Abstract
This study investigated pupillary and behavioral responses to an emotional word valence identification paradigm among 32 pre/early and 34 mid/late pubertal typically developing children and adolescents. Participants were asked to identify the valence of positive, negative, and neutral words while pupil dilation was assessed using an eye-tracker. Mid-to-late pubertal children showed greater peak pupillary reactivity to words presented during the emotional word identification task than pre-to-early pubertal children, regardless of word valence. Mid-to-late pubertal children also showed smaller sustained pupil dilation than pre-to-early pubertal children after the word was no longer on screen. These findings were replicated controlling for participants’ age. Additionally, mid-to-late pubertal children had faster reaction times to all words, and rated themselves as more emotional during their laboratory visit compared to pre-to-early pubertal children. Greater recall of emotional words following the task was associated with mid-to-late pubertal status, and greater recall of emotional words was also associated with higher peak pupil dilation. These results provide physiological, behavioral, and subjective evidence consistent with a model of puberty-specific changes in neurobehavioral systems underpinning emotional reactivity.
This study examines puberty-related changes in neurobehavioral systems underpinning emotional reactivity and regulation using pupillary and behavioral measures of emotionally-salient information processing. Gaining a better understanding of normative changes in emotional information processing across pubertal development may help us to understand a paradox in adolescent health (Dahl, 2004; Steinberg et al., 2006). Although adolescence is one of the healthiest periods of the life span with respect to physical health, overall morbidity and mortality rates increase 200–300% (Ozer, Macdonald, & Irwin, 2002; Resnick et al., 1997). Accidents, suicide, homicide, depression, anxiety, alcohol and substance use, eating disorders, HIV, STD’s, and unwanted pregnancies all increase sharply in this developmental period (Ozer et al., 2002; U.S. Preventive Services Task Force, 1996). Furthermore, many of the most costly, chronic and impairing disorders of adulthood—including mood disorders, anxiety disorders, and drug and alcohol abuse, typically onset during adolescence (Alpert, Maddocks, Rosenbaum, & Fava, 1994; Chung, Martin, & Winters, 2006; Pine, Cohen, Gurley, Brook, & Ma, 1998).
Many of these problems and disorders have been linked to the onset of puberty or early pubertal maturation, particularly in females (Angold, Costello, & Worthman, 1998; Caspi, Lynam, Moffitt, & Silva, 1993; Ge, Conger, & Elder, 1996; Graber, Lewinsohn, Seeley, & Brooks-Gunn, 1997; Orr & Ingersoll, 1995). This puberty-linked increase in adolescent mental and behavioral health problems appears to be related to difficulties with the control of emotions and behavior (Dahl, 2004). In fact, many health problems that increase during this period involve dysregulation of emotion (e.g. bipolar disorder, depression, anxiety disorder) or risk-taking and poor decision-making in the face of emotional influence (e.g. unsafe sex, peer pressure to engage in substance abuse or drunk driving). Consistent with this model, there is initial evidence that problems in emotion regulation during adolescence are linked to the development of depressive symptoms and problem behaviors (Silk, Steinberg, & Morris, 2003), although the role of puberty in this relationship is not well understood. Thus, gaining a better understanding of normative puberty-specific changes in systems involved in emotional reactivity and regulation is critical to elucidating the nature of adolescent health problems and refining prevention and intervention approaches.
Development of Neurobehavioral Systems Subserving Emotional Information Processing During Adolescence
Recent research in developmental neuroscience suggests that problems in emotion regulation during adolescence may result from dyssynchronous development in neurobehavioral systems involved in both emotional reactivity and regulation (Dahl, 2004; Ernst, Pine, & Hardin, 2005; Steinberg, 2005; Steinberg et al., 2006). There is some evidence that change in regions of the brain that subserve primarily socio-emotional functions, such as responding to social stimuli, and the perception and evaluation of risk and reward, may be directly linked to pubertal maturation (Nelson, Leibenluft, McClure, & Pine, 2005; Steinberg, 2007; Steinberg et al., 2006). These areas undergo significant reorganization in early adolescence that appears to be triggered, at least in part, by the hormonal changes of puberty (see Nelson et al., 2005; Patton & Viner, 2007). Emerging evidence from animal and human studies suggests that, presumably as a result of this reorganization, the brain systems that regulate rewards, social information, and emotions become more sensitive and/or active during puberty (Chambers, Taylor, & Potenza, 2003; Dahl, 2001; Nelson et al., 2005; Spear, 2000; Steinberg, 2005, 2007). Consequently, pubertal adolescents seem to seek experiences that create high-intensity feelings. For example, one study found that among adolescents of a similar age, those who were more advanced in puberty were more likely to seek exciting experiences and show risk-taking behavior (Martin et al., 2002). Additionally, Quevedo, Benning, Gunnar, and Dahl (this section) found that pubertal adolescents showed appetitive potentiation of the postauricular reflex, a marker of appetitive motivation, while their same age prepubertal peers showed no modulation of this reflex. Quevedo et al. also found that puberty was associated with an increase in the magnitude of the eye-blink startle response during an affective picture viewing paradigm regardless of the valence of the picture, suggesting that puberty may be associated with a general increase in the reactivity of neural systems underlying the startle response, possibly including the amygdala and fear-related circuitry.
Increased sensation seeking and/or affective intensity could interact with the social-contextual changes that accompany puberty to lead to a spiraling of both positive and negative emotions. The transition through adolescence involves a period of flux and renegotiation (Cicchetti & Rogosch, 2002), including dramatic changes in the social environment, such as the increasing importance of peer and romantic relationships, changing family relationships, and entry into quasi-adult roles such as working and driving (Steinberg & Morris, 2000; Steinberg & Silk, 2002). Evidence suggests that changes in the parent-child relationship, including increased frequency and intensity of parent-adolescent conflict, may actually be directly linked to pubertal maturation (Steinberg, 1987). Other changes are indirectly triggered by puberty because changes in physical appearance lead to changes in the way children and adolescents are treated by parents, other adults, and peers, especially those of the opposite gender. A wide array of affective challenges accompany these new experiences, including increased self-consciousness, social anxieties, igniting romantic interests, academic pressures, and attempts to balance a desire for immediate gratification with an understanding of the importance of long-term goals and consequences. These changes can be both exhilarating and overwhelming, creating new challenges in the integration of cognitive and emotional processes in ways that require skills in emotion regulation.
Some evidence suggests that the adolescent brain may not be fully prepared to handle these challenges. Brain regions that subserve cognitive control functions mature more gradually over the course of adolescence and young adulthood, and are believed to be largely independent of pubertal timing (Steinberg et al., 2006). For example, regions of the prefrontal cortex that underpin higher cognitive-executive functions show functional changes that continue well into late adolescence and even early adulthood (Jernigan & Sowell, 1997; Lewis, 1997; Sowell & Jernigan, 1998). Perhaps as a result of this continued maturation, adolescents appear to perform cognitive tasks less efficiently than adults (Luna et al., 2001) and utilize a different set of brain structures than adults to accomplish certain attentional tasks in the context of emotion (Monk et al., 2003).
Thus, it is believed that puberty directly affects neural systems involved in social and emotional responsivity, whereas development of systems mediating higher cognitive functions occurs slowly, continues well beyond pubertal maturation, and is relatively independent of pubertal timing (Dahl, 2004; Nelson et al., 2002). With pubertal onset occurring at earlier ages (Herman-Giddens, 2006; Worthman, 1999), adolescents may now find themselves in emotionally challenging situations as much as a decade before having developed the full capacity to regulate the associated emotion. This has led to a metaphor for the historical advancement of puberty as “starting the engines with an unskilled driver” (Dahl, 2004). This dilemma may explain, in part, the fact that adolescents often make poor decisions (e.g. high-rates of participation in dangerous activities such as automobile accidents, drug use, and unprotected sex) despite understanding cognitively the risks involved (Cauffman & Steinberg, 1995; Reyna & Farley, 2006; Slovic, 1987, 2000). These decisions may reflect an imbalance in cognitive and emotional influences on decision-making and behavior during adolescence, with the scale temporarily tipped toward emotional influences until skills in cognitive control gradually “catch-up” (Ernst et al., 2005).
Despite this compelling model, few empirical studies have examined puberty-specific changes in affective systems in human populations. There is a need to better understand normative changes in emotional information processing across pubertal development (Steinberg et al., 2006). In the current study, we address this question by examining whether pubertal differences exist in pupillary reactivity to emotional stimuli among a sample of typically developing children and adolescents.
The Pupillometric Approach to Studying Emotional Information Processing
Pupillometry is a novel approach to studying emotional information processing that holds promise for understanding puberty-specific changes in affective systems. An ancient proverb holds that the eye is “the window to the soul.” In fact, research suggests that the pupil does provide a window into the activity of the mind. Pupil dilation provides a peripheral index of brain activation in response to a specific stimulus. The pupil becomes more dilated in response to stimuli that require greater cognitive load or that have greater emotional intensity (Beatty, 1982; Beatty & Lucero Wagoner, 2000; Siegle, Steinhauer, Carter, Ramel, & Thase, 2003a; Siegle, Steinhauer, & Thase, 2004). For example, research has carefully demonstrated incremental increases in pupil dilation with conditions requiring greater memory or cognitive effort, such as remembering more digits, up until a person reaches the limits of his or her mental capacity (Granholm, Asarnow, Sarkin, & Dykes, 1996; Kahneman & Beatty, 1966).
This response occurs because the pupil is innervated by brain structures involved in both cognitive and emotional processing. Inhibition of the constrictor muscle occurs through parasympathetic innervation of the Edinger Whestphal nucleus which receives extensive inputs from cortical and limbic regions. Stimulation of the dilator muscle occurs through a hypothalamic pathway which also receives corticolimbic inputs. Thus, stimulation of limbic regions such as the amygdala increases pupil dilation (Koikegami & Yoshida, 1953), as does stimulation of the midbrain reticular formation (Beatty, 1986). The midbrain reticular formation receives afferent projections from the frontal cortex, particularly, structures such as the anterior cingulate cortex, which are implicated in emotion regulation (Szabadi & Bradshaw, 1996), and sends efferent projections to the ocular motor nuclei. Concurrent pupillary/fMRI studies suggest that pupil dilation provides a summative index of task-related cognitive/affective brain activity (Siegle, Steinhauer, Stenger, Konecky, & Carter, 2003b). Pupil dilation also provides information about the time-course of brain activation in response to a stimulus. The pupil remains dilated as long as the processing demand persists and, because pupil dilation can be sampled every 16 ms, provides a dynamic measure of changes in cognitive/affective load following exposure to the stimulus.
Pupillometry has long been used in cognitive science to understand cognitive processes (see Beatty & Lucero Wagoner, 2000). The role of the pupil in emotional processing has also been recognized for a long time (Janisse, 1973), although remarkably little research has been conducted in this area. Early studies of the pupillary response to emotion refuted the original theory put forward by Hess (1965) that the pupil dilates in response to attractive stimuli and constricts in response to aversive stimuli, instead demonstrating that the pupil dilates to both positive and negative emotional stimuli (Janisse & Peavler, 1974; Stelmack & Mandelzys, 1975). Emotion researchers have recently shown renewed interest in utilizing the pupillometric approach in emotional information processing paradigms. For example, Partala and Surakka (2003) found that the pupils of normal adults dilated more to positive and negative sounds than to neutral sounds. In a study using an emotion regulation paradigm, Urry et al. (2006) recently demonstrated that when participants are asked to intentionally regulate their affect, initial and sustained pupil dilation is increased.
Siegle and colleagues have shown that patterns of pupil dilation to emotional stimuli are altered in depressed adults relative to normal controls. For example, depressed adults show increased and sustained pupil dilation on a task requiring identification of the valence of emotional words for up to 30 seconds after their responses compared to normal controls (Siegle, Granholm, Ingram, & Matt, 2001; Siegle et al., 2003a). This finding has been replicated in both medicated and unmedicated adults with MDD and has been shown to be specific to emotional rather than purely cognitive information processing (Siegle et al., 2001; Siegle, Steinhauer, Carter, & Thase, submitted; Siegle et al., 2004). It is correlated with self-reported rumination and thus appears to reflect sustained elaboration on emotional information (Siegle et al., 2003a).
Although researchers have used pupil dilation as an index of cognitive processing in children and adolescents (Gardner, Philp, & Radacy, 1978; Juris & Velden, 1977), we are only aware of one study that has used pupillometry to examine emotional information processing in children and adolescents. Silk et al. (2007) examined depressed and typically developing children’s pupil dilation to emotional words on a valence identification task similar to the task completed by depressed adults in Siegle et al.’s (2001, 2003a) studies. We found that depressed children and adolescents exhibited a diminished late pupil dilation response to negative emotional words relative to controls that was related to greater depressive severity. We also found that children with lower levels of late pupil dilation to negative words in the laboratory reported experiencing higher levels of negative emotion, lower positive emotion, and a lower ratio of positive to negative emotion in their everyday lives using Ecological Momentary Assessment, suggesting that the finding related to problems in emotion regulation in depressed youth. These findings were unique to sustained processing. Overall, the Silk et al. (2007) findings suggested that depressed children might be avoiding negative emotional information, blunting their emotional response, or engaging in less regulation in response to negative emotional information. Importantly, this pattern differed from Siegle et al.’s (2001, 2003a) findings for depressed adults, which showed increased pupil dilation to negative words in depressed subjects relative to controls. This discrepancy highlighted the need for normative data on the developmental trajectory of pupillary reactivity to emotional information, which the present study was designed, in part, to address.
The Current Study
This study investigates pupil dilation to emotional stimuli among a new sample of pre/early and mid/late pubertal typically developing children and adolescents in the seconds following presentation of emotional words. We examined both initial pupillary reactivity to emotional words as well as sustained pupil dilation after the word was no longer on screen. Because of theorized increases in sensitivity to affective stimuli, we hypothesized that mid/late pubertal children would show greater initial pupillary reactivity to emotional words than pre/early pubertal children. We also conducted exploratory analyses to examine whether sustained processing of emotional words differed across pubertal development. Finally, we hypothesized that mid/late pubertal children would have a slower reaction time to label the emotional words than pre/early pubertal children due to emotional interference effects, and would rate themselves as more emotional during the laboratory visit.
Method
Participants
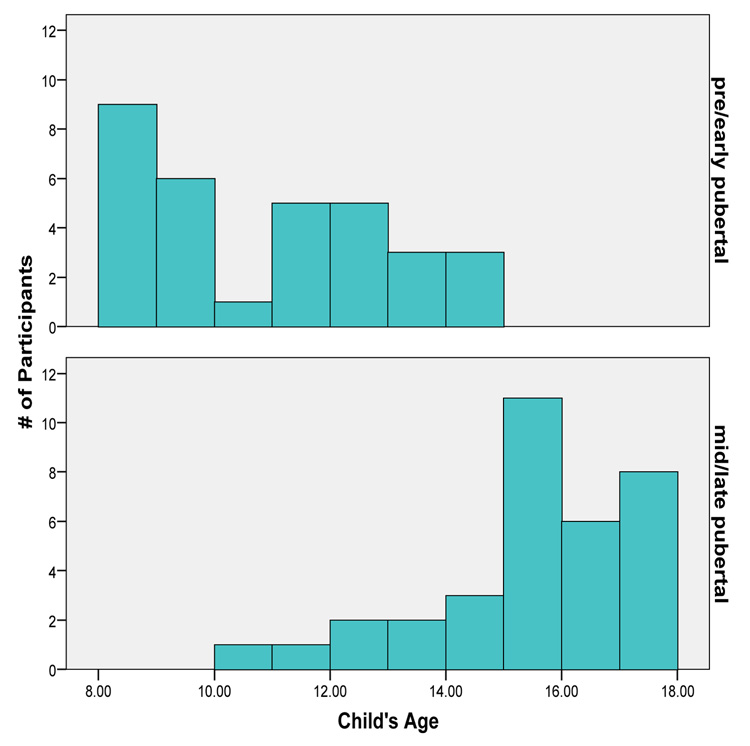
Participants were 64 typically developing children and adolescents. Participants (39 female) ranged in age from 8.1 to 17.9 (M = 13.2; SD = 3.0). 34 participants were in the mid/late pubertal group (ages 10.96 – 17.91, M = 15.43; SD = 1.74), and 32 were in the pre/early pubertal group (ages 8.06 – 14.75, M = 10.83; SD = 2.08). Figure 1 displays the distribution of age as a function of pubertal status group and additional demographic characteristics are presented in Table 1. Exclusion criteria for the study included: (a) symptoms suggestive of an Axis I psychiatric disorder based on the Child or Adolescent Symptom Inventory-4 (Gadow & Sprafkin, 1998a, 1998b), (b) the existence of a major systemic medical illness, (c) a history of serious head injury, or (d) having eye problems or difficulties in vision not corrected by the use of glasses or contact lenses.
Figure 1.
Distribution of participants’ age as a function of pubertal status group.
Table 1.
Demographic Characteristics of Participants
| Pre/Early-Pubertal | Mid/Late-Pubertal | t/χ2 | |
|---|---|---|---|
| (n = 32) | (n = 34) | ||
| Sex (% Female) | 50.00 | 67.6.00 | x2 [1]= 2.12 |
| Ethnicity (% White) | 77.40 | 88.20 | x2[1]= 1.35 |
| SES (Hollingshead) | |||
| M | 52.64 | 50.97 | t[63] = .66 |
| SD | 10.56 | 10.01 | |
| Age | |||
| M | 10.83 | 15.43 | t[64] = −9.78** |
| SD | 2.08 | 1.74 | |
| PDS+ | |||
| M | 1.91 | 3.29 | t[49] = −12.68** |
| SD | .52 | .32 |
p < .01
SES = Socioeconomic Status
PDS = Pubertal Development Scale
see revised PDS scoring criteria in method section.
Procedure
The University’s Institutional Review Board approved the study. All participants were recruited from community advertisements and normal control samples in existing research projects. To participate in the study, children and their parents were required to sign assents and informed consents, respectively. Because this study focused on a normative sample of children, participants’ psychiatric symptomatology was assessed using a questionnaire that screens for DSM-IV Axis I disorders, the Child or Adolescent Symptom Inventory-4 (Gadow & Sprafkin, 1998a, 1998b), with the parent or guardian serving as the informant. Participants completed an initial phone screen and one 2 hour laboratory visit at the Child and Adolescent Neurobehavioral Laboratory, during which event-related potential and pupil dilation assessments were completed using methods described previously (Ladouceur et al., 2005; Siegle et al., 2003a; Silk et al., 2007).
Self-Report Measures
Current affect
The Positive and Negative Affect Schedule for Children (PANAS-C; Laurent et al., 1999) was used as a child-report measure of current feelings of positive and negative affect at the beginning of the testing session. 30 emotions were rated on a five-point scale, with positive emotions summed to form a Positive Affect scale and negative emotions summed to form a Negative Affect scale. The PANAS-C possesses high internal consistency and good convergent and divergent validity (Laurent et al., 1999).
Demographic information
A Sociodemographic questionnaire was used to obtain sociodemographic information from parent and child. This questionnaires included information regarding basic socio-demographic information such as age, gender, family history of affective disorders, and socio-economic status (Hollingshead, 1975).
Psychiatric symptomatology
To screen for Axis 1 psychiatric disorders in this normative sample of children, parents completed a paper and pencil psychiatric screening inventory about child symptomatology. Parents of children 12 years of age and older completed the Adolescent Symptom Inventory 4 (ASI-4; Gadow & Sprafkin, 1998a) and parents of children less than 12 completed the Child Symptom Inventory 4 (CSI-4; Gadow & Sprafkin, 1998b). The ASI-4 and CSI-4 both inquire about child behavior over 17 categories related to DSM-IV (American Psychiatric Association, 1994) diagnostic categories. The ASI-4 and CSI-4 demonstrate convergent and discriminant validity with corresponding scales of the Child Behavior Checklist (Achenbach, 1991) and with clinician diagnoses (Gadow & Sprafkin, 1998a, 1998b). The CSI-4 has also been shown to correlate with DSM-IV diagnoses (Gadow, Devincent, Pomeroy, & Azizian, 2005).
Pubertal development
Pubertal status was measured using the Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988), a self-report measure of pubertal status completed by each child that has been shown to have good psychometric properties (Petersen et al., 1988). To score the PDS, we only included questions that indexed the three main developmental axes of puberty: growth (item 1), adrenal (items 2, 3), and gonadal (items 4, 5-males only, 6-females only; see Dorn, Dahl, Woodward, & Biro, 2006). The questions that met these criteria required participants to rate puberty-related physiological changes. The excluded questions asked for subjective measurements of height, weight and pubertal completion as well as the assessment of pubertal development in relation to peers. A composite measure was created on a 4 point scale with a median score of 2.8. A median split was used to place participants into pre-to-early and mid-to-late pubertal groups. These decisions were based on earlier studies in our laboratory that included Tanner staging by physical exam and hormonal measures of puberty in conjunction with the PDS. The PDS has been shown to correlate with Tanner Stages on physical exam (Brooks Gunn, Warren, Rosso, & Gargiulo, 1987). Recently, Shirtcliff et al. (2007) found that the PDS was correlated with Tanner stages based on physical exam and was also a good predictor of basal hormones responsible for advancing pubertal development.
Pupil Dilation Assessment
The pupil dilation testing occurred in a moderately lit room in which the experimenter was present. Stimuli were displayed in dark gray on a light gray computer screen. Participants sat approximately 122 cm from the bottom of the stimulus. Stimuli were lowercase letters approximately 1 cm high, subtending .45° of visual angle. Reaction times were recorded using a game pad capable of reading reaction times with millisecond resolution. The game pad was modified to have only three working buttons, arranged in a triangle, so that respondents’ fingers were nearly equidistant from each possible response. To account for differential response latencies to different buttons, the mapping of game-pad buttons to responses was counterbalanced across participants.
Pupil dilation was recorded using methods previously described and tested (Siegle et al., 2001; Siegle et al., 2003a). In brief, data were collected using a table-mounted RK-726 eye-tracker. The eye-tracker consisted of a video camera and infrared light source that were pointed at a participant’s eye, and a device that tracked the location and size of the pupil using these tools. Pupil size was recorded at 60 Hz (every 16 ms) and passed digitally from the eye-tracker to a computer that stored the acquired data along with signals marking the beginning of trials, the end of fixation, stimulus onset time, and reaction time. The resolution for a typical participant was better than .05 mm pupil diameter.
Word Valence Identification Task and Word Recall
Pupil dilation was collected while participants completed a Word Valence Identification Task (VID). Participants were instructed to identify the emotional valence of 66 words by pressing a corresponding button as quickly and accurately as possible. Using the three button game-pad described above, participants pressed the button that corresponded with their rating of the word as positive, negative, or neutral. The positive (i.e. birthday), negative (i.e. divorce), and neutral (i.e. lamp) words used in the VID task were chosen from a corpus of emotional words normed for use with children and adolescents (Neshat-Doost, Moradi, Taghavi, Yule, & Dalgleish, 1999). Twenty-two words from each valence category were selected and balanced for word length and frequency. Each trial included a 1 second fixation mask (a row of X’s that they were instructed to look at), the presentation of the word for 5 seconds, and a mask (another row of X’s) for the 6 second inter-trial interval. A word recall assessment was administered following the Word Valence Identification Task. Children were given one minute and thirty seconds to recall as many of the words from the task as possible. For the purposes of this analysis, we calculated indices of number of emotional (positive or negative) and neutral words recalled.
Data Selection, Cleaning, and Reduction
Trials with reaction times below 100 ms and above 4.9 s were discarded as outliers (Matthews & Southall, 1991). Data were cleaned using our laboratory’s standard procedures (derived from Granholm et al., 1996). Blinks were identified as large changes in pupil dilation occurring too rapidly to signify actual dilation or contraction. Trials comprised of over 50% blinks were removed from consideration. These procedures resulted in the elimination of M = 16 trials per subject. Linear interpolations replaced blinks throughout the data set. Data were smoothed using a 10-point weighted average filter. Then, linear trends in pupil dilation calculated over blocks of trials were removed from pupil dilation data to eliminate effects of slow drift in pupil diameter not related to trial characteristics. The final pupil dilation variable used in analyses was a measure of change in pupil diameter from baseline calculated for each sample (every 16 ms) and then averaged across samples for each second in a trial. Baseline pupil diameter was calculated separately for each trial based on the average dilation over the 167ms (10 samples) preceding the onset of the stimulus. Baseline pupil diameter was subtracted from pupil diameter after stimulus onset to produce the final pupil dilation index. This index therefore represents a change in millimeters from baseline. Pupil dilation was then averaged across seconds in a trial to create pupil dilation waveforms depicting the average time course of pupil dilation across a 12 second trial by word valence (positive, negative, or neutral) or puberty group.
Plan of Analyses
Pupil dilation analyses were conducted using mixed effects models with an autoregressive (AR1) covariance structure, using pupil dilation as the dependent variable. Models included pupil dilation at each second for each valence per subject, with subject treated as a random effect and time and valence as repeated measures. Fixed effects included pubertal group, valence, waveform segment, and all interactions. Based on previous studies (e.g. Siegle et al., 2003a; Silk et al., 2007), we focused on two specific regions of interest in the pupil dilation waveform: (1) a “peak” segment defined as the period 2–4 seconds following word presentation, and (2) a “late” segment defined as the latter 3 seconds of the period during which the word was off-screen (9–12 secs). The peak segment represents initial reactivity and the late segment represents sustained processing. Because the pupil continues to grow throughout childhood and adolescence, peaking somewhere between the ages of 6 and 20 (Boev et al., 2005; Kohnen, Zubcov, & Kohnen, 2004; MacLachlan & Howland, 2002), baseline pupil size could introduce a confound, as having a larger pupil would allow for a greater range of reactivity. For this reason, we also included a covariate for baseline pupil diameter to account for potential differences in pupillary reactivity based on differences in pupillary size across pubertal development.
To follow up significant effects from the mixed models, group and valence contrasts on pupil dilation were examined at each sample along pupil dilation waveforms. Guthrie and Buchwald’s (1991) contiguity threshold technique was used to control type I error across these tests. This technique defines the size of a temporal window over which a series of contiguous point-by-point tests could be considered significant, controlling error across all tests at p < .05. Autocorrelation is accounted for via Monte Carlo simulations of the maximum length of adjacent significant tests present in less than 5% of simulated data with a similar autocorrelation structure to acquired empirical data. Using this technique, regions of the waveforms were considered significantly different when over .78 secs of consecutive tests were statistically significant at p < .1 in the peak (2–4 second) region and when over .95 secs of consecutive tests were statistically significant at p < .1 in the late (9–12 second) region.
Results
Table 1 shows demographic characteristics of the sample. As shown in Table 1, there were no gender, race, or socio-economic status differences between the pre/early- and mid/late-pubertal groups.
PANAS-C Ratings and Reaction Times
Table 2 presents comparison of puberty groups on behavioral and subjective measures. We first examined whether puberty groups differed on self-reported emotionality in the lab. We found that mid/late-pubertal children rated their current negative affect on the PANAS-C higher (M = 1.82, SD = .53) than pre/early-pubertal children (M = 1.58, SD = .38). There were no significant differences in ratings of positive affect on the PANAS-C, although mid/late-pubertal children were higher (M = 2.74, SD = .36) than pre/early-pubertal children (M = 2.53, SD = .40) on a global measure of emotionality that included the average of all positive and negative items on the PANAS-C.
Table 2.
Pubertal Differences in Behavioral and Subjective Measures of Emotional Processing
| Pre/Early-Pubertal | Mid/Late Pubertal | t | Cohen’s d | |
|---|---|---|---|---|
| (n = 32) | (n = 34) | |||
| PANAS-C | ||||
| Positive | 1.16 | .27 | ||
| M | 3.48 | 3.65 | ||
| SD | .73 | .49 | ||
| Negative | 2.07* | .52 | ||
| M | 1.58 | 1.82 | ||
| SD | .38 | .53 | ||
| Emotional | 2.19* | .55 | ||
| M | 2.53 | 2.74 | ||
| SD | .40 | .36 | ||
| Word Recall | ||||
| Neutral | .90 | .22 | ||
| M | 2.06 | 2.62 | ||
| SD | 2.37 | 2.64 | ||
| Emotional | 2.84** | .70 | ||
| M | 5.72 | 7.94 | ||
| SD | 2.98 | 3.37 | ||
| Reaction Time | ||||
| Positive | 2.84** | 1.27 | ||
| M | 1741.77 | 1311.92 | ||
| SD | 399.33 | 264.79 | ||
| Negative | 3.68** | .90 | ||
| M | 1805.31 | 1395.56 | ||
| SD | 539.58 | 351.87 | ||
| Neutral | 4.52** | 1.11 | ||
| M | 1762.73 | 1385.71 | ||
| SD | 380.44 | 294.31 |
p < .05
p < .01
PANAS-C = Positive and Negative Affect Schedule for Children
Next, we examined participants’ harmonic mean reaction times (time taken to classify words) on the valence identification task to test whether puberty groups differed in overall reaction time or reaction time to words of a specific valence. A repeated measures ANOVA indicated that there were no differences in reaction times by word valence and no pubertal group X valence interactions, but mid/late-pubertal children responded more quickly to all words (M = 1364.40, SD = 274.65) than pre/early-pubertal children (M = 1769.94, SD = 400.20).
Pupil Dilation
We examined pupil dilation waveforms to test for group differences in pupil dilation during the KVID task. Mixed effects analyses indicated a main effect on pupil dilation for word valence (F(2,877) = 3.44; p < .05) and a main effect for waveform segment (F(1,489) = 59.50; p < .001) that was qualified by a group × segment interaction (F(1,489) = 4.64; p < .05). The group × valence × segment interaction did not reach statistical significance (F(1,922) = 2.15; p = .12). There was no effect of baseline pupil diameter on pupil dilation during the task (F(1,394) = .03; p = .86).
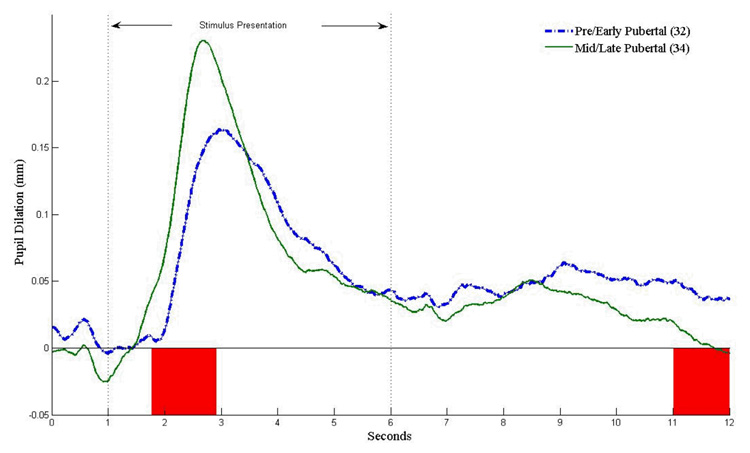
Pairwise comparisons of estimated marginal means for the valence effect revealed that pupil dilation across the waveform was greater to neutral words (M = .07 mm) than to positive words (M = .05 mm); however, this difference was not significant using Guthrie and Buchwald’s (1991) contiguity threshold criterion. To follow up the group × segment interaction, group contrasts on pupil dilation were examined at each point along pupil dilation waveforms within the peak and late regions. Results of these analyses, shown in Figure 2, indicate that pubertal groups differed in their peak pupil dilation, with mid/late-pubertal children showing greater peak pupil dilation than pre/early-pubertal children, regardless of word valence (1.78 to 2.93s: t(64) = 2.40, p < .05, d = .59). Pubertal groups also differed in late pupil dilation, with mid-late pubertal children showing decreased late pupil dilation compared to pre/early-pubertal children in the final second of the task (11.03 to 12.00s: t(64) = −2.05, p < .05, d = −.50).
Figure 2.
Pubertal group differences in pupil dilation to all words. Shaded regions are statistically significant at p < .05.
We also conducted exploratory analyses to examine specificity of these findings to pubertal status. Because age is measured more precisely than puberty, it will typically wash out the effects of puberty when it is included in the same model; thus, this is a conservative analysis. However, the high number of degrees of freedom provided by measuring pupil dilation repeatedly over time (one measurement per second for 12 measurements per participant per condition), and the analysis of this data in a mixed effects model accounting for temporal autocorrelation among measurements, yielded sufficient degrees of freedom to conduct exploratory analyses including both age and puberty. To do so, we recomputed the mixed effects model described above controlling for participant’s age. The pubertal group × segment interaction remained significant controlling for age (F(1,489) = 4.75; p < .05). Because there was a significant main effect for age (F(1,397) = 9.24; p < .01), we recomputed the mixed effects model using participants’ age rather than pubertal status. This model included a random effect for subject, repeated effects for time and valence, and fixed effects for age, valence, waveform segment, and all interactions, as well as a covariate for baseline pupil diameter. The effect for valence remained significant (F(2,877) = 4.73; p < .01); however, the age × segment interaction was not significant (F(1,489) = 2.74; p = .10). Furthermore, there were not significant correlations between peak pupil dilation and age (r = .18; p = .15) or between late pupil dilation and age (r = .02; p = .88), suggesting that the pupil dilation findings were not driven by age.
Word Recall
To examine whether word recall on the free recall test immediately following the word valence identification task was associated with pubertal status, we conducted a multinomial logistic regression to predict puberty group membership from recall of emotional and neutral words. Number of emotional words recalled emerged as a predictor of pubertal status (B = −.26, p < .01), but not number of neutral words recalled (B = −.15, p = .18). Follow-up t-tests indicated that mid/late-pubertal children remembered more emotional words (M = 7.94, SD = 3.37) than pre/early-pubertal children (M = 5.72, SD = 2.98; t = 2.84; p < .01). There were no differences in the number of neutral words recalled by mid/late-pubertal children (M = 2.62, SD = 2.64) and pre/early-pubertal children (M = 2.06, SD = 2.37; t = .90, p = .37).
Relations between Pupil Dilation and Word Recall, PANAS-C Ratings, and Reaction Time
We examined relations between behavioral and subjective measures using bivariate correlations. Table 3 shows intercorrelations between PANAS-C ratings, reaction times, and participants’ word recall. These correlations reveal that current positive affect ratings on the PANAS-C were not related to other indices of emotional information processing, but ratings of current negative affect and overall emotionality on the PANAS-C were associated with remembering more emotional words on the free recall task. Additionally, there were inverse relationships between emotional words and reaction time on all conditions of the task, with children who responded faster remembering more emotional words. Relationships between reaction time and memory for neutral words were less strong, with significant associations between memory for neutral words and reaction times to positive words but not to negative or neutral words. Together, the findings suggest that youth higher in emotionality had faster reaction times on the KVID task as well as biased memory for emotional words.
Table 3.
Inter-Correlations between Behavioral and Subjective Measures of Emotional Processing
| PANAS PA | PANAS NA | PANAS EMOT | WR Neutral | WR EMOT | RT Positive | RT Negative | RT Neutral | |
|---|---|---|---|---|---|---|---|---|
| 1. PANAS PA | - | |||||||
| 2. PANAS NA | .02 | |||||||
| 3. PANAS EMOT | .80** | .62** | ||||||
| 6. WR Neutral | .09 | −.02 | .06 | |||||
| 7. WR EMOT | .13 | .25* | .25* | −.15 | ||||
| 8. RT Positive | −.05 | −.06 | −.08 | −.35** | −.40** | |||
| 9. RT Negative | −.07 | −.03 | −.07 | −.22 | −.40** | .81** | ||
| 10. RT Neutral | −.05 | .07 | .09 | −.14 | −.38** | .78** | .78** | - |
p < .05
p < .01
p < .001
PANAS= Positive and Negative Affect Schedule for Children
PA = Positive affect
NA= negative affect
EMOT= Emotional
WR= Word Recall
RT= Reaction Time
We also conducted exploratory analyses examining how the initial pupillary response was related to behavioral and subjective measures of emotionality. As we needed a single peak value for use in correlations, we extracted each participant’s average peak pupil dilation. This was computed by calculating the highest pupil dilation value within the 2 to 4 second window for each trial and averaging this peak value across trials. As puberty groups did not differ in pupil dilation by word valence, peak pupil dilation was collapsed across word valence categories. Table 4 summarizes the correlations between peak pupil dilation and the behavioral and subjective measures. First, as shown in Table 4, there were no relationships between peak pupil dilation and current PANAS-C ratings.
Table 4.
Bivariate Correlations between Initial Peak and Late Pupil Dilation and Behavioral and Subjective Measures
| Pupil Dilation |
||
|---|---|---|
| Peak | Late | |
| PANAS-C | ||
| Positive | .19 | −.08 |
| Negative | .02 | .06 |
| Emotional | .17 | −.03 |
| Word Recall | ||
| Neutral | −.08 | .17 |
| Emotional | .31* | −.03 |
| Reaction Time | ||
| Positive | −.28* | .03 |
| Negative | −.26* | −.00 |
| Neutral | −.35** | −.02 |
| All | −.32* | .00 |
p < .05
p < .01
PANAS-C= Positive and Negative Affect Schedule for Children
Peak =maximum dilation from 2 – 4 secs
Late = average dilation from 9 –12 secs.
Second, we examined whether peak pupil dilation was associated with word recall and reaction time. We conducted a repeated measures regression in which word-recall was a repeated measure and peak pupil dilation was a continuous explanatory variable. There was a significant peak pupil dilation × valence interaction for recall for emotional vs. neutral words (F(1,64) = 5.14; p < .05). To interpret this effect, we examined correlations between pupil dilation and word recall separately for emotional and neutral words. As shown in Table 4, overall peak pupil dilation was associated with greater recall of emotional words (r = .31, p < .05), but was not associated with recall of neutral words (r = −.08, n.s).
Third, we conducted a repeated measures regression in which reaction time was a repeated measure and peak pupil dilation was a continuous explanatory variable. There was a significant main effect for peak pupil dilation that was not moderated by valence (F(1,64) = 7.05; p < .01). As shown in Table 4, greater initial peak pupil dilation was associated with faster reaction times on the emotional word valence identification task regardless of word valence (rall valences = −.32, p < .05).
We also examined whether late (9–12 seconds) pupil dilation to all words was associated with behavioral or subjective ratings of emotional information processing. As shown in Table 4, late pupil dilation was not correlated with subjective ratings of emotionality in the lab. Repeated measures regressions indicated that late pupil dilation was not associated with word recall or reaction time, either alone or in interaction with word valence.
Discussion
In this study, we found evidence that cognitive and affective processes involved in classifying the emotionality of words appear to undergo alterations during pubertal maturation. These cognitive-affective changes were apparent in measures of psychophysiology, memory bias, and subjective ratings of emotion among children in the mid or late stages of puberty compared to children and adolescents who were prepubertal or in the beginning stages of puberty. First, mid-to-late pubertal children and adolescents showed greater peak pupillary reactivity to words during an emotional word identification paradigm than pre-to-early pubertal children and adolescents. This peak pupil dilation was associated with greater memory for emotional but not neutral words on an unexpected free recall task, suggesting that it may be linked to emotionally-relevant processing. This finding was replicated controlling for participants’ age, and was not found using age rather than puberty as a predictor, suggesting that the effect is puberty-specific. Second, mid-to-late pubertal children and adolescents showed a bias toward remembering more emotional words than pre-to-early pubertal children on an unexpected free recall task following the emotional word identification paradigm. Third, we also found that mid-to-late pubertal children rated themselves as higher in emotionality, especially negative affect, during their laboratory visit than pre-to-early pubertal children.
These results are generally consistent with models suggesting that puberty is associated with greater reactivity of neurobehavioral systems involved in emotional information processing (Dahl, 2001; Nelson et al., 2005; Spear, 2000; Steinberg, 2005; Steinberg et al., 2006). However, we did not find that the increased initial pupillary response on the emotional word valence identification task with puberty was moderated by word valence. This parallels Quevedo, Benning, Gunnar, & Dahl’s (this special section) finding of increased startle response with puberty during an affective pictures paradigm irrespective of picture valence. Our finding of increased puberty-linked pupillary reactivity on the emotional word identification task could have occurred through at least two mechanisms: 1) increased devotion of general cognitive processing resources in the pubertal group, or 2) increased affective reactivity in the pubertal group. Because pupil dilation provides a summative index of task-related cognitive and affective brain activity (Siegle et al., 2003b), and because we did not include a purely cognitive task in our protocol, we cannot rule out the possibility that the increased pupillary reactivity is driven primarily by more cognitive processes, such as reading and classifying the word. However, given that pubertal children and adolescents were generally older, it would be expected that these participants would have an easier time performing the cognitive aspects of the task. Thus, we would expect them to devote less mental resources to cognitive aspects of the task such as reading and classifying words than pre-and early pubertal children, resulting in smaller mental effort-related pupil dilation relative to pre and early pubertal participants.
Additionally, we found that participants’ whose pupils initially dilated more in response to the words presented in the emotional valence identification task remembered more emotional words in the unexpected recall task. Initial pupil dilation was not associated with memory for neutral words. This suggests that initial pupil dilation was related to emotional biases in word retrieval, an important late-stage component of information processing models (e.g. Anderson, Qin, Jung, & Carter, 2007). This finding, in conjunction with the emotional context of the task— in which participants’ attention was explicitly directed toward evaluating the emotional qualities of the words presented—suggest that the pubertal difference in peak pupil dilation is at least partially driven by affective processes.
There may also be important maturational changes in cognitive systems related to these findings, such as changes in reactivity to verbal stimuli or engagement in reading. Our use of verbal stimuli is particularly relevant here. (Booth et al. 2004, 2001) have proposed that cerebral maturation occurring after puberty could lead to the automatization of reading routines. Specifically, they hypothesize that brain maturation, along with increased practice and exposure, leads to specialization of a network of left brain regions involved in reading including the angular or supramarginal gyrus. However, the extent of changes in reading-related neural activity beyond late childhood and the timing of such changes relative to puberty is still poorly understood (see Proverbio & Zani, 2005). Future research is also needed to examine whether pubertal differences in pupil dilation are found in more purely cognitive information processing tasks, as well as affective tasks with both verbal and nonverbal stimuli.
Even more likely is the possibility that puberty is linked with changes in processes that are at the interface of cognitive and emotional processing. The valence identification task, which requires youth to make cognitive decisions about potentially emotional stimuli is an example of this type of integration. It requires participants to cognitively process (i.e., pay attention to and classify words according to valence) emotionally salient information. Although tasks that require the integration of cognitive and emotional functions need specific methodological designs in order to be able to tease apart and draw firm conclusions about cognitive versus affective systems, these types of tasks are representative of the types of processes implicated in the decisions and dilemmas that adolescents are faced with on a daily basis. These decisions rarely require pure cognitive processing or pure emotional processing —they require an integration of the two— and this integration may be the adolescents’ biggest challenge. For example, Reyna and Farley (2006) reported that although adolescents are capable of arriving at the same judgments about risky decisions (e.g., drunk driving) as adults, they are somewhat slower in their response. Baird, Fugelsang, and Bennett (2005) demonstrated that such behavioral differences were related to differences in the neural circuitry recruited in adolescents compared to adults. Future studies are needed that are designed to explicitly test the maturation of the neural systems involved in cognitive-emotional integration, particularly the impact of puberty.
The lack of valence moderation in the present study appears to be explained by the fact that children in our sample had a strong pupillary reaction to neutral words as well as to emotional words. In fact, there was a trend for pupil dilation across the waveform to be greater to neutral words than to emotional words, although it was not replicated across a long enough segment of the waveform to be considered significant. There are at least two potential explanations for this relatively strong pupillary reactivity to neutral words. First, because participants’ attention was directed toward evaluating the emotional aspects of each word, participants may have been more likely to view neutral words in an emotional context. Research has shown that the interpretation of neutral stimuli can vary depending on the valences of the other stimuli presented during the experimental context (Russell & Fehr, 1987). For example, the word “pen” when viewed alone may appear relatively neutral; but when asked to evaluate its emotionality an adolescent may begin to think about school and homework and attach a negative evaluation to this word. In fact, we conducted debriefing interviews on a small number of subjects and were struck by the idiosyncratic positive and negative qualities several youth attached to seemingly neutral words such as “pen” and “sheep” and “tree.” It may be that there are few inherently neutral words. Consistent with this explanation, Siegle et al. also found that unmedicated depressed adults’ initial pupil dilation does not differ as a function of word valence when attention is directed toward identifying emotional valence of words (Siegle et al., 2001), although valence specificity was observed in medicated depressed adults (Siegle et al., 2003a).
Second, stimuli that are novel and ambiguous can be arousing (Schwartz et al., 2003; Whalen, 1998). For example, Schwartz et al. (2003) have shown increased amygdala activity in novel versus familiar faces, all with a neutral expression. This is theorized to occur because the amygdala acts to detect and process unexpected or unfamiliar information that might have potential biological importance (Schwartz et al., 2003; Whalen, 1998). Because neutral words may be ambiguous in terms of their valence, these words might elicit greater reactivity of neural systems.
There is some evidence that youth in particular may have a tendency to show affective reactions to neutral stimuli during experimental procedures. For example, Thomas et al. (2001) found that children had greater amygdala reactivity to neutral faces than adults. Youth may thus be more sensitive to the ambiguity of neutral stimuli, or, especially among pubertal children, have a tendency to see emotion even in non-emotional stimuli. Reactivity to neutral stimuli based on either ambiguity or overgeneralization of emotion would likely be intensified when children are asked to classify the valence of neutral words, a task which is much easier for words that are obviously positive or negative than for ambiguous neutral words. These findings do, however, differ from our previous study, which included a small sample of 8–17 year old typically developing children as well as children with Major Depressive Disorder. In this study, we found that children’s initial pupil dilation was greater following negative words than neutral or positive words (Silk et al., 2007). Although both studies used the same words, there were a number of differences in the context in which the task was completed in the two studies, such as time of day, distance from computer screen, and length of overall study (participants in the MDD study stayed in the laboratory overnight). Future research with larger samples of both typically developing and clinical populations of children is needed to resolve this issue.
There are several potential brain mechanisms that might underlie this increased initial pupil dilation during the emotional word identification task. Pupil dilation results from extensive inputs from both cortical and limbic regions of the brain mediated via sympathetic and parasympathetic pathways (Steinhauer, Siegle, Condray, & Pless, 2004). Pupil dilation has been associated with stimulation of the amygdala (Koikegami & Yoshida, 1953) as well stimulation of the frontal cortex and anterior cingulate cortex via connections from the midbrain reticular formation (Beatty, 1986). Thus, increased initial pupil dilation could indicate increased limbic reactivity or increased engagement of regulatory structures. The use of pupil dilation alone cannot distinguish between purely emotional reactions and other processes, such as emotion regulation, that also involve cognitive components (Urry et al., 2006). This lack of specificity is a limitation to the pupillometry approach that could be addressed in future research using concurrent collection of pupillometric and neuroimaging data, which is now available in many fMRI environments (see Siegle et al., 2003b).
One of the strengths of this study is that we were able to show that increased pupil dilation to negative words was specific to the effects of puberty above and beyond participants’ age. Disentangling pubertal and age effects is an important but challenging task given that the two are highly related. There is a need for future studies to be designed in ways that will facilitate the identification of puberty-specific effects, such as selecting youth matched on age but differing on pubertal status (i.e, Quevedo et al., this special section). Using this approach, the researcher can attribute group differences to pubertal effects without having to include a statistical covariate for age in the model. Obtaining the most precise measure of puberty that is possible, such as Tanner staging via physical examination, will also enhance researchers’ ability to detect puberty-specific effects.
We also found that sustained pupillary responses among children in mid-to-late puberty were smaller compared to children and adolescents who were prepubertal or in the initial stages of puberty. Mid-to-late pubertal children and adolescents showed decreased pupil dilation to all words on average in the last second of the trial compared to pre-to-early pubertal children and adolescents, regardless of word valence. Unlike the peak pupil dilation response, this sustained response was not related to memory for emotional words or reaction time. We have previously observed a more pronounced decrease in late pupil dilation response to below baseline levels in depressed children and adolescents relative to controls that is specific to negative words and is correlated with other indices of emotionality (Silk et al. 2007); however, this difference among typically developing children and adolescents is more subtle, occurs over a shorter region of the waveform, and is less clearly associated with emotional processes. The present finding might indicate that children further along in puberty are disengaging their attention from the task more than pre and early pubertal children when the word is no longer on screen. These adolescents might be more bored, or might be demonstrating improved skills in refocusing attention away from emotional material relative to pre and early pubertal children. Although studies have investigated differences in attentional control between adolescents and adults (e.g. Monk et al., 2003), little is known about changes in attentional control as a function of puberty, and further research is needed in this area.
Contrary to our hypotheses, we also found that mid-to-late pubertal children and adolescents had faster reaction times to all words than pre-to-early pubertal children and adolescents. This finding may be a function of faster reading speed among children further along in puberty, as these children are also older. Nevertheless, faster reaction time was associated with remembering more emotional words, suggesting that faster reactions may be reflecting an attentional bias toward the emotional words.
Strengths and Limitations
Several limitations of the present study should be noted. The sample was small, limiting our power to detect small-to-moderate effects. The small sample also limited our ability to examine gender by puberty interactions and to explore differences between prepubertal and early pubertal children and between mid and late pubertal children. Reliance on child-report to assess puberty is another limitation; however, there is evidence that the PDS correlates with Tanner Stages on physical exam and hormonal measures of puberty (Brooks Gunn et al., 1987; Shirtcliff et al., 2007). We also acknowledge that the median split used to create pubertal status groups is relatively arbitrary. As discussed above, future research would benefit from obtaining measures of pubertal development (e.g. physical exam) that are more precise and thus can be included in analyses with age and can be used to create groupings that are less arbitrary.
Finally, we relied on verbal affective stimuli. We chose to use words because they are less visually complex and thus can be more easily balanced for visual characteristics, such as luminosity, that can affect pupil dilation. Despite this advantage, words are subject to developmental differences in reading speed and may also be less ecologically valid than affective faces or pictures. However, affective words have now been used successfully in several studies with child and adolescent samples, and have been useful in delineating developmental pattern of emotional processing (Perez Edgar & Fox, 2003) as well as revealing alterations from normative patterns of emotional information processing in studies youth with and at risk for mood disorders (Gotlib, Traill, Montoya, Joormann, & Chang, 2005; Neshat-Doost, Taghavi, Moradi, Yule, & Dalgleish, 1998; Silk et al., 2007) and anxious youth (Taghavi, Dalgleish, Moradi, Neshat Doost, & Yule, 2003; Vasey, El-Hag, & Daleiden, 1996).
This study also has several notable strengths. It utilized a multi-method approach including physiological, behavioral, and subjective measures of emotional information processing in conjunction with pubertal assessment, a domain often overlooked in developmental research. This is the first study of which we are aware to assess pupil dilation to emotional stimuli in typically developing children and adolescents. Findings suggest that this novel approach is a promising method for understanding emotional information processing in children and adolescents. It provides strong temporal precision regarding the pattern of early and late emotional processing that is not available by self-report or even by many other psychophysiological measurement approaches. Furthermore, pupil dilation is measured using non-invasive and affordable techniques that were easily tolerated by children. Research with clinical samples of depressed adults (Siegle et al., 2001; Siegle et al., 2003a) as well as our initial work with depressed children (Silk et al., 2007) suggest that pupil dilation to emotional words may have clinical relevance, which is particularly exciting since pupil dilation could eventually be measured in clinical settings. The finding that the pubertal differences in pupil dilation remained significant controlling for age suggests that this method may be indexing an important neurobehavioral change that is specific to pubertal maturation. Future concurrent pupil/fMRI studies may be able to combine the temporal specificity of the pupillometry approach with the spatial specificity of the fMRI approach to delineate the specific neural systems underlying these puberty-linked changes in the time course of pupillary response to emotional words.
Clinical Implications
The findings of the present study support models that view puberty as a period of enhanced sensitivity to emotional stimuli (Dahl, 2001; Spear, 2000; Steinberg, 2005; Steinberg et al., 2006). Given evidence that prefrontal systems subserving regulatory control are still developing throughout adolescence (Sowell & Jernigan, 1998), the pubertal window is a critical period for parents, teachers, and other adults to support children in practicing and refining skills for emotion regulation. Paradoxically, this occurs at a time when parents may be inclined to distance themselves from their children emotionally. Despite more physically mature appearances and more conflictual parent-child relationships (Steinberg, 1987), parents remain critical sources of support for adolescents during the emotional journey through puberty (Steinberg & Silk, 2002). The pubertal period may also provide a window of opportunity for intervening in clinical and vulnerable populations of children. Early intervention during this period of relative plasticity could lead to long-term reductions in health-related cost and suffering in adulthood.
Author Note
We are grateful to Joanna Prout for her assistance in this project. We also thank the participants and their families. This research was supported by a National Institute of Mental Health (NIMH) R24 research network MH67346 (Ronald E. Dahl, PI) and K01 MHO73077 (Jennifer S. Silk, PI).
References
- Achenbach TM. Manual for the child behavior checklist/4-18 and 1991 profile. Burlington: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Alpert JE, Maddocks A, Rosenbaum JF, Fava M. Childhood psychopathology retrospectively assessed among adults with early onset major depression. Journal of Affective Disorders. 1994;31:165–171. doi: 10.1016/0165-0327(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Qin Y, Jung K-J, Carter CS. Information-processing modules and their relative modality specificity. Cognitive Psychology. 2007;54:185–217. doi: 10.1016/j.cogpsych.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Worthman CM. Puberty and depression: The roles of age, pubertal status and pubertal timing. Psychological Medicine. 1998;28:51–61. doi: 10.1017/s003329179700593x. [DOI] [PubMed] [Google Scholar]
- American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, D.C.: Author; 1994. [Google Scholar]
- Baird A, Fugelsang J, Bennett C. What Were You Thinking: an fMRI Study of Adolescent Decision-Making. New York, USA: Poster presented at Cognitive Neuroscience Society Meeting; 2005. Apr, [Google Scholar]
- Beatty J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological Bulletin. 1982;91:276–292. [PubMed] [Google Scholar]
- Beatty J. The pupil system. In: Coles MGH, editor. Psychophysiology systems processes and applications. New York: Guilford; 1986. pp. 44–50. [Google Scholar]
- Beatty J, Lucero Wagoner B. The pupillary system. In: Cacioppo JT, editor. Handbook of psychophysiology. 2nd ed. New York, NY: Cambridge University Press; 2000. pp. 142–162. [Google Scholar]
- Boev AN, Fountas KN, Karampelas I, Boev C, Machinis TG, Feltes C, et al. Quantitative pupillometry: Normative data in healthy pediatric volunteers. Journal of Neurosurgery. 2005;103:496–500. doi: 10.3171/ped.2005.103.6.0496. [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Mesulam MM. Development of brain mechanisms for processing orthographic and phonologic representations. Journal of Cognitive Neuroscience. 2004;16:1234–1249. doi: 10.1162/0898929041920496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Van Santen FW, Harasaki Y, Gitelman DR, Parrish TB, et al. The development of specialized brain systems in reading and oral-language. Child Neuropsychology. 2001;7:119–141. doi: 10.1076/chin.7.3.119.8740. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls' pubertal status. Child Development. 1987;58:829–841. [PubMed] [Google Scholar]
- Caspi A, Lynam D, Moffitt TE, Silva PA. Unraveling girls' delinquency: Biological, dispositional, and contextual contributions to adolescent misbehavior. Developmental Psychology. 1993;29:19–30. [Google Scholar]
- Cauffman E, Steinberg L. The cognitive and affective influences on adolescent decision-making. Temple Law Review. 1995;68:1763–1789. [Google Scholar]
- Chambers R, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: A critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung T, Martin CS, Winters KC. Diagnosis, course and assessment of alcohol abuse and dependence in adolescents. In: Galanter M, editor. Alcohol problems in adolescents and young adults: Epidemiology, neurobiology, prevention, and treatment. New York: Springer US; 2006. pp. 5–27. [Google Scholar]
- Cicchetti D, Rogosch FA. A developmental psychopathology perspective on adolescence. Journal of Consulting and Clinical Psychology. 2002;70:6–20. doi: 10.1037//0022-006x.70.1.6. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Affect regulation, brain development, and behavioral/emotional health in adolescence. CNS Spectrums. 2001;6:1–12. doi: 10.1017/s1092852900022884. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: A period of vulnerabilities and opportunities. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Dahl RE, Woodward HR, Biro F. Defining the boundaries of early adolescence: A user's guide to assessing pubertal status and pubertal timing in research with adolescents. Applied Developmental Science. 2006;10:30–56. [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2005;35:1–14. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadow KD, Devincent CJ, Pomeroy J, Azizian A. Comparison of DSM-IV symptoms in elementary school-age children with PDD versus clinic and community samples. Autism. 2005;9:392–415. doi: 10.1177/1362361305056079. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J. Adolescent symptom inventory - 4: Norms manual. Stony Brook, NY: Checkmate Plus; 1998a. [Google Scholar]
- Gadow KD, Sprafkin J. Child symptom inventory - 4: Screening manual. Stony Brook, NY: Checkmate Plus; 1998b. [Google Scholar]
- Gardner RM, Philp P, Radacy S. Pupillary changes during recall in children. Journal of Experimental Child Psychology. 1978;25:168–172. doi: 10.1016/0022-0965(78)90046-2. [DOI] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH., Jr Coming of age too early: Pubertal influences on girls' vulnerability to psychological distress. Child Development. 1996;67:3386–3400. [PubMed] [Google Scholar]
- Gotlib IH, Traill SK, Montoya RL, Joormann J, Chang K. Attention and memory biases in the offspring of parents with bipolar disorder: Indications from a pilot study. Journal of Child Psychology and Psychiatry. 2005;46:84–93. doi: 10.1111/j.1469-7610.2004.00333.x. [DOI] [PubMed] [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36:1768–1776. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Granholm E, Asarnow RF, Sarkin AJ, Dykes KL. Pupillary responses index cognitive resource limitations. Psychophysiology. 1996;33:457–461. doi: 10.1111/j.1469-8986.1996.tb01071.x. [DOI] [PubMed] [Google Scholar]
- Guthrie D, Buchwald JS. Significance testing of difference potentials. Psychophysiology. 1991;28:240–244. doi: 10.1111/j.1469-8986.1991.tb00417.x. [DOI] [PubMed] [Google Scholar]
- Herman-Giddens ME. Recent data on pubertal milestones in united states children: The secular trend toward earlier development. International Journal of Andrology. 2006;29:241–246. doi: 10.1111/j.1365-2605.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- Hess EH. Attitude and pupil size. Scientific American. 1965;212(4):1965. doi: 10.1038/scientificamerican0465-46. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven: Yale University Sociology Department; 1975. Unpublished manuscript. [Google Scholar]
- Janisse MP. Pupil size and affect: A critical review of the literature since 1960. The Canadian Psychologist. 1973;14:311–329. [Google Scholar]
- Janisse MP, Peavler WS. Pupillary response today: Emotion in the eye. Psychology Today. 1974;7:60–63. [Google Scholar]
- Jernigan TL, Sowell ER. Magnetic resonance imaging studies of developing brain. In: Keshvan MS, Murray RM, editors. Neurodevelopment and Adult Psychopathology. Cambridge University Press; 1997. pp. 63–70. [Google Scholar]
- Juris M, Velden M. The pupillary response to mental overload. Physiological Psychology. 1977;5:421–424. [Google Scholar]
- Kahneman D, Beatty J. Pupil diameter and load on memory. Science. 1966;154:1583–1585. doi: 10.1126/science.154.3756.1583. [DOI] [PubMed] [Google Scholar]
- Kohnen EM, Zubcov AA, Kohnen T. Scotopic pupil size in a normal pediatric population using infrared pupillometry. Graefes Archive for Clinical & Experimental Ophthalmology. 2004;242:18–23. doi: 10.1007/s00417-003-0735-4. [DOI] [PubMed] [Google Scholar]
- Koikegami H, Yoshida K. Pupillary dilation induced by stimulation of amygdaloid nuclei. Folia Pychiatrica Neurologica Japonica. 1953;7:109–125. doi: 10.1111/j.1440-1819.1953.tb00600.x. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Ryan ND, Casey B. Altered emotional processing in pediatric anxiety, depression, and comorbid anxiety-depression. Journal of Abnormal Child Psychology. 2005;33:165–177. doi: 10.1007/s10802-005-1825-z. [DOI] [PubMed] [Google Scholar]
- Laurent J, Catanzaro SJ, Joiner TE, Jr, Rudolph KD, Potter KI, Lambert S, et al. A measure of positive and negative affect for children: Scale development and preliminary validation. Psychological Assessment. 1999;11:326–338. [Google Scholar]
- Lewis DA. Development of the prefrontal cortex during adolescence: Insights into vulnerable neural circuits in schizophrenia. Neuropsychopharmacology. 1997;16:385–398. doi: 10.1016/S0893-133X(96)00277-1. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, Merriam EP, Garver KE, Minshew NJ, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- MacLachlan C, Howland HC. Normal values and standard deviations for pupil diameter and interpupillary distance in subjects aged 1 month to 19 years. Ophthalmic & Physiological Optics. 2002;22:175–182. doi: 10.1046/j.1475-1313.2002.00023.x. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, et al. Sensation seeking, puberty and nicotine, alcohol and marijuana use in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- Matthews G, Southall A. Depression and the processing of emotional stimuli: A study of semantic priming. Cognitive Therapy and Research. 1991;15:283–302. [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Bloom FE, Cameron JL, Amaral D, Dahl RE, Pine D. An integrative, multidisciplinary approach to the study of brain-behavior relations in the context of typical and atypical development. Development & Psychopathology. 2002;14:499–520. doi: 10.1017/s0954579402003061. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure E, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Neshat-Doost HT, Moradi AR, Taghavi MR, Yule W, Dalgleish T. The development of a corpus of emotional words produced by children and adolescents. Personality and Individual Differences. 1999;27:433–451. [Google Scholar]
- Neshat-Doost HT, Taghavi MR, Moradi AR, Yule W, Dalgleish T. Memory for emotional trait adjectives in clinically depressed youth. 1998 doi: 10.1037//0021-843x.107.4.642. [DOI] [PubMed] [Google Scholar]
- Orr DP, Ingersoll GM. The contribution of level of cognitive complexity and pubertal timing to behavioral risk in young adolescents. Pediatrics. 1995;95:528–533. [PubMed] [Google Scholar]
- Ozer EM, Macdonald T, Irwin CE., Jr . Adolescent health care in the united states: Implications and projections for the new millennium. In: Mortimer JT, Larson RW, editors. The changing adolescent experience: Societal trends and the transition to adulthood. New York: Cambridge University Press; 2002. pp. 129–174. [Google Scholar]
- Partala T, Surakka V. Pupil size variation as an indication of affective processing. International Journal of Human-Computer Studies. 2003;59:185–198. [Google Scholar]
- Patton GC, Viner R. Pubertal transitions in health. The Lancet. 2007;369:1130–1139. doi: 10.1016/S0140-6736(07)60366-3. [DOI] [PubMed] [Google Scholar]
- Perez Edgar K, Fox NA. Individual differences in children's performance during an emotional stroop task: A behavioral and electrophysiological study. Brain and Cognition. 2003;52:33–51. doi: 10.1016/s0278-2626(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Proverbio AM, Zani A. Developmental changes in the linguistic brain after puberty. Trends in Cognitive Sciences. 2005;9:164–167. doi: 10.1016/j.tics.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Quevedo KM, Benning SD, Gunnar MR, Dahl RE. The onset of puberty: Effects on the psychophysiology of defensive and appetitive motivation. Development & Psychopathology. doi: 10.1017/S0954579409000030. (this special section) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick MD, Bearman PS, Blum RW, Bauman KE, Harris KM, Jones J, et al. Protecting adolescents from harm. Findings from the national longitudinal study on adolescent health. JAMA. 1997;278:823–832. doi: 10.1001/jama.278.10.823. [DOI] [PubMed] [Google Scholar]
- Reyna VF, Farley F. Risk and rationality in adolescent decision making: Implications for theory, practice, and public policy. Psychological Science in the Public Interest. 2006;7:1–44. doi: 10.1111/j.1529-1006.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Russell JA, Fehr B. Relativity in the perception of emotion in facial expressions. Journal of Experimental Psychology: General. 1987;116:223–237. [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, et al. Differential amygdalar response to novel versus newly familiar neutral faces: A functional mri probe developed for studying inhibited temperament. Biological Psychiatry Vol 53(10) May 2003, 854-862. 2003;53:854–862. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Heiligenstein M, Hoornstra L, Squires K, Pollak SD. Raging hormones? Stages of pubertal development largely capture underlying hormonal processes in early adolescence; Boston, MA. Paper presented at the Society for Research on Child Development.2007. [Google Scholar]
- Siegle GJ, Granholm E, Ingram RE, Matt GE. Pupillary and reaction time measures of sustained processing of negative information in depression. Biological Psychiatry. 2001;49:624–636. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer S, Carter CS, Thase ME. Is sustained processing specific to emotional information in depression? Evidence from pupil dilation. (submitted) [Google Scholar]
- Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognitive Therapy and Research. 2003a;27:365–382. [Google Scholar]
- Siegle GJ, Steinhauer SR, Stenger VA, Konecky R, Carter CS. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. Neuroimage. 2003b;20:114–124. doi: 10.1016/s1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Steinhauer SR, Thase ME. Pupillary assessment and computational modeling of the stroop task in depression. International Journal Psychophysiology. 2004;52:63–76. doi: 10.1016/j.ijpsycho.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Silk JS, Dahl RE, Ryan ND, Forbes EE, Axelson DA, Birmaher B, et al. Pupillary reactivity to emotional information in child and adolescent depression: Links to clinical and ecological measures. American Journal of Psychiatry. 2007;164:1873–1880. doi: 10.1176/appi.ajp.2007.06111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Steinberg L, Morris AS. Adolescents' emotion regulation in daily life: Links to depressive symptoms and problem behavior. Child Development. 2003;74:1869–1880. doi: 10.1046/j.1467-8624.2003.00643.x. [DOI] [PubMed] [Google Scholar]
- Slovic P. Perception of risk. Science. 1987;236:280–285. doi: 10.1126/science.3563507. [DOI] [PubMed] [Google Scholar]
- Slovic P. What does it mean to know a cumulative risk? Adolescent's perceptions of short-term and long-term consequences of smoking. Journal of Behavioral Decision Making. 2000;13:259–266. [Google Scholar]
- Sowell ER, Jernigan TL. Further MRI evidence of late brain maturation: Limbic volume increases and changing asymmetries during childhood and adolescence. Developmental Neurophysiology. 1998;14:599–617. [Google Scholar]
- Spear LP. Neurobehavioral changes in adolescence. Current Directions in Psychological Science. 2000;9:111–114. [Google Scholar]
- Steinberg L. Impact of puberty on family relations: Effects of pubertal status and pubertal timing. Developmental Psychology. 1987;27:451–460. [Google Scholar]
- Steinberg L. Cognitive and affective development in adolescence. Trends in Cognitive Sciences. 2005;9:69–74. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk-taking in adolescence: New perspectives from brain and behavioral science. Current Directions in Psychological Science. 2007;16(2):55–59. [Google Scholar]
- Steinberg L, Dahl R, Keating D, Kupfer DJ, Masten AS, Pine DS. The study of developmental psychopathology in adolescence: Integrating affective neuroscience with the study of context. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology, Vol 2: Developmental Neuroscience. 2nd ed. Hoboken, NJ: John Wiley & Sons Inc.; 2006. p. xvii.p. 876. [Google Scholar]
- Steinberg L, Morris A. Adolescent development. Annual Review of Psychology. 2000;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Silk JS. Parenting adolescents. In: Bornstein MH, editor. Handbook of parenting: Vol. 1: Children and parenting. 2nd ed. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. pp. 103–133. [Google Scholar]
- Steinhauer SR, Siegle GJ, Condray R, Pless M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. International Journal of Psychophysiology. 2004;52:77–86. doi: 10.1016/j.ijpsycho.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Stelmack RM, Mandelzys N. Extraversion and pupillary response to affective and taboo words. Psychophysiology. 1975;12:536–540. doi: 10.1111/j.1469-8986.1975.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Szabadi E, Bradshaw CM. Autonomic pharmacology of 2-adrenoceptors. Journal of Psychopharmacology. 1996;10 S.3:6–18. [Google Scholar]
- Taghavi MR, Dalgleish T, Moradi AR, Neshat Doost HT, Yule W. Selective processing of negative emotional information in children and adolescents with generalized anxiety disorder. British Journal of Clinical Psychology. 2003;42:221–230. doi: 10.1348/01446650360703348. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, et al. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force. Guide to clinical preventive services. 2nd ed. Alexandria, VA: International Medical Publishing; 1996. [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasey MW, El-Hag N, Daleiden EL. Anxiety and the processing of emotionally threatening stimuli: Distinctive patterns of selective attention among high- and low-test-anxious children. Child Development. 1996;67:1173–1185. [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7:177–188. [Google Scholar]
- Worthman CM. Epidemiology of human development. In: Panter-Brick, Worthman CM, editors. Hormones, health, and behavior: A socio ecological and lifespan perspective. New York, NY: Cambridge University Press; 1999. pp. 47–104. [Google Scholar]