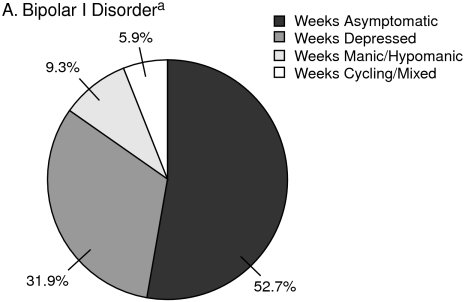
Bipolar disorder is associated with increased lost days from work,1 reduced vocational and residential status,2 and increased psychosocial impairment.3 People with bipolar disorders are symptomatic about half of the time, and depressive symptoms occur much more frequently than hypomania/mania or cycling (Figure 1).4,5 Evidence suggests that the index episode tends to predict the polarity of the next episode,6 which could suggest that, in the modern era, relapse is more common than recurrence. Disability and illness recurrence early in the course of the illness may be, in part, attributable to a delay in recognition and appropriate treatment, as the first course of treatment typically occurs about 6 years after the onset of bipolar episodes.7 A group of experts reviewed therapies for acute and maintenance treatment of bipolar disorder and explained the pathophysiology of mood disorders and the mechanism of action of medications.
Figure 1.
Symptomatology in Patients With Bipolar I and Bipolar II Disorders
aData from Judd et al.4 Percentages are weeks spent at specific affective symptom categories during a mean of 12.8 years follow-up in 146 patients with bipolar I disorder.
bData from Judd et al.5 Percentages are weeks spent at specific affective symptom categories during a mean of 13.4 years of prospective follow-up in 86 patients with bipolar II disorder.
Effective Agents in Treating Bipolar Depression
Because patients with bipolar disorder spend more time with depression than mania, Andrew A. Nierenberg, M.D., reviewed evidence for the efficacy of acute and maintenance treatments for bipolar depression. He emphasized that not all treatments discussed are approved by the U.S. Food and Drug Administration (FDA; Table 1),8 and some studies were inconclusive or underpowered. Dr. Nierenberg mentioned that adjunctive psychosocial interventions such as cognitive-behavioral therapy, family-focused therapy, interpersonal and social rhythm therapy, and some group psychoeducation can also be effective treatments for bipolar depression.9
Table 1.
Agents Approved for Use in Adults With Bipolar I Disorder in the United Statesa
| Acute Mania |
Maintenance |
Acute Depression |
|||
| Year | Drug | Year | Drug | Year | Drug |
| 1970 | Lithium | 1974 | Lithium | 2003 | Olanzapine-fluoxetine combination |
| 1973 | Chlorpromazine | 2003 | Lamotrigine | ||
| 1994 | Divalproex | 2004 | Olanzapine | 2006 | Quetiapine |
| 2000 | Olanzapine* | 2005 | Aripiprazole | ||
| 2003 | Risperidone* | 2008 | Quetiapine** | ||
| 2004 | Quetiapine* | ||||
| 2004 | Ziprasidone | ||||
| 2004 | Aripiprazole* | ||||
| 2004 | Carbamazepine | ||||
Adapted with permission from Ketter.8
Approved as adjunctive treatment as well as monotherapy.
Approved only as adjunctive treatment.
Acute Bipolar Depression
Antidepressants.
A fundamental question is whether antidepressants are effective in bipolar depression. Although a meta-analysis10 included studies of antidepressants used alone, Dr. Nierenberg concluded that insufficient evidence is available to determine the efficacy of antidepressant monotherapy for acute bipolar depression. The risk of a switch to mania is a concern with antidepressant monotherapy.11
Antidepressant plus mood stabilizer.
The combination of the mood stabilizer lithium with the antidepressants paroxetine or imipramine was compared with lithium plus placebo in a study of patients in a major depressive episode stabilized with lithium.11 Dr. Nierenberg commented that this study was underpowered and differences in efficacy between the 3 groups were not statistically significant. However, the study suggested that paroxetine and imipramine were superior to placebo in treating depression in patients with a low serum lithium level, and the placebo effect was reduced in patients with low serum lithium levels. Mania developed in none of the paroxetine-treated patients, 2.3% of the placebo-treated patients, and in 7.7% of the imipramine-treated patients.
A large, well-conducted study12 within the National Institute of Mental Health–sponsored Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) examined whether adjunctive antidepressant therapy (bupropion or paroxetine) reduced bipolar depression without increasing the risk of mania. Notably, this study used rigorously defined outcome and effectiveness measures consistent with the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition13 definitions. In patients taking mood stabilizers or atypical antipsychotics, adjunctive antidepressant medication did not increase the percentage of patients who experienced durable recovery (adjunctive placebo, 27.3% vs. adjunctive antidepressant, 23.5%), but adjunctive antidepressantsalso failed to increase the risk of treatment-emergent affective switch (placebo, 10.7% vs. antidepressant, 10.1%). Dr. Nierenberg commented that the adjunctive antidepressant seemed to confer no benefit but also to do no harm.
A study14 of 2 mood stabilizers (lithium plus divalproex sodium) versus an antidepressant plus a mood stabilizer (paroxetine plus lithium or divalproex sodium) was inconclusive because it was not powered to show a difference between the treatment groups.
Mood stabilizer alone.
Few data compare lithium with placebo for the treatment of bipolar depression. In a review15 of 8 studies of lithium versus placebo in depressed bipolar patients, lithium appeared to be more effective than placebo, but Dr. Nierenberg observed that the study was small and the definition of response was loosely defined.
Calabrese et al.16 found lamotrigine to be effective for acute treatment of bipolar I depression compared with placebo. Patients who were treated with 50 or 200 mg/day of lamotrigine demonstrated significant (p < .05) reductions in Montgomery-Asberg Depression Rating Scale (MADRS) scores compared with those who took placebo. However, 4 of 5 recent studies failed to find similar efficacy.17
Antipsychotics.
Tohen and colleagues18 compared the atypical antipsychotic olanzapine with olanzapine-fluoxetine treatment or placebo in depressed patients with bipolar I disorder. At week 8, MADRS scores were lower by 11.9 points with placebo, 15.0 points with olanzapine, and 18.5 points with olanzapine-fluoxetine. Response rates for the 3 groups were 30.4%, 39.0%, and 56.1%, respectively. The olanzapine-fluoxetine combination showed a robust decrease in acute bipolar I depressive symptoms.
A robust response was also found for the atypical antipsychotic quetiapine compared with placebo for bipolarI or II depression.19 Although a 600-mg/day dose was slightly more effective than a 300-mg/day dose, 300 mg/day was better tolerated.
Maintenance Treatment
A review20 of studies of lithium, lamotrigine, carbamazepine, divalproex, and olanzapine maintenance therapies in bipolar disorder found that lithium remained the gold standard for overall preventive efficacy. However, lithium was more effective for prevention of mania than depression.
Lithium, divalproex, and placebo were compared for bipolar I relapse prevention after an index manic episode.21 No significant difference was found between the 3 agents in time to recurrence of any mood episode over 12 months. In an 18-month trial,22 lithium and lamotrigine monotherapies were compared for prevention of relapse or recurrence of depression in recently manic or hypomanic bipolar I patients. Both lithium and lamotrigine were more effective than placebo at delaying recurrence of any mood episode; lamotrigine was significantly superior to placebo for prevention of depression (p = .02), while lithium was significantly superior to placebo for prevention of mania (p = .006). Similarly, in a comparison23 between lamotrigine, lithium, and placebo maintenance treatment over 18 months in recently depressed patients with bipolar I disorder, lamotrigine was statistically more effective than placebo in time to intervention for depression (p = .047), while lithium was significantly more effective than placebo at delaying mania (p = .026).
Lithium and olanzapine monotherapy for maintenance treatment were compared in patients with bipolar disorder for 12 months.24 Fewer patients treated with lithium than with olanzapine experienced syndromic recurrence of depression (8.3% vs. 13.9%, respectively). Dr. Nierenberg noted that a meta-analysis25 showed a consistent effect of lithium against suicidality, which may be a reason to combine lithium with other medications when treating bipolar depression.
Summary
Dr. Nierenberg emphasized that more research is needed comparing monotherapies and combination treatments for acute bipolar depressive episodes and their recurrence. Insufficient evidence is available for many of the medications that are used without FDA-approved indications. Besides pharmacotherapy, structured psychosocial interventions should be part of everyday practice.
Monotherapy Versus Combination Treatment in Bipolar Disorder
Terence A. Ketter, M.D., cautioned that, although the use of combination therapy in the treatment of bipolar disorder is growing, clinicians need to carefully weigh the efficacy, safety, and tolerability of combined treatments. Since 1970, multiple medications have been approved by the FDA for the treatment of bipolar disorder (see Table 1), some of which are also approved for use in combination therapy.26 The percentage of patients with treatment-resistant bipolar or unipolar depression who are discharged taking 3 or more medications has increased from 3.3% between 1974 and 1979 to 43.8% between 1990 and 1995.27 Monotherapy is not always sufficient for acute or maintenance treatment, but, while combined medications can provide therapeutic synergy, they can also have additive adverse effects as well as drug interactions. Dr. Ketter emphasized that the FDA-approved combination therapies have been proven in rigorous trials to have efficacy in specific situations and have extensive safety data, whereas the efficacy and toxicity of other combinations lack such rigorous scientific support.
Monotherapy Versus Combination Therapy for Mania
Combination therapy appears to be more effective than monotherapy for acute mania. A review28 compared the response rates of patients with acute mania treated with monotherapy or combination therapy (Figure 2). Patients treated with monotherapy had a higher response rate than those who received placebo, and a higher response rate was found among the combination-therapy patients compared with patients receiving monotherapy. In both analyses, the addition of an active agent increased the response rate by approximately 20%.
Figure 2.
Monotherapy Versus Placebo or Combination Therapy for Acute Bipolar Mania: Response Rates in 20 Studiesa
aReprinted with permission from Ketter et al.28
Abbreviations: CBZ = carbamazepine, DVPX = divalproex, Li = lithium.
In a European study29 of patients with acute mania, treatment with antipsychotics such as haloperidol and/or perazine plus placebo was compared with treatment with these antipsychotics plus valproate sodium. The antipsychotic plus valproate combination produced more responders compared with antipsychotic monotherapy (70% vs. 46%; p = .005), according to improvement in Young Mania Rating Scale (YMRS) scores. This result indicates a comparable level of increased response with combination therapy compared with monotherapy as the previously described review.28
As shown in Table 1, the only combination treatments that have FDA approval for use in mania involve atypical antipsychotics. Dr. Ketter compared the efficacy of specific combinations using these 4 atypical antipsychotics for acute mania.
Olanzapine combination therapy has proved effective for acute mania.30 Adjunctive olanzapine in patients taking lithium or valproate was associatedwith significantly higher response rates than adjunctive placebo (67.7% vs. 44.7%, p < .001). Dr. Ketter noted that blood serum levels of lithium and valproate in the patients were slightly lower than is typically used in monotherapy. Therapeutic synergy between the agents may make lower concentrations of both medications possible and enhance tolerability. In contrast, a recent study31 found that adding olanzapine to carbamazepine failed to yield improved efficacy in patients with acute mania.
In a study by Sachs et al.,32 patients with a current manic or mixed episode treated with lithium or divalproex were given adjunctive risperidone, haloperidol, or placebo. Compared with placebo, the adjunctive risperidone and haloperidol showed approximately a 20% advantage in response rate according to Clinical Global Impressions ratings of much or very much improved; however, adverse events such as movement disorders were experienced by more patients taking haloperidol. Mean doses of risperidone and haloperidol were 3.8 mg/day and 6.2 mg/day, respectively. Dr. Ketter warned that higher doses of risperidone are associated with more extrapyramidal side effects and that the mood stabilizer carbamazepine decreases plasma risperidone concentrations by about 40%.33
In 2 double-blind, placebo-controlled studies,34 more patients treated for acute mania with lithium or divalproex and adjunctive quetiapine responded (≥ 50% decrease in YMRS score) than those who received adjunctive placebo (55.7% vs. 41.6%, p < .01) after 3 weeks. Dr. Ketter pointed out that the mean quetiapine dose in responders (approximately 500 mg/day) and serum mood stabilizer concentrations indicated that lower doses are effective with combination therapy. Also, quetiapine requires some titration, but many patients can tolerate a regimen that starts with 100 mg on the first day and increases by 100-mg increments each day to reach adequate dosage.
Aripiprazole monotherapy was found to be more effective than placebo in the treatment of acute manic or mixed episodes in patients with bipolar disorder.35 A statistically significantly higher response rate was found in patients treated with aripiprazole compared with placebo (40% vs. 19%). Aripiprazole has also been found to be effective in combination therapy with valproate or lithium in patients with treatment-resistant bipolar I disorder.36 Adjunctive aripiprazole produced significantly more responders by endpoint(6 weeks) than valproate or lithium plus placebo (62.8% vs. 48.5%, p < .01).
A small study37 of patients with treatment-resistant bipolar I disorder and schizoaffective disorder, bipolar type, with a history of mania found that adjunctive clozapine produced additional benefit on all measures except depression compared with treatment as usual. The dose of clozapine was much lower in patients with bipolar disorder than schizoaffective disorder. Over the 12-month study, medication use in patients who received adjunctive clozapine plus treatment as usual decreased. Dr. Ketter explained that clozapine does not have FDA approval for the treatment of bipolar disorder, as large placebo-controlled studies are currently lacking.
Monotherapy Versus Combination Therapy for Bipolar Depression
Although patients with bipolar disorder spend more time depressed than manic (see Figure 1),4,5 fewer treatments have FDA approval for bipolar depression than mania, noted Dr. Ketter (see Table 1). A review38 of placebo-controlled treatment trials in acute bipolar depression provided evidence of differential efficacy over placebo for various monotherapy and combination treatments (Figure 3). Dr. Ketter reiterated Dr. Nierenberg's observation that the role of adjunctive antidepressants is controversial in bipolar depression treatment.12
Figure 3.
Monotherapy Versus Combination Therapy for Acute Bipolar Depression: Response Rates in 4 Studiesa
aReprinted with permission from Sachs.38
The efficacy of augmentation with lamotrigine, inositol, or risperidone was examined in a STEP-BD study39 of 66 patients with bipolar depression who were resistant to treatment with a mood stabilizer (lithium, valproate, or carbamazepine) plus an antidepressant. Although recovery rates were not significantly different across treatments (perhaps because of the small sample size), lamotrigine yielded numerically greater recovery rates compared with inositol and risperidone (23.8%, 17.4%, and 4.6% respectively). Larger studies are needed.
Monotherapy Versus Combination Therapy for Bipolar Maintenance
Quetiapine combined with lithium or valproate was recently approved for bipolar maintenance treatment based on 2 recent 24-month, multicenter, randomized, double-blind, placebo-controlled studies40 of patients with bipolar I disorder with a recent manic, mixed, or depressive episode. Combining the results from these trials,40 adjunctive quetiapine (N = 646) compared with adjunctive placebo (N = 680) yielded significantly lower overall (19% vs. 51%), depressive (10% vs. 27%), and manic (9% vs. 23%) relapse rates (all p < .0001).
Safety and Tolerability
All of the approved treatments for bipolar disorder carry boxed warnings in the prescribing information about serious side effects. Safety and tolerability of monotherapy or combination therapy should be factored into treatment decisions for individual patients. For example, a study41 of the olanzapine-fluoxetine combination versus lamotrigine monotherapy for acute bipolar I depression found that, although the combination was more effective in reducing depression rating scale scores than lamotrigine monotherapy, it was associated with significantly greater weight gain and increase in total cholesterol and triglyceride levels (p ≤ .001).
Conclusion
Dr. Ketter stated that physicians have a growing choice of agents for the treatment of bipolar disorder. Monotherapy may suffice for some patients, but most will require combination treatments. Although combinations may offer therapeutic synergy, they also risk drug interactions and a wider range of adverse effects. When selecting pharmacotherapy for patients with bipolar disorder, clinicians should match individual agents to the patient's risk factors and ability to tolerate the medications. Finally, physicians should always exercise caution when treating patients with combination therapy.
Treatments for Mania: From Efficacy to Effectiveness
Roy H. Perlis, M.D., M.Sc., discussed the efficacy and tolerability of individual medications and considered how clinicians might choose between the available treatment options for bipolar mania.
Guidelines for Treating Mania
The American Psychiatric Association (APA) 2002 guidelines42 for the treatment of acute mania in patients with bipolar disorder recommended the combination of lithium or valproate and an atypical antipsychotic as first-line treatment for severely ill patients. In less severely ill patients, the recommended first-line treatment is monotherapy with lithium, valproate, or an atypical antipsychotic.42 Illness severity was not clearly defined, but severely ill patients usually require hospitalization while less ill patients can be managed as outpatients.
For patients who experience breakthrough manic symptoms during treatment, the APA guidelines recommended that the primary mood-stabilizing medication be optimized and then an atypical antipsychotic be added, if necessary. Dr. Perlis explained that optimizing a mood stabilizer—ensuring that the dose is in the therapeutic range or sometimes is at the higher end of the therapeutic range—is a common clinical practice and a standard recommendation. However, little evidence addresses the value of optimization.
In evidence-based guidelines, it is difficult to specify how clinicians should choose among treatment options across and within classes, which specific medication should be prescribed first, or how safety and tolerability issues should be weighed in selecting treatments during acute treatment for mania. Dr. Perlis remarked that more information is needed to answer these questions.
Efficacy
Dr. Perlis reviewed data for the efficacy of monotherapies for acute bipolar mania. A meta-analysis43 compared results from 12 placebo-controlled monotherapy trials of individual atypical antipsychotics in patients with acute manic or mixed episodes. Importantly, pooled data (Figure 4) showed that the atypical antipsychotics studied had similar efficacy, which was not substantially different from that of lithium or haloperidol. Dr. Perlis noted that individual differences in efficacy between these medications are likely to be modest. Atypical antipschoics may have differences in time to onset of action, but these possible differences are difficult to determine without direct comparisons.
Figure 4.
Monotherapy Efficacy Relative to Placebo in Acute Bipolar Mania: Pooled Trial Drug Effectsa–c
aReprinted with permission from Perlis et al.43
bBars represent 95% confidence intervals.
cThe dotted line on the left indicates the pooled difference from placebo among all monotherapy and combination trials.
Abbreviation: YMRS = Young Mania Rating Scale.
Newer anticonvulsants have attracted interest for the treatment of bipolar disorder. Unfortunately, a review44 of these drugs found that none have shown significant efficacy for treating mania, although some have benefit in treating other aspects of bipolar disorder. Adequately powered trials of topiramate, lamotrigine, and gabapentin were negative, and smaller trials of tiagabine and zonisamide did not suggest efficacy. Studies45 have demostrated that carbamazepine extended-release is superior to placebo for the treatment of acute manic or mixed episodes, but the carbamazepine derivative oxcarbazepine showed no benefit in a large study46 in children and adolescents with manic or mixed states.
Few head-to-head studies of newer agents for mania exist to help clinicians distinguish between them for efficacy. Olanzapine and risperidone were compared in a 3-week trial47 in hospitalized patients with nonpsychotic acute manic or mixed episodes. No discernible difference in YMRS scores was found between risperidone and olanzapine. Olanzapine and divalproex were compared in 2 head-to-head studies48,49: one48 suggested a modest but significant difference in efficacy favoring olanzapine (p = .03), and the second study,49 which was not powered to show a significant difference, suggested a small numericdifference in efficacy in favor of olanzapine.
Tolerability
Efficacy cannot be the only consideration in medication choice, especially when drugs appear to have similar efficacy. Dr. Perlis advised that tolerability and safety are important factors in drug effectiveness; drugs that have efficacy but not tolerability are not effective or clinically useful.
Dropout rates in clinical trials give an indication of tolerability. A meta-analysis50 of 26 studies of atypical antipsychotics in patients with acute mania (N = 6187) found that, among monotherapy trials, dropout rates for the atypical antipsychotics aripiprazole, olanzapine, quetiapine, risperidone, and ziprasidone were similar to or less than dropout rates for the placebo-treated groups. Rates of dropout due to adverse events were similar not only among the atypical antipsychotics but also in comparison with lithium and divalproex, despite differences in specific side effect profiles. However, the dropout rate due to side effects was higher for haloperidol than the atypical antipsychotics, a noteworthy point according to Dr. Perlis, because the APA's recommendation42 in favor of atypical rather than typical antipsychotics has been questioned. When tolerability data are considered, atypical antipsychotics may have an advantage in effectiveness compared with typical antipsychotics, a possibility that merits direct study in bipolar disorder.
Extrapyramidal symptoms (EPS) are often a concern in the short-term treatment of mania. Dr. Perlis commented that, while the rates of EPS are generally lower with atypical than with typical antipsychotics, some variation occurs within the class. Scherk and colleagues' meta-analysis50 showed increased EPS in patients treated with atypical antipsychotics compared with placebo-treated patients, but the risk varied between medications. The incidence of EPS in patients taking haloperidol was greater than with atypical antipsychotics, either individually or as a group (p < .001). Dr. Perlis reminded clinicians that, since treatment for acute mania is increasingly continued as maintenance treatment, consideration should be given to long-term adverse effects.
Conclusion
Dr. Perlis concluded that the efficacy data across the drugs with FDA-approval for treatment of acute mania do not indicate a clear best choice. Therefore, clinicians should consider other criteria, such as time to onset of action, tolerability, and long-term adverse effects of the agents, in the context of the needs and concerns of the individual patient. All of these factors contribute to the effectiveness of the treatment.
New Understanding of Mechanisms of Action of Bipolar Medications
The classic model of mood disorder pathophysiology attributed the disorders to neurochemical deficits in the monoamine neurotransmitters norepinephrine and/or serotonin. Gerard Sanacora, M.D., Ph.D., explained that the model has been reconceptualized to one in which mood disorders are attributed to changes in neuroplasticity. Neuroplasticity is the lifelong ability of the brain to reorganize itself by forming new neural networks and connections. This ability allows neurons in the brain to adjust their activities in response to new situations or environmental changes and to compensate for injury and disease.
Concepts related to neuroplasticity are apoptosis, neuroprotection, neurotoxicity, resiliency, and neurotrophic factors. Apoptosis is a form of programmed cell death in multicellular organisms, and anything that prevents apoptosis is called antiapoptotic. Mechanisms within the nervous system that protect neurons and glia from apoptosis and other degeneration (including that associated with injury or neurodegenerative diseases) provide neuroprotection. Events or treatments that damage the nervous system have neurotoxicity. Resiliency is the brain's ability to withstand or recover from cellular stressors. Substances called neurotrophic factors are responsible for the growth and survival of neurons during childhood development and maintenance of neurons in adulthood.
Evidence of Neuroplastic Changes in Patients With Mood Disorders
Imaging studies have shown that structural and neuroplastic changes occur in the brains of patients with mood disorders. Reduced metabolic activity and cortical volume in the subgenual prefrontal cortex was found in patients with bipolar or unipolar depression.51 Reduced hippocampal volume was shown in patients with unipolar depression.52 Reductions in neuronal and glial cell volume and density in the dorsolateral prefrontal cortex were found in patients with bipolar disorder.53 Glial cell density and neuronal size were reduced in patients with major depressive disorder (MDD).54 Similar reductions were reported in patients with schizophrenia, but no difference was found in patients with bipolar disorder compared with control subjects.54 Dr. Sanacora observed that, of particular relevance for mood disorders, reduced levels of brain-derived neurotrophic factor (BDNF) have been found at autopsy in the hippocampus and prefrontal cortex55,56 of suicide victims and in serum of live depressed patients.57
Effects of Stress on Neuroplasticity
Major depressive episodes are known to be triggered by stressful life events.58 Observing the effects of stress on the brain forms a model for describing the effects of depression on the brain, stated Dr. Sanacora. Stress has been shown to cause neuroplastic changes by inducing atrophy of pyramidal neurons in the medial prefrontal cortex of animals.59 Chronic psychosocial stress also decreased hippocampal volume and reduced the size and number of glial cells in animals,60 similar to the changes seen in several brain regions of patients with MDD.54
Neurobiological Mechanisms Affected by Stress and Targeted by Mood Disorder Treatment
Chronic stress can affect cellular mechanisms and processes, causing harmful neuroplastic changes.61 Normally, activation of receptors found on the cellular membrane leads to stimulation of signal transduction cascades, the mechanism by which cells transcribe their effects from the synaptic membrane into the cytosol inside the cell. These cascades can lead to the phosphorylation of kinases and enzymes that have multiple effects on the brain. Activation of some signal transduction cascades can activate kinases that lead to apoptosis or neurotoxicity; activation of other cascades can activate kinases or enzymes that are antiapoptotic. A healthy brain maintains a delicate balance of apoptotic and antiapoptotic activity, and this balance of plasticity is constantly changed throughout life by the activation of membrane receptors. Processes inside the nucleus, including gene expression and chromatin remodeling, can also be affected by these cascades.
Chronic stress can have a negative effect on the brain by attacking several different mechanisms. Understanding those mechanisms can aid in the development and use of drugs that act in various ways to counteract the effects of stress, thereby treating mood disorders.62,63 Dr. Sanacora proceeded to describe systems thought to be affected by stress and therefore may be treatment targets.
Glutamatergic system.
The glutamatergic system is believed to be a treatment target because it is heavily affected by stress. Glutamate is the major excitatory neurotransmitter in the brain; it is necessary for most forms of learning and is involved in most aspects of human behavior. Excessive stimulation of glutamate leads to increased calcium influx, which ultimately leads to an increase in free radicals, increased expression of apoptotic factors, and cell damage and atrophy. Research has shown that stress can cause an increase in glutamate release in the prefrontal cortex64 and hippocampus,65 potentially causing neurotoxicity in both of these areas.66 Some agents that target the glutamatergic system and may help balance glutamate release are N-methyl-D-aspartate (NMDA) antagonists such as ketamine, group I metabotropic antagonists, group II metabotropic modulators, agents that act on the voltage-dependent neuronal sodium channels that modulate glutamate release such as lamotrigine, agents that activate the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors, and agents that increase glutamate uptake.
Glucocorticoid system.
Stress also affects the glucocorticoid system, specifically the hypothalamic-pituitary-adrenal axis, one of the central stress-response systems. Activation of the cortisol glucocorticoid receptor has direct effects on gene expression, leading to many apoptotic pathways. Several drugs are now thought to modulate this effect, in 3 possible ways. First, glucocorticoid antagonists such as mifepristone are thought to directly antagonize the effects of glucocorticoid at various sites both inside and outsidethe brain. Other agents such as metyrapone are thought to affect the synthesis of glucocorticoids and may have some effects on mood. Third,corticotropin-releasing factor antagonists seem to block the effect of corticotropin-releasing hormone within the brain and regulate the release in the adrenal gland through activation of the pituitary gland.
Monoaminergic system.
The monoaminergic system has long been considered a main source of stress-triggered adverse events such as mood disorders. The regulation of monoamine transmission can be targeted by the classic monoaminergic agents, such as selective serotonin reuptake inhibitors, tricyclic antidepressants, monoamine oxidase inhibitors, and atypical antipsychotics. The downstream signal transduction cascades associated with these neurotransmitter systems may also be targets using a phosphodiesterase inhibitor like rolipram.
Neurotrophic factor system.
The neurotrophic factor system, specifically BDNF, is also affected by stress and is a target for treatment. Activation of BDNF leads to antiapoptotic activity that is involved in cell maintenance and survival. Increases in BDNF expression in the hippocampus have been shown in animals and humans with antidepressant agents, exercise, and electroconvulsive therapy (ECT).67,68 Antidepressant treatment has also been shown to increase serum BDNF levels.69
Apoptotic and antiapoptotic kinases.
Stress affects the brain through apoptotic and antiapoptotic kinases or enzymes. The apoptotic glycogen synthase kinase (GSK-3β) and the antiapoptotic protein Bcl-2 have gained prominence in the field in relation to mood disorders. Two of the most clinically useful drugs in treating bipolar disorder—lithium and valproic acid—inhibit GSK-3β activity and increase Bcl-2 levels in the frontal cortex.70,71
Gene expression.
While all of the targeted mechanisms described above can affect gene expression, gene expression can also be affected in the long term by change within the nucleus. Chromatin remodeling within the nucleus can chronically alter gene expression through histone acetylation and methylation, changing the structure of the DNA and determining which genes are expressed and which are silenced. This process might explain how early childhood trauma could have long-lasting consequences into adulthood. Valproic acid72 and ECT73 are known to have effects on histone acetylation and may, therefore, target this mechanism.
Stress.
Finally, Dr. Sanacora emphasized that a target of treatment for mood disorders is stress itself. The direct effects of stress or perceived stress may be modulated with psychotherapies such as cognitive-behavioral therapy, interpersonal therapy, and social rhythm therapy. Exercise has many effects consistent with neuroresiliency that also may be useful in directly treating stress and providing antidepressant effects.
Conclusion
Neuroplastic changes in the brain are believed to play a major role in the pathogenesis and pathophysiology of mood disorders, summarized Dr. Sanacora. The conceptualization of mood disorders in terms of neuroplastic mechanisms represents a break from the classic psychiatric model of neurochemical deficits. Using the neuroplasticity model of mood disorders, specific neurobiological mechanisms can be targeted for treatment.
Selecting Appropriate Maintenance Treatments for Bipolar Disorder
The long-term management of bipolar disorder is a crucial part of treatment, said Michael E. Thase, M.D., because no curative treatments exist. The medications available can be seen as symptom-controlling or illness-suppressing.
Ideal treatments for long-term management of bipolar disorder would effectively treat and delay recurrence of both manic and depressive episodes. Preventing early relapse may lead to a more benign course of illness, so long-term treatment should be considered after a single severe manic episode.42 Recurrences of the illness devastate patients' working and home lives, increase the risk of suicide, and affect health.74
Patients need to understand that abrupt discontinuation of, or nonadherence to, medication can increase the risk of recurrence and that treatment is long-term.42 Clinicians may be able to help patients accept treatment by calling it indefinite rather than lifelong because lifelong treatment may become unnecessary in the future as genetic vulnerabilities and gene-environment interactions are better understood and more curative treatments are developed.
Treatment Options
Medications used to initiate treatment may be continued as maintenance therapy, or different treatment may be used in the maintenance phase than was used acutely. Dr. Thase reminded clinicians that the polarity of the index episode tends to predict the polarity of the next episode,6 which he suggested could reflect that, in the modern era, relapse of the index episode is more common than recurrence. He then summarized potential maintenance treatment options, although not all have FDA approval for maintenance (see Table 1).
Lithium.
Dr. Thase stated that the mood stabilizer lithium has been shown to be about 20% more effective in the prevention of bipolar relapse than placebo, but, as Dr. Nierenberg noted, lithium is more effective in preventingrelapse to mania than relapse to depression.75 Although lithium is a good example of a treatment that can be initiated for acute treatment and continued as maintenance treatment, it has a relatively narrow therapeutic window, and some patients find lithium therapy hard to maintain over months or years of preventive therapy. A naturalistic study76 of 402 patients found that after 5 years of treatment, 27.9% of patients had stopped taking lithium, 38.1% still took lithium but had experienced at least 1 relapse, and 23.4% continued to take lithium with no recurrences; 10.7% were not available for interview at 5-year follow-up. Among patients with therapeutic blood levels of lithium, 88.0% spent at least 50% less time in the hospital yearly than they did pretreatment, and 43.0% of these patients had no recurrences of illness.76
Valproate.
Since its approval for treatment of acute mania in bipolar disorder, valproate has overtaken lithium in terms of market share, even though valproate is not formally indicated for recurrence prevention. For example, the ratio of initial filled prescriptions for lithium versus divalproex changed from about 6:1 in 1994 to about 1:2 in 2001.77 However, whereas the prophylactic effects of lithium for prevention of suicidal behaviors is well accepted, the data are less certain for valproate and, in one study,77 the risk of suicide attempt or death was 1.5 to 3 times higher in patients treated with divalproex than it was in those treated with lithium.
Atypical antipsychotics.
Dr. Thase noted that, since the introduction of atypical antipsychotics, clinicians have moved away from monotherapy for both short-term therapy and long-term prophylaxis. Among patients treated for 1 year in the Stanley Foundation Bipolar Network, a mean number of 4.1 psychotropic medications was taken.78 Atypical antipsychotics are increasingly added to mood stabilizers early in treatment for breakthrough mania.
Olanzapine was the first atypical antipsychotic to be FDA-approved for maintenance treatment of bipolar disorder (see Table 1). In a 47-week study,48 previously manic patients treated with olanzapine or divalproex showed no significant difference in rates of relapse into mania or depression (42.3% vs. 56.5%, respectively).
An 18-month study79 of relapse prevention in patients with bipolar I disorder compared olanzapine in combination with lithium or valproate to treatment with lithium or valproate monotherapy; no significant differences were found in the rates of syndromic or symptomatic relapse to either mania or depression or in time to syndromal relapse to either mania or depression. However, treatment with combination therapy significantly increased time to symptomatic relapse (monotherapy, 42 days; combination therapy, 163 days; p = .023). If a patient taking a mood stabilizer experiences relapse, adding an antipsychotic might be clinically useful.
Aripiprazole is also an approved maintenance treatment. In a 100-week relapse prevention study,80 patients who were originally stabilized on aripiprazole treatment (over 26 weeks) were randomly assigned to aripiprazole or placebo for maintenance therapy (over 74 weeks). Aripiprazole monotherapy was found effective for relapse prevention and maintained good tolerability and safety profiles.
In 2008, quetiapine received FDA approval for use in combination with the conventional mood stabilizers lithium and valproate on the basis of 2 controlled preventive studies.40
Clinicians need to be vigilant for side effects of antipsychotics, note Dr. Thase. Besides EPS, weight gain and other signs of metabolic syndrome should also be monitored. Early in the course of therapy, a baseline waist size and fasting glucose level should be established. Vigorous early interventionfor weight gain as little as 5 pounds within a few weeks can improve outcomes.
Antidepressants.
Long-term antidepressant therapy may be effective for a subset of patients. A study81 of 84 patients successfully treated for acute bipolar depression with a mood stabilizer and adjunctive antidepressant followed the patients for 1 year. Of these patients, 43 (51%) stopped antidepressant treatment within 6 months after remission. Depressive relapse occurred in 70% of patients who discontinued antidepressant treatment within 6 months and in 36% of patients who continued antidepressant treatment beyond 6 months.
Lamotrigine.
Lamotrigine is the only agent approved for maintenance treatment in bipolar disorder that is not also approved for acute phase therapy. Compared with placebo, lamotrigine showed significant (p = .034) efficacy for prevention of manic episodes and a more robust effect in prevention of depressive episodes (p = .009; Figure 5).82 Dr. Thase noted that although lamotrigine is relatively weak for the prevention of mania, it is an important medication to consider for long-term prevention when depression prophylaxis is the primary goal. Limitations of lamotrigine include lack of approval for acute phase therapy and a relatively high risk of dermatologic reactions that can lead to severe systemic conditions such as Stevens-Johnson syndrome. To minimize this risk, lamotrigine should be started at a low dose and slowly titrated upwards.83 Combinations with other medications such as valproate that might result in higher blood lamotrigine levels should be used with caution.
Figure 5.
Time to Intervention for (A) Depressive Episode and (B) Manic, Hypomanic, or Mixed Episode With Lamotrigine, Lithium, and Placeboa
aReprinted with permission from Goodwin et al.82
bLamotrigine vs. placebo, p = .009; lithium vs. placebo, p = .120; lamotrigine vs. lithium, p = .325.
cLamotrigine vs. placebo, p = .034; lithium vs. placebo, p < .001; lamotrigine vs. lithium, p = .030.
Factors that Contribute to Relapse
Several factors appear to contribute to relapse of bipolar episodes. A large study over 2 years84 showed that 34.7% of subjects had a depressive relapse, while 13.8% had a manic, hypomanic, or mixed episode. Each residual manic symptom at recovery increased the risk of depressive relapse by about 20%. Dr. Thase suggested that clinicians be alert for subtle mixed states as targets for ongoing treatment.
Obesity is another risk factor for failure of preventive treatment.85 Patients with obesity experienced shorter times to depressive recurrence and higher rates of recurrence than patients without obesity. Dr. Thase remarked that obesity may interfere with antidepressant treatment, higher doses of medication may be needed, and lifestyle adjustments associated with obesity may have a depressing effect.
Summary
Dr. Thase reiterated the importance of helping patients with bipolar disorder stay well after initial treatment and of maximizing patients' functional capacity. Several medications are available for maintenance treatment, and clinicians should consider the individual patient characteristics when selecting therapies. Dr. Thase stressed that psychoeducation and focused psychotherapy should not be overlooked. Clinicians should also monitor the patient for long-term treatment safety and tolerability.
Drug names: aripiprazole (Abilify), bupropion (Aplenzin, Wellbutrin, and others), carbamazepine (Carbatrol, Equetro, and others), clozapine (FazaClo, Clozaril, and others), divalproex (Depakote and others), gabapentin (Neurontin and others), haloperidol (Haldol and others), imipramine (Tofranil and others), ketamine (Ketlar and others), lamotrigine (Lamictal and others), lithium (Eskalith, Lithobid, and others), metyrapone (Metopirone), mifepristone (Mifeprex), olanzapine (Zyprexa), olanzapine-fluoxetine (Symbyax), oxcarbazepine (Trileptal and others), paroxetine (Paxil, Pexeva, and others), quetiapine (Seroquel and others), risperidone (Risperdal and others), tiagabine (Gabitril), topiramate (Topamax), valproate sodium (Depacon and others), valproic acid (Depakene and others), ziprasidone (Geodon), zonisamide (Zonegran and others).
Disclosure of off-label usage: The chair has determined that, to the best of his knowledge, gabapentin, ketamine, oxcarbazepine, tiagabine, topiramate,and zonisamide are not approved by the U.S. Food and Drug Administration for the treatment of bipolar disorder.
Financial disclosure: Dr. Nierenberg has received research support from Bristol-Myers Squibb, Cederroth, Cyberonics, Forest, Eli Lilly, GlaxoSmithKline, Janssen, Lichtwer, the National Alliance for Research on Schizophrenia and Depression (NARSAD), the National Institute of Mental Health (NIMH), Pfizer, the Stanley Foundation, and Wyeth-Ayerst; is a member of the speakers bureaus for Bristol-Myers Squibb, Cyberonics, Forest, Eli Lilly, GlaxoSmithKline, and Wyeth-Ayerst; and is a consultant or a member of the advisory board for Abbott, BrainCells, Bristol-Myers Squibb, Cederroth, Eli Lilly, GlaxoSmithKline, Genaissance, Innapharma, Janssen, Novartis, Pfizer, Sepracor, Shire, and Somerset. Dr. Ketter is a consultant for Abbott, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, Janssen, Jazz, Novartis, Solvay, and Wyeth; has received grant/research support from Abbott, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, GlaxoSmithKline, Pfizer, Repligen, and Wyeth; and has received lecture honoraria from Abbott, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, and GlaxoSmithKline; and his spouse/partner is an employee of Johnson & Johnson. Dr. Perlis is a consultant for, has received grant/research support and honoraria from, and is a member of the speakers/advisory boards for Eli Lilly, Pfizer, Bristol-Myers Squibb, GlaxoSmithKline, AstraZeneca, and Proteus. Dr. Sanacora is a consultant for AstraZeneca, Sepracor, Roche, and Ruxton; has received grant/research support from NIMH, NARSAD, the Donahue Foundation, Yale/Pfizer Imaging Alliance, the Stanley Foundation, AstraZeneca, Sepracor, and Ruxton; has received honoraria from Abbott, AstraZeneca, Bristol-Myers Squibb, Lundbeck, and Sepracor; has given expert testimony for Shook, Hardy & Bacon; and has received patents for US patent application PCTWO06108055A1. Dr. Thase is an advisor or consultant for AstraZeneca, Bristol-Myers Squibb, Cephalon, Cyberonics, Eli Lilly, GlaxoSmithKline, Janssen, MedAvante, Neuronetics, Novartis, Organon, Sepracor, Shire, Supernus, and Wyeth; is a member of the speakers bureaus for AstraZeneca, Bristol-Myers Squibb, Cyberonics, Eli Lilly, GlaxoSmithKline, Organon, Sanofi Aventis, and Wyeth; has given expert testimony for Jones Day (Wyeth litigation) and Phillips Lytle (GlaxoSmithKline litigation); has equity holdings in MedAvante; and has received royalties from American Psychiatric Publishing, Inc., Guilford Publications, and Herald House; currently has research funding from the National Institute of Mental Health, Eli Lilly, Sepracor, and GlaxoSmithKline; and his spouse/partner is an employee of Advogent.
Pretest and Objectives

Instructions and Posttest

Registration Form

Footnotes
This ACADEMIC HIGHLIGHTS section of The Primary Companion to The Journal of Clinical Psychiatry presents the highlights of the planning teleconference series “Easing the Burden of Bipolar Disorder: From Urgent Situations to Remission,” which was held in February and March 2008. This report was prepared by the CME Institute of Physicians Postgraduate Press, Inc., and was supported by an educational grant from Eli Lilly and Company.
The planning teleconference series was chaired by Andrew A. Nierenberg, M.D., from the Depression Clinical and Research Program and the Bipolar Clinic and Research Program, Massachusetts General Hospital and Harvard Medical School, Boston. The faculty were Terence A. Ketter, M.D., from the Department of Psychiatry and Behavior Sciences and the Bipolar Disorders Clinic, Stanford University School of Medicine, Stanford, Calif.; Roy H. Perlis, M.D., M.Sc., from the Department of Psychiatry, Harvard Medical School, and the Bipolar Clinic and Research Program, Massachusetts General Hospital, Boston; Gerard Sanacora, M.D., Ph.D., from the Yale Depression Research Program, Department of Psychiatry, Yale University School of Medicine, New Haven, Conn.; and Michael E. Thase, M.D., from the Department of Psychiatry, the University of Pennsylvania School of Medicine, the Philadelphia Veterans Affairs Medical Center, and the University of Pittsburgh Medical Center, Philadelphia and Pittsburgh, Pa. Financial disclosure appears at the end of this article.
The opinions expressed herein are those of the faculty and do not necessarily reflect the views of the CME provider and publisher or the commercial supporter.
REFERENCES
- 1.Kleinman NL, Brook RA, Rajagopalan K, et al. Lost time, absence costs, and reduced productivity output for employees with bipolar disorder. J Occup Environ Med. 2005;47:1117–1124. doi: 10.1097/01.jom.0000177048.34506.fc. [DOI] [PubMed] [Google Scholar]
- 2.Tohen M, Zarate CA, Jr, Hennen J, et al. The McLean-Harvard First-Episode Mania Study: prediction of recovery and first recurrence. Am J Psychiatry. 2003;160:2099–2107. doi: 10.1176/appi.ajp.160.12.2099. [DOI] [PubMed] [Google Scholar]
- 3.Judd LL, Akiskal HS, Schettler PJ, et al. Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Arch Gen Psychiatry. 2005;62:1322–1330. doi: 10.1001/archpsyc.62.12.1322. [DOI] [PubMed] [Google Scholar]
- 4.Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 5.Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60:261–269. doi: 10.1001/archpsyc.60.3.261. [DOI] [PubMed] [Google Scholar]
- 6.Calabrese JR, Vieta E, El-Mallackh R, et al. Mood state at study entry as predictor of the polarity of relapse in bipolar disorder. Biol Psychiatry. 2004;56:957–963. doi: 10.1016/j.biopsych.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 7.Wang PS, Berglund P, Olfson M, et al. Failure and delay in initial treatment contact after first onset of mental disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:603–613. doi: 10.1001/archpsyc.62.6.603. [DOI] [PubMed] [Google Scholar]
- 8.Ketter TA. Washington, DC: American Psychiatric Publishing; 2005. Advances in Treatment of Bipolar Disorder: Review of Psychiatry, vol 24, no 3; p. 2. [Google Scholar]
- 9.Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry. 2007;64:419–426. doi: 10.1001/archpsyc.64.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gijsman HJ, Geddes JR, Rendell JM, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry. 2004;161:1537–1547. doi: 10.1176/appi.ajp.161.9.1537. [DOI] [PubMed] [Google Scholar]
- 11.Nemeroff CB, Evans DL, Gyulai L, et al. Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry. 2001;158:906–912. doi: 10.1176/appi.ajp.158.6.906. [DOI] [PubMed] [Google Scholar]
- 12.Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;356:1711–1722. doi: 10.1056/NEJMoa064135. [DOI] [PubMed] [Google Scholar]
- 13.American Psychiatric Association. Fourth Edition. Washington, DC: American Psychiatric Association; 1994. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 14.Young LT, Joffe RT, Robb JC, et al. Double-blind comparison of addition of a second mood stabilizer versus an antidepressant to an initial mood stabilizer for treatment of patients with bipolar depression. Am J Psychiatry. 2000;157:124–126. doi: 10.1176/ajp.157.1.124. [DOI] [PubMed] [Google Scholar]
- 15.Zornberg GL, Pope HG. Treatment of depression in bipolar disorder: new directions for research. J Clin Psychopharmacol. 1993;13:397–408. [PubMed] [Google Scholar]
- 16.Calabrese JR, Bowden CL, Sachs GS, et al. for the Lamictal 602 Study Group A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry. 1999;60:79–88. doi: 10.4088/jcp.v60n0203. [DOI] [PubMed] [Google Scholar]
- 17.Calabrese JR, Huffman RF, White RL, et al. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disord. 2008;10:323–333. doi: 10.1111/j.1399-5618.2007.00500.x. [DOI] [PubMed] [Google Scholar]
- 18.Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Correction. Arch Gen Psychiatry. 2003;2004;6061:1079–1088. 176. doi: 10.1001/archpsyc.60.11.1079. [DOI] [PubMed] [Google Scholar]
- 19.Calabrese JR, Keck PE, Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005;162:1351–1360. doi: 10.1176/appi.ajp.162.7.1351. [DOI] [PubMed] [Google Scholar]
- 20.Muzina DJ, Calabrese JR. Maintenance therapies in bipolar disorder: focus on randomized controlled trials. Aust N Z J Psychiatry. 2005;39:652–661. doi: 10.1080/j.1440-1614.2005.01649.x. [DOI] [PubMed] [Google Scholar]
- 21.Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Arch Gen Psychiatry. 2000;57:481–489. doi: 10.1001/archpsyc.57.5.481. [DOI] [PubMed] [Google Scholar]
- 22.Bowden CL, Calabrese JR, Sachs G, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Correction. Arch Gen Psychiatry. 2003;2004;60:392–400. 61–680. doi: 10.1001/archpsyc.60.4.392. [DOI] [PubMed] [Google Scholar]
- 23.Calabrese JR, Bowden CL, Sachs G, et al. for the Lamictal 605 Study Group. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry. 2003;64:1013–1024. doi: 10.4088/jcp.v64n0906. [DOI] [PubMed] [Google Scholar]
- 24.Tohen M, Greil W, Calabrese JR, et al. Olanzapine versus lithium in the maintenance treatment of bipolar disorder: a 12-month, randomized, double-blind, controlled clinical trial. Am J Psychiatry. 2005;162:1281–1290. doi: 10.1176/appi.ajp.162.7.1281. [DOI] [PubMed] [Google Scholar]
- 25.Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders: a systematic review of randomized trials. Am J Psychiatry. 2005;162:1805–1819. doi: 10.1176/appi.ajp.162.10.1805. [DOI] [PubMed] [Google Scholar]
- 26.Ketter TA, Sachs GS, Bowden CL, et al. Introduction. In: Ketter TA, editor. Advances in Treatment of Bipolar Disorder: Review of Psychiatry, vol 24, no 3. Washington, DC: American Psychiatric Publishing; 2005. pp. 1–9. [Google Scholar]
- 27.Frye MA, Ketter TA, Leverich GS, et al. The increasing use of polypharmacotherapy for refractory mood disorders: 22 years of study. J Clin Psychiatry. 2000;61:9–15. doi: 10.4088/jcp.v61n0104. [DOI] [PubMed] [Google Scholar]
- 28.Ketter TA, Wang PW, Nowakowska C, et al. Treatment of acute mania in bipolar disorder. In: Ketter TA, editor. Advances in Treatment of Bipolar Disorder: Review of Psychiatry; vol 24, no 3. Washington, DC: American Psychiatric Publishing; 2005. pp. 11–55. [Google Scholar]
- 29.Muller-Oerlinghausen B, Retzow A, Henn FA, et al. Valproate as an adjunct to neuroleptic medication for the treatment of acute episodes of mania: a prospective, randomized, double-blind, placebo-controlled, multicenter study. J Clin Psychopharmacol. 2000;20:195–203. doi: 10.1097/00004714-200004000-00012. [DOI] [PubMed] [Google Scholar]
- 30.Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry. 2002;59:62–69. doi: 10.1001/archpsyc.59.1.62. [DOI] [PubMed] [Google Scholar]
- 31.Tohen M, Bowden CL, Smulevich AB, et al. Olanzapine plus carbamazepine versus carbamazepine alone in treating manic episodes. Br J Psychiatry. 2008;192:135–143. doi: 10.1192/bjp.bp.107.041301. [DOI] [PubMed] [Google Scholar]
- 32.Sachs GS, Grossman F, Ghaemi SN, et al. Combination of a mood stabilizer with risperidone or haloperidol for treatment of acute mania: a double-blind, placebo-controlled comparison of safety and efficacy. Am J Psychiatry. 2002;159:1146–1154. doi: 10.1176/appi.ajp.159.7.1146. [DOI] [PubMed] [Google Scholar]
- 33.Yatham LN, Grossman F, Augustyns I, et al. Mood stabilisers plus risperidone or placebo in the treatment of acute mania: international, double-blind, randomized controlled trial. Br J Psychiatry. 2003;182:141–147. doi: 10.1192/bjp.182.2.141. [DOI] [PubMed] [Google Scholar]
- 34.Yatham LN, Paulsson B, Mullen J, et al. Quetiapine versus placebo in combination with lithium or divalproex for the treatment of bipolar mania. J Clin Psychopharmacol. 2004;24:599–606. doi: 10.1097/01.jcp.0000144887.66319.2f. [DOI] [PubMed] [Google Scholar]
- 35.Keck PE, Jr, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry. 2003;160:1651–1658. doi: 10.1176/appi.ajp.160.9.1651. [DOI] [PubMed] [Google Scholar]
- 36.Vieta E, T'joen C, McQuade R, et al. Efficacy of adjunctive aripiprazole to either valproate or lithium in bipolar mania patients partially nonresponsive to valproate/lithium monotherapy: a placebo-controlled study [published online ahead of print May 9, 2008] Am J Psychiatry. 2008 doi: 10.1176/appi.ajp.2008.07101560. [DOI] [PubMed] [Google Scholar]
- 37.Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and history of mania. Am J Psychiatry. 1999;156:1164–1169. doi: 10.1176/ajp.156.8.1164. [DOI] [PubMed] [Google Scholar]
- 38.Sachs GS. Treatment of acute depression in bipolar disorder. In: Ketter TA, editor. Advances in Treatment of Bipolar Disorder: Review of Psychiatry, vol 24, no 3. Washington, DC: American Psychiatric Publishing; 2005. pp. 57–109. [Google Scholar]
- 39.Nierenberg AA, Ostacher MJ, Calabrese JR, et al. Treatment-resistant bipolar depression: a STEP-BD equipoise randomized effectiveness trial of antidepressant augmentation with lamotrigine, inositol, or risperidone. Am J Psychiatry. 2006;163:210–216. doi: 10.1176/appi.ajp.163.2.210. [DOI] [PubMed] [Google Scholar]
- 40.Brecher M, Anderssen H, Paulsson B. Third Biennial International Conference on Bipolar Disorder. suppl 1. Vol. 10. Delhi, India: Bipolar Disorders; Jan–30. 2008. 2008. Quetiapine in the maintenance treatment in bipolar I disorder: combined data from two long-term phase III studies [abstract] p. 40. [Google Scholar]
- 41.Brown EB, McElroy SL, Keck PE, Jr, et al. A 7-week, randomized, double-blind trial of olanzapine/fluoxetine combination versus lamotrigine in the treatment of bipolar I depression. J Clin Psychiatry. 2006;67:1025–1033. doi: 10.4088/jcp.v67n0703. [DOI] [PubMed] [Google Scholar]
- 42.American Psychiatric Association. Practice Guideline for the Treatment of Patients With Bipolar Disorder [Revision] Am J Psychiatry. 2002;159(suppl 4):1–50. [PubMed] [Google Scholar]
- 43.Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67:509–516. doi: 10.4088/jcp.v67n0401. [DOI] [PubMed] [Google Scholar]
- 44.Yatham LN, Kusumakar V, Calabrese JR, et al. Third generation anticonvulsants in bipolar disorder: a review of efficacy and summary of clinical recommendations. J Clin Psychiatry. 2002;63:275–283. doi: 10.4088/jcp.v63n0402. [DOI] [PubMed] [Google Scholar]
- 45.Weisler RH, Hirschfeld R, Cutler AJ, et al. Extended-release carbamazepine capsules as monotherapy in bipolar disorder: pooled results from two randomised, double-blind, placebo-controlled trials. CNS Drugs. 2006;20:219–231. doi: 10.2165/00023210-200620030-00004. [DOI] [PubMed] [Google Scholar]
- 46.Wagner KD, Kowatch RA, Emslie GJ, et al. A double-blind, randomized, placebo-controlled trial of oxcarbazepine in the treatment of bipolar disorder in children and adolescents. Erratum. Am J Psychiatry. Am J Psychiatry. 2006;2006;163163:1179–1186. 1843. doi: 10.1176/ajp.2006.163.7.1179. [DOI] [PubMed] [Google Scholar]
- 47.Perlis RH, Baker RW, Zarate CA, et al. Olanzapine versus risperidone in the treatment of manic or mixed states in bipolar I disorder: a randomized, double-blind trial. J Clin Psychiatry. 2006;67:1747–1753. doi: 10.4088/jcp.v67n1112. [DOI] [PubMed] [Google Scholar]
- 48.Tohen M, Ketter T, Zarate C, et al. Olanzapine versus divalproex sodium for the treatment of acute mania and maintenance of remission: a 47-week study. Am J Psychiatry. 2003;160:1263–1271. doi: 10.1176/appi.ajp.160.7.1263. [DOI] [PubMed] [Google Scholar]
- 49.Zajecka JM, Weisler R, Sachs G, et al. A comparison of the efficacy, safety, and tolerability of divalproex sodium and olanzapine in the treatment of bipolar disorder. J Clin Psychiatry. 2002;63:1148–1155. doi: 10.4088/jcp.v63n1210. [DOI] [PubMed] [Google Scholar]
- 50.Scherk H, Pajonk FG, Leucht S. Second-generation antipsychotic agents in the treatment of acute mania: a systematic review and meta-analysis of randomized controlled trials. Arch Gen Psychiatry. 2007;64:442–455. doi: 10.1001/archpsyc.64.4.442. [DOI] [PubMed] [Google Scholar]
- 51.Drevets WC, Price JL, Simpson JR, Jr., et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 52.Videbach P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 53.Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 54.Cotter D, Mackay D, Landau S, et al. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- 55.Dwivedi Y, Rizavi HS, Conley RR, et al. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 56.Karege F, Vaudan G, Schwald M, et al. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 57.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications [published online ahead of print June 19, 2008] Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- 59.Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci U S A. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Czéh B, Simon M, Schmelting B, et al. Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology. 2006;31:1616–1626. doi: 10.1038/sj.npp.1300982. [DOI] [PubMed] [Google Scholar]
- 61.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 62.Gervasoni N, Aubry JM, Bondolfi G, et al. Partial normalization of serum brain-derived neurotrophic factor in remitted patients after a major depressive episode. Neuropsychobiology. 2005;51:234–238. doi: 10.1159/000085725. [DOI] [PubMed] [Google Scholar]
- 63.Gonul AS, Akdeniz F, Taneli F, et al. Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. Eur Arch Psychiatry Clin Neurosci. 2005;255:381–386. doi: 10.1007/s00406-005-0578-6. [DOI] [PubMed] [Google Scholar]
- 64.Moghaddam B. Stress activation of glutamate neurotransmission in the prefrontal cortex: implication for dopamine-associated psychiatric disorders. Biol Psychiatry. 2002;51:775–787. doi: 10.1016/s0006-3223(01)01362-2. [DOI] [PubMed] [Google Scholar]
- 65.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000;48:755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 66.Krystal JH, Sanacora G, Blumberg H, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7:S71–S80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 67.Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Russo-Neustadt AA, Alejandre H, Garcia C, et al. Hippocampal brain-derived neurotrophic factor expression following treatment with reboxetine, citalopram, and physical exercise. Neuropsychopharmacology. 2004;29:2189–2199. doi: 10.1038/sj.npp.1300514. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu E, Hashimoto K, Okamura N, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with of without antidepressants. Biol Psychiatry. 2003;54:70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 70.Chen G, Huang LD, Jiang YM, et al. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem. 1999;72:1327–1330. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- 71.Chen G, Zeng W-Z, Yuan P-X, et al. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- 72.Phiel CJ, Zhang F, Huang EY, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- 73.Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McIntyre RS, Woldeyohannes HO, Yasgur BS, et al. Maintenance treatment in bipolar disorder: a focus on aripiprazole. Expert Rev Neurother. 2007;7:919–925. doi: 10.1586/14737175.7.8.919. [DOI] [PubMed] [Google Scholar]
- 75.Geddes JR, Burgess S, Hawton K, et al. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry. 2004;161:217–222. doi: 10.1176/appi.ajp.161.2.217. [DOI] [PubMed] [Google Scholar]
- 76.Maj M, Pirozzi R, Magliano L, et al. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30–35. doi: 10.1176/ajp.155.1.30. [DOI] [PubMed] [Google Scholar]
- 77.Goodwin FK, Fireman B, Simon GE, et al. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290:1467–1473. doi: 10.1001/jama.290.11.1467. [DOI] [PubMed] [Google Scholar]
- 78.Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J Clin Psychiatry. 2003;64:680–690. doi: 10.4088/jcp.v64n0610. [DOI] [PubMed] [Google Scholar]
- 79.Tohen M, Chengappa KNR, Suppes T, et al. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v mood stabiliser alone. Br J Psychiatry. 2004;184:337–345. doi: 10.1192/bjp.184.4.337. [DOI] [PubMed] [Google Scholar]
- 80.Keck PE, Calabrese JR, McIntyre RS, et al. Aripiprazole monotherapy for maintenance therapy in bipolar I disorder: a 100-week, double-blind study versus placebo. J Clin Psychiatry. 2007;68:1480–1491. doi: 10.4088/jcp.v68n1003. [DOI] [PubMed] [Google Scholar]
- 81.Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry. 2003;160:1252–1262. doi: 10.1176/appi.ajp.160.7.1252. [DOI] [PubMed] [Google Scholar]
- 82.Goodwin GM, Bowden CL, Calabrese JR, et al. A pooled analysis of 2 placebo-controlled 18-month trials of lamotrigine and lithium maintenance in bipolar I disorder. J Clin Psychiatry. 2004;65:432–441. doi: 10.4088/jcp.v65n0321. [DOI] [PubMed] [Google Scholar]
- 83.Wong IC, Mawer GE, Sander JW. Factors influencing the incidence of lamotrigine-related skin rash. Ann Pharmacother. 1999;33:1037–1042. doi: 10.1345/aph.18422. [DOI] [PubMed] [Google Scholar]
- 84.Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder:primary outcomes from the SystematicTreatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2006;163:217–224. doi: 10.1176/appi.ajp.163.2.217. [DOI] [PubMed] [Google Scholar]
- 85.Fagiolini A, Kupfer DJ, Houck PR, et al. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry. 2003;160:112–117. doi: 10.1176/appi.ajp.160.1.112. [DOI] [PubMed] [Google Scholar]