Abstract
The anterior medial prefrontal (AMPFC) and retrosplenial (RSC) cortices are active during self-referential decision-making tasks such as when participants appraise traits and abilities, or current affect. Other appraisal tasks requiring an evaluative decision or mental representation, such as theory of mind and perspective-taking tasks, also involve these regions. In many instances, these types of decisions involve a subjective opinion or preference, but also a degree of ambiguity in the decision, rather than a strictly veridical response. However, this ambiguity is generally not controlled for in studies that examine self-referential decision-making. In this functional MRI (fMRI) experiment with seventeen healthy adults, we examined neural processes associated with subjective decision-making with and without an overt self-referential component. The task required subjective decisions about colors—regarding self-preference (internal subjective decision) or color similarity (external subjective decision) under conditions where there was no objectively correct response. Results indicated greater activation in the AMPFC, RSC, and caudate nucleus during internal subjective decision-making. The findings suggest that self-referential processing, rather than subjective judgments among ambiguous response alternatives, accounted for the AMPFC and RSC response.
Introduction
An increasing number of studies in the cognitive and affective neurosciences are focused on the subjective self (Vogeley & Fink, 2003). Functional neuroimaging studies on self-referential tasks are finding similar activation patterns involving the anterior medial prefrontal cortex (AMPFC) and the retrosplenial cortex (RSC) (Northoff & Bermpohl, 2004; Vogeley & Fink, 2003). Relevant experimental imaging paradigms include those in which the participant rates the self or an ‘other’ on abilities/traits (Johnson et al., 2002; Kelley et al., 2002; Schmitz, Kawahara-Baccus, & Johnson, 2004; Zysset, Huber, Ferstl, & von Cramon, 2002; Zysset, Huber, Samson, Ferstl, & von Cramon, 2003), rates one's affective response to connotative pictures (Gusnard, Akbudak, Shulman, & Raichle, 2001; Lane, Fink, Chau, & Dolan, 1997) or words (Cato et al., 2004; Maddock, Garrett, & Buonocore, 2003), or evaluates personal preferences (Paulus & Frank, 2003; Seger, Stone, & Keenan, 2004). Theory of mind paradigms (Fletcher et al., 1995; Frith & Frith, 1999; Goel, Grafman, Sadato, & Hallett, 1995) in which the participant infers the mind content/intentions of another, have also found activation in the AMPFC and RSC. All of these tasks have in common a meta-cognitive component (Stuss, Picton, & Alexander, 2001), comprising the participant's subjective appraisal rather than a veridical criterion-based decision (see also Zysset et al., 2002). For example, Seger et al. (2004) presented 144 food names and asked participants to decide with a ‘yes’ or ‘no’ response whether they preferred the food. In another condition the subjects decided whether someone they were familiar with preferred the food. This type of task is dependent on the completeness of knowledge about the self and the other, and secondly on the strength of the preference. In forced-choice scenarios such as this, when veridical knowledge may be incomplete, or when there is a degree of uncertainty among response alternatives, the choice selection becomes subjective because each response alternative has some degree of plausibility. Similarly, when strong beliefs or preferences (like or dislike) have not yet been formed, or when there is ambivalence between the choices, the response again becomes more subjective or arbitrary, and less criterion-based. Ambiguity or uncertainty between response alternatives is therefore a possible component of many self-referential or other meta-representational functional imaging experiments. Although the studies cited above have found common regions of activation, the problem of ambiguity among choice alternatives (and thus subjectivity in responses) has not been adequately addressed as a factor that may be accounting for AMPFC and RSC activity. Thus, strong conclusions regarding the functional nature of this network cannot yet be made.
In the present study we sought to test whether nonveridical (i.e. subjective) decisions, with or without a salient self-referential component, could account for AMPFC and/or RSC involvement. We therefore asked seventeen healthy young adult participants to make decisions regarding color preference and color similarity during fMRI, using a paradigm in which subjective choice between two equally plausible alternatives was required. A color preference condition is described here as an ‘internal’ subjective decision (ISD) task because it had an inward or self-referential context. A subjective color similarity condition was termed the ‘external’ subjective decision (ESD) because the choice between two equivocal alternatives was based on the external color properties of the color stimuli rather than on self-preference. Because blood oxygen dependent (BOLD) fMRI is a relative measure, the choice of the baseline task is critical to making valid interpretations of the results. Recent research has indicated that low-level baseline conditions (such as rest or crosshair fixation) activate the MPFC and RSC, perhaps due to the participant being more self-aware of their immediate surroundings or performance in the experiment during nondemanding activities (Greicius & Menon, 2004; Gusnard & Raichle, 2001; Raichle et al., 2001). Thus, a low-level baseline task such as crosshair fixation was avoided in this study, as it may implicitly be self-referential. The baseline condition for this study involved making veridical color similarity decisions in which one of the choice alternatives was unambiguously correct. This was termed the external veridical decision (EVD) condition. The key comparison between the two ambiguous conditions was achieved with a random-effects group analysis. Our hypothesis was that the midline structures would be more active during internal (self-referential) subjective decision-making.
Results
Reaction times for the three conditions were as follows EVD: Avg = .977 ms, SD = .280; ESD: Avg = 1.717 ms, SD =.388; ISD: Avg= 1.671 ms, SD=.446. Repeated measures ANOVA indicated the means were significantly different (p<.001). The two subjective conditions were significantly different from the veridical condition (ESD vs EVD: t[16]=10.71, p<.001; ISD vs EVD: t[16]=7.25, p<.001), but they were not significantly different from each other (ESD versus ISD t[16]=.865, p=ns).
ESD vs. EVD Activation
The fMRI signal difference between the two external color similarity conditions was computed using a paired t-test random-effects model. The result is shown in the statistical parametric map in Figure 2. The voxel locations and associated t-statistics and FDR corrected p-values of the maxima are in Table 1. The contrast resulted in activation of the dorsal medial frontal cortex, just dorsal to the mid-cingulate. The intraparietal sulcus was active bilaterally, as was the dorsolateral and inferolateral frontal lobe and anterior insula. Neither the anterior medial prefrontal cortex or the retrosplenial cortex were significant in this comparison.
Figure 2.
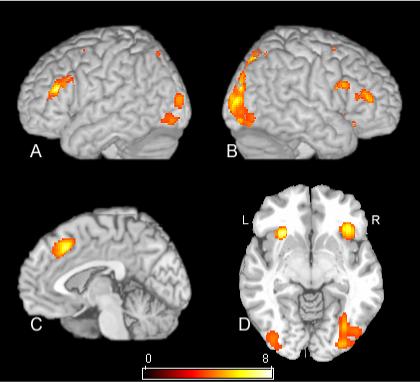
SPM[t] contrast of ESD versus EVD conditions. The map in this figure has a statistical threshold of p<.02 using the false discovery rate correction procedure described in the text. Rendered views of the left and right hemisphere are shown in A and B respectively. C is a mid-sagittal view, and D is an axial view just below the commissures. See Table 1 for cluster locations and voxel level statistics.
Table 1.
Statistics and locations for ESD versus EVD contrast.
| p(FDR) | T | x,y,z (mm) | Region |
|---|---|---|---|
| 0.003 | 9.35 | −44 28 18 | Left inferior frontal gyrus |
| 0.003 | 7.67 | −30 22 −8 | Left anterior insula |
| 0.003 | 7.29 | 0 24 44 | Dorsomedial frontal cortex |
| 0.003 | 7.27 | 24 −76 40 | Right posterior parietal and lateral occipital cortex |
| 0.004 | 6.41 | 32 −64 −16 | Right inferior temporal-occipital cortex |
| 0.004 | 6.52 | 36 26 −8 | Right anterior insula |
| 0.004 | 6.31 | −26 −70 36 | Left intraparietal sulcus |
| 0.004 | 5.96 | 48 10 22 | Right inferior frontal gyrus |
| 0.005 | 5.59 | 32 0 54 | Right middle frontal gyrus |
| 0.007 |
4.96 |
−38 −80 −12 |
Left inferior temporal-occipital cortex |
ISD vs. EVD Activation
A very similar pattern was shown when the ISD condition was compared to the baseline EVD condition. This contrast is presented in Figure 3 and Table 2.
Figure 3.
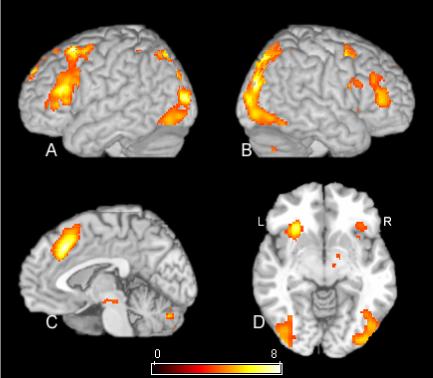
SPM[t] contrast of ISD versus EVD conditions. The statistical map in this figure has a statistical threshold of p<.02 using the false discovery rate correction procedure described in the text. Rendered views of the left and right hemisphere are shown in A and B respectively. C is a mid-sagittal view, and D is an axial view just below the commissures. See Table 2 for cluster locations and voxel level statistics.
Table 2.
Statistics and locations for ISD versus EVD contrast.
| p(FDR-cor) | T | x,y,z (mm) | Region |
|---|---|---|---|
| <.001 | 10.25 | 30 −74 36 | Right posterior parietal and lateral occipital |
| <.001 | 8.38 | −48 20 16 | Left inferior frontal gyrus |
| <.001 | 8.22 | −32 18 58 | Left middle frontal gyrus |
| <.001 | 8.28 | −28 −72 34 | Left posterior parietal and lateral occipital |
| <.001 | 8.11 | 34 2 54 | Right middle frontal gyrus |
| <.001 | 8.06 | 2 26 42 | Dorsomedial frontal cortex |
| <.001 | 7.47 | −30 26 −6 | Left anterior insula |
| 0.001 | 7.03 | 48 10 22 | Right inferior frontal gyrus |
| 0.001 | 6.84 | 8 −78 −28 | Cerebellum near midline |
| 0.001 | 6.20 | 36 −72 −30 | Right cerebellum |
| 0.001 | 5.55 | 48 40 8 | Right inferior frontal gyrus |
| 0.004 |
4.49 |
34 18 −2 |
Right anterior insula |
ISD vs. ESD Activation: Internal versus external subjective decisions
The key comparison in this study was between the two subjective conditions. The result is shown in the statistical parametric map in Figure 4 and plot in Figure 5. Table 3 contains the voxel locations and statistics. The left anterior medial prefrontal cortex and the retrosplenial cortex were each differentially more active during the internal subjective decision compared to the external subjective decision. Also active in this contrast were the head of the caudate bilaterally, the left posterior temporal cortex, and the left anterior hippocampus.
Figure 4.
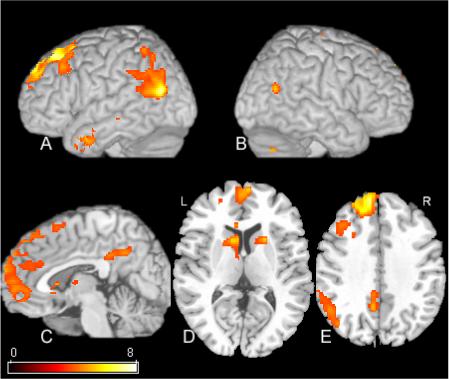
SPM[t] contrast of ISD versus ESD conditions. The results are thresholded at a statistical threshold of .02 FDR corrected. Rendered views of the left and right hemisphere are shown in A and B respectively. C is a mid-sagittal view depicting AMPFC and RSC activation. D-E are axial views. See Table 3 for cluster locations and voxel-level statistics.
Figure 5.
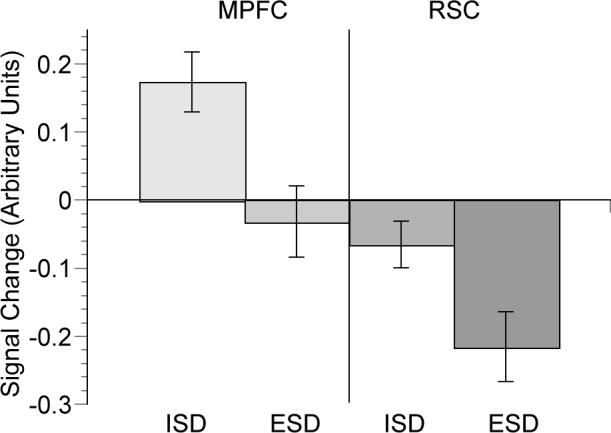
Plot of the eigenvariate of signal change averaged over seven voxels defined by a 2mm radius sphere in the anterior medial prefrontal cortex (coordinate −12, 50, 34) and retrosplenial cortex (−8, −40, 32) for ISD and ESD conditions relative to the EVD condition. Error bars represent the standard error.
Table 3.
Statistics and locations for ISD versus ESD contrast
| p(FDR) | T | x,y,z (mm) | Region |
|---|---|---|---|
| 0.005 | 7.71 | −12 50 34 | Left AMPFC (superior frontal gyrus) |
| 0.005 | 7.51 | −52 −70 18 | Left posterior middle and superior temporal gyri |
| 0.005 | 7.10 | −20 10 14 | Left caudate head |
| 0.005 | 5.92 | 30 −78 −34 | Right cerebellum |
| 0.005 | 5.76 | −48 6 −30 | Left temporal pole |
| 0.005 | 5.74 | 20 16 −2 | Right caudate head and anterior acumbens |
| 0.007 | 5.13 | −8 −40 32 | Left retrosplenial cortex; RSC |
| 0.009 |
4.87 |
−24 −10 −18 |
Left anterior hippocampus |
Discussion
In this experiment we found that self-referential (internal) subjective decision-making activated the AMPFC and RSC significantly more than an external subjective decision-making comparison task. These results suggest that the cerebral response to subjective decision-making is dependent on the referent of the task but not ambiguity.
Self-referential decision-making (ISD)
In the key comparison of ISD versus ESD, the AMPFC was relatively more active on the left, as was the caudate, RSC, and posterolateral temporal lobe. The AMPFC and RSC findings supported our primary hypothesis; the regions were active when a subjective choice had a self-referent, but not when the referent was external. This finding supports the growing body of literature for a meta-representational network invoked by self-referential tasks (Frith & Frith, 1999; Gallagher & Frith, 2003; Goel et al., 1995; Gusnard et al., 2001; Gusnard et al., 2003; Johnson et al., 2002; Maddock, 1999; Schmitz et al., 2004; Vogeley et al., 2001; Zysset et al., 2002). In addition, the posterolateral temporal lobe has also been reported to be active for these types of tasks (Frith & Frith, 1999). Why the left hemisphere was more active than the right is not completely clear. Prior studies (in which ambiguity was not controlled, e.g. Johnson et al., 2002) have not found strongly lateralized effects. However, Turk and colleagues (Turk et al., 2002; Turk, Heatherton, Macrae, Kelley, & Gazzaniga, 2003) have studied self recognition in split brain patients and suggest that the integrative and interpretive functions of the left hemisphere (Gazzaniga, 2000) provide an advantage for self-recognition performance. That finding is consistent with the self-referential effect observed in the present study, but clearly more work is needed in this regard.
Goldberg and Podell have postulated that common goal-directed but nonveridical self-referential decisions (e.g. selecting from a menu at a restaurant or choosing which clothes to wear) involve the frontal lobes (Goldberg & Podell, 1999, 2000). Furthermore they have shown with an elegant behavioral paradigm that frontal lobe injury can be distinguished from normal functioning on a cognitive bias task, in which subjective choice preference is measured (Goldberg & Podell, 2000; Podell, Lovell, Zimmerman, & Goldberg, 1995). Although our experiment was quite different from Goldberg's in many respects including behavioral versus imaging methodology, subject population, and paradigm, there is some conceptual similarity. Further, the results of the present study demonstrate robust dorsomedial as well as dorsolateral frontal lobe activation in each of the subjective conditions relative to the EVD veridical baseline decision task. This is consistent with Goldberg's findings in patients with frontal lobe lesions (Goldberg & Podell, 1999).
Regarding the contrast of the two external conditions, we note that a variety of regions were active. The ESD condition likely differed from the EVD condition in more than one way. First, the reaction times differed; the ESD condition had longer reaction times, suggesting greater difficulty and attentional demand. The dorsal medial frontal and intraparietal findings have been commonly implicated in studies of attention, inclusive of working memory (Nelson, Reuter-Lorenz, Sylvester, Jonides, & Smith, 2003; Smith & Jonides, 1999), conflict monitoring and cognitive control (Carter, MacDonald, Ross, & Stenger, 2001; Kerns et al., 2004; van Veen, Cohen, Botvinick, Stenger, & Carter, 2001). Dorsomedial prefrontal activation has also been reported by Gusnard et al. (2001) during an ‘external’ decision making task in which the decision was whether a visual scene was indoors or outdoors.
The finding in the inferior frontal gyrus, left more than right on both the subjective tasks relative to the EVD baseline, may reflect a verbal reasoning strategy during those conditions that may not have been employed during the less difficult EVD condition. This is, however, a speculative interpretation based on the location of the finding around Broca's area, and the longer reaction times for the subjective conditions.
The bilateral anterior insula response during each of the subjective tasks is intriguing. A recent fMRI report found activation here, right more than left, when subjects were asked to perform an interoceptive awareness task—being mindful of their own heartbeat (Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004). This region has been suggested by Craig (2003) to be involved in the meta-representation of interoceptive feelings and is more active when the stimuli are perceived as more emotionally intense (Craig, 2002). Damasio (1999) also supports this conclusion. In addition, Phan et al. (2004) report bilateral anterior insula activation during a self-referential task, more so when stimuli were perceived as more emotionally intense. As Phan et al. (2004) discuss, the anterior insula may be evoked by the emotional salience of a task. In the current study, although the task was not intended to be emotionally evocative, perhaps the ambiguity of the subjective conditions resulted in an unintended affective response, thus explaining insula activity during both subjective (ESD and ISD) conditions compared to the EVD condition. A limitation of the current study is that we did not have other physiological measures to parallel the fMRI paradigm. Galvanic skin conductance for example may have helped determine whether the affective arousal of either of the subjective tasks was correlated with the cerebral response in the insula.
Retrieval and the Retrosplenial Cortex
The RSC is consistently active during episodic retrieval (Cabeza, Dolcos, Graham, & Nyberg, 2002; Piefke, Weiss, Zilles, Markowitsch, & Fink, 2003). Since prior studies of self-referential decision making also commonly activate the RSC, we sought to avoid retrieval demands in our task design. For example, Johnson et al. (2002) asked participants to rate themselves on attributes and abilities and found activation in the RSC. Potentially, participants in that study could have engaged in retrieval of schemata they have about themselves regarding the trait or ability in question (analogous to calling to mind ‘personal’ semantic knowledge); or they could have called to mind specific episodes from the past to support their answers. Other relevant studies have similar potential retrieval demands that could account for the retrosplenial activation (Kelley et al., 2002; Schmitz et al., 2004; Zysset et al., 2002). By avoiding explicit retrieval of schematic and episodic memory in the current task design, we can state more confidently that the RSC finding in this study is attributable to the referent rather than incidental episodic retrieval processes.
The interaction between episodic retrieval and self-referential processing is deserving of further work since the RSC is often reported as activating to both types of tasks. The importance of gaining this understanding is underscored by the fact that the RSC is hypometabolic in Alzheimer's Disease (AD) (Matsuda, 2001; Minoshima, Foster, & Kuhl, 1994; Minoshima et al., 1997) as well as in people with risk factors for AD (Reiman et al., 1996; Reiman et al., 2004; Small et al., 2000). In addition to diminished memory and cognitive abilities, there is a concomitant lack of appreciation, or self-awareness, for such memory and cognitive deficits (Duke, Seltzer, Seltzer, & Vasterling, 2002; Rymer et al., 2002; Tabert et al., 2002). A diminished sense of awareness is also frequently observed in other diseases with a predominant frontal lobe etiology such as frontal lobe dementia (Miller et al., 2001), traumatic brain injury (Flashman, Amador, & McAllister, 1998; Flashman & McAllister, 2002; Prigatano, 1996; Sherer et al., 1998) and schizophrenia (Flashman et al., 2001). One potentially fruitful area of investigation will be to determine whether diseases with predominant RSC involvement will show a different pattern of self-referential deficits than diseases with predominant frontal lobe etiology (Rombouts et al., 2003). These important issues have yet to be studied.
A default network for self
Task induced deactivations (TIDs) have been reported recently to occur in the AMPFC and RSC. TIDs refer to increased activity during low-demand baseline tasks relative to the experimental task in fMRI and PET studies (Greicius & Menon, 2004). A low level baseline has many conceptual advantages, if one can assume that it is a physiologically and psychologically neutral condition. The plot in Figure 5 indicates that this was not the case for our baseline condition; the easier EVD condition evoked more signal in the RSC than the subjective tasks. Several investigators (Gusnard & Raichle, 2001; Lustig et al., 2003; Raichle et al., 2001) have interpreted TIDs as resulting from a default mode in which the participant is more self-aware of the ‘here and now’ of the experiment, their surroundings, and their performance. Easier task conditions, including rest, in fMRI and PET studies may facilitate this type of awareness (D'Argembeau et al., 2005). Many studies of observer effects on performance (e.g. Kazdin, 1982) demonstrate that performance differs base on whether one is aware of being observed, perhaps due to increased self-monitoring. Thus, it may be that AMPFC and RSC ‘default mode’ findings are explainable by these social psychological processes. This hypothesis remains to be studied with modern brain mapping techniques and integrated social psychological theory (Cacioppo et al., 2003). Research with quantitative blood flow imaging methods are also needed since BOLD fMRI only measures relative signal change between conditions and is therefore not suited to fully explain the concept of deactivation.
Limitations
This study had some limitations that should be considered. The experimental design of this study did not allow us to completely test the self-referential effect. The design had veridical and subjective conditions for external decisions, but the internal decisions were always subjective. Although the results of the study allow us to conclude that there was a self-referential effect while controlling for ambiguity, an additional condition for veridical self-referential decisions would have allowed us to determine whether there was a main effect for self-referential processing or an interaction with ambiguity in the midline structures. A second limitation is our ability to isolate self-referential processing to the internal (preference) condition. The external subjective decision, which was always a choice between two equally plausible alternatives, may have also involved implicit self-referential decision-making to some degree. While this cannot be ruled out, any self-referential processing in the ESD condition was likely to be less than the explicitly self-referential ISD condition. This is borne out by the result of the key comparison between ISD and ESD showing regions relatively more active during the overtly self-referential condition.
Conclusions
In conclusion, this study sought to determine the neural response during subjective decision-making with and without a self-referential component. The findings confirmed our hypothesis that subjective decision making alone does not account for the frequently observed AMPFC and RSC results in prior self-referential brain activation paradigms. Thus, the results provide further support for a self-referential brain network (Gusnard & Raichle, 2001; Northoff & Bermpohl, 2004). Future functional imaging studies involving patients with either impaired self-referential capacities, or known damage within this network, will likely facilitate our understanding of this system. Such work is underway in our laboratory.
Methods
Seventeen healthy young adult right-handed participants (9 male and 8 female) were included in this study (mean age= 21.3, SD=2.4; mean education =15.3, SD=1.3). The participants were recruited from the University of Wisconsin—Madison campus via advertisement. Prior to study procedures, participants were screened in a phone interview for MRI safety/compatibility and questioned regarding general health history. The exclusion criteria consisted of color blindness, any chronic medical condition (e.g. neurological, cardiovascular, cancer), prior invasive surgical procedures, incidence of head trauma involving loss of consciousness for more than 5 minutes, prior diagnosis of a major psychiatric disorder, history of alcohol/substance abuse, learning disability, or vision/hearing impairment. All prescription medications (with the exception of stable oral contraceptives) were excluded. Participants who met criteria provided written informed consent prior to engaging in study procedures. On the day of the scan, participants were administered a brief set of neuropsychological tests including the Wide Range Achievement Test-Third Edition (WRAT-III) Reading Recognition subtest (a quick estimate of verbal intelligence) to verify that broad cognitive abilities were within normal ranges. The average score from these participants was 111, SD= 5. The population average on this test is 100 with a population SD of 15. The participants were compensated for taking part in this Institutional Review Board approved study.
Task
Stimulus Characteristics
The general configuration of the stimuli is depicted in Figure 1. Three colored squares were presented simultaneously on the screen. A new set of squares appeared every 4 seconds for the course of the experiment, but the general stimulus characteristics, spatial layout and motoric and perceptual demands were the same for all three conditions in the task. The only factors that differed were the type of choice to be made (color similarity or preference decision). The color luminance was always equal for the two choice alternatives in all three conditions. This was achieved by manipulating the Red, Green and Blue (RGB) components of the colors.
Figure 1.
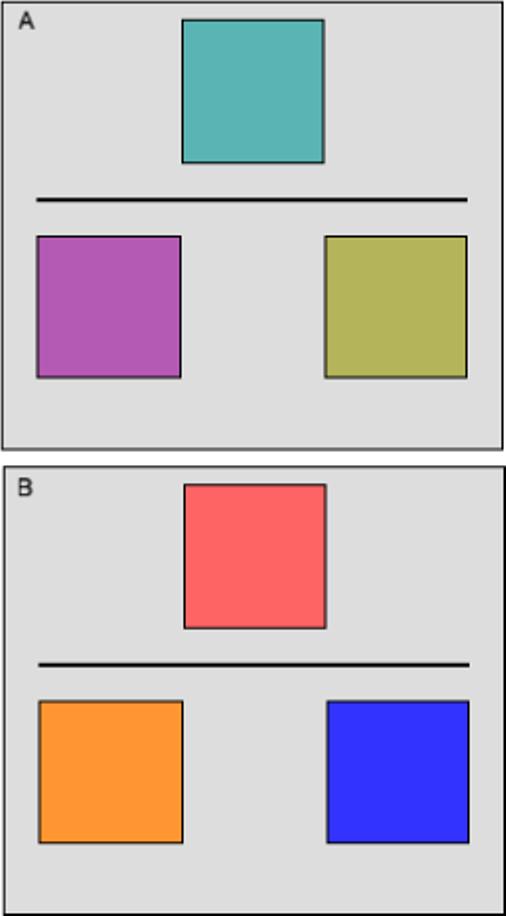
The spatial layout of the stimuli for this experiment. In frames A and B, the square above the horizontal line is the target or referent; the bottom two squares are the choice alternatives that must be matched to the target based on color preference, or color similarity. The colors are actual examples from the experiment. The choice alternatives in Frame A are equidistant from each other, and equally similar to the referent. The saturation and luminance were also equal. This type of ambiguous stimulus is representative of the two subjective conditions ISD and ESD. The red green blue (RGB) values for the colors are as follows (counterclockwise from the top): 90,180,180; 180,90,180; 180,180,90. Frame B is representative of the EVD control condition in which the participant made a veridical decision about which of the two color choices was most similar to the target. The RGB values in Frame B are: 255,100,100; 255,150,150; 51,51,255.
On a 800 × 600 screen layout, the target or referent square was centered 150 pixels above the two choice alternatives, which were situated 120 pixels to the left and right of center. The squares were all 90 × 90 in size. A semantic cue was present at the bottom of the screen to remind the subject of the current condition. The response selection of the left or right square was made on a two-button response panel placed in the right hand. The match was either by similarity or preference depending on the condition. Three conditions were presented:
Condition 1
Internal Subjective Decision or ISD: During this condition the colors were always equally distant in color hue and luminance from the referent. The match decision was based on self-preference, in the moment, rather than on color similarity. The decision was thus subjective and ‘internal’ or self-referential.
Condition 2
External Subjective Decision or ESD: During this condition the two alternatives were different from each other, but equidistant from the target in color hue and luminance. Thus, the choice of which of the two alternatives was more similar to the target was entirely ambiguous and subjective since there was no apparent correct answer based on external color features.
Condition 3
External Veridical Decision or EVD: During the EVD condition, a correct answer was always apparent. The correct answer occurred equally often in the left and right position. This was the baseline condition and was employed to control for visuospatial complexity, color identification and processing, the motoric and attentional components of choice selection.
Task Design
The conditions occurred in epochs of 20 seconds and five decision trials (four seconds each) were presented in each epoch. The order of the three conditions was pseudorandom and all three occurred within each of five 60-second cycles. The task was 5 minutes 40 seconds duration. The condition order was the same for all subjects. A semantic reminder was always at the bottom of the screen: “which is more similar” or “which do you prefer.” In addition, a four-second instructional cue was presented whenever the task switched from/to similarity to/from preference. The instruction cue at the beginning of the similarity conditions (conditions 2 or 3) was “which of the bottom boxes is more similar in color to the top box?” The instruction set for condition 1 was “which of the bottom boxes do you prefer with the top box.” Participants were instructed to make their decision as rapidly as possible using the MRI-compatible response device.
Setup Procedures and Hardware
Participants were provided with instruction and practice prior to scanning. They were then situated on the bed of a GE long bore 3.0 Tesla scanner and outfitted with the MR-compatible button-box and a high-resolution goggle system, set at 800 × 600 from Resonance Technology (Northridge, CA, USA). Head motion was constrained by foam padding around the head. The software Presentation (http://www.neurobs.com) was used to deliver visual stimuli from a PC computer via the goggle system and also record responses through the button-box connected to the serial port of the stimulus-delivery computer. A coaxial cable connecting the scanner control computer to the stimulus delivery computer enabled Presentation to monitor the pulses from the scanner, thereby enabling precise synchrony between slice acquisition and stimulus delivery.
Imaging protocol
Prior to the functional MRI scan, higher order shimming was performed using software provided by the manufacturer. The routine is run iteratively 2−3 times per subject resulting in field homogeneity over the brain in the range of 15−23 Hz RMS.
Field Mapping
Even after high-order shimming, there is residual magnetic field (Bo) inhomogenities across the brain that cause regional image distortions in echo planar images such as in the inferior prefrontal regions near the frontal and ethmoid sinuses. Since this was a relevant region for this experiment, image distortions were corrected by measuring 3D field maps across the brain (co-planar with the fMRI slices). This was accomplished by measuring the phase of non-EPI gradient echo images at two echo times (7 and 10 ms). The phase difference between the two echo images is proportional to the static field inhomogeneity (Jezzard & Balaban, 1995). The warp correction was performed using custom software developed in Matlab. A 3D phase-unwrapping algorithm (based on Jenkinson, 2003) was used to estimate the continuous field map. Image unwarping was performed using a nonlinear pixel shifting and B splines interpolation algorithm.
Image Acquisition
For each subject, a T2* weighted gradient-echo echo-planar image (EPI) pulse sequence was prescribed for the functional trials. The EPI parameters were as follows: echo time (TE) = 30 ms; repetition time (TR) = 2000 ms; flip angle = 90°; acquisition matrix = 64 × 64 voxels; field of view (FOV) = 240 mm. Thirty sagittal slices of the brain were acquired within the TR at each time point, with a voxel resolution of 3.75 × 3.75 × 4 mm, and a 1 mm skip between slices. Over a 5 min 40 s scanning run, 170 time points were collected, of which 3 images acquired during the first 6 s were discarded. Subsequent to the functional scans, a 3D IR-prepped fast gradient echo pulse sequence was administered (7.5 minutes) to provide high-resolution T1-weighted structural images.
Image Processing
Following echo-planar image reconstruction the files were converted to Analyze7.5 file format and reoriented to the axial plane. The 4D image time-series was motion-corrected to overcome minor head movement during the scan. The field map described above was then applied to each image in the time series to correct for echo-planar related distortions in image space. The images were then normalized into MNI standard atlas space (using the EPI template provided through SPM2), and then smoothed with an 8 mm full-width at half-maximum Gaussian kernel.
Data Analysis
Statistical analyses of the time-series data were performed on individual participants using a boxcar model convolved with the canonical hemodynamic response function. The statistical model included high frequency signal filtering (high pass filter = 128 seconds) and low-pass filtering with the hemodynamic response function. The time series data were statistically analyzed using the general linear model using SPM2 (Friston et al., 1995). Three contrasts were specified per single-participant analysis: ESD versus EVD, ISD versus EVD, and ISD versus ESD.
The resultant single-participant contrast images were then entered into second-level random effects analyses for this group of 17 subjects. Inference on the statistical significance of these group analyses used corrected p-values with the False Discovery Rate method (Genovese, Lazar, & Nichols, 2002) of p < 0.05 or lower.
Acknowledgements
This study was supported by MH65723 (SCJ). The assistance of Natalie Rahming, Justin Dunker, Michael Anderle and Ron Fisher is greatly appreciated.
References
- Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage. 2002;16(2):317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Lorig TS, Norris CJ, Rickett E, Nusbaum H. Just because you're imaging the brain doesn't mean you can stop using your head: a primer and set of first principles. J Pers Soc Psychol. 2003;85(4):650–661. doi: 10.1037/0022-3514.85.4.650. [DOI] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158(9):1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Cato MA, Crosson B, Gokcay D, Soltysik D, Wierenga C, Gopinath K, et al. Processing Words with Emotional Connotation: An fMRI Study of Time Course and Laterality in Rostral Frontal and Retrosplenial Cortices. J. Cogn. Neurosci. 2004;16(2):167–177. doi: 10.1162/089892904322984481. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio A. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. Harcourt Brace; New York: 1999. [Google Scholar]
- D'Argembeau A, Collette F, Van der Linden M, Laureys S, Del Fiore G, Degueldre C, et al. Self-referential reflective activity and its relationship with rest: a PET study. Neuroimage. 2005;25(2):616–624. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Duke LM, Seltzer B, Seltzer JE, Vasterling JJ. Cognitive components of deficit awareness in Alzheimer's disease. Neuropsychology. 2002;16(3):359–369. doi: 10.1037//0894-4105.16.3.359. [DOI] [PubMed] [Google Scholar]
- Flashman LA, Amador X, McAllister TW. Lack of Awareness of Deficits in Traumatic Brain Injury. Semin Clin Neuropsychiatry. 1998;3(3):201–210. [PubMed] [Google Scholar]
- Flashman LA, McAllister TW. Lack of awareness and its impact in traumatic brain injury. NeuroRehabilitation. 2002;17(4):285–296. [PubMed] [Google Scholar]
- Flashman LA, McAllister TW, Johnson SC, Rick JH, Green RL, Saykin AJ. Specific frontal lobe subregions correlated with unawareness of illness in schizophrenia: a preliminary study. J Neuropsychiatry Clin Neurosci. 2001;13(2):255–257. doi: 10.1176/jnp.13.2.255. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, et al. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57(2):109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, Frackowiak RS, et al. Analysis of fMRI time-series revisited. Neuroimage. 1995;2(1):45–53. doi: 10.1006/nimg.1995.1007. [see comments] [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds--a biological basis. Science. 1999;286(5445):1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123(Pt 7):1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Goel V, Grafman J, Sadato N, Hallett M. Modeling other minds. Neuroreport. 1995;6(13):1741–1746. doi: 10.1097/00001756-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Goldberg E, Podell K. Adaptive versus veridical decision making and the frontal lobes. Conscious Cogn. 1999;8(3):364–377. doi: 10.1006/ccog.1999.0395. [DOI] [PubMed] [Google Scholar]
- Goldberg E, Podell K. Adaptive decision making, ecological validity, and the frontal lobes. J Clin Exp Neuropsychol. 2000;22(1):56–68. doi: 10.1076/1380-3395(200002)22:1;1-8;FT056. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Menon V. Default-Mode Activity during a Passive Sensory Task: Uncoupled from Deactivation but Impacting Activation. J Cogn Neurosci. 2004;16(9):1484–1492. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Ollinger JM, Shulman GL, Cloninger CR, Price JL, Van Essen DC, et al. Persistence and brain circuitry. Proc Natl Acad Sci U S A. 2003;100(6):3479–3484. doi: 10.1073/pnas.0538050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49(1):193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34(1):65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(Pt 8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kazdin A. Observer effects: Reactivity of direct observation. New Directions for Methodology of Social & Behavioral Science. 1982;14:5–19. [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ. Neural activation during selective attention to subjective emotional responses. Neuroreport. 1997;8(18):3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O'Brien KC, McAvoy M, Raichle ME, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100(24):14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22(7):310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp. 2003;18(1):30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda H. Cerebral blood flow and metabolic abnormalities in Alzheimer's disease. Ann Nucl Med. 2001;15(2):85–92. doi: 10.1007/BF02988596. [DOI] [PubMed] [Google Scholar]
- Miller BL, Seeley WW, Mychack P, Rosen HJ, Mena I, Boone K. Neuroanatomy of the self: evidence from patients with frontotemporal dementia. Neurology. 2001;57(5):817–821. doi: 10.1212/wnl.57.5.817. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Foster NL, Kuhl DE. Posterior cingulate cortex in Alzheimer's disease. Lancet. 1994;344(8926):895. doi: 10.1016/s0140-6736(94)92871-1. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42(1):85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- Nelson JK, Reuter-Lorenz PA, Sylvester CY, Jonides J, Smith EE. Dissociable neural mechanisms underlying response-based and familiarity-based conflict in working memory. Proc Natl Acad Sci U S A. 2003;100(19):11171–11175. doi: 10.1073/pnas.1334125100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Frank LR. Ventromedial prefrontal cortex activation is critical for preference judgments. Neuroreport. 2003;14(10):1311–1315. doi: 10.1097/01.wnr.0000078543.07662.02. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21(2):768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126(Pt 3):650–668. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- Podell K, Lovell M, Zimmerman M, Goldberg E. The Cognitive Bias Task and lateralized frontal lobe functions in males. J Neuropsychiatry Clin Neurosci. 1995;7(4):491–501. doi: 10.1176/jnp.7.4.491. [DOI] [PubMed] [Google Scholar]
- Prigatano G. Behavioral limitations TBI patients tend to underestimate: a replication and extension to patients with lateralized dysfunction. Clinical Neuropsychologist. 1996;10:191–201. [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci U S A. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts SA, van Swieten JC, Pijnenburg YA, Goekoop R, Barkhof F, Scheltens P. Loss of frontal fMRI activation in early frontotemporal dementia compared to early AD. Neurology. 2003;60(12):1904–1908. doi: 10.1212/01.wnl.0000069462.11741.ec. [DOI] [PubMed] [Google Scholar]
- Rymer S, Salloway S, Norton L, Malloy P, Correia S, Monast D. Impaired awareness, behavior disturbance, and caregiver burden in Alzheimer disease. Alzheimer Dis Assoc Disord. 2002;16(4):248–253. doi: 10.1097/00002093-200210000-00006. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22(2):941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Seger CA, Stone M, Keenan JP. Cortical Activations during judgments about the self and an other person. Neuropsychologia. 2004;42(9):1168–1177. doi: 10.1016/j.neuropsychologia.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Sherer M, Bergloff P, Levin E, High WM, Jr., Oden KE, Nick TG. Impaired awareness and employment outcome after traumatic brain injury. J Head Trauma Rehabil. 1998;13(5):52–61. doi: 10.1097/00001199-199810000-00007. [DOI] [PubMed] [Google Scholar]
- Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2000;97(11):6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Stuss D, Picton T, Alexander M. Consciousness, self-awareness and the frontal lobes. In: Duffy J, editor. The Frontal Lobes and Neuropsychiatric Illness. American Psychiatric Press; Washington DC: 2001. [Google Scholar]
- Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, et al. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58(5):758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]
- Turk DJ, Heatherton TF, Kelley WM, Funnell MG, Gazzaniga MS, Macrae CN. Mike or me? Self-recognition in a split-brain patient. Nat Neurosci. 2002;5(9):841–842. doi: 10.1038/nn907. [DOI] [PubMed] [Google Scholar]
- Turk DJ, Heatherton TF, Macrae CN, Kelley WM, Gazzaniga MS. Out of contact, out of mind: the distributed nature of the self. Ann N Y Acad Sci. 2003;1001:65–78. doi: 10.1196/annals.1279.005. [DOI] [PubMed] [Google Scholar]
- van Veen V, Cohen JD, Botvinick MM, Stenger VA, Carter CS. Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage. 2001;14(6):1302–1308. doi: 10.1006/nimg.2001.0923. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happe F, Falkai P, et al. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14(1 Pt 1):170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Fink GR. Neural correlates of the first-person-perspective. Trends Cogn Sci. 2003;7(1):38–42. doi: 10.1016/s1364-6613(02)00003-7. [DOI] [PubMed] [Google Scholar]
- Zysset S, Huber O, Ferstl E, von Cramon DY. The anterior frontomedian cortex and evaluative judgment: an fMRI study. Neuroimage. 2002;15(4):983–991. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]
- Zysset S, Huber O, Samson A, Ferstl EC, von Cramon DY. Functional specialization within the anterior medial prefrontal cortex: a functional magnetic resonance imaging study with human subjects. Neurosci Lett. 2003;335(3):183–186. doi: 10.1016/s0304-3940(02)01196-5. [DOI] [PubMed] [Google Scholar]


