randomized controlled trials represent a “gold standard” in research methods, but these trials are impossible in the case of EHS. An institutional review board would not allow research that induces EHS in human volunteers. Also, it is unethical for medical professionals to deny a scientifically justifiable treatment approach for a medical emergency. Therefore, the studies described below focus on exercise-induced hyperthermia, that is, elevated body temperature (greater than 38.5°C [101.3°F]) resulting from exercise, as a research model despite the fact that some of the test participants did not experience EHS. Clearly, a body temperature of 38.5°C is not dangerous, but it serves as a means of testing the efficacy of cooling modalities.
The purpose of this systematic review was 2-fold. First, we sought to evaluate and summarize the data regarding whole-body cooling modalities used in the treatment of exercise-induced hyperthermia. Second, we wanted to rank the various means of whole-body cooling, so that clinicians could identify their modality of choice in different settings.
Methods
We searched the following databases without limits on language: MEDLINE, SportDiscus, CINAHL, the Cochrane Reviews database, and the Physiotherapy Evidence Database. All dates (which varied according to database) were included, and the search was performed in April 2007. Previously known cases, review articles, and reference lists of available studies were cross-referenced for possible articles meeting inclusion criteria. Key words used for searches were cooling, cryotherapy, water immersion, cold-water immersion, ice-water immersion, icing, fanning, bath, baths, cooling modality, heat illness, heat illnesses, exertional heatstroke, exertional heat stroke, heat exhaustion, hyperthermia, hyperthermic, hyperpyrexia, exercise, exertion, running, football, military, runners, marathoner, physical activity, marathoning, soccer, and tennis. This original search revealed a total of 89 possible studies.
Study Selection
Specific inclusion criteria identified before data analysis included (1) exercise-associated hyperthermia, (2) pretreatment hyperthermia greater than 38.5°C (101.3°F), (3) a valid core body temperature measurement to characterize hyperthermia, (4) detailed explanation, sufficient for repeatability of the cooling modality methods, and (5) original research studies with human participants. Passive hyperthermia was excluded so that results from exercising conditions would reflect traditional athletic training and military settings. Individuals with classic heat stroke may respond differently to whole-body cooling than do EHS victims. Core body temperatures exceeding 38.5°C (101.3°F) normally occur in some athletic settings, and this cutoff point was selected to appropriately generalize research findings to common athletic training settings, even though rapid cooling is not necessary.3 Valid core body temperature measurement techniques included rectal thermistors, ingestible telemetric sensors, bladder catheters, and esophageal thermistors. Investigations using other temperature measures were excluded because these devices are known to report invalid results for exercising individuals.14–16 Animal studies were not included. Case reports were not included in the overall data analysis but appear in a separate section.
The time between maximum core body temperature and treatment initiation is one determinant of the overall effectiveness of treatment. The main outcome for an EHS patient is based on the amount of time a victim remains above a critical temperature threshold. Also, if the time to treatment is 15 minutes versus 1 minute, this affects the cooling rate, because the body can gain or lose heat via radiation to the environment. The time to treatment was not considered an exclusionary criterion in our review, because authors offered enough information to provide a reasonable estimate of this measure. All groups explained their methods similarly, which led us to assume that less than 10 minutes separated the hyperthermia and initiation of treatment. We tried to contact authors of previous research to assess this variable if it was not reported originally.
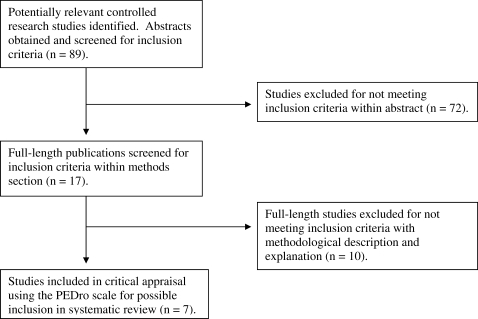
Abstracts of the 89 identified papers were included in this review only if they met all inclusion criteria previously identified (Figure 1). A total of 17 abstracts met these criteria. In their abstracts, some authors did not report complete methods or describe measures taken. For this reason, 1 reviewer completed a full-text evaluation of methods to assure that all inclusion criteria were met. This review identified 7 articles that met the inclusion criteria; a quality assessment review of these studies followed.
Figure 1. Selection process for articles included in the systematic review. PEDro indicates Physiotherapy Evidence Database.
Quality Assessment
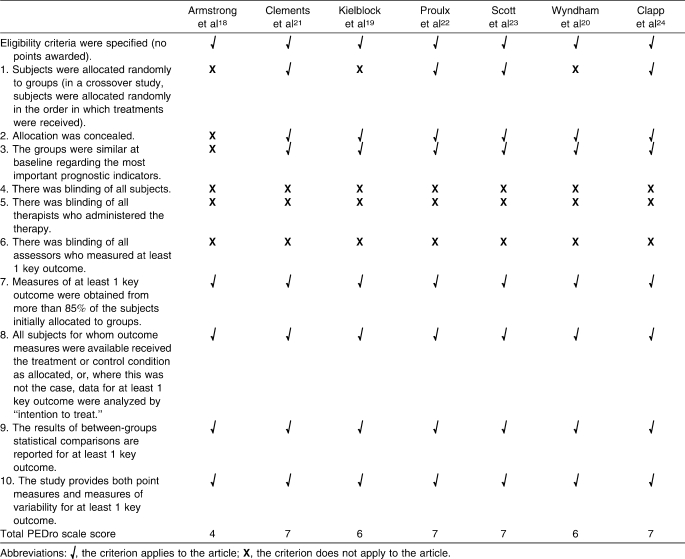
Two reviewers independently assessed the methodologic quality of the studies based on the Physiotherapy Evidence Database (PEDro) scale.17 An a priori inclusion score of 4/10 was selected for an article to be included in data analysis. If discrepancies existed between reviewers, an open discussion took place to ensure that one had not missed or misinterpreted an aspect of the study. After discussion, if an agreement was not reached, a third independent reviewer assessed the article based on the scale. This reviewer was independent and was blinded to previous reviews and discussions. Initial κ statistics revealed strong agreement of 0.984. After a consensus meeting, the κ statistic for agreement was 1.000. A third independent reviewer was not needed for unresolved disagreement on PEDro scores. Final PEDro scale scores for the 7 articles that met the inclusion criteria are shown in Table 1.
Table 1.
Physiotherapy Evidence Database (PEDro) Scale Scores of Critically Reviewed Articles
A score of 4/10 on the PEDro scale was chosen because complete blinding of participants and therapists is impossible when assessing whole-body cooling modalities. Thus, the maximum score obtainable for this research is 7/10. Case reports and epidemiologic studies were not included because they achieved a zero score on the PEDro scale.
None of the studies included blinding of participants, assessors, or researchers. Three groups18–20 did not randomly allocate volunteers to groups. One set of investigators18 was unable to conceal allocation to groups or control the fact that important prognostic indicators were not similar at the beginning of whole-body cooling. All studies that met the inclusion criteria, however, met the criterion score of 4/10.18–24
To determine if recommendations for certain modalities were warranted, we identified 3 categories of cooling based on efficiency. Category A required approximately 20 minutes of cooling for an EHS patient with a maximum core body temperature of 42.2°C (108°F) to be cooled to 38.89°C (102°F). Category B required up to 40 minutes for the same body temperature reduction, and category C required more than 40 minutes of cooling. The cooling rates identified were category A, equal to or greater than 0.155°C · min−1; category B, greater than 0.078°C · min−1 but less than 0.155°C · min−1; and category C, less than or equal to 0.078°C · min−1.
Only cooling rate data were synthesized for this review. Some authors included other outcome variables, but because our original research question focused specifically on cooling efficiency after exercise-induced hyperthermia, we included only data related to these criteria in the current review.
Data Synthesis
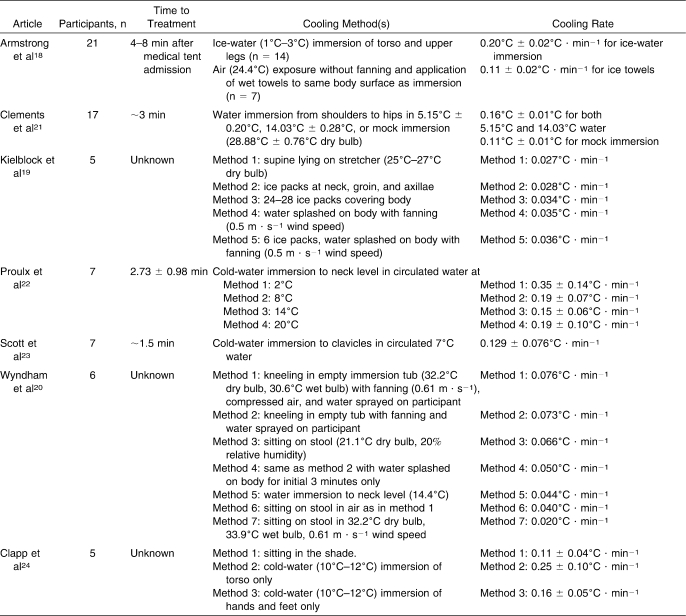
Overall results for the 7 studies in this review are presented in Table 2. Mean cooling rates ranged from 0.020°C · min−1 to 0.35°C · min−1.20,22 The fastest cooling rates were reported for ice-water and cold-water immersion.22 The slowest cooling rates were reported with no cooling or control conditions included in comparison studies.19,20 A brief description of each research study follows.
Table 2.
Results From Published Articles
Armstrong et al18 compared treatments of EHS using wet towels applied to the torso and ice-water immersion. Category A cooling efficiency (0.20°C · min−1) was reported with ice-water immersion, whereas category B cooling (0.110°C · min−1) was found with wet towels. The participants included in this comparison were EHS patients in a medical tent after a road race.
Two groups21,22 evaluated the effects of water temperature on immersion after exercise-induced hyperthermia. Category A cooling rates (0.16°C to 0.35°C · min−1) were reported for all water temperatures ranging from 2°C to 20°C. Of the experiments including ice-water or cold-water immersion as a treatment, category A cooling rates were seen with cold-water immersion of either the torso area or the entire body up to the clavicular level.18,21,22 Other reported protocols are summarized in Table 2 and include all studied methods.
The time between maximum core body temperature and the initiation of treatment was reported in 4 of the 7 included studies.18,21–23 When reported, time to treatment ranged from 1.5 to 8 minutes. An author of another study24 was contacted and stated that the average time to treatment was approximately 5 minutes. The other 2 studies19,20 were published more than 20 years before our search, and we were not able to contact those authors. However, information provided in those articles led us to safely assume that the time to treatment was less than 10 minutes (ie, once maximum body temperature was reached, participants were placed in the treatment condition).
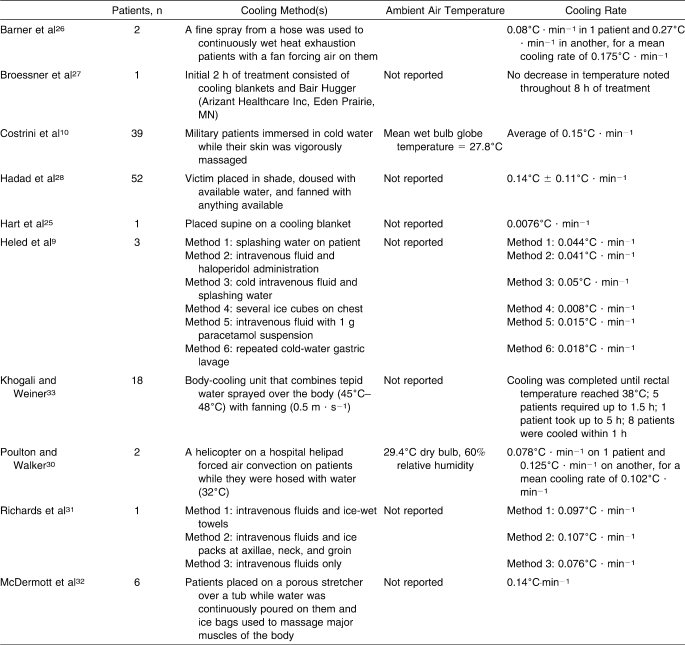
A variety of whole-body cooling methods has been described in case reports, with cooling rates ranging from 0.0076°C to 0.270°C · min−1 (Table 3).25–28 Category A mean cooling rates included fanning and hosing patients continually (0.175°C · min−1).26 Category B cooling rates were reported with cold-water immersion (0.150°C · min−1),29 helicopter downdraft with continual hosing (0.102°C · min−1),30 cold intravenous fluid administration with ice packs on the major arteries of the body (0.107°C · min−1),31 continually dousing patients with tepid water and fanning them with available means (0.140°C · min−1),11 pouring water on patients while massaging major muscle groups with ice bags (0.140°C · min−1),32 and intravenous fluids with ice-wet towels over the body (0.097°C · min−1).31 One set of authors33 did not report cooling rates but provided total time to cool patients to 38°C; these times ranged from approximately 30 minutes to more than 5 hours with the use of a body-cooling unit and were clearly unacceptable.
Table 3.
Case Report Cooling Rates
Discussion
Ice-Water and Cold-Water Immersion
Ice-water immersion currently is recommended by the National Athletic Trainers' Association2 and American College of Sports Medicine3 for the treatment of EHS. This method has been challenged in the past due to speculation that peripheral vasoconstriction and shivering cause a paradoxical rise in body temperature.19,20,25,30 The potential negative consequences of peripheral vasoconstriction and shivering have been refuted in a recent review,34 which also showed that these factors do not negatively affect cooling rates for EHS victims. In fact, these physiologic responses did not cause a rise in core body temperature in hyperthermic individuals.21,22 Peripheral vasoconstriction and shivering occur in normothermic individuals, but EHS patients either do not exhibit these physiologic responses or if they do, rapid cooling is not impeded.34
Various methods were used in the studies included in this review. Two groups18,21 included immersion from the neck to the upper thighs, whereas others immersed participants completely to the clavicles or used larger cooling tubs.20,22,23 The fastest cooling rates were reported when the largest proportion of the body was immersed in cold water.22 Category A or B cooling rates were reported in all but 1 experiment involving the use of cold-water immersion.20
Wyndham et al20 reported a cooling rate roughly 3 times slower (0.044°C · min−1) than the next slowest study using cold-water immersion (0.129°C · min−1).23 Reported cooling rates in this study were determined by fitting straight lines for curves when body temperatures were above a certain point.20 This method was not otherwise reported and was not justified. The methods were not completely clear, representing an obvious flaw in the results. Actual data points of average starting and finishing temperatures were not reported, so we were unable to calculate actual cooling rates and relied on reported results. Other groups divided the change in body temperature by total cooling time.
The coldest circulated water (2°C) showed the fastest cooling rate (Figure 2).22 A variety of circulated water temperatures were compared by Proulx et al.22 Of note, the second degree of cooling for participants (the decrease in body temperature after the first decrease of 1°C to 2°C from maximum) occurred at a mean cooling rate of 0.51°C · min−1. It takes a moment to begin cooling the body, but cooling rates increase for the subsequent degree of cooling with cold-water immersion. Ice-water immersion with circulated water is the superior whole-body cooling treatment for exercise-induced hyperthermia.18,21,22
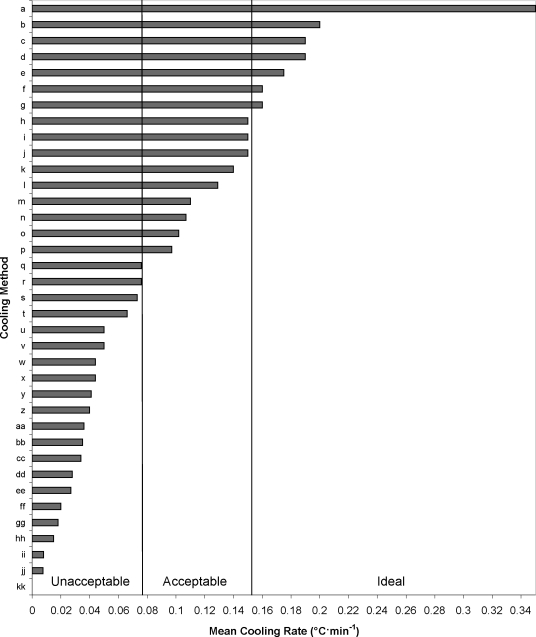
Figure 2. Mean cooling rates from case reports and critically reviewed articles. Mean cooling rates defined as unacceptable are <0.078°C · min−1, acceptable are 0.078°C to 0.154°C · min−1, and ideal are ≥0.155°C · min−1. IV indicates intravenous.
a Ice-water immersion, 2°C (n = 7): 0.35°C · min−1.22
b Ice-water immersion, 1–3°C (n = 14): 0.2°C · min−1.18
c Cold-water immersion, 20°C (n = 7): 0.19°C · min−1.22
d Cold-water immersion, 8°C (n = 7): 0.19°C · min−1.22
e Fine spray (temperature not reported) (n = 2): 0.175°C · min−1.26
f Cold-water immersion, 14.03°C (n = 17): 0.16°C · min−1.21
g Ice-water immersion, 5.15°C (n = 17): 0.16°C · min−1.21
h Dousing with water while fanning (n = 52): 0.15°C · min−1.28
i Cold-water immersion (temperature not reported) (n = 39): 0.15°C · min−1.10
j Cold-water immersion, 14°C (n = 7): 0.15°C · min−1.22
k Continual dousing with ice-bag massage (n = 5): 0.14°C · min−1.32
l Cold-water immersion, 7°C (n = 7): 0.129°C · min−1.23
m Ice-wet towels (n = 7): 0.11°C · min−1.18
n IV fluids and ice packs at major arteries (n = 1): 0.107°C · min−1.31
o Helicopter downdraft with spraying (n = 2): 0.102°C · min−1.30
p IV fluids and ice-wet towels (n = 1): 0.097°C · min−1.31
q IV fluids (n = 1): 0.076°C · min−1.31
r Fine spray, compressed air, and fanning (n = 6): 0.076°C · min−1.20
s Fine spray with fanning (n = 6): 0.073°C · min−1.20
t Sitting on stool, 21.1°C (n = 6): 0.066°C · min−1.20
u Fine spray for 3 minutes with fanning (n = 6): 0.05°C · min−1.20
v Cold IV and dousing with water (n = 1): 0.05°C · min−1.9
w Dousing with water (n = 1): 0.044°C · min−1.9
x Cold-water immersion, 14.4°C (n = 6): 0.044°C · min−1.20
y IV fluid with haloperidol (n = 1): 0.041°C · min−1.9
z Fanning and compressed air (n = 6): 0.04°C · min−1.20
aa Ice packs at major arteries and dousing with fanning (n = 5): 0.036°C · min−1.19
bb Dousing with water while fanning (n = 5): 0.035°C · min−1.19
cc Ice packs covering body (n = 5): 0.034°C · min−1.19
dd Ice packs at major arteries (n = 5): 0.028°C · min−1.19
ee Lying on stretcher (n = 5): 0.027°C · min−1.19
ff Fanning only (n = 6): 0.02°C · min−1.20
gg Repeated gastric lavage (n = 1): 0.018°C · min−1.9
hh IV fluid with paracetamol (n = 1): 0.015°C · min−1.9
ii Ice cubes on chest (n = 1): 0.008°C · min−1.9
jj Cooling blankets (n = 1): 0.0076°C · min−1.25
kk Cooling blankets (n = 1): 0.0°C · min−1.27
Ice-Pack Application
Some medical professionals recommend applying ice packs or ice bags to the major arteries of the body as a means of whole-body cooling.35,36 The most common placement sites are the neck, groin, and axillae. The goal of this treatment is to cool the blood as it is pumped through the carotid, axillary, and femoral arteries. However, even when ice bags were applied to the entire body (24 to 28 bags of ice), cooling rates were in category C (0.028°C · min−1).19 In fact, these results suggest that it would take approximately 110 minutes to cool an EHS patient from 42.2°C (108°F) to 38.89°C (102°F) with this method. Given proper planning and available resources for category A treatments, the use of ice packs or ice bags for the treatment of EHS should be discontinued, because the extraction of heat from the body is ineffective for the body temperatures typically associated with EHS.19
Showering the Body with Water and Fanning
The Israeli Defense Forces recommend the use of a cooling modality beginning immediately upon soldier collapse.1,32 Military personnel are trained to douse a collapsed soldier with available water and to fan using any means necessary. When continual dousing was coupled with fanning and compressed air, cooling rates fell short of category B (0.076°C · min−1).19,20 Although controlled trials showed a limited cooling rate using this method, with rapid treatment initiation success rates have been adequate for 95% of EHS patients.
Fanning the Body
Fanning attempts to expedite convection and evaporation by increasing the turnover of air near the body's periphery. The data reported do not support this method alone for the treatment of exercise-induced hyperthermia based on cooling rates.20 Conclusions from Wyndham et al20 support the use of this modality, but their results have not been replicated and their cooling rates represent inefficient cooling. When fanning was not accompanied by continual dousing of the patient with water, cooling rates markedly decreased. Moving an athlete or soldier to the shade and in front of a fan does not provide effective cooling.20
Wet-Towel Application
Wet-towel application was only reported in 1 study.18 Medical researchers based the treatment of either cold-water immersion or ice-wet towel application on the degree of hyperthermia. Runners who were more hyperthermic (41.7°C ± 0.2°C) were treated with ice-water immersion, and hyperthermic runners with lower rectal temperatures (40.4°C ± 0.3°C) were treated with ice-wet towels applied to the thorax and abdomen. This difference may have influenced the results, because a higher initial temperature normally results in faster cooling rates. However, this method of whole-body cooling still resulted in category B cooling rates for hyperthermic runners.18
Future investigators may validate ice-towel application as an alternative to ice-water immersion in athletic training settings. The ease with which this modality can be set up makes it a possible alternative for certified athletic trainers. An extra cooler can be prepared with plenty of ice, a little water, and a few towels packed in it. In the field, the treatment could be initiated within seconds of collapse, making for an effective overall treatment.
Combined Ice Packs, Continual Dousing, and Fanning
One group19 used ice packs at the major arteries plus fanning while water was splashed on the body. The goal of this combined treatment was to encourage convection, conduction, and evaporation, thereby maximizing efficiency. Yet the cooling rate data did not support a recommendation for this treatment for EHS patients.19 The methods used in this study did not provide adequate cooling for a recommendation in the field.19
Control Methods
Not all studies in the current review included a control treatment as a means of comparing cooling modalities. Some control methods of cooling were reported to cool patients faster than some modalities, as in the case of Clements et al21 (0.11°C · min−1). Regardless, no control modality provided a category B cooling rate that can be recommended. Therefore, research fails to support the solitary treatment of moving an athlete with EHS to a shaded area with circulated air as acceptable.
However, the air temperature in studies including a control method of cooling varied. This variability makes comparisons between control conditions impossible, because a participant resting in 10°C air temperature cannot be compared with a participant resting at 35°C. Of note, though, is that in all studies with reports of control methods, air temperatures matched at least 1 other modality. This finding allows for comparison within a study using the control as baseline cooling that would have occurred in the absence of the whole-body cooling modality.
Case Report Data
Because it is unethical to deliberately cause human EHS in a laboratory setting, researchers are forced to look at case report data regarding actual EHS. The only other way to research EHS cases is an observational approach, in which patients are treated in multiple ways and the efficiency of treatments is compared. This approach is difficult, because EHS often happens in the presence of a previously established protocol or emergency action plan. Thus, treatment cannot be altered unless the plan could be changed temporarily, which is difficult if the plan has shown success.
The most conclusive results from case series stem from epidemiologic studies of multiple patients.11,29 Two such reports include 39 and 52 EHS patients, respectively.11,29 Clinical significance must be attributed to these results because no fatalities were reported in either study. Similarly, Costrini10 reported successful treatment using cold-water immersion for 252 cases of EHS over a 15-year period. Cold-water immersion29 and continually dousing a patient while fanning11 are successful whole-body cooling modalities for the treatment of EHS patients. Continual dousing of a patient, implemented immediately, seems a viable alternative when cold-water immersion is not possible. Another cold-water immersion case series by Brodeur et al37 showed success with an average of 10 to 12 EHS patients per year. Although not strictly controlled, these results provide important considerations for sports medicine personnel.
Because the thermoregulatory system of an EHS patient is overwhelmed by metabolic and environmental heat, he or she may show a different response than a volunteer in a study whose core temperature reaches 40°C (104°F). A greater initial core body temperature may translate into a faster cooling rate. It was impossible for us to pool data and determine a correlation or correction factor with the available data based on the variety of confounding variables for such calculations. However, this possibility may explain why controlled research studies have not supported the use of continual dousing with fanning, whereas case report data have suggested that it provides efficient cooling.9,20
Some have opposed the use of cold-water immersion due to the difficulty of maintaining intravenous access, keeping the airway accessible, and having the patient ready for defibrillation if needed.34 To avoid these complications, the patient can be placed on a gurney that rests on an immersion tub. The water in the tub does not immerse or cover any portion of the patient. The patient is continually doused with water while others massage the major muscle groups (ie, quadriceps, gastrocnemii, pectorals) with ice bags.32 This simultaneously allows intravenous access and oxygen administration and avoids concerns regarding defibrillation, because the patient only needs to be dried before rhythm analysis is performed. This combination of modalities has shown category B results. A drawback worth noting is the requirement of at least 3 to 4 staff members for treatment.32
Numerous whole-body cooling methods have been described in case reports with various results (Table 3); most are not feasible for use by certified athletic trainers. For example, Poulton et al30 proposed hosing a patient while he or she is fanned by a helicopter rotor. Obviously, it is not practical to have a helicopter at every setting where EHS is a possibility. Expense should also be a concern. Khogali and Weiner33 described the body-cooling unit, which continually sprays a fine mist of tepid water on the patient while he or she is fanned. The unit costs approximately $18,000.33 Cooling rates with this expensive unit were so slow that some patients were not cooled to appropriate levels within 5 hours. Cooling blankets have shown ineffective cooling rates, which disqualifies them from our recommendation.25
The only category B cooling methods proposed in case reports include cold-water immersion,29 continually dousing patients with water while fanning by available measures,11 and dousing and ice-bag massage.32 To date, the only treatment that is supported by research experiments and case report data is cold-water immersion.
Practical Recommendations
Based on the available research, some recommendations can be offered regarding the treatment of exertional heat illness that requires rapid cooling. When setting up a plan, cold-water immersion should be included if possible. Ice in coolers adjacent to tubs filled with water provides a rapid-cooling tub for EHS patients. Some athletic events are located in areas not conducive to having cold-water tubs available. In these rare instances, athletic trainers should plan to have a cooler available containing ice, water, and towels. The cold, wet towels should be changed every 2 to 3 minutes. Alternately, a large water supply should be available for the continual dousing of a patient (either from a hose or multiple water containers). These treatments can be completed while more aggressive cooling is en route or while the patient is being moved to an area where this is possible (eg, athletic training room with cold whirlpool). In extreme cases, when individuals may complete training runs at remote locations and EHS is suspected, cooling should be implemented as soon as possible using a water source of some sort (eg, garden hose, stream, lake, pond). The recommendation of cooling first and transporting second cannot be overemphasized.4,7
Limitations
Two important limitations exist in the extrapolation of these results: the data regarding body size and internal temperatures of test participants are limited. Well-controlled studies rely on volunteers. Therefore, a majority of the research findings are based on average-sized individuals. It is not clear how larger individuals (eg, football linemen) may respond to the cooling modalities described in this review. However, it bears noting that controlled studies support the use of cold-water immersion. Case reports including a total of 276 EHS patients also show no fatalities with the use of cold-water immersion.10,29 More than 300 cases of EHS have been reported elsewhere to have no fatalities associated with cold-water immersion.37
Our current evidence base on this topic is limited as well by the amount of hyperthermia in controlled studies. As previously noted, studies were included if they induced a body temperature of more than 38.5°C (101.3°F) before cooling participants. Individuals may respond differently to whole-body cooling modalities in this state than if they had reached a body temperature of 42°C (107.6°F) with EHS. Despite these limitations, the data have been critically analyzed and some conclusions can be drawn until future researchers confirm or discredit the literature available to date.
Conclusions
Published research specific to cooling modalities for treating exercise-induced hyperthermia is lacking. We identified only 7 studies that met a priori inclusion criteria and PEDro scale assessment. The current National Athletic Trainers' Association and American College of Sports Medicine recommendations agree with the most up-to-date research regarding heat illness treatment.
The available results allow 2 basic deductions. First, ice-water or cold-water immersion seems to provide the most efficient cooling treatment for exercise-induced hyperthermia in the populations tested and is recommended as the definitive treatment for EHS. Second, we found no other whole-body cooling modality supported by multiple studies, although some showed category B cooling rates and could be used if cold-water immersion is not feasible. Whenever possible, certified athletic trainers should use ice-water or cold-water immersion to treat severe exertional heat illnesses. When this is not feasible, case reports show that immediate and continual dousing of the patient, combined with fanning and continually rotating cold, wet towels, represents a viable alternative until advanced cooling is possible. Given that the amount of time above a critical temperature determines eventual outcome after EHS, patients should be cooled first and transported second.4,7 Practical guidelines for the implementation of emergency action plans for EHS can be found in multiple locations.1–7,34
Footnotes
Brendon P. McDermott, MS, ATC, and Douglas J. Casa, PhD, ATC, FNATA, FACSM, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting, critical revision, and final approval of the article. Matthew S. Ganio, MS, and Rebecca M. Lopez, MS, ATC, contributed to conception and design; analysis and interpretation of the data; and drafting, critical revision, and final approval of the article. Susan W. Yeargin, PhD, ATC, contributed to acquisition and analysis and interpretation of the data and drafting, critical revision, and final approval of the article. Lawrence E. Armstrong, PhD, FACSM, and Carl M. Maresh, PhD, FACSM, contributed to acquisition and analysis and interpretation of the data and drafting, critical revision, and final approval of the article.
References
- 1.McDermott B.P, Casa D.J, Ganio M.S, Yeargin S.W, Armstrong L.E, Maresh C.M. Recovery and return to activity following exertional heat stroke: considerations for the sports medicine staff. J Sport Rehabil. 2007;16(3):163–181. doi: 10.1123/jsr.16.3.163. [DOI] [PubMed] [Google Scholar]
- 2.Binkley H.M, Beckett J, Casa D.J, Kleiner D.M, Plummer P.E. National Athletic Trainers' position statement: exertional heat illnesses. J Athl Train. 2002;37(3):329–343. [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong L.E, Casa D.J, Millard-Stafford D, Moran D, Pyne S.W, Roberts W.O. American College of Sports Medicine position stand: exertional heat illnesses during training and competition. Med Sci Sports Exerc. 2007;39(3):556–572. doi: 10.1249/MSS.0b013e31802fa199. [DOI] [PubMed] [Google Scholar]
- 4.Casa D.J, Almquist J, Anderson S, et al. Inter-Association Task Force on Exertional Heat Illnesses consensus statement. NATA News. Jun, 2003. pp. 24–29.
- 5.Casa D.J, Armstrong L.E, Ganio M.S, Yeargin S.W. Exertional heat stroke in competitive athletes. Curr Sports Med Rep. 2005;4(6):309–317. doi: 10.1097/01.csmr.0000306292.64954.da. [DOI] [PubMed] [Google Scholar]
- 6.Casa D.J, Armstrong L.E. Exertional heatstroke: a medical emergency. In: Armstrong L.E, editor. Exertional Heat Illnesses. Champaign, IL: Human Kinetics; 2003. pp. 29–56. [Google Scholar]
- 7.Casa D.J, Anderson J.M, Armstrong L.E, Maresh C.M. Survival strategy: acute treatment of exertional heat stroke. J Strength Cond Res. 2006;20(3):462. doi: 10.1519/1533-4287(2006)20[462:SSATOE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Epstein Y, Moran D.S, Shapiro Y. Medical Aspects of Harsh Environments. Washington, DC: US Government Printing Office; 2002. Exertional heat stroke in the Israeli Defense Forces; pp. 281–292. Vol 1. [Google Scholar]
- 9.Heled Y, Rav-Acha M, Shani Y, Epstein Y, Moran D.S. The “golden hour” for heat stroke treatment. Mil Med. 2004;169(3):184–186. doi: 10.7205/milmed.169.3.184. [DOI] [PubMed] [Google Scholar]
- 10.Costrini A. Emergency treatment of exertional heatstroke and comparison of whole body cooling techniques. Med Sci Sports Exerc. 1990;22(1):15–18. [PubMed] [Google Scholar]
- 11.Hadad E, Rav-Acha M, Heled Y, Epstein Y, Moran D.S. Heat stroke: a review of cooling methods. Sports Med. 2004;34(8):501–511. doi: 10.2165/00007256-200434080-00002. [DOI] [PubMed] [Google Scholar]
- 12.Smith J.E. Cooling methods used in the treatment of exertional heat illness. Br J Sports Med. 2005;39(8):503–507. doi: 10.1136/bjsm.2004.013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steves R, Hootman J.M. Evidence-based medicine: what is it and how does it apply to athletic training. J Athl Train. 2004;39(1):83–87. [PMC free article] [PubMed] [Google Scholar]
- 14.Ganio M.S, Brown C.M, Casa D.J, et al. Assessing the validity of devices measuring core body temperature during exercise in a heat chamber. J Athl Train. In press. [PMC free article] [PubMed]
- 15.Casa D.J, Becker S.L, Ganio M.S, et al. Validity of devices that assess body temperature during exercise in the heat. J Athl Train. 2007;42(3):333–342. [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S.M, Williams W.J, Fortney Schneider S.M. Core temperature measurement during supine exercise: esophageal, rectal, and intestinal temperatures. Aviat Space Environ Med. 2000;71(9):939–945. [PubMed] [Google Scholar]
- 17.PEDro Scale. Physiotherapy Evidence Database. http://www.pedro.fhs.usyd.edu.au/. Accessed April 8, 2007.
- 18.Armstrong L.E, Crago A.E, Adams R, Roberts W.O, Maresh C.M. Whole-body cooling of hyperthermic runners: comparison of two field therapies. Am J Emerg Med. 1996;14(4):355–358. doi: 10.1016/S0735-6757(96)90048-0. [DOI] [PubMed] [Google Scholar]
- 19.Kielblock A.J, Van Rensburg J.P, Franz R.M. Body cooling as a method for reducing hyperthermia: an evaluation of techniques. S Afr Med J. 1986;69(6):378–380. [PubMed] [Google Scholar]
- 20.Wyndham C.H, Strydom N.B, Cooke H.M, et al. Methods of cooling subjects with hyperpyrexia. J Appl Physiol. 1959;14(5):771–776. doi: 10.1152/jappl.1959.14.5.771. [DOI] [PubMed] [Google Scholar]
- 21.Clements J.M, Casa D.J, Knight J.C, et al. Ice-water immersion and cold-water immersion provide similar cooling rates in runners with exercise-induced hyperthermia. J Athl Train. 2002;37(2):146–150. [PMC free article] [PubMed] [Google Scholar]
- 22.Proulx C.I, Ducharme M.B, Kenny G.P. Effect of water temperature on cooling efficiency during hyperthermia in humans. J Appl Physiol. 2003;94(4):1317–1323. doi: 10.1152/japplphysiol.00541.2002. [DOI] [PubMed] [Google Scholar]
- 23.Scott C.G, Ducharme M.B, Haman F, Kenny G.P. Warming by immersion or exercise affects initial cooling rate during subsequent cold water immersion. Aviat Space Environ Med. 2004;75(11):956–963. [PubMed] [Google Scholar]
- 24.Clapp A.J, Bishop P.A, Muir I, Walker J.L. Rapid cooling techniques in joggers experiencing heat strain. J Sci Med Sport. 2001;4(2):160–167. doi: 10.1016/s1440-2440(01)80026-8. [DOI] [PubMed] [Google Scholar]
- 25.Hart L.E, Egier B.P, Shimizu A.G, Tandan P.J, Sutton J.R. Exertional heat stroke: the runner's nemesis. Can Med Assoc J. 1980;122(10):1146–1150. [PMC free article] [PubMed] [Google Scholar]
- 26.Barner H.B, Wettach G.E, Masar M, Wright D.W. Field evaluation of a new simplified method for cooling of heat casualties in the desert. Mil Med. 1984;149(2):95–97. [PubMed] [Google Scholar]
- 27.Broessner G, Beer R, Franz G, et al. Case report: severe heat stroke with multiple organ dysfunction. A novel intravascular treatment approach. Crit Care. 2005;9(5):R498–R501. doi: 10.1186/cc3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadad E, Moran D.S, Epstein Y. Cooling heat stroke patients by available field measures. Intensive Care Med. 2004;30(2):338. doi: 10.1007/s00134-003-2084-5. [DOI] [PubMed] [Google Scholar]
- 29.Costrini A.M, Pitt H.A, Gustafson A.B, Uddin D.E. Cardiovascular and metabolic manifestations of heat stroke and severe heat exhaustion. Am J Med. 1979;66(2):296–302. doi: 10.1016/0002-9343(79)90548-5. [DOI] [PubMed] [Google Scholar]
- 30.Poulton T.J, Walker R.A. Helicopter cooling of heatstroke victims. Aviat Space Environ Med. 1987;58(4):358–361. [PubMed] [Google Scholar]
- 31.Richards D, Richards R, Schofield P.J, Sutton J.R. Management of heat exhaustion in Sydney's the Sun City-To-Surf Run runners. Med J Aust. 1979;2(9):457–461. doi: 10.5694/j.1326-5377.1979.tb125754.x. [DOI] [PubMed] [Google Scholar]
- 32.McDermott B.P, Casa D.J, Adams B, et al. Examination of cold water-ice massage therapy for exertional heat stroke. J Athl Train. 2007;42(suppl 2):S53. [Google Scholar]
- 33.Khogali M, Weiner J.S. Heat stroke: report on 18 cases. Lancet. 1980;2(8189):276–278. doi: 10.1016/s0140-6736(80)90232-9. [DOI] [PubMed] [Google Scholar]
- 34.Casa D.J, McDermott B.P, Lee E, Yeargin S.W, Armstrong L.E, Maresh C.M. Cold-water immersion: the gold standard for exertional heat stroke treatment. Exerc Sport Sci Rev. 2007;35(3):141–149. doi: 10.1097/jes.0b013e3180a02bec. [DOI] [PubMed] [Google Scholar]
- 35.Guyton A.C, Hall J.E. Textbook of Medical Physiology. Philadelphia, PA: WB Saunders; 2006. pp. 899–900. 11th ed. [Google Scholar]
- 36.Wexler R.K. Evaluation and treatment of heat-related illnesses. Am Fam Phys. 2002;65(11):2307–2314. [PubMed] [Google Scholar]
- 37.Brodeur V.B, Dennett S.R, Griffin L.S. Exertional hyperthermia, ice baths, and emergency care at the Falmouth Road Race. J Emerg Nurs. 1989;15(4):304–312. [PubMed] [Google Scholar]