Abstract
Vitamin D, the sunshine vitamin, is important for childhood bone health. Over the past two decades, it is now recognized that vitamin D not only is important for calcium metabolism and maintenance of bone health throughout life, but also plays an important role in reducing risk of many chronic diseases including type I diabetes, multiple sclerosis, rheumatoid arthritis, deadly cancers, heart disease and infectious diseases. How vitamin D is able to play such an important role in health is based on observation that all tissues and cells in the body have a vitamin D receptor, and, thus, respond to its active form 1,25-dihydroxyvitamin D. However, this did not explain how living at higher latitudes and being at risk of vitamin D deficiency increased risk of these deadly diseases since it was also known that the 1,25-dihydroxyvitamin D levels are normal or even elevated when a person is vitamin D insufficient. Moreover, increased intake of vitamin D or exposure to more sunlight will not induce the kidneys to produce more 1,25-dihydroxyvitamin D. The revelation that the colon, breast, prostate, macrophages and skin among other organs have the enzymatic machinery to produce 1,25-dihydroxyvitamin D provides further insight as to how vitamin D plays such an essential role for overall health and well being. This review will put into perspective many of the new biologic actions of vitamin D and on how 1,25-dihydroxyvitamin D is able to regulate directly or indirectly more than 200 different genes that are responsible for a wide variety of biologic processes.
1. Introduction
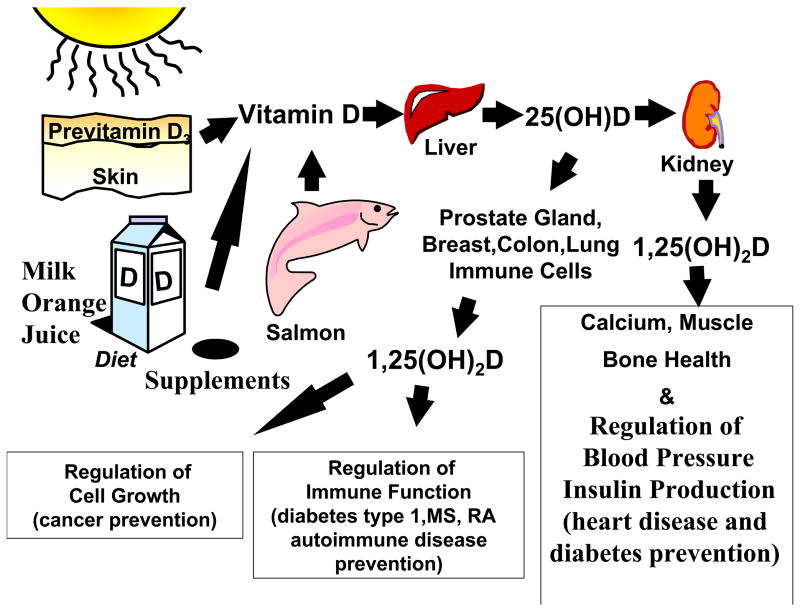
Most humans throughout evolution have depended on the sun for their vitamin D requirement. (Holick, 2007; Holick, 2003) When the skin is exposed to sunlight, the ultraviolet B radiation (UVB; 290-315 nm) is absorbed by 7-dehydrocholesterol in the epidermis and dermis and is converted to previtamin D3. (Fig 1) Once formed, previtamin D3 rapidly undergoes an isomerization induced by the body’s temperature to form vitamin D3. The zenith angle of the sun is critically important for the production of vitamin D3. Ozone efficiently absorbs the vitamin D3 producing UVB radiation, and, thus, in the early morning and late afternoon and evening, very few UVB photons reach the earth’s surface, thus, little, if any, vitamin D is produced in the skin no matter where one lives on the globe. (Holick, 2004) During the winter living above 35° latitude, the zenith angle is more oblique, and, thus, few, if any UVB photons reach the earth’s surface minimizing or completely preventing the production of vitamin D3 from October through March. Living at higher latitudes increases that period by as much as six months from September through April. (Moan et al., 2008; Holick, 2003)
Figure 1.
Once vitamin D is made in the skin or ingested from the diet, it travels to the liver where it is converted to 25-hydroxyvitamin D [25(OH)D]. This major circulating form of vitamin D is then converted in the kidneys to its active form 1,25-dihydroxyvitamin D [1,25(OH)2D]. The renal production of 1, 25(OH)2D is for regulating calcium and phosphorus metabolism. 25(OH)D is also converted in many tissues including prostate, colon, breast, lung, immune cells including monocytes and macrophages to 1, 25(OH)2D. The local production of 1, 25(OH)2D is to regulate cell growth, control immune function as well as affect up to 200 different genes responsible for health. Reproduced with permission. Holick copyright 2008.
Very few foods naturally contain vitamin D3. These include oily fish, such as salmon, mackerel and herring, and liver oils from cod, tuna and shark. (Holick, 2007) Sun-dried mushrooms also contain variable amounts of vitamin D2. Few foods are fortified with vitamin D (D represents D2 or D3). In the United States, milk, breads, some yogurts and cheeses are fortified with vitamin D. In Europe, some margarines, cereals, and in Sweden and Finland, milk is also fortified with vitamin D. (Holick, 2007) However, the amount of fortification of 100 IU per serving will help prevent the development of rickets in children, but will not prevent vitamin D deficiency either in children or adults. For every 100 IU of vitamin D ingested, the blood level of the major circulating form of vitamin D, 25-hydroxyvitamin D [25(OH)D] increases by 1 ng/ml. (Heaney et al., 2003; Holick et al, 2008) Thus, many experts have reported that at least 1,000 – 2,000 of vitamin D/d is required to sustain a blood level of 25(OH)D of > 30 ng/ml. Vitamin D deficiency has been defined as a 25(OH)D < 20 ng/ml; vitamin D insufficiency as 21–29 ng/ml and vitamin D sufficiency as > 30 ng/ml. (Holick, 2007; Vieth et al., 2007)
2. The Vitamin D Deficiency Pandemic
It has been assumed that eating a balanced diet or living near the equator would be all that was required to guarantee vitamin D sufficiency. Although it was well known that institutionalized elderly were at very high risk of vitamin D deficiency, it has come as a surprise to many health care professionals that essentially everyone who does not get an adequate amount of sun exposure or who intentionally ingests at least 1,000 IU of vitamin D/d is at high risk for vitamin D deficiency and its skeletal and non-skeletal health consequences. No one is immune from vitamin D deficiency. Thus, high rates of vitamin D deficiency have been reported in children and adults living in the United States, Europe, Middle East, India, Australia, New Zealand and Asia. (Holick, 2007; Vieth et al., 2007; McKenna, 1992; Maalouf et al., 2008; Gordon et al., 2008; Marwaha et al., 2005; Harris et al, 2000)
3. Antiproliferative Activity of Vitamin D and its Clinical Utility
There is strong epidemiologic data that living at higher latitudes and being at higher risk of vitamin D deficiency or being vitamin D deficient increases risk of not only developing but dying of deadly cancers including cancers of the colon, prostate, breast, esophagus among other cancers. (Gorham et al., 2005; Grant 2002; Abbas et al., 2008; Giovannucci et al., 2006) These epidemiologic studies have been supported by the observations of Woo et al (Woo et al, 2005) who reported that men who had metastatic prostate cancer and received 2,000 IU of vitamin D/d either had a decrease or no chang in their prostate-specific antigen levels after 21 months. Lappe et al (Lappe et al, 2007) reported that women who ingested 1,100 IU of vitamin D/d along with 1,500 milligrams of calcium reduced risk of developing all cancers by 66% after four years. Women in the Women’s Health Initiative who had a 25(OH)D of < 12 ng/ml at baseline and who were ingesting an inadequate amount of 400 IU of vitamin D/d had a 253% increase of developing colorectal cancer when compared to women who started out with a baseline of > 23 ng/ml and followed for eight years. (Holick, 2006) These data are also supported by Tangpricha et al (Tangpricha et al., 2005) who reported that mice who were vitamin D deficient and received a mouse colon tumor subcutaneously had more aggressive tumor growth of as much as 40% compared to mice that were receiving an adequate amount of dietary vitamin D.
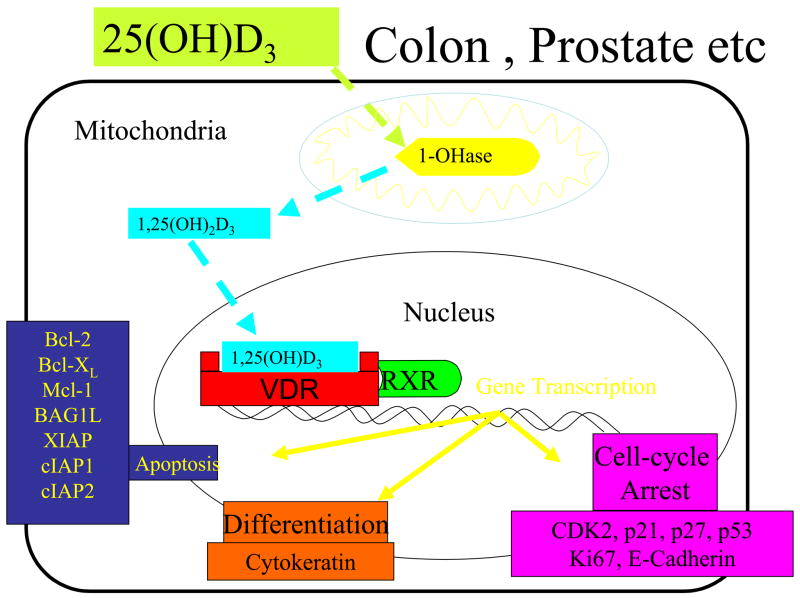
In the early 1980’s, it was first observed that malignant cells that had a vitamin D receptor (VDR) responded to 1,25(OH)2D3 with marked inhibition of proliferation and induction of terminal differentiation. (Tanaka et al., 1982) Since this initial observation, a wide variety of genes that regulate cellular proliferation and differentiation, have either been identified as target genes for 1,25(OH)2D because they have a vitamin D responsive element in their promotor region or the gene is indirectly influenced by 1,25(OH)2D3. (Fig 2) These genes include P21, P27 and genes responsible for differentiation, apoptosis and angiogenesis. (Nagpal et al., 2005; Feldman et al., 2000; Chen et al., 2003; Mantell et al., 2000) (Fig 2)
Figure 2.
Colon, prostate, skin, monocytes among other tissues and cells can convert 25-hydroxyvitamin D [25(OH)D] to 1, 25-dihydroxyvitamin D [1,25(OH)2D]. Once formed within the cell, it can induce a wide variety of genes by interacting with its vitamin D receptor (VDR) in the nucleus to regulate proliferation, differentiation and apoptosis. Once it completes this process, it then induces its own destruction by enhancing the 24-hydroxylase activity (24-OHase). Reproduced with permission. Holick copyright 2008.
Initially it was thought that 1,25(OH)2D3 and its analogues could be the magic bullet for the treatment of deadly cancers. However, it is recognized that tumors have developed several strategies to prevent the antiproliferative activity of 1,25(OH)2D3. One example is that some human prostate cancer cell lines have a marked up regulation of the 25-hydroxyvitamin D-24-hydroxylase (CYP24R; 24-OHase). (Feldman et al., 2000; Chen et al., 2003) The enhanced 24-OHase activity results in the rapid degradation of 1,25(OH)2D3, thus, making it ineffective in regulating genes for controlling cellular proliferation and differentiation. 1,25(OH)2D3 also inhibits the Wnt/β-catenin pathway in colon cancer cells. 1,25(OH)2D3-VDR complex binds β-catenin preventing it from inducing proliferation. Palmer et al (Palmer et al., 2004) observed that SNAIL-1 which induces epithelial-to-mesenchymal transition does so by inhibiting the expression of both VDR and E-cadherin which inhibit cellular proliferation.
The one clinical application for the antiproliferative activity of 1,25(OH)2D3 and it analogs is for the treatment of hyperproliferative skin disease psoriasis. Human keratinocytes have a VDR and produces 1,25(OH)2D3 which inhibits keratinocyte proliferation and induces their differentiation. When 1,25(OH)2D3 or one of its analogues is topically applied to psoriatic skin, there is marked reduction in the proliferative activity of the epidermis and restoration of normal differentiation. Thus, 1,25(OH)2D3 and its analogues have proven effective as a first line treatment for psoriasis. (Perez et al., 1996; Kragballe, 1989)
Several thousand vitamin D analogues (Bouillon et al., 1995) and novel vitamin D receptor modulators (Ma et al., 2006) have been synthesized. Some of these compounds have been evaluated in vivo (Bouillon et al., 1995; Koeffler et al., 1985; Beer et al., 2007) and have demonstrated some promise in cancer therapy although the calcemic activity continues to be a concern. Novel 1,25(OH)2D3 analogues that have two side arms known as gemini analogues have been designed to prevent 24-OHase degradation while increasing its binding activity within the VDR and having minimal calcemic activity. (Spina et al 2006) These analogues have potentially great promise either as primary or as adjunctive therapy for treating some deadly cancers.
4. Non-renal Production of 1,25-dihydroxyvitamin D3
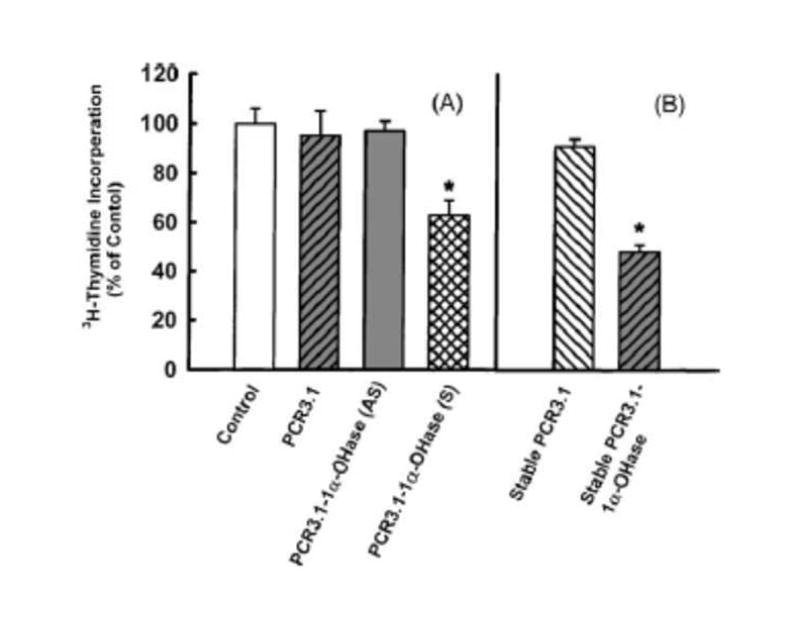
Bikle et al (Bikle, 2005) reported more than 20 years ago that cultured keratinocytes expressed the 25-hydroxyvitamin D-1α-hydroxylase (CPY27B; 1-OHase). Since that initial observation, it is now recognized that colon, prostate, breast, brain, β-islet cells, vascular smooth muscle cells as well as macrophages are able to produce 1,25(OH)2D3. (Schwartz et al., 1998; Cross et al., 2001; Holick, 2007) It is recognized that both normal and malignant cells including keratinocytes, the human prostate cancer cell lines DU145 and PC-3 and a wide variety of other human and mouse cell lines express the 1-OHase, and, thus, 25(OH)D3 is able to inhibit proliferation similar to 1,25(OH)2D3. (Feldman et al., 2000; Chen et al., 2003) However, to be certain that the expression of the 1-OHase was important for the antiproliferative activity of 25(OH)D3, the human prostate cancer cell line LNCaP which does not express the 1-OHase did not respond to the antiproliferative activity of 25(OH)D were transfected with a plasmid that contained the 1-OHase gene or its antisense counterpart. The LNCaP cells transfected with the 1-OHase plasmid that contained a green fluorescent protein probe demonstrated that the transfected cells expressed the gene and the fluorescent tagged 1-OHase was present in the mitochrondia and was able to convert 25(OH)D3 to 1,25(OH)2D3. (Whitlatch et al., 2002) LNCaP cells transfected either with the green fluorescent protein plasmid or the anti-sense 1-OHase gene demonstrated no 1-OHase activity. When LNCaP cells transfected with 1-OHase plasmid were exposed to 25(OH)D3, the antiproliferative activity was restored. (Fig 3b) This data demonstrates at least in this cell line that restoration of 1-OHase restores the antiproliferative activity of 25(OH)D3. These data are also supported by the observation that human cultured keratinocytes that were transfected with the 1-OHase-plasmid had marked increase antiproliferative activity when exposed to 25(OH)D3. (Flanagan et al., 1999)
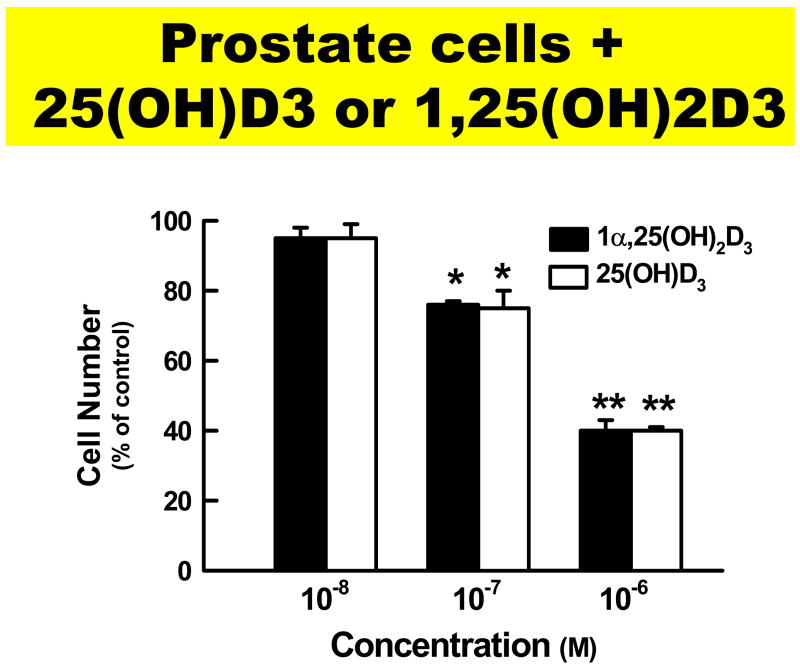
Figure 3.
Figure 3a. Prostate cells that have 25-hydroxyvitamin D-1-hydroxylase activity when incubated with either 25(OH)D3 or 1,25(OH)2D3 are able to inhibit proliferation in a dose dependent fashion. Reproduced with permission. Holick copyright 2008.
Figure 3b. Panel A, effect of 25(OH)D3 (10-8M) on 3H-thymidine incorporation into DNA of LNCaP cells with or without transient transfection with PCR 3.1 vector, anti-sense (AS) or sense PCR 3.1-1α-OHase cDNA (S). Panel B, effect of 25(OH)D3 (10-8M) on 3H-thymidine incorporation into DNA of LNCaP cells stably transfected with vector PCR 3.1 or with sense PCR 3.1-1α-OHase cDNA. Bars indicate the standard deviation of eight determinations, *P < 0.05. Reproduced with permission. Whitlatch, et. al, 2002.
5. Autoimmune Diseases
There is a latitudinal association risk of many autoimmune diseases including multiple sclerosis, rheumatoid arthritis, Crohn’s disease and type I diabetes. (Ponsonby et al., 2002; Mohr et al., 2008). Hypponen et al (4Hypponen et al., 2001) observed that 10,366 children in Finland who received on average 2,000 IU of vitamin D/d in the 1960’s and followed for 31 years had a 78% reduced risk of developing type I diabetes. Living above 35° latitude for the first 10 years of life increased risk of developing multiple sclerosis by 100%. (Ponsonby et al., 2002; Embry et al., 2000) Women who ingested more than 400 IU of vitamin D/d had a 42% reduced risk of developing MS (Munger et al., 2004) and a 40% reduced risk of developing rheumatoid arthritis (Merlino et al, 2004) and osteoarthritis. (McAlindon et al., 1996)
Resting T and B lymphocytes do not have a VDR, but when activated, both induce the expression of VDR. (Tsoukas et al., 1984) Dendritic cells which are important for immunosurveillance have a VDR and respond to 1,25(OH)2D3. (Gauzzi et al., 2005) 1,25(OH)2D3 regulates the expression and production of several cytokines including IL-2, TGF-B1 (Mathieu et al., 2002) and enhances immunoglobin synthesis in activated B cells. (Adams et al., 1986) Thus, 1,25(OH)2D3 is a potent immunomodulator. (Fig 4)
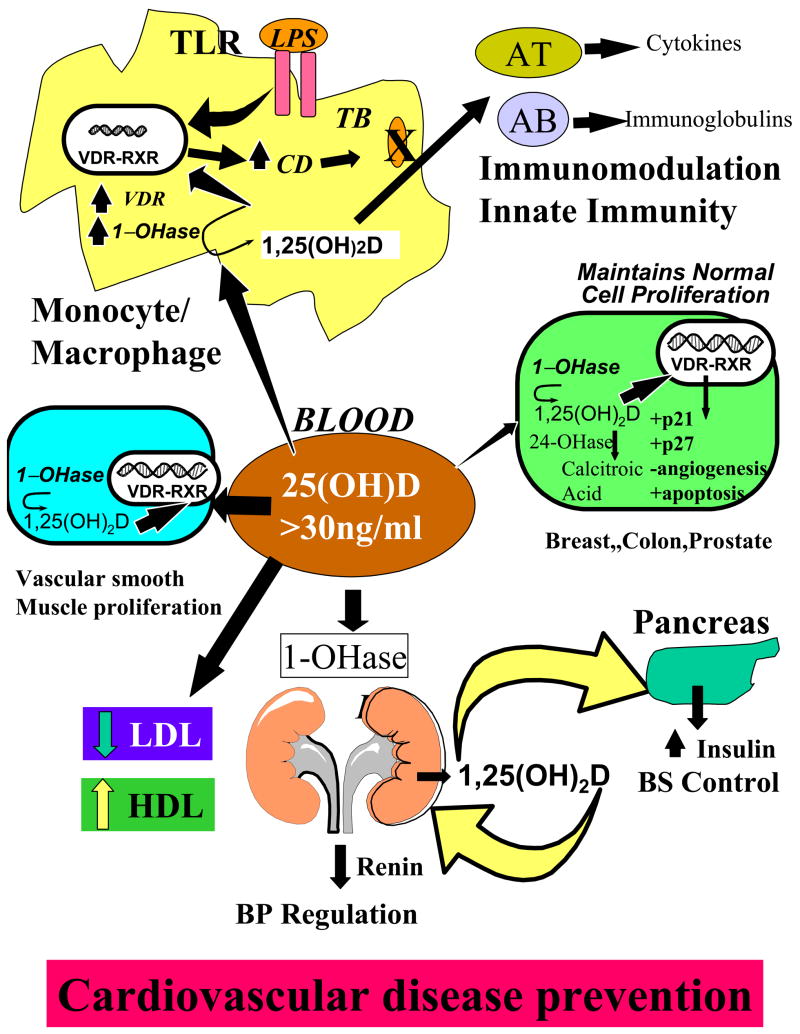
Figure 4.
When the 25(OH)D is > 30 ng/ml, it is believed to have a multitude of effects including regulating activated T and B cell activity, enhancing the destruction of infectious agents by increasing the production of cathelicidin (CD). It alters smooth muscle cell proliferation, decreases low density lipoproteins (LDL) and increases high density lipoproteins (HDL) as well as decreasing renin production and enhancing insulin production all for helping preventing cardiovascular disease. Reproduced with permission. Holick copyright 2008.
It is also recognized that the macrophage expresses the 1-OHase. (Adams et al., 1986; Adams et al., 2006) Thus, patients with chronic granulomatous disorders that have activated macrophages produce an excess of 1,25(OH)2D3 that causes hypercalcuria and hypercalcemia. (Holick, 2007; Adams et al., 2006) Only recently is it understood why the macrophage produces 1,25(OH)2D3. Liu et al (Liu et al., 2006) observed that when a macrophage is infected with Microbacterium tuberculosis (TB), toll-like receptors respond with a signal transduction to the nucleus to increase the expression not only of the 1-OHase but also the VDR. The macrophage production of 1,25(OH)2D3 results in expression of the cathelicidin gene. Cathelicidin, one of the defensin proteins, is responsible for killing infective agents such as TB within the macrophage. (Fig 4) Liu et al (Liu et al., 2006) also demonstrated that macrophages incubated in serum with a 25(OH)D of 8 ng/ml were unable to mount an immune response when infected with TB and were quickly killed. However, when macrophages were incubated in serum that contained 25(OH)D at 18 ng/ml, the macrophages were able to produce 1,25(OH)2D3 resulting in an increase in the cathelicidin production causing the destruction of the TB. It has also been speculated that the increase production of 1,25(OH)2D3 in the macrophage is also released and acts in a paracrine fashion to interact with activated T and B lymphocytes to induce local immunomodulation. (Holick, 2007) It’s been suggested that both type I diabetes and multiple sclerosis may be caused by a viral infection early in life. (Ponsonby et al., 2002; Mohr et al., 2008; Cannell et al., 2006) Thus, by macrophages producing 1,25(OH)2D3, they can destroy infective agents including viruses as well as modulate both T and B lymphocyte activity. These mechanisms may be responsible for why vitamin D sufficiency decreases risk of developing these autoimmune diseases. In addition, vitamin D deficiency has been associated with increase risk of being infected with TB (Liu et al., 2006) and having more invasive disease as well as increase risk of developing upper respiratory tract infections, (Aloia et al., 2007) influenza (Cannell et al., 2006) and wheezing disorders (Camargo et al., 2006).
6. Cardiovascular Disease
Rostand (Rostand, 1997) reported that living at higher latitudes increase risk of having hypertension. This was followed by the observation that hypertensive patients exposed to UVB radiation who had on average an 180% increase in their circulating 25(OH)D level reduced their systolic and diastolic by blood pressure 6 mm Hg into the normal range. A similar group of subjects exposed to ultraviolet A radiation under the similar circumstances had no change in their circulating 25(OH)D level and there was no benefit for their hypertension. (Krause, et al., 1998) Li et al (Li et al., 2002) reported in a mouse model that 1,25(OH)2D3 was a potent regulator of renin production helping to explain the antihypertensive activity of 1,25(OH)2D3. (Fig 4) These observations have been supported by the recent reports that vitamin D deficiency increases risk of developing first myocardia infarction by more than 50% (Wang et al., 2008) and that vitamin D deficiency increases risk of dying from a cardiovascular event. (Dobnig et al., 2008) In addition, it is recognized that vitamin D deficiency increases risk of developing type II diabetes which can exacerbate lipoprotein disorders increasing risk of a cardiac event. (Pittas et al., 2006; Carbone et al., 2008; Scragg et al., 1990; Poole et al., 2006; Cigolini et al., 2006; Melamed et al., 2008; Martins et al., 2007; Scragg et al., 2007) Recently, it was reported that exposure to UVB radiation raising blood levels of 25(OH)D > 30 ng/ml decreased low density lipoprotein levels and improved high density lipoprotein levels (Carbone et al., 2008). Adults with no history of cardiovascular disease followed for 5.4 years found that the rate of fatal or nonfatal myocardial infarction, ischemia stroke or heart failure was 53–80% higher in people with low 25(OH)D levels of < 20 ng/ml. (Scragg et al., 2007) It is also recognized that patients with chronic kidney disease (CKD) are at much higher risk of dying of a cardiovascular event. Vitamin D deficiency has been associated with increased mortality rates in CKD patients and repleating their vitamin D improves outcomes. (Wolf et al., 2007) Besides 1,25(OH)2D3 reducing renin production, it also decreases the proliferation of myocardial and vascular smooth muscle cells (Fig 4) which may play a role in reducing risk of congestive heart failure and atherosclerosis (Zitterman, 2006).
7. Conclusion
There is a great need globally to make health care professionals and regulatory agencies responsible for the overall health and welfare of their populations to be aware of the vitamin D deficiency pandemic and insidious consequences for non-skeletal health. It is been estimated that the body can use up to 5,000 IU of vitamin D/d. (Heaney et al., 2003) The likely reason for such a high requirement is because every tissue and cell in the body has a vitamin D receptor, and if supplied with enough 25(OH)D3 can locally produce 1,25(OH)2D3 in order to carry out its important health-promoting biologic functions. From an evolutionary perspective, it has been estimated that vitamin D has been synthesized on this earth in the earliest phytoplankton and zooplankton life forms for more than 0.5 billion years. (Holick, 2003) Vitamin D became critically important in early evolution for calcium metabolism and continues this role in most vertebrates including humans. There appears to be a hierarchy in the utilization of vitamin D by the body. First and foremost is that the body maintains its extracellular ionized calcium within the physiologic range in order to maintain signal transduction and most metabolic functions. To accomplish this, 25(OH)D is converted in the kidneys to 1,25(OH)2D which in turn acts as a hormone to increase the efficiency of intestinal calcium absorption. If this is inadequate to sustain the blood levels of calcium, then 1,25(OH)2D acts on the bone to increase the number of osteoclasts to mobilize precious calcium from the skeleton. (Holick, 2007) Only when the body’s extracellular ionized calcium requirement is satisfied, will 25(OH)D be used by other tissues and cells in the body and be converted locally to 1,25(OH)2D3. 1,25(OH)2D3 produced in non-calcium regulating tissues such as the prostate and breast helps to maintain normal cell proliferation and differentiation. It prevents malignancy by inducing apoptosis to prevent the cell from becoming malignant and metastasizing. It also has a strategy to prevent angiogenesis so that if a cell becomes malignant it has little nutrition to support its rapid proliferative activity. Once 1,25(OH)2D3 carries out is autocrine functions, it then induces its own destruction by increasing the expression of the 24-OHase. Thus, it never leaves the non-calcemic tissues into the blood stream to influence calcium metabolism. This is the likely explanation for why increasing exposure to sunlight or raising blood levels of 25(OH)D has been associated with reduced risk not only of developing deadly cancers but dying from them. Knight et al (Knight et al., 2007) observed that young women who had the most sun exposure were less likely to develop breast cancer.
1,25(OH)2D3 enhances renin, insulin, cytokine, immunoglobin, neurotransmitter and cathelicidin synthesis. These well documented functions of 1,25(OH)2D3 may help explain why vitamin D deficiency has now been recognized to be associated with many chronic diseases that plagued humans throughout their lives. There needs to be a reevaluation of vitamin D fortification not only to increase the amount per serving but to increase the number of foods fortified with vitamin D to help increase vitamin D intake in diverse populations. In addition, an appreciation for sensible sun exposure as a major source of vitamin D needs to be reinstituted. Most children and adults have the serum 25(OH)D reach their maximum level at the end of the summer and their nadar at the end of the winter. (Holick et al., 2007; Brot et al., 2001) Even in the skin capital of the world, Australia, it is now recognized that vitamin D deficiency is a common problem as a result of the campaign to encourage people to avoid all direct sun exposure without either wearing a sunscreen or sun protection from clothing. (Holick, 2007) A sunscreen with a sun protection factor of 15 reduces the production of vitamin D3 in the skin by 99%. (Matsuoka et al., 1987) The Australian Council for Dermatology and the Australian Cancer Council have now recommended that their needs to be an appreciation for some exposure to ultraviolet radiation to enhance vitamin D production while preventing excessive exposure with increased risk of non-melanoma skin cancer.
Acknowledgments
Sources of Support: This work was supported in part by NIH grants M01RR00533 and the UV Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas S, Linseisen J, Slanger T, Kropp S, Mutschelknauss EJ, Flesch-Janys D, Chang-Claude J. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer-results of a large case-control study. Carcinogenesis. 2008;29(1):93–99. doi: 10.1093/carcin/bgm240. [DOI] [PubMed] [Google Scholar]
- Adams JS, Gacad MA, Anders A, Endres DB, Sharma OP. Biochemical indicators of disordered vitamin D and calcium homeostasis in sarcoidosis. Sarcoidosis. 1986;3:1–6. [PubMed] [Google Scholar]
- Adams JS, Hewison M. Hypercalcemia caused by granuloma-forming disorders. In: Favus MJ, editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 6. Washington, DC: American Society for Bone and Mineral Research; 2006. pp. 200–202. [Google Scholar]
- Aloia JR, Li-Ng M. Epidemic influenza and vitamin D. Epidemiol Infect. 2007;12:1–4. doi: 10.1017/S0950268807008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beer TM, Ryan CW, Venner PM, Petrylak DP, Chatta GS, Ruether JD, Redfern CH, Fehrenbacher L, Saleh MN, Waterhouse DM, Carducci MA, Vacario D, Dreicer R, Higano CS, Ahmann FR, Chi KN, Henner WD, Arroyo A, Clow FW. Double-blinded randomized study of igh-dose calcitriol plus docetaxel compared with placebo plus docetaxel in androgen-independent prostate cancer: a report from the ASCENT investigators. J Clin Oncol. 2007;25:669–674. doi: 10.1200/JCO.2006.06.8197. [DOI] [PubMed] [Google Scholar]
- Bikle DD. Vitamin D: Role in skin and hair. In: Feldman, et al., editors. Vitamin D. New Jersey: Elsevier Academic Press; 2005. pp. 609–630. [Google Scholar]
- Bouillon R, Okamura WH, Norman AW. Structure-function relationships in the vitamin D endocrine system. Endocrine Rev. 1995;16:200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- Brot C, Vestergaard P, Kolthoff N, Gram J, Hermann AP, Sorensen OH. Vitamin D status and its adequacy in healthy Danish perimenopausal women: relationships to dietary intake, sun exposure and serum parathyroid hormone. British Journal of Nutrition. 2001;86(1):S97–103. doi: 10.1079/bjn2001345. [DOI] [PubMed] [Google Scholar]
- Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, Gillman MW. Prospective study of maternal intake of vitamin D during pregnancy and risk of wheezing illnesses in children at age 2 years. J Allergy Clin Imunol. 2006;117:721–722. [Google Scholar]
- Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone LD, Rosenberg EW, Tolley EA, Holick MF, Hughes TA, Watsk MA, Barrow KD, Chen TC, Wilkin NK, Bhattacharya SK, Dowdy JC, Sayre RM, Weber KT. 25-Hydroxyvitamin D, cholesterol, and ultraviolet irradiation. Metabolism Clinical and Experimental. 2008;57:741–748. doi: 10.1016/j.metabol.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Chen TC, Holick MF. Vitamin D and prostate cancer prevention and treatment. Trends in Endocrinol Metabol. 2003;14:423–430. doi: 10.1016/j.tem.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Cigolini M, Iagulli MP, Miconi V, Galiotto M, Lombard iS, Targher G. Serum 25-hydroxyvitamin D3 concentrations and prevalence of cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2006;29:722–4. doi: 10.2337/diacare.29.03.06.dc05-2148. [DOI] [PubMed] [Google Scholar]
- Cross HS, Bareis P, Hofer H, Bischof MG, Bajna E, Kriwanek S. 25-Hydroxyvitamin D3-1-hydroxylase and vitamin D receptor gene expression in human colonic mucosa is elevated during early cancerogenesis. Steroids. 2001;66:287–292. doi: 10.1016/s0039-128x(00)00153-7. [DOI] [PubMed] [Google Scholar]
- Diesel B, Radermacher J, Bureik M, Bernhardt R, Seifert M, Reichrath J, Fischer U, Meese E. Vitamin D3 metabolism in human glioblastoma multiforme: functionality of CYP27B1 splice variants, metabolism of calcidiol, and effect of calcitriol. Clin Cancer Res. 2005;11(15):5370–5380. doi: 10.1158/1078-0432.CCR-04-1968. [DOI] [PubMed] [Google Scholar]
- Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. Independent association of low serum 25-hydroxyvitamin D and 1, 25-dihyroxyvitamin D levels with all-cause and cardiovascular mortality. Arch Intern Med. 2008;168(12):1340–1349. doi: 10.1001/archinte.168.12.1340. [DOI] [PubMed] [Google Scholar]
- Embry AF, Snowdon LR, Vieth R. Vitamin D and seasonal fluctuations of gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol. 2000;48:271–272. [PubMed] [Google Scholar]
- Feldman D, Zhao XY, Krishnan AV. Editorial/Mini-review: Vitamin D and prostate cancer. Endocrinology. 2000;141:5–9. doi: 10.1210/endo.141.1.7341. [DOI] [PubMed] [Google Scholar]
- Flanagan JN, Whitlatch LW, Rudoph T, Xuehong P, Kong X, Chen TC, Holick MF. Development of gene therapy with the 25-OH-1-α-hydroxylase gene: In vitro and in vivo enhancement of 1-α-hydroxylase activity in cultured prostate cancer cells and in the skin of mice. J Bone Miner Res. 1999;14:1145. [Google Scholar]
- Gauzzi MC, Purificato C, Donato K, Jin X, Wang L, Daniel KC, Maghazachi AA, Belardelli F, Adorini L, Gessani S. Suppressive effect of 1α,25-dihydroxyvitamin D3 on type 1 IFN-mediated monocyte differentiation into dendritic cells: impairment of functional activities and chemotaxis. The Journal of Immunology. 2005;174:270–276. doi: 10.4049/jimmunol.174.1.270. [DOI] [PubMed] [Google Scholar]
- Giovannucci E, Liu Y, Willett WC. Cancer incidence and mortality and vitamin D in black ad white male health professionals. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2467–72. doi: 10.1158/1055-9965.EPI-06-0357. [DOI] [PubMed] [Google Scholar]
- Gordon CM, Feldman HA, Sinclair L, Williams AL, Kleinman PK, Perez-Rossello J, Cox JE. Prevalence of vitamin D deficiency among healthy infants and toddlers. Arch Pediatr Adolesc Med. 2008;162(6):505–512. doi: 10.1001/archpedi.162.6.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, Newmark HL, Giovannucci E, Wei M, Holick MF. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol. 2005;97(1–2):179–194. doi: 10.1016/j.jsbmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Grant WB. An estimate of premature cancer mortality in the US due to inadequate doses of solar ultraviolet-B radiation. 2002;94(6):1867–1875. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- Harris SS, Soteriades E, Coolidge JA, Mudgal S, Dawson-Hughes B. Vitamin D insufficiency and hyperparathyroidism in a low income, multiracial, elderly population. J Clin Endocrinol Metab. 2000;85:4125–4130. doi: 10.1210/jcem.85.11.6962. [DOI] [PubMed] [Google Scholar]
- Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: A millennium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D: Importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362–71. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- Holick MF. Calcium plus vitamin D and the risk of colorectal cancer. N Engl J Med. 2006;354(21):2287. doi: 10.1056/NEJMc060753. [DOI] [PubMed] [Google Scholar]
- Holick MF. Vitamin D Deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Holick MF, Chen TC, Sauter ER. Vitamin D and Skin Physiology: A D-Lightful Story. J Bone Miner Res. 2007;22(S2):V28–V33. doi: 10.1359/jbmr.07s211. [DOI] [PubMed] [Google Scholar]
- Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J. Clin. Endocrinol Metab. 2008;93(3):677–681. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hypponen E, Laara E, Reunanen A, Jarvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: a birth-cohort study. Lancet. 2001;358:1500–3. doi: 10.1016/S0140-6736(01)06580-1. [DOI] [PubMed] [Google Scholar]
- Knight JA, Lesosky M, Barnett H, Raboud JM, Vieth R. Vitamin D and reduced risk of breast cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16(3):422–499. doi: 10.1158/1055-9965.EPI-06-0865. [DOI] [PubMed] [Google Scholar]
- Koeffler HP, Hirjik J, Iti L the Southern California Leukemia Group. 1, 25-Dihydroxyvitamin D3: in vivo and in vitro effects on human preleukemic and leukemic cells. Cancer Treat Rep. 1985;69:1399–1407. [PubMed] [Google Scholar]
- Kragballe K. Treatment of psoriasis by the topical application of the novel vitamin D3 analogue MC 903. Arch Dermatol. 1989;125:1647–1652. [PubMed] [Google Scholar]
- Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet. 1998;352(9129):709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85(6):1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- Li Y, Kong J, Wei M, Chen ZF, Liu S, Cao LP. 1, 25-dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik S, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor Triggering of a vitamin D-mediated human antimicrobial response. Sciencexpress. 2006;3:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Ma Y, Khalif aB, Yee YK, Lu J, Mernezawa A, Savkur RS, Yamamoto Y, Chintalacharuva SR, Yamaoka K, Stayrook KR, Bramlett KS, Zeng ZZ, Chandrasekhar S, Yu XP, Linebarger JH, Iturria ST, Burris TP, Kato S, Chin WW, Nagpal S. Identification and characterization of noncalcemic, tissue-selective, nonsecosteroidal vitamin D receptor modulators. J Clin Invest. 2006;116(4):892–904. doi: 10.1172/JCI25901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalouf J, Nabulsi M, Vieth R, Kimball S, El-Rassi R, Mahfoud Z, Fuleihan GE. Short term and long term safety of weekly high dose of vitamin D3 supplementation in school children. J Clin Endocrin Metab. 2008 doi: 10.1210/jc.2007–2530. First published ahead of print April 29, 2008 as. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1α, 25-dihydroxyvitamin D3 inhibits angiogenesis in vitro and in vivo. Circ Res. 2000;87:412–220. doi: 10.1161/01.res.87.3.214. [DOI] [PubMed] [Google Scholar]
- Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, Felsenfeld A, Levine B, Mehrotra R, Norris K. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–65. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- Marwaha RK, Tandon N, Reddy D, Aggarwal R, Singh R, Sawhney RC, Saluja B, Ganie M, Singh S. Vitamin D and bone mineral density status of healthy schoolchildren in northern India. Am J Clin Nutr. 2005;82:477–482. doi: 10.1093/ajcn.82.2.477. [DOI] [PubMed] [Google Scholar]
- Mathieu C, Adorini L. The coming of age of 1, 25-dihydroxyvitamin D3 analogs as immunomodulatory agents. Trends in Molecular Medicine. 2002;8(4):174–179. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- Matsuoka LY, Ide L, Wortsman J, MacLaughlin J, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64:1165–1168. doi: 10.1210/jcem-64-6-1165. [DOI] [PubMed] [Google Scholar]
- McAlindon TE, Felson DT, Zhang Y, Hannan MT, Aliabadi P, Weissman B, Rush D, Wilson PW, Jacaques P. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med. 1996;125(5):353–359. doi: 10.7326/0003-4819-125-5-199609010-00001. [DOI] [PubMed] [Google Scholar]
- McKenna MJ. Differences in vitamin D status between countries in young adults and the elderly. Am J Med. 1992;93:9–77. doi: 10.1016/0002-9343(92)90682-2. [DOI] [PubMed] [Google Scholar]
- Melamed ML, Muntner P, Michos ED, Uribarri J, Weber C, Sharma J, Raggi P. Serum 25-Hydroxyvitamin D Levels and the Prevalence of Peripheral Arterial Disease. Results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol. 2008;28(6):1179–85. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA, Saag KG Iowa Women’s Health Study. Vitamin D intake is inversely associated with rheumatoid arthritis: results from the Iowa Women’s Health Study. Arthritis Rheum. 2004;50(1):72–77. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- Moan J, Porojnicu AC, Dahlback A, Setlow RB. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proc Natl Acad Sci USA. 2008;105(2):668–673. doi: 10.1073/pnas.0710615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr SB, Garland CF, Gorham ED, Garland FC. The association between ultraviolet B irradiance, vitamin D status and incidence rates of type 1 diabetes in 51 regions worldwide. Diabetologia. 2008 2008 June 12; doi: 10.1007/s00125-008-1061-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Munger KL, Zhang SM, O’Reilly E, Hernan MA, Olek MJ, Willett WC, Ascherio A. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62(1):60–5. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocrine Reviews. 2005;26:662–87. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- Palmer HG, Larriba MJ, Garcia JM, Ordonez-Moran P, Pena C, Peiro S, Puig I, Rodriguez R, de la Fuente R, Bernad A, Pollan M, Bonilla F, Camallo C, Garcia de Herreros A, Munoz A. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nature Medicine. 2004;10:917–919. doi: 10.1038/nm1095. [DOI] [PubMed] [Google Scholar]
- Perez A, Chen TC, Turner A, Raab R, Bhawan J, Poche P, Holick MF. Efficacy and safety of topical calcitriol (1, 25-dihydroxyvitamin D3) for the treatment of psoriasis. Br J Dermatol. 1996;134:238–246. [PubMed] [Google Scholar]
- Pittas AG, Dawson-Hughes B, Li T, Van Dam RM, Willett WC, Manson JE, Hu FB. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29(3):650–56. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- Ponsonby A-L, McMichael A, van der Mei I. Ultraviolet radiation and autoimmune disease: insights from epidemiological research. Toxicology. 2002:181–182. 71–78. doi: 10.1016/s0300-483x(02)00257-3. [DOI] [PubMed] [Google Scholar]
- Poole KE, Loveridge N, Barker PJ, Halsall DJ, Rose C, Reeve J, Warburton EA. Reduce vitamin D in acute stroke. Stroke. 2006;37(1):243–5. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–6. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- Schwartz GG, Whitlatch LW, Chen TC, Lokeshwar BL, Holick MF. Human prostate cells synthesize 1, 25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Cancer Epidemiol Biomarkers Prev. 1998;7:391–395. [PubMed] [Google Scholar]
- Scragg R, Jackson R, Holdaway IM, Lim T, Beaglehole R. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: a community-based study. Int J Epidemiol. 1990;19:559–63. doi: 10.1093/ije/19.3.559. [DOI] [PubMed] [Google Scholar]
- Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713–9. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Spina CS, Tangpricha V, Uskokovic M, Adorinic L, Maehr H, Holick MF. Vitamin D and cancer. Anticancer Res. 2006;26(4a):2515–2524. [PubMed] [Google Scholar]
- Tanaka H, Abe E, Miyaura C, Kuribayashi T, Konno K, Nishi Y, Suda T. 1, 25-Dihydroxycholeciferol and human myeloid leukemia cell line (HL-60): The presence of cytosol receptor and induction of differentiation. Biochem J. 1982;204(3):713–719. doi: 10.1042/bj2040713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangpricha V, Flanagan JN, Whitlatch LW, Tseng CC, Chen TC, Holt RP, Lipkin MS, Holick MF. 25-hydroxyvitamin D-1α-hydroxylase in normal and malignant colon tissue. Lancet. 2001;357(9269):1673–1674. doi: 10.1016/S0140-6736(00)04831-5. [DOI] [PubMed] [Google Scholar]
- Tangpricha V, Spina C, Yao M, Chen TC, Wolfe MM, Holick MF. Vitamin D deficiency enhances the growth of MC-26 colon cancer xenografts in Balb/c mice. J Nutr. 2005;135(10):2350–2354. doi: 10.1093/jn/135.10.2350. [DOI] [PubMed] [Google Scholar]
- Tsoukas CD, Provvedine DM, Manolagas SC. 1, 25-Dihydroxyvitamin D3, a novel immuno-regulatory hormone. Science. 1984;221:1438–1440. doi: 10.1126/science.6427926. [DOI] [PubMed] [Google Scholar]
- Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland C, Heaney RP, Holick MF, Hollis BW, Lamberg-Allardt C, McGrath JJ, Norman AW, Scragg R, Whiting SJ, Willett WC, Zittermann A. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85(3):649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlatch LW, Young MV, Schwartz GG, Flanagan JN, Burnstein KL, Lokeshwar BL, Rich ES, Holick MF, Chen TC. 25-hydroxyvitamin D-1-α-hydroxylase activity is diminished in human prostate cancer cells and is enhanced by gene transfer. J Steroid Biochem Mol Biol. 2002;81(2):135–140. doi: 10.1016/s0960-0760(02)00053-5. [DOI] [PubMed] [Google Scholar]
- Wolf M, Shah A, Gutierrez O, Ankers, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr, Tonelli M, Thadhani R. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney International. 2007 doi: 10.1038/sk.ki.5002451. advance online publication 8 August 2007. [DOI] [PubMed] [Google Scholar]
- Woo TCS, Choo R, Jamieson M, Chander S, Vieth R. Pilot study: potential role of vitamin D (cholecalciferol) in patients with PSA relapse after definitive therapy. Nutrition and Cancer. 2005;51(1):32–36. doi: 10.1207/s15327914nc5101_5. [DOI] [PubMed] [Google Scholar]
- Zittermann A. Vitamin D and Disease prevention with Special Reference to Cardiovascular Disease. Biophysics and Molecular Biology. 2006;92:39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]