Abstract
Objective
To develop a model using individual and lesion characteristics to help surgeons choose lesions with a high probability of containing histologically-confirmed endometriosis.
Design
Secondary analysis of prospectively collected information. Setting: Government research hospital.
Patients
Healthy women aged 18–45 with chronic pelvic pain and possible endometriosis, enrolled in a clinical trial.
Intervention
All participants underwent laparoscopy, and information was collected on all visible lesions. Lesion data were randomly allocated to a training and test dataset.
Main Outcome Measures(s)
Predictive logistic regression with the outcome of interest being histologic diagnosis of endometriosis.
Results
After validation, the model was applied to the complete dataset with a sensitivity of 88.4% and specificity of 24.6%. The positive predictive value was 69.2% and the negative predictive value was 53.3%, equating to correct classification of a lesion of 66.5%. Mixed color, larger width and location in the ovarian fossa, colon, or appendix were most strongly associated with the presence of endometriosis.
Conclusions
This model identified characteristics which indicated a high and low probability of biopsy-proven endometriosis. It is useful as a guide in choosing appropriate lesions for biopsy, but is not sufficiently robust to use alone.
Keywords: Endometriosis, Prediction, Logistic Regression Modeling
Introduction
According to the 2005 ESHRE guidelines (1), the 2000 ACOG Practice Bulletin(2), and the 2005 ACOG Committee Opinion (3), in some circumstances it is acceptable to diagnose endometriosis based on the visual appearance of lesions at laparoscopy. In questionable cases, biopsy with histologic confirmation of the disease is recommended, but positive histology is not a requisite for diagnosis in all cases. The responsibility of identifying endometriosis rests with the surgeon, who must recognize endometriosis using only visual assessment at surgery (4).
Unfortunately, visual assessment of lesions has poor intra-physician agreement and high rates of misdiagnosis (5) and correlates poorly with actual histologic findings (4, 6–14). Presurgical screening tests to increase the probability of a woman having endometriosis at surgery are one way to improve the likelihood of accurate diagnosis. However, questionnaires, MRI, ultrasound and adjunctive lab tests all lack the sensitivity and specificity to significantly increase the pre-test probability of disease (1, 8, 11, 15, 16), except for women with severe endometriosis (Stage III or IV) (6, 17–19). Based on these facts, some method to assess which lesions are most likely to represent true disease is needed, particularly if visual appearance is used.
At surgery, endometriosis lesions present with a variety of colors, sizes, and locations (8, 10, 11, 15, 20). The heterogeneity of lesion characteristics suggests that visual inspection may not be sufficient to identify endometriosis (9), especially when subtle lesions are present. Thus development of a model synthesizing multiple factors might improve the accuracy of diagnosis or at least guide the surgeon regarding which lesions are most likely to be positive on biopsy. By correlating patient and individual lesion characteristics with pathologic findings in a multivariate regression model, we sought to assess which lesions are most likely to be biopsy-confirmed as endometriosis. Knowing this fact might improve on the poor histologic correlation with surgical assessment (4, 6–14).
Methods
This is a secondary data analysis of a clinical study which received IRB approval from both the NIH and University of North Carolina-Chapel Hill. Data were collected from 119 women aged 18–45 who were enrolled in a clinical trial for the treatment of chronic pelvic pain and endometriosis, from 1999 to 2004, that combined laparoscopic excision of all lesions with medical treatment. Women were healthy with the exception of pelvic pain lasting at least three months.
Data Collection
After providing informed consent, all participants underwent laparoscopy. At surgery, information was collected on all visible lesions as previously described (11). Lesions suspected of being endometriosis were excised with contact neodymium:yttrium-aluminum-garnet laser (Surgical Laser Technologies, The Oaks, PA); no lesions were ablated and all were sent for histology. When cysts were present, the walls were stripped from the surrounding tissue. An appendectomy was performed if there was chronic inflammation or endometriosis involving this structure. Peritoneal defects were excised in-toto whenever possible. To minimize unreasonable risks to the patient, deep implants were not resected if found in the recto-vaginal septum or transmural to the bowel wall.
The following categories were used to identify the location of the lesion: bladder/peritoneum, colon/appendix, cul-de-sac, ovarian fossa, ovary, sidewall, utero-sacral ligament, and the uterus/fallopian tube. Each lesion was measured across two diameters, and averaged. If lesions were closely spaced (within 0.5 cm of each other) the measurement included the distance across all lesions. Color categories included red, white, black, mixed, and endometriomas, and the type of lesions as judged by the surgeon (endometriotic vs. nonendometriotic) was also recorded. Finally, the revised American Fertility Society (revASRM) classification system was used to estimate the severity or stage of the disease in the pelvis (21).
Histology was only considered to be positive if both endometrial glands and stroma were identified.
All lesions identified as endometriosis by the surgeon were eligible for inclusion in the model. To remain in the dataset, the observation for each lesion had to have complete covariate data of lesion characteristics, as well as histology results, revASRM disease stage, and patient demographics.
Data Analysis
For the model development, data were randomly allocated to one of two datasets (designated the training and test dataset) using Stata Version 8.2 (Stata Corporation, College Station, TX). The model was developed in the training dataset and validated in the test dataset. Univariate analysis was done for each variable to assess distribution and the need for transformation.
The model was developed using logistic regression with the histologic diagnosis of endometriosis as the outcome of interest. Variance estimates for individual parameters were adjusted for multiple observations taken from single participants and the non-independence of these observations. Lesion color, location, stage, and race were treated as categorical variables. Age, BMI, and lesion width were evaluated for linearity in the logit before being included as continuous variables. Transformations were not required for these variables. Categorical variables were condensed based on similarities of the estimates in the model and tests of heterogeneity across categories. Therefore, categories were constructed based on mathematical, not clinical criteria.
The Hosmer-Lemeshow goodness-of-fit test was used to test the predictive ability (calibration) of the model. The area under the receiver-operator characteristic (ROC) curve evaluated the discrimination of the model fit. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), as well as percent of lesions correctly classified were calculated for the combined data. Analyses were done using Stata Version 8.2 (College Station, TX).
Results
There were a total of 530 observations from 119 women that were potentially eligible for inclusion in the model. Forty-three observations (8.1% of the complete dataset) were dropped during modeling because of missing covariate information. A total of 114 women contributing complete data on 487 lesions, of which 320 (65.7%) had histologically confirmed endometriosis. Approximately 2/3 of the observations (n = 334) were included in the training dataset and the remainder (n = 153) were included in the test dataset. Since each lesion was considered separately during the allocation process, the same woman could contribute lesions to both datasets. Thus, 104 women contributed to the training dataset, and 77 women to the validation dataset.
Of women in the complete dataset, 92 reported their race as white (80.7%). Forty (35%) had stage I disease, 46 (40%) had stage II disease, 17 (6%) had stage III disease, and 11 (9%) had stage IV disease. The mean age of the study participants was 31.4 ± 7.2 years (range 18–45) and the mean BMI was 25 ± 4.7 (range 17.2–44.5). Forty percent of lesions were located in the cul-de-sac or utero-sacral ligaments. The majority of the lesions were subtle lesions (red or white, 51.6%)(Table 1). The distributions of age, race, BMI, stage, lesion location, color, width and histologically confirmed endometriosis did not differ significantly between the training and validation datasets, suggesting that participant characteristics were reasonably well-balanced.
Table 1.
Distribution of lesion characteristics, odds ratios for association of lesion characteristics with histologically-confirmed endometriosis, and calculated probability of histologically-confirmed endometriosis of each characteristic (derived from the model after adjustment of all other variables).
| Lesion Characteristic | N (%) | Odds Ratio (95% CI) | Adj. Prob. Endo+ (95% CI) | % Change from referent |
|---|---|---|---|---|
| Locationa | ||||
| Bladder/peritoneum, round/broad, sidewall | 129 (26.5) | 0.42 (0.25, 0.71) | 0.44 (0.15, .078) | −22 |
| Uterus, ovary, fallopian tube | 106 (21.8) | 0.50 (0.26, 0.97) | 0.49 (0.18, 0.81) | −17 |
| Cul-de-sac, utero-sacrals ligaments (referent) | 196 (40.3) | ------ | 0.66 (0.30, 0.89) | ------ |
| Ovarian fossa, colon, appendix | 56 (11.5) | 1.18 (0.70, 1.98) | 0.69 (0.34, 0.91) | 3 |
| Color Groupsb | ||||
| Red or white | 251 (51.6) | --- | 0.66 (0.30, 0.89) | --- |
| Blue, black, brown, endometrioma | 136 (27.9) | 1.21 (0.76, 1.93) | 0.69 (0.33, 0.91) | 3 |
| Mixed colors | 100 (20.5) | 1.87 (1.05, 3.34) | 0.78 (0.44, 0.94) | 12 |
| Racec | ||||
| Nonwhite | 82 (16.8) | 0.67 (0.40, 1.12) | 0.56 (0.22, 0.85) | 10 |
| White (referent) | 405 (83.2) | --- | 0.66 (0.30, 0.89) | --- |
| Staged | ||||
| I | 99 (20.3) | 0.49 (0.31, 0.79) | 0.48 ( 0.17, 0.81) | −18 |
| II, III, IV | 388 (79.7) | --- | 0.66 (0.30, 0.89) | --- |
| Median (range) | OR per unit increase (95%CI) | |||
| Width of lesion (mm)d | 5 (1–90) | 1.05 (1.02, 1.09) | ||
| 2.7 | 0.59 (0.25, 0.87) | −7 | ||
| 7.7 (mean, referent) | 0.66 (0.30, 0.89) | --- | ||
| 12.7 | 0.71 (0.35, 0.92) | 4 | ||
| 17.7 | 0.76 (0.40, 0.94) | 8 | ||
| 22.7 | 080 (0.45, 0.95) | 10 | ||
| BMI (kg/m2)e | 24.3 (17.2-4.5) | 0.97 (0.93, 1.01) | ||
| 35 | 0.59 (0.19, 0.90) | −4 | ||
| 30 | 0.62 (0.24, 0.90) | −2 | ||
| 25 (mean, referent) | 0.66 (0.30, 0.89) | --- | ||
| 20 | 0.68 (0.36, 0.89) | 2 | ||
| 15 | 0.71 (0.43, 0.89) | 4 | ||
| Age (yrs)f | 31 (17–46) | 1.01 (0.98, 1.04) | ||
| 21.4 | 0.63 (0.32, 0.87) | −2 | ||
| 26.4 | 0.64 (0.31, 0.88) | 0 | ||
| 31.4 (mean, referent) | 0.66 (0.30, 0.89) | --- | ||
| 36.4 | 0.66 (0.29, 0.91) | 1 | ||
| 41.4 | 0.67 (0.28, 0.92) | 2 | ||
For each calculation using location, color group=red or white (reference level), race=white (reference level), width=7.7, stage=II, III, IV (reference level), BMI=25 (reference level), Age=31.4 (reference level)
For each calculation using color group, location= Cul-de-sac, utero-sacrals ligaments (referent), race=white (reference level), width=7.7, stage=II, III, IV (reference level), BMI=25 (reference level), Age=31.4 (reference level)
For each calculation using race, location= Cul-de-sac, utero-sacrals ligaments (referent), color group=red or white (reference level), width=7.7, stage=II, III, IV (reference level), BMI=25 (reference level), Age=31.4 (reference level)
For each calculation using width, location= Cul-de-sac, utero-sacrals ligaments (referent), color group=red or white (reference level), race=white (reference level, stage=II, III, IV (reference level), BMI=25 (reference level), Age=31.4 (reference level)
For each calculation using stage, location= Cul-de-sac, utero-sacrals ligaments (referent), color group=red or white (reference level), race=white (reference level), width=7.7, BMI=25 (reference level), Age=31.4 (reference level)
For each calculation using Age, location= Cul-de-sac, utero-sacrals ligaments (referent), color group=red or white (reference level), race=white (reference level), width=7.7, stage=II, III, IV (reference level), BMI=25 (reference level)
The Hosmer-Lemeshow goodness-of-fit test for the training dataset was used to validate the model. The p value was estimated at 0.78, indicating good calibration of the model to the dataset. The test dataset had a p value of 0.30, again showing good calibration. The area under the ROC curve for the training dataset was 0.70 indicating only fair discrimination of the model which was similar to the test dataset at 0.69.
After validating the model, it was applied to the complete dataset to determine characteristics predictive of endometriosis. Lesions located on the ovarian fossa, colon, or appendix were 25% more likely than those on the uterus, ovary, fallopian tubes, cul-de-sac, or utero-sacral ligaments to contain histologically-confirmed endometriosis (Table 1). The odds that a given lesion was confirmed to be endometriosis increased by 5% per millimeter of lesion width (OR=1.05, 95% CI 1.02, 1.09). Lesions from women classified as having Stage I disease were significantly less likely to contain endometriosis than women with Stage II-IV disease (OR=0.49, 95% CI 0.31, 0.79). The odds of lesions of mixed color truly containing endometriosis were 87% greater than those that were red or white (OR=1.87; 95% CI 1.05, 3.34), yet, red or white lesions were as likely as those that were blue, black, brown, or endometriomas to be confirmed as endometriosis. Age and BMI were not associated with changes in the odds of histologically-confirmed endometriosis.
The model was also used to explore the change in the probability of confirming endometriosis by adjusting each variable in relation to the referent (most common) value for each characteristic. The largest change in percentage occurred among different locations (from −22% to +3%), and the smallest change was observed with age (from −2% to +2%). Using the characteristics with the highest probability of confirming endometriosis, a nearly 3 cm wide, mixed color lesion in the ovarian fossa from a 42 year old Caucasian woman with a BMI of 15 and at least stage II disease had the highest probability of endometriosis (92.9%; 95% CI: 0.73, 0.98). By contrast, a small red lesion on the bladder peritoneum from a 22 year old non-Caucasian woman with stage I endometriosis had the lowest probability of confirmed endometriosis (22.0%; CI: 0.09, 0.45).
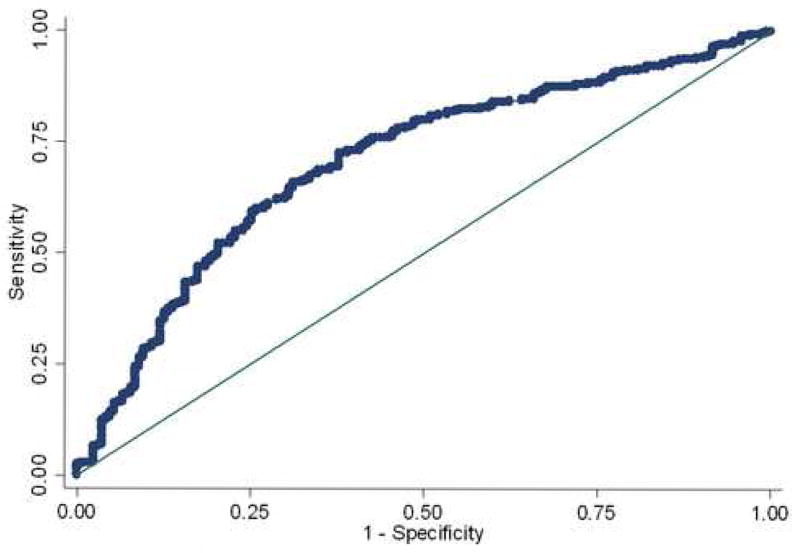
We also determined the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the final model. The model predicted the presence of endometriosis with a sensitivity of 88.8% and predicted the absence of endometriosis with a specificity of 24.6%. The PPV was 69.3% and the NPV 53.3%. This equated to correct classification of a lesion of 66.5% (Figure 1).
Figure 1.
ROC curve. Model correctly predicts endometriosis in 66.7% of the time. Area under ROC curve=0.7026
Discussion
Overall, this model had a modest ability to predict endometriosis from individual lesion characteristics. We found moderate associations between the characteristics of size, location and mixed color with endometriosis, but, as expected, no other single lesion characteristic predicted endometriosis with a high accuracy. Lesions of any single color had similar probabilities of being confirmed as endometriosis. A mixed color lesion increased the probability of confirming endometriosis from 66% to 78%.
Lesion size and location were more helpful in predicting biopsy-positive endometriosis lesions. This model confirmed that wider lesions had a greater probability of containing endometriosis, with an increase of 17% noted in comparing a 2.7mm to a 27.7mm lesion. Likewise, there was a 17% increase in the probability of confirming biopsy-proven endometriosis in identical lesions located in the ovarian fossa compared to the uterus. However, while endometriosis was commonly found in the cul-de-sac or on the utero-sacral ligaments, location alone did not correlate well with the presence of endometriosis on biopsy.
This model provides guidance about appropriate lesions to biopsy for confirmation of the disease. Information about subtle lesions may be useful to direct the surgeon to biopsy atypical, low probability lesions to ensure that endometriosis is the true diagnosis. For instance, a large lesion in the ovarian fossa that is of mixed color has a 93% probability of being endometriosis and may not need to be biopsied unless definitive diagnosis is needed, such as would be required for entry into a study protocol. However, a small red or white lesion on the uterus has just a 22% chance of containing endometriosis, and if other high probability lesions were not seen, a biopsy of this lesion would be appropriate to confirm the presence of the disease.
Overall, basing the diagnosis of endometriosis on subtle lesions alone is problematic because of the variable appearance of these lesions. (4, 10–13). Martin et al (4) reported a 30% increase in the diagnosis of endometriosis in women overall when biopsies were taken from all abnormal appearing tissue (subtle findings), and not just from tissue suspected to be endometriosis. Until another method of easily identifying endometriosis in these lesions at surgery is found, our results indicate it is prudent to confirm the diagnosis of endometriosis by biopsy in women with atypical lesions.
Our model aptly described results in the study group, but may not be generalizable to the population at large or to women with infertility, but not pain. All subjects had symptoms consistent with pain associated with endometriosis; therefore the findings at laparoscopy may be most relevant for lesions likely to cause pain but not with infertility. Had we sampled women with pain-free endometriosis or those with infertility, we might have found different associations. However, random laparoscopies in asymptomatic women present ethical issues and women with endometriosis related infertility undergoing in vitro fertilization may not undergo laparoscopy. Also, the clinical trial was not designed to define lesion characteristics, and normal peritoneum was not routinely sampled as a control. This may account for the low specificity and low negative predictive value since lesions believed to be negative were not routinely sampled.
In conclusion, our findings are consistent with previously published reports that use of a lesion color is a poor predictor for the presence of endometriosis and that endometriosis is found in lesions of any single colors with similar frequencies. Width, location, mixed color, and race were modestly predictive of endometriosis, whereas stage of disease was more strongly predictive. Overall, our model increased the sensitivity of identifying endometriosis in individual lesions from 65.7% to 88%, but had a poor specificity, meaning that we could not reliably predict lesions that did not contain endometriosis. This model was able to identify lesions with a high (93%) or low (22%) probability of containing endometriosis, but also demonstrated the need for disease confirmation by biopsy in women with subtle lesions. The information we present provides useful guidelines to aid in decision making about the likelihood of a patient having endometriosis at the time of surgery and to help differentiate between high and low probability lesions for biopsy if this diagnosis is in question, thus supporting excising rather than ablating any high probability lesion. This model augments, but does not replace, clinical judgment.
Acknowledgments
This research was supported, in part, by the intramural research program of the Reproductive Biology and Medicine Branch of the NICHD, National Institutes of Health, and the National Institute of Health Clinical Center and by the NIH-sponsored “Training in Epidemiology and Clinical Trials” training grant (T32 HD40672) at the University of North Carolina, Chapel Hill, NC.
Footnotes
Poster presented at the Society for Gynecologic Investigation Annual Meeting, Toronto, Canada, March 21–25, 2006
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kennedy S, Bergqvist A, Chapron C, D’Hooghe T, Dunselman G, Greb R, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20:2698–704. doi: 10.1093/humrep/dei135. [DOI] [PubMed] [Google Scholar]
- 2.ACOG practice bulletin. Medical management of endometriosis. Number 11, December 1999 (replaces Technical Bulletin Number 184, September 1993). Clinical management guidelines for obstetrician-gynecologists. Int J Gynaecol Obstet. 2000;71:183–96. doi: 10.1016/s0020-7292(00)80034-x. [DOI] [PubMed] [Google Scholar]
- 3.ACOG Committee Opinion. Number 310, April 2005, Endometriosis in adolescents. Obstet Gynecol. 2005;105:921–7. doi: 10.1097/00006250-200504000-00058. [DOI] [PubMed] [Google Scholar]
- 4.Martin DC. Endometriosis: correlation between histologic and visual findings at laparoscopy. Am J Obstet Gynecol. 2003;188:1663. doi: 10.1067/mob.2003.426. author reply -4. [DOI] [PubMed] [Google Scholar]
- 5.Buchweitz O, Wulfing P, Malik E. Interobserver variability in the diagnosis of minimal and mild endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;122:213–7. doi: 10.1016/j.ejogrb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Brosens I, Puttemans P, Campo R, Gordts S, Brosens J. Non-invasive methods of diagnosis of endometriosis. Curr Opin Obstet Gynecol. 2003;15:519–22. doi: 10.1097/00001703-200312000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Buchweitz O, Poel T, Diedrich K, Malik E. The diagnostic dilemma of minimal and mild endometriosis under routine conditions. J Am Assoc Gynecol Laparosc. 2003;10:85–9. doi: 10.1016/s1074-3804(05)60240-x. [DOI] [PubMed] [Google Scholar]
- 8.Donnez J, Squifflet J, Casanas-Roux F, Pirard C, Jadoul P, Van Langendonckt A. Typical and subtle atypical presentations of endometriosis. Obstet Gynecol Clin North Am. 2003;30:83–93. viii. doi: 10.1016/s0889-8545(02)00054-2. [DOI] [PubMed] [Google Scholar]
- 9.Marchino GL, Gennarelli G, Enria R, Bongioanni F, Lipari G, Massobrio M. Diagnosis of pelvic endometriosis with use of macroscopic versus histologic findings. Fertil Steril. 2005;84:12–5. doi: 10.1016/j.fertnstert.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 10.Nezhat F, Allan CJ, Nezhat C, Martin DC. Nonvisualized endometriosis at laparoscopy. Int J Fertil. 1991;36:340–3. [PubMed] [Google Scholar]
- 11.Stratton P, Winkel CA, Sinaii N, Merino MJ, Zimmer C, Nieman LK. Location, color, size, depth, and volume may predict endometriosis in lesions resected at surgery. Fertil Steril. 2002;78:743–9. doi: 10.1016/s0015-0282(02)03337-x. [DOI] [PubMed] [Google Scholar]
- 12.Stripling MC, Martin DC, Chatman DL, Zwaag RV, Poston WM. Subtle appearance of pelvic endometriosis. Fertil Steril. 1988;49:427–31. doi: 10.1016/s0015-0282(16)59767-2. [DOI] [PubMed] [Google Scholar]
- 13.Walter AJ, Hentz JG, Magtibay PM, Cornella JL, Magrina JF. Endometriosis: correlation between histologic and visual findings at laparoscopy. Am J Obstet Gynecol. 2001;184:1407–11. 11–3. doi: 10.1067/mob.2001.115747. [DOI] [PubMed] [Google Scholar]
- 14.Wykes CB, Clark TJ, Khan KS. Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. Bjog. 2004;111:1204–12. doi: 10.1111/j.1471-0528.2004.00433.x. [DOI] [PubMed] [Google Scholar]
- 15.Donnez J, Van Langendonckt A. Typical and subtle atypical presentations of endometriosis. Curr Opin Obstet Gynecol. 2004;16:431–7. doi: 10.1097/00001703-200410000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am. 2003;30:1–19. vii. doi: 10.1016/s0889-8545(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 17.Stratton P, Winkel C, Premkumar A, Chow C, Wilson J, Hearns-Stokes R, et al. Diagnostic accuracy of laparoscopy, magnetic resonance imaging, and histopathologic examination for the detection of endometriosis. Fertil Steril. 2003;79:1078–85. doi: 10.1016/s0015-0282(03)00155-9. [DOI] [PubMed] [Google Scholar]
- 18.Kinkel K, Frei KA, Balleyguier C, Chapron C. Diagnosis of endometriosis with imaging: a review. Eur Radiol. 2006;16:285–98. doi: 10.1007/s00330-005-2882-y. [DOI] [PubMed] [Google Scholar]
- 19.Arrive L, Hricak H, Martin MC. Pelvic endometriosis: MR imaging. Radiology. 1989;171:687–92. doi: 10.1148/radiology.171.3.2717739. [DOI] [PubMed] [Google Scholar]
- 20.Martin DC, Hubert GD, Vander Zwaag R, el-Zeky FA. Laparoscopic appearances of peritoneal endometriosis. Fertil Steril. 1989;51:63–7. doi: 10.1016/s0015-0282(16)60429-6. [DOI] [PubMed] [Google Scholar]
- 21.Damario MA, Rock JA. New considerations for the classification of endometriosis. Int J Gynaecol Obstet. 1993;40(Suppl):S9–20. [PubMed] [Google Scholar]