Abstract
Recent studies have revealed that in G protein-coupled receptor signalings switching between G protein- and β-arrestin (βArr)-dependent pathways occurs. In the case of opioid receptors, the signal is switched from the initial inhibition of adenylyl cyclase (AC) to an increase in AC activity (AC activation) during prolonged agonist treatment. The mechanism of such AC activation has been suggested to involve the switching of G proteins activated by the receptor, phosphorylation of signaling molecules, or receptor-dependent recruitment of cellular proteins. Using protein kinase inhibitors, dominant negative mutant studies and mouse embryonic fibroblast cells isolated from Src kinase knock-out mice, we demonstrated that μ-opioid receptor (OPRM1)-mediated AC activation requires direct association and activation of Src kinase by lipid raft-located OPRM1. Such Src activation was independent of βArr as indicated by the ability of OPRM1 to activate Src and AC after prolonged agonist treatment in mouse embryonic fibroblast cells lacking both βArr-1 and -2. Instead the switching of OPRM1 signals was dependent on the heterotrimeric G protein, specifically Gi2 α-subunit. Among the Src kinase substrates, OPRM1 was phosphorylated at Tyr336 within NPXXY motif by Src during AC activation. Mutation of this Tyr residue, together with mutation of Tyr166 within the DRY motif to Phe, resulted in the complete blunting of AC activation. Thus, the recruitment and activation of Src kinase by OPRM1 during chronic agonist treatment, which eventually results in the receptor tyrosine phosphorylation, is the key for switching the opioid receptor signals from its initial AC inhibition to subsequent AC activation.
Classical G protein-coupled receptor (GPCR)2 signaling involves the activation of specific heterotrimeric G proteins and the subsequent dissociation of α- and βγ-subunits. These G protein subunits serve as the activators and/or inhibitors of several effector systems, including adenylyl cyclases, phospholipases, and ion channels (1). However, recent studies have shown that GPCR signaling deviates from such a classical linear model. For example, in kidney and colonic epithelial cells, protease-activated receptor 1 can transduce its signals through either Gαi/o or Gαq subunits via inhibition of small GTPase RhoA or activation of RhoD. Thus, RhoA and RhoD act as molecular switches between the negative and positive signaling activity of protease-activated receptor 1 (2). Another example is the ability of β2-adrenergic receptor to switch from Gs-dependent pathways to non-classical signaling pathways by coupling to pertussis toxin-sensitive Gi proteins in a cAMP-dependent protein kinase/protein kinase C phosphorylation-dependent manner. In this case, the phosphorylation-induced switch in G protein coupling provides the receptor access to alternative signaling pathways. For β2-adrenergic receptors, this leads to a Gi-dependent activation of MAP kinase (3, 4). Furthermore the involvement of protein scaffolds, such as β-arrestins in the MAP kinase cascade, could also alter the GPCR signaling (5–8). Hence the formation of “signaling units” or “receptosomes” would influence the GPCR signaling process and destination.
For opioid receptors, which are members of the rhodopsin GPCR subfamily receptors, signal switching is also observed. Normally opioid receptors inhibit AC activity, activate the MAP kinases and Kir3 K+ channels, inhibit the voltage-dependent Ca2+ channels, and regulate other effectors such as phospholipase C (9). However, during prolonged agonist treatment, not only is there a blunting of these cellular responses but also a compensatory increase in intracellular cAMP level, which is particularly significant upon the removal of the agonist or the addition of an antagonist such as naloxone (10–12). This compensatory adenylyl cyclase activation phenomenon has been postulated to be responsible for the development of drug tolerance and dependence (13). The observed change from receptor-mediated AC inhibition to receptor-mediated AC activation reflects possible receptor signal switching. Although the exact mechanism for such signal changes has yet to be elucidated, activation of specific protein kinases and subsequent phosphorylation of AC isoforms (14, 15) and other signaling molecules (16) have been suggested to be the key for observed AC activation. Among all the protein kinases studied, involvement of protein kinase C, MAP kinase, and Raf-1 has been implicated in the activation of AC (17–19). Alternative mechanisms, such as agonist-induced receptor internalization and the increase in the constitutive activities of the receptor, also have been suggested to play a role in increased AC activity after prolonged opioid agonist treatment (20). Earlier studies also implicated the switching of the opioid receptor from Gi/Go to Gs coupling during chronic agonist treatment (21). Regardless of the mechanism, the exact molecular events that lead to the switching of opioid receptor from an inhibitory response to a stimulatory response remain elusive.
Src kinases, which are members of the nonreceptor tyrosine kinase family, have been implicated in GPCR function because several Src family members such as cSrc, Fyn, and Yes have been reported to be activated by several GPCRs, including β2- (22) and β3 (23)-adrenergic, M2- (24) and M3 (25)-muscarinic, and bradykinin receptors (26). The GPCRs that are capable of activating Src predominantly couple to Gi/o family G proteins (27). Src kinases appear to associate with, and be activated by, GPCRs themselves either through direct interaction with intracellular receptor domains or by binding to GPCR-associated proteins, such as G protein subunits or β-arrestins (27). Src kinase has been reported to be activated by κ- (28) and δ (29)-opioid receptors and regulate the c-Jun kinase and MAP kinase activities. Src kinase within the nucleus accumbens has been implicated in the rewarding effect and hyperlocomotion induced by morphine in mice (30). However, it is not clear whether the Src kinase is activated and involved in the signal transduction in AC activation after chronic opioid agonist administration.
Previously we reported that the lipid raft location of the receptor and the Gαi2 proteins are two prerequisites for the observed increase in AC activity during prolonged agonist treatment (31, 32). Because various protein kinases including Src kinases and G proteins have been shown to be enriched in lipid rafts (33), the roles of these cellular proteins in the eventual switching of opioid receptor signals from inhibition to stimulation of AC activity were examined in the current studies. We were able to demonstrate that the association with and subsequent activation of Src kinase by the μ-opioid receptor (OPRM1), which leads to eventual tyrosine phosphorylation of OPRM1, are the cellular events required for the switching of opioid receptor signaling upon chronic agonist treatment.
EXPERIMENTAL PROCEDURES
Cell Culture—HEK293 cells stably expressing either wild type OPRM1 with the hemagglutinin (HA) epitope at the amino terminus (HA-OPRM1HEK293) or HA-OPRM1 with Tyr mutations were cultured with advanced modified Eagle's medium (Invitrogen) supplemented with 5% fetal bovine serum (HyClone), 2 mm glutamine (GlutaMAX™, Invitrogen), 100 units/ml penicillin, 100 μg/ml streptomycin (Invitrogen), and 0.2 mg/ml G418 (Geneticin, Invitrogen) in a 5% CO2 atmosphere at 37 °C. Wild type mouse embryonic fibroblast cells (MEF WT-1) and MEF cells with Src/Fyn/Yes knock-out (SYF; obtained from ATCC) (34) were cultured under similar conditions in advanced Dulbecco's modified Eagle's medium (Invitrogen) without the addition of G418 in a 10% CO2 atmosphere at 37 °C. SYF cells with expression of cSrc (SYF-cSrc; obtained from ATCC) (34) were cultured in the same Dulbecco's modified Eagle's medium as the SYF cells with the addition of 400 μg/ml hygromycin B. Neuroblastoma2A cells stably expressing HA-OPRM1 (HA-OPRM1Neuro2A), MEF with β-arrestin2 knock-out (MEF-βArr2KO) and MEF with β-arrestin2 and two double knock-out (MEF-βArr1&2KO) cells were cultured in the same Dulbecco's modified Eagle's medium with the addition of 0.2 mg/ml G418.
cAMP Assay—For acute morphine inhibition of the AC activity, cells were seeded on a 96-well plate the day before the assay. The culture medium was then removed and replaced with 100 μl of incubation buffer (0.5 mm isobutylmethylxanthine and 10 μm forskolin in Krebs-Ringer-HEPES buffer with or without various morphine concentrations. The cells were incubated for 15 min at 37 °C and then lysed at 85 °C for 6 min to release the intracellular cAMP content. The cAMP level was determined with an AlphaScreen cAMP Detection kit (BioSignal) and Biomek 2000 Automation Workstation (Beckman Coulter) as described previously (31). For prolonged agonist treatment, 1 μm morphine or etorphine was added to the culture medium 4 h prior to the assays. The culture medium then was aspirated, cells were washed once prior to the addition of 100 μl of incubation buffer with or without agonist or antagonist (naloxone), and the incubation was carried out as above. The results were analyzed using Prism 4.0 software (GraphPad).
Mutagenesis of Tyr Residues in OPRM1—Mutations of the Tyr residues in OPRM1 facing the cytosol were generated using QuikChange™ site-directed mutagenesis methods as outlined by Stratagene. Point mutation primers were designed as follows: for Y96F, 5′-GTATGTGATTGTAAGATTCACCAAAATGAAGACTGCCACC-3′; for Y166F, 5′-GTCGACTGCACCATGAGCGTCGACCGCTTCATTGCTGTCTGC-3′; and for Y336F, 5′-CTCTTCGCCTGAATCCAGTTCTCTTCGCCTTCCTGGATGAAAAC-3′.
Discontinuous Gradient Centrifugation and Lipid Raft Fraction Separation—Two confluent T-75-cm2 flasks of cells were washed twice with phosphate-buffered saline (pH 7.4) at 4 °C and scraped into 2 ml of 500 mm sodium carbonate (pH 11.0) solution with 1× Complete™ Protease Inhibitor Mixture and 1× PhosSTOP™ Phosphatase Inhibitor Mixture (Roche Applied Science) followed by 10 strokes of homogenization with pestle A of a Dounce homogenizer. The lysate then was sonicated with one 30-s burst at setting 4 and one 30-s burst at setting 7 (model W-220F, Ultrasonics, Inc.). The homogenate was adjusted to 45% sucrose by adding 2 ml of 90% sucrose prepared in MBS buffer (25 mm MES, pH 6.5, and 0.15 m NaCl) and placed at the bottom of an ultracentrifuge tube. Then 4 ml of 35% sucrose and 4 ml of 5% sucrose (both in MBS buffer containing 250 mm sodium carbonate) were loaded above the sample accordingly to form a 5–45% discontinuous sucrose gradient. The sample was centrifuged at 160,030 × g for 16 h in a Beckman L7 ultracentrifuge with an SW-41Ti rotor. Fractions of 1 ml each were collected from the top. Fractions 4 and 5 with a light-scattering band between 5 and 35% sucrose gradient were considered to be lipid raft-rich fractions (35).
Immunoprecipitation and Western Blotting—The prepared lipid raft fractions were combined and diluted with 15 ml of buffer A (100 mm NaCl and 10 mm Tris, pH 7.4) and concentrated with a Centriplus™ YM-30 centrifugal filter column (Millipore) to 1 ml at a speed of 3,500 × g. The concentrated solution was transferred to a new tube with the addition of 0.1% digitonin (Sigma) and mouse monoclonal anti-HA antibody (1:200; Covance), and the mixture was incubated at 4 °C overnight with slow rotation. Sixty microliters of protein G-agarose beads (Invitrogen) were added, and the mixture was incubated at 4 °C for 3 h with slow rotation. The protein G-agarose beads were then pelleted by centrifuging at 12,000 × g for 15 min at 4 °C and washed five times with buffer A. Protein samples were eluted from the beads with 1× SDS sample buffer (75 mm Tris, pH 6.8, 100 mm dithiothreitol, 2% SDS, 0.1% bromphenol blue, and 10% glycerol) by heating at 65 °C for 5 min, resolved in a 10% SDS-polyacrylamide gel, and transferred to polyvinylidene difluoride membrane (0.45 μm; Amersham). The membrane was blocked overnight at 4 °C in a blocking solution (10% powdered nonfat milk in 0.1% TTBS (0.1% Tween 20, 25 mm Tris, and 150 mm NaCl, pH 7.6)) and then probed with various specific antibodies for 1 h at room temperature in the blocking solution. After three 15-min washings with 0.1% TTBS, the membrane was incubated with secondary antibody conjugated with alkaline phosphatase (1:5,000; Bio-Rad) for 1 h in the same blocking solution. The protein bands were detected by ECF substrate (GE Healthcare) and scanned in the Storm 860 Imaging System (GE Healthcare). The band intensities were quantified and analyzed with the ImageQuant software (GE Healthcare).
In Vitro Src Kinase Activity Assay—After various treatments, HA-OPRM1HEK293 cells were lysed in lysis buffer containing 1% Triton X-100, 10 mm Tris, pH 7.5, 120 mm NaCl, 25 mm KCl, and 1× Complete protease inhibitor (Roche Applied Science). The cell lysate was centrifuged at 800 × g for 5 min. The supernatant was incubated with 2 μg of anti-cSrc polyclonal antibody (Santa Cruz Biotechnology, Inc.) overnight at 4 °C followed by addition of 20 μl of protein G-agarose beads (Invitrogen) and incubation for another 3 h. The agarose beads were washed once with lysis buffer, then three times with washing buffer (0.5 m LiCl and Tris-HCl, pH 7.5), and one time with 25 mm Tris-HCl, pH 7.5. The immunoprecipitates were incubated at 30 °C with 5 μg of Src substrate peptide (KVEKIGEGTYGVVYK, corresponding to amino acids 6–20 of p34cdc2; Upstate) in kinase buffer (total volume of 50 μl) containing 5 μCi of [γ-32P]ATP (PerkinElmer Life Sciences), 50 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 10 mm MnCl2, 25 μm ATP, 1 mm dithiothreitol, and 100 μm Na3VO4. Ten-microliter aliquots were taken out at time points of 0, 2, 5, 10, and 20 min, and the reaction was immediately terminated by the addition of 10 μl of 40% trichloroacetic acid, and the sample was spotted onto P81 cellulose phosphate paper (Upstate). The paper was washed extensively with 1% phosphoric acid three times and one time with acetone. Radioactivity retained on the P81 paper was quantified by liquid scintillation counting.
RESULTS
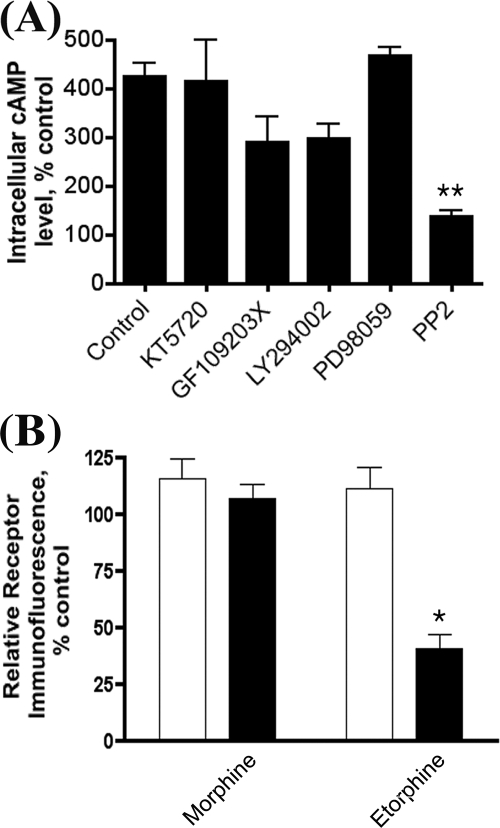
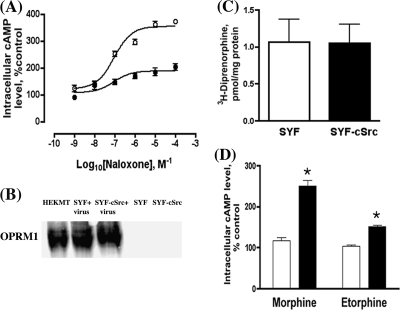
Protein kinases, including cAMP-dependent protein kinase, protein kinase C, phosphatidylinositol 3-kinase, and MAP kinase, and the alteration of receptor intrinsic activities have been suggested to be involved in the activation of AC during chronic opiate agonist treatment (9, 36). Because these protein kinases are located within lipid rafts and opioid receptor lipid raft location is essential for the observed increase in AC activity (32), we initially examined the effects of various protein kinase inhibitors on chronic morphine-induced AC activation (Fig. 1A). Among the inhibitors tested, PP2, a Src kinase inhibitor, was the only one that totally blocked AC activation. The involvement of Src kinase in agonist-induced opioid receptor internalization has been reported (37), and the relationship between receptor endocytosis and AC activity increase during chronic agonist treatment has been implicated (38). However, the ability of the Src kinase inhibitor to attenuate morphine-induced AC activity is unrelated to receptor internalization. Although morphine treatment did not cause receptor internalization, whereas etorphine treatment did (Fig. 1B), both agonist-induced AC activity increases could be blocked by the Src kinase inhibitor PP2 (data not shown). To further substantiate the effect of the Src kinase inhibitor in AC activation, dominant negative cSrc construct was transiently overexpressed in HA-OPRM1HEK293 (HEKMT) cells prior to prolonged agonist treatment. As shown in Fig. 2A, expression of the dominant negative cSrc mutant reduced the maximal increase in AC activation to 208 ± 24.0% after morphine treatment, whereas a 382 ± 22.5% increase in AC activity was observed in HEKMT cells transfected with the control vector. The requirement of Src activity in the morphine-induced AC activity increase was further demonstrated in the SYF cells, the embryonic fibroblast cells isolated from mice in which three Src kinase subtypes, cSrc, Yes, and Fyn, were knocked out (34). The expression of HA-OPRM1 in these cells, delivered by the adenovirus constructs, was compared with that in SYF-cSrc cells, which are SYF cells with cSrc reintroduced. The receptor expression levels were similar in both SYF and SYF-cSrc cells as determined by Western analysis (Fig. 2B). The [3H]diprenorphine binding assays also confirmed that the OPRM1 expression level on the cell surface is similar in these two cell lines (Fig. 2C). Prolonged morphine or etorphine treatment-induced AC activation was absent in SYF cells but was observed in SYF-cSrc cells (Fig. 2D). Although etorphine causes receptor internalization and results in a fewer number of receptors on the cell surface (39), the difference in the maximal level of AC activation between morphine and etorphine was not because of the abilities of the agonists to induce receptor internalization (32). Further the ability to restore morphine-induced AC activity was not restricted to cSrc activity. Transient expression of Fyn in SYF cells also restored morphine-induced AC activation in these cells (data not shown).
FIGURE 1.
A, the effect of various protein kinase inhibitors on chronic morphine-induced AC activation. HEKMT cells (HA-OPRM1HEK293) were seeded into 96-well plates the day before the assay. Cells were treated with 1 μm morphine for 4 h, and then cAMP assays were performed in the presence of 10 μm naloxone as described under “Experimental Procedures.” cAMP-dependent protein kinase inhibitor KT 5720 (6 μm), protein kinase C kinase inhibitor GF109203X (5 μm), or phosphatidylinositol 3-kinase inhibitor LY294002 (25 μm) was added 30 min prior to the assay, whereas MAP kinase inhibitor PD98059 (50 μm) or Src kinase inhibitor PP2 (2 μm) was added 1 h prior to the assay. Experiments were repeated three times and values represent averages ± S.E. ** denotes p < 0.01 when compared with the control using unpaired t test. B, the differential effects of morphine and etorphine treatments on OPRM1 internalization. HEKMT cells were treated with 1 μm morphine or etorphine for 4 h. Cells were washed and then incubated at 4 °C for 1 h with anti-HA antibody (Covance; 1:500). Afterward cells were washed twice and incubated with Alexa 488-labeled goat anti-mouse IgG secondary antibody (Invitrogen; 1:400) at 4 °C for 1 h. Cells were then washed and fixed with 3.7% formaldehyde prior to quantifying the cell surface receptor immunoreactivity with fluorescence flow cytometry (FACScan, BD Biosciences). The fluorescence intensity of 10,000 cells was collected for each sample. Cell Quest software (BD Biosciences) was used to calculate the mean fluorescence intensity of the cell population. All fluorescence-activated cell sorting assays were repeated three times in triplicate in each experiment ± S.E. * denotes p < 0.05 when compared with control using unpaired t test.
FIGURE 2.
Chronic agonist treatment-induced AC activation is Src kinase-dependent. A, transient expression of dominant negative cSrc blocks the AC activation. HEKMT cells were transiently transfected with 0.5 μg of pcDNA3 (control, ○) or dominant negative cSrc cDNA (•) for 12 h prior to 4-h morphine (1 μm) pretreatment followed by cAMP assay in the presence of various concentration of naloxone as described under “Experimental Procedures.” B, Western blotting analysis of OPRM1 receptor expression in SYF (SYF+virus) and SYF-cSrc (SYF-cSrc+virus) cells with recombinant HA-OPRM1 adenovirus infection and in both cell lines without virus infection. Cells were infected with HA-OPRM1 adenoviruses 48 h prior to the assay at a multiplicity of infection sufficient for expression of the receptor around 300–500 fmol/mg of proteins. HEKMT cells were used as positive control. Anti-HA antibodies (1:1,000) were used to detect OPRM1. C, whole cell binding assays (67) with 2 nm [3H]diprenorphine were carried out to determine the expression level of OPRM1 in SYF and SYF-cSrc cells after 48-h HA-OPRM1 adenovirus infection. The values represent the averages ± S.E. of triplicate binding assays from three separate 25-mm plates of cells. D, AC activation is totally absent in SYF cells, whereas it is restored in SYF-cSrc cells. Cells were infected with recombinant HA-OPRM1 adenoviruses 48 h prior to agonist treatment. The HA-OPRM1 adenovirus-infected SYF (open bars) or SYF-cSrc cells (filled bars) were pretreated with 1 μm morphine or etorphine for 4 h. AC activation was then determined after removal of agonists and in the presence of 10 μm naloxone. Error bars represent the S.E. calculated across experiments and * denotes p < 0.05 when compared with control using unpaired t test.
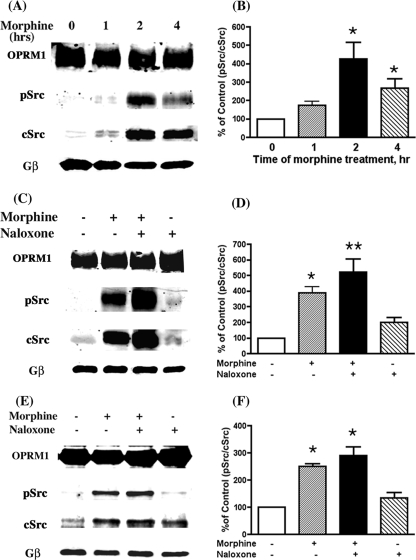
Activation of Src kinase by opioid receptors has been reported (40). Whether the requirement of Src for morphine-induced AC activity reflects the actual activation of Src was examined. We utilized antibodies that detect autophosphorylation at Tyr416, reflecting Src activation (41), to monitor Src activity. Instead of examining the total cellular Src kinase activation, the OPRM1 receptor complex within the lipid rafts was immunoprecipitated, and the amount of Tyr416-phosphorylated Src (pSrc) and the amount of cSrc associated with the complex were determined. The ratio of pSrc versus cSrc was used for comparison between the control and drug treatment. G protein β-subunits (Gβ) were used as a loading control in all the immunoprecipitation analyses. As shown in Fig. 3, A and B, there was a time-dependent increase in the total Src associated with OPRM1 with a parallel increase in Src activity (pSrc). The ratio of pSrc versus cSrc associated with OPRM1 reached a peak level of 425.4 ± 91.7% above the control level after about 2 h of morphine treatment. There was minimal increase in pSrc associated with OPRM1 when HEKMT cells were exposed to morphine acutely, i.e. <15 min (data not sown). The time course for the pSrc increase parallels that of the morphine-induced AC activation. It could be demonstrated further that after 2 h of morphine treatment, the pSrc associated with OPRM1 was significantly enhanced when naloxone was added to displace the morphine from the receptor (Fig. 3, C and D); this is coincident with the characteristic phenomenon of compensatory increase of AC activity in the AC activation (11, 42). Treatment with naloxone itself did not result in any Src association with and activation by OPRM1. The association and activation of Src after chronic morphine treatment was also observed in the SYF-cSrc cells (Fig. 3, E and F). In contrast, in the SYF cells, which are missing three subtypes of Src kinase, the transient expression and immunoprecipitation of OPRM1 did not reveal the presence of any pSrc (data not shown), demonstrating the specificity of the pSrc antibodies used in our current studies.
FIGURE 3.
Chronic morphine treatment induces Src kinase activation. After HEKMT or SYF-cSrc cells were treated as indicated, lipid raft fractions were prepared and used in the following immunoprecipitation analyses. A, Src activation was determined at 0, 1, 2, or 4 h after 1 μm morphine pretreatment in HEKMT cells. C, effect of morphine and/or naloxone on Src activation. HEKMT cells were treated either with (+) or without (–)1 μm morphine for 2 h followed with (+) or without (–)10 μm naloxone for 15 min. E, specificity of Src association with OPRM1. SYF-cSrc cells were infected with HA-OPRM1 adenoviruses 48 h prior to the drug treatment and immunoprecipitation assay as described above. B, D, and F, quantitative analysis of A, C, and E, respectively. The density of pSrc versus the density of cSrc in control cells (without drug treatment) was considered to be 100%. All the experiments were repeated three times and values represent averages ± S.E. * denotes p < 0.05 and ** denotes p < 0.01 when compared with control using unpaired t test. In all experiments, the immunoprecipitation antibody was anti-HA (1:200) and the immunoblotting antibodies were anti-pSrc (Upstate; Tyr416, 1:500), anti-cSrc (Santa Cruz Biotechnology, Inc.; 1:500), or anti-Gβ (Santa Cruz Biotechnology, Inc.; 1:500).
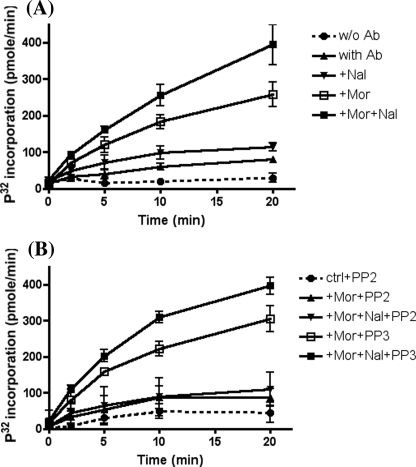
To examine the activity of the Src kinase in a more direct manner, Src kinase signal complex was immunoprecipitated by anti-cSrc antibodies after various drug treatments, and Src kinase-catalyzed phosphorylation of synthetic target peptides was quantified by using [γ-32P]ATP. In HEKMT cells pretreated with morphine for 2 h or morphine for 2 h followed by naloxone for 15 min, incorporation of 32P into the Src-specific substrate peptide (corresponding to p34cdc2 amino acids 6–20) (43) was significantly greater than in the controls without addition of cSrc antibodies, without morphine pretreatment, or only pretreated with naloxone (Fig. 4A) at 10 or 20 min of incubation with [32P]ATP. Furthermore Src kinase inhibitor PP2 totally blocked the 32P incorporation in the substrate peptide, whereas pretreatment with PP3, the inactive analogue of PP2, did not show any effect on the Src-catalyzed phosphorylation of the substrate peptide after incubation with the immunoprecipitates from cells pretreated with morphine or morphine plus naloxone (Fig. 4B). Coincidentally the same result was observed from the immunoprecipitation experiment in which PP2 totally blocked the morphine-induced Src activation (supplemental figure), whereas PP3 did not (data not shown). These results further confirmed that Src kinase was activated within the OPRM1 complex in which AC activation was observed after chronic morphine treatment.
FIGURE 4.
Kinetic study of Src kinase activity after chronic morphine treatment. A, HEKMT cells were treated with 1 μm morphine for 2 h and then with or without 10 μm naloxone for 15 min. B, cells were treated with morphine and/or naloxone the same as A. Src kinase inhibitor PP2 (2 μm) or its inactive analogue PP3 (2 μm) was added 1 h prior to the assay. Cells were lysed in lysis buffer, and cell lysate was incubated with 2 μg of anti-cSrc antibody (Santa Cruz Biotechnology, Inc.) overnight at 4 °C followed by addition of 20 μl of protein G-agarose beads (Invitrogen) and incubation for another 3 h. The immunoprecipitates were incubated at 30 °C with 5 μg of Src substrate peptide (Upstate) in 50 μl of kinase buffer containing 5 μCi of [γ-32P]ATP (PerkinElmer Life Sciences). Ten-microliter aliquots were taken out at time points of 0, 2, 5, 10, and 20 min; the reaction was immediately terminated by the addition of 10μl of 40% trichloroacetic acid; and each sample was spotted onto P81 cellulose phosphate paper (Upstate). The paper was washed extensively with 1% phosphoric acid, and the radioactivity retained on the paper was measured with a scintillation counter. The blank counts (without cell lysate) were subtracted from each result, and the cpm was converted to pmol/min. Each experiment was repeated three times. w/o, without; Ab, antibody; Nal, naloxone; Mor, morphine; ctrl, control.
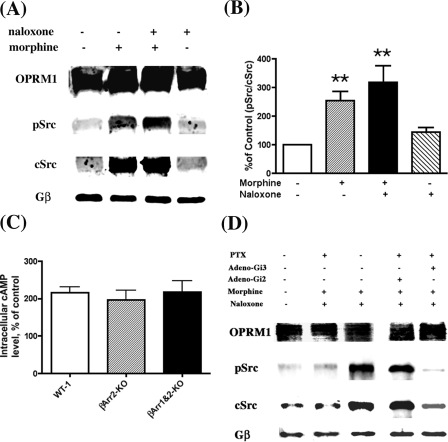
The switching of OPRM1 signaling during chronic morphine treatment could be the consequence of protein scaffolding. Recent studies suggest the important role of β-arrestins (βArrs) in scaffolding signaling proteins, thereby regulating GPCR signaling (44, 45). For example, βArrs have been reported to recruit Src kinases to form signaling complexes that include the MAP kinases with activated β2-adrenergic receptors (46). Whether βArrs have similar roles in scaffolding Src kinases and OPRM1 in mediating morphine-induced AC activation is unknown. Thus, MEF cells isolated from βArr-2 knock-out (βArr2KO) mice or βArr-1 and -2 double knock-out (βArr1&2KO) mice were used in subsequent studies. Transient expression of OPRM1 in these MEF cells was accomplished by using adeno-OPRM1 viruses. The level of AC activation after 4 h of morphine treatment in both the βArr2KO and βArr1&2KO MEF cells compared favorably to that observed in wild type (WT-1) MEF cells: 196 ± 10.4 and 218 ± 11.8% for βArr2KO and βArr1&2KO cells, respectively, versus 216 ± 5.8% for WT-1 cells (Fig. 5C). This observed increase in AC activity was accompanied by an increase in pSrc associated with OPRM1 in the βArr1&2KO cells (Fig. 5, A and B). The amount of pSrc associated with OPRM1 increased by 254.4 ± 31.0% after chronic morphine treatment and was further increased to 317.9 ± 58.9% after morphine removal and the addition of naloxone. These data suggest that the Src association with and subsequent activation by OPRM1 is independent of βArrs. If βArrs are not involved in scaffolding and activating Src by OPRM1, the heterotrimeric G protein could be a probable candidate for such roles. The activation of Src kinase by Gα has been reported previously (47). Our reported studies indicate that Gαi2 is essential for opioid agonist-induced AC activation. The involvement of Gαi2 was demonstrated by the ability of pertussis toxin (PTX)-insensitive mutants of the Gi/Go α-subunits to restore the AC activation after PTX treatment (31). If Src activation is a prerequisite for morphine-induced AC activation, then using a PTX-insensitive mutant of the Gi2 α-subunit should restore OPRM1-mediated Src activation after PTX treatment. Because HEK293 cells express adenovirus Early Region1 (E1) gene that has been eliminated from the recombinant adenoviruses (48), expression of such viruses in HEK293 cells will lead to the eventual lysis of the cells. Therefore, the following experiments were carried out in Neuroblastoma2A cells stably expressing HA-OPRM1 (N2AMT), which had been successfully used in our previous studies (31). As shown in Fig. 5D, pretreating N2AMT cells with PTX completely blocked the interaction between OPRM1 and pSrc after chronic morphine treatment and naloxone challenge (second lane). When the PTX-insensitive Gi2 α-subunit mutant was transiently expressed in these cells (31), chronic morphine-induced Src association with OPRM1 and subsequent Src activation were observed even after PTX pretreatment (fourth lane). Meanwhile transient expression of the PTX-insensitive Gi3 α-subunit mutant did not restore the interaction between OPRM1 and Src (fifth lane). The requirement of Gi2 but not Gi3 α-subunit in Src activation parallels the Gi2 α-subunit-dependent AC activation after chronic morphine treatment (31).
FIGURE 5.
Chronic morphine-induced Src kinase activation is β-arrestin-independent but Gαi2-dependent. A, association of OPRM1 and activation of Src in the absence of β-arrestins. βArr1&2KO MEF cells were infected with HA-OPRM1 adenoviruses for 48 h and then treated with 1 μm morphine for 2 h and/or 10 μm naloxone for 15 min. The lipid raft preparation and co-immunoprecipitation were carried out as described under “Experimental Procedures.” The anti-HA antibodies were used to pull down the OPRM1-Src complex. The immunoblotting antibodies were anti-pSrc (Tyr416), anti-cSrc, or anti-Gβ. B, quantitative analysis of A. The density of pSrc versus the density of cSrc in control cells (without drug treatment) was used as the 100% reference. Each experiment was repeated three times and values represent averages ± S.E. ** denotes p < 0.01 when compared with control using unpaired t test. C, morphine-induced AC activation in the absence of β-arrestins. Wild type (WT-1), β-arrestin2 knock-out (βArr2-KO), or β-arrestin1 and -2 double knock-out (βArr1&2-KO) MEF cells were seeded into 96-well plates 24 h after HA-OPRM1 adenovirus infection. Twenty-four hours later, cells were treated with 1 μm morphine for 4 h, and cAMP assays were carried out in the presence of 10 μm naloxone. Error bars represent the S.E. calculated across experiments. D, N2AMT cells were infected with Gαi2 C353L or Gαi3 C351L PTX-insensitive mutant adenoviruses (31, 68). After 24 h, some cells were treated with PTX (0.1 μg/ml; Tocris) for another 12 h. The cells were further treated with (+) or without (–) 1 μm morphine for 2 h followed by the addition of 10 μm naloxone for 15 min. Cells were collected, the lipid raft fractions were prepared as described under “Experimental Procedures,” and immunoprecipitation analyses were carried out using anti-HA antibody. Lanes 1–3 and lanes 4 and 5 were from two membrane blots and were probed with anti-pSrc (Tyr416), anti-cSrc, or anti-Gβ antibodies at the same time.
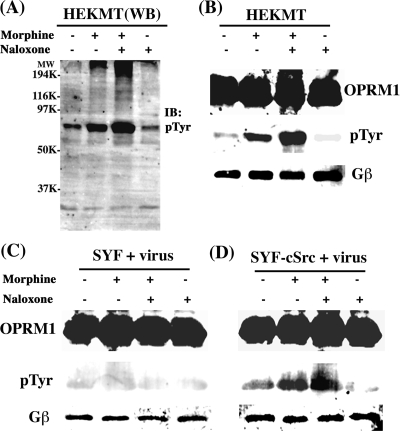
Phosphorylation of signaling proteins such as AC has been reported to be the key for morphine-induced AC activation (49). Because our data indicate a correlation between Src kinase activation and morphine-induced AC activation, whether the activated Src would phosphorylate signaling molecules within the receptor complex was investigated. As Fig. 6A shows, among the Tyr-phosphorylated proteins, the protein band around 66 kDa was Tyr-phosphorylated during chronic morphine treatment and was significantly enhanced after morphine removal and the addition of naloxone. Because OPRM1 migrates within such molecular mass in SDS-PAGE, further immunoprecipitation analysis with HA antibody suggests that this Tyr-phosphorylated protein is OPRM1 (Fig. 6B). Moreover OPRM1 Tyr phosphorylation could be demonstrated to be mediated by Src kinase. In SYF cells transiently expressing OPRM1, Tyr phosphorylation of OPRM1 was not detected in all paradigms tested (Fig. 6C). In contrast, after treatment of SYF-cSrc cells with morphine for 2 h, Tyr phosphorylation of OPRM1 was detected (Fig. 6D). The fact that PP2 can totally block this morphine-induced Tyr phosphorylation (supplemental figure) further indicates that Src kinase activity is responsible for the OPRM1 phosphorylation. These data suggest that OPRM1 is a probable target for activated Src during chronic morphine treatment, and such OPRM1 phosphorylation increased significantly upon removal of morphine and the addition of naloxone, which parallels the compensatory increase of AC activity under the same treatment condition.
FIGURE 6.
Src kinase is responsible for the tyrosine phosphorylation of OPRM1. A, Western blotting (WB) analysis. HEKMT cells were treated with (+) or without (–)1 μm morphine for 2 h and then with (+) or without (–)10 μm naloxone for 15 min. The lipid raft separation and Western blotting were carried out as described under “Experimental Procedures.” Molecular weight markers (MW) are labeled on the left. IB, immunoblot. B–D, immunoprecipitation analyses. SYF and SYF-cSrc cells were infected with HA-OPRM1 adenoviruses 48 h prior to drug treatment. HEKMT (B), SYF (C), or SYF-cSrc (D) cells were treated with (+) or without (–)1 μm morphine for 2 h followed by treatment with (+) or without (–)10 μm naloxone for 15 min. Then cells were washed and collected followed by lipid raft separation and immunoprecipitation assay as described under “Experimental Procedures.” All experiments were repeated three times. Immunoprecipitation of OPRM1 in the lipid raft fractions was carried out using anti-HA antibody, and anti-phospho-Tyr antibody (Upstate; 1:500) was used to examine OPRM1 Tyr phosphorylation.
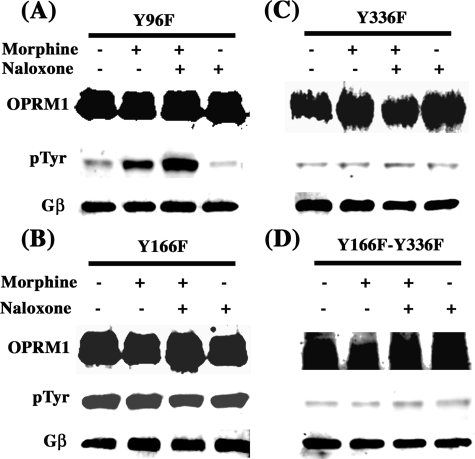
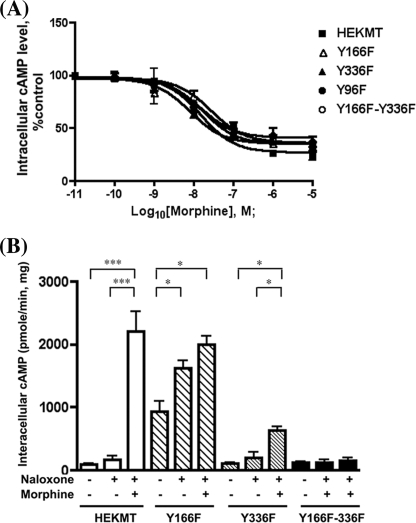
In the OPRM1 sequence, three Tyr residues face the inside of the cell that could be the potential phosphorylation targets of Src kinase: Tyr96 in the first intracellular loop, Tyr166 within the DRY motif at the second intracellular loop, and Tyr336 within the NPXXY motif right after the seventh transmembrane domain, which is conserved among this subfamily of GPCRs. To examine the target of Src phosphorylation and subsequent effects on AC activation, these Tyr residues were mutated to Phe to generate the single mutants Y96F, Y166F, and Y336F and double mutants Y166F/Y336F. All of these mutants were stably expressed in HEK293 cells, and the colonies with similar receptor expression levels were chosen for further biochemical and functional studies. The mutation of Tyr96 to Phe did not affect the Tyr phosphorylation of the receptor (Fig. 7A). Surprisingly the Y166F mutant exhibited hyperphosphorylation even without morphine pretreatment or only with naloxone pretreatment (Fig. 7B). Such hyperphosphorylation was observed in multiple clones of this OPRM1 mutant. In cells expressing the Y336F mutant, morphine-induced Tyr phosphorylation was totally eliminated (Fig. 7C). In cells expressing Y166F/Y336F double mutations, prolonged morphine treatment also failed to induce Tyr phosphorylation of OPRM1 (Fig. 7D). Thus, the results from these mutants suggest that the probable Src kinase phosphorylation site is the Tyr336 residue. The mutations of all of these Tyr residues, whether single or double, did not alter the receptor lipid raft location (data not shown). To further investigate the functional effects of the mutants Y96F, Y166F, and Y336F and the double mutant Y166F/Y336F, the cAMP assay was performed. The mutation of all of these Tyr residues did not affect the acute morphine-mediated inhibition of AC activity (Fig. 8A). However, under the chronic morphine treatment condition, the changes of intracellular cAMP concentration showed remarkable differences among the cells expressing these mutants. In control HEKMT cells, 10 μm forskolin induced cAMP production at the level of 91.8 ± 20.2 pmol/min/mg of protein. The intracellular level of cAMP in these cells in the presence of naloxone and forskolin increased to 171.5 ± 58.6 pmol/min/mg of protein. Chronic morphine and naloxone treatment induced a 12.8-fold increase in the intracellular cAMP level in the presence of forskolin (2,203.0 ± 20.15 pmol/min/mg of protein) when compared with acute naloxone and forskolin treatments. The cells with mutant Y96F showed cAMP production levels similar to those in HEKMT cells in all the categories of treatment (data not shown). In Y166F mutant cells, the intracellular cAMP level in the presence of 10 μm forskolin was 10.2-fold higher than that in HEKMT cells (935.0 ± 165.0 pmol/min/mg of protein). Addition of naloxone in the presence of forskolin further increased the intracellular cAMP level to 1,624.0 ± 115.0 pmol/min/mg of protein. Under such activated AC conditions, chronic morphine treatment followed by acute naloxone only resulted in a 1.2-fold increase in the intracellular cAMP level to 1,995.0 ± 20.2 pmol/min/mg of protein compared with that with forskolin and naloxone treatment. In contrast, cells expressing Y336F mutant exhibited intracellular cAMP levels similar to those of the HEKMT cells in the presence of forskolin and in the presence of forskolin and naloxone: 102.8 ± 14.2 and 203.2 ± 82.1 pmol/min/mg of protein, respectively. Chronic morphine treatment and acute naloxone resulted in a much lower level of intracellular cAMP (633.5 ± 52.50 pmol/min/mg of protein) compared with the HEKMT cells (3.1-fold as compared with 12.8-fold in HEKMT). Cells expressing Y166F/Y336F double mutants did not show any difference in the intracellular cAMP level whether the cells were stimulated with forskolin, with forskolin and naloxone, or with forskolin and naloxone after chronic morphine treatment (116.8 ± 22.9, 128.3 ± 39.5, and 159.4 ± 44.7 pmol/min/mg of protein, respectively). These data suggest that the Tyr residue in the DRY motif, although it is not phosphorylated by Src, can regulate the Tyr phosphorylation of the receptor at Tyr336 within the NPXXY motif. The phosphorylation of Tyr336 is functionally important for opioid receptor-mediated AC activation and, thus, is the key for switching the receptor signals from AC inhibition to AC activation.
FIGURE 7.
Tyr336 of OPRM1 is the phosphorylation target of Src kinase. HEK293 cells stably expressing OPRM1 with single Tyr mutant of Y96F (A), Y166F (B), Y336F (C) or double mutants (Y166F/Y336F, (D)) were treated with (+) or without (-) 1 μm morphine for 2 h, followed by the treatment with (+) or without (-) 10 μm naloxone for 15 min. Then, cells were washed and collected followed by lipid raft separation and immunoprecipitation assay as described under “Experimental Procedures.” All experiments were repeated three times. Immunoprecipitation of OPRM1 in the lipid raft fractions was carried out using anti-HA antibody and anti-phospho-Tyr (pTyr) antibody was used to examine OPRM1 Tyr-phosphorylation.
FIGURE 8.
The effect of Tyr mutations on AC activities. A, wild type HEKMT (▪); single mutant Y96F (•), Y166F (▴), or Y336F (▵); or double mutant Y166F/Y336F (○) cells were cultured on 96-well plates. For the acute inhibition assay, the abilities of various concentrations of morphine to inhibit the 10 μm forskolin-stimulated intracellular cAMP production were determined as described under “Experimental Procedures.” B, for the chronic activation assay, HEKMT and Y166F, Y336F, and Y166F/Y336F mutant cells were cultured on 96-well plates. Some cells were pretreated with 1 μm morphine for 4 h, and then cAMP assays were performed in Krebs-Ringer-HEPES buffer with 10 μm forskolin and in some cases as indicated with 10 μm naloxone. The intracellular cAMP level is presented as pmol/min/mg of protein. Each analysis was repeated at least three times with triplicate measurements in each experiment and values represent averages ± S.E. * denotes p < 0.05 and *** denotes p < 0.001 when compared using one-way analysis of variance.
DISCUSSION
It is becoming increasingly clear that signaling via GPCRs is a diverse phenomenon involving receptor interaction with a variety of signaling partners that results in activation or inhibition of signal pathways. Signal switching may be a key in controlling the receptor to choose the specific pathway. The new concept of “ligand bias,” which refers to the ability of ligands to selectively stabilize receptor conformations that stimulate or inhibit subsets of receptor activities (50), is also a plausible explanation for coupling specificity among the signaling complexity of the receptor. It is also clear that GPCR desensitization, classically viewed only as a mechanism of signal termination, is itself a means to confer unique signaling properties on the receptor. For opioid receptors, agonists induce acute inhibition of AC activity, which is gradually changed to an increase of AC activity and intracellular cAMP accumulation upon prolonged treatment and is particularly significant upon the removal of the agonist or the addition of an antagonist; this has been postulated to be the molecular basis for the development of opiate tolerance and dependence (10–12). This dramatic change from receptor-mediated AC inhibition to receptor-mediated AC activation reflects possible intracellular signal switching. Our current studies demonstrate that Src kinase activation could be the key for such signal switching.
It has been known that numerous GPCRs activate Src family kinases (24–26). For example, in β3-adrenergic receptors, direct binding of activated Src is required for MAP kinase activation (23). In opioid receptors, agonist activation of Src kinase has been reported (29), and the Src kinase activation regulates the time course of ERK activation by δ-opioid receptor ligands (37). In the present studies, by using protein kinase inhibitors (Fig. 1A), dominant negative cSrc (Fig. 2A), and SYF/SYF-cSrc cells (Fig. 2D), the involvement of Src kinase in chronic morphine-induced AC activation was confirmed. Our previous studies showed that only the receptors located in the lipid rafts are functional during AC activation. Coincidentally we found that OPRM1 formed the signaling complex with activated Src (pSrc) only in lipid raft fractions. The key to our current observation is that Src was recruited to the opioid receptor complex within the lipid rafts during the chronic morphine treatment and the addition of naloxone after chronic and not after acute agonist treatment (Fig. 3A). The recruitment of Src resulted in an additional significant increase of Src kinase activity. Prolonged naloxone treatment itself failed to induce any Src activation (Fig. 3C). The involvement of Src kinase was further confirmed by using SYF-cSrc cells, which specifically express cSrc (Fig. 3E). It needs to be pointed out that this Src kinase activation by chronic opioid agonist treatment was not limited to the cSrc subtype as we also observed similar kinase activation in SYF cells transiently expressing Fyn (data not shown). Furthermore the fact that Src kinase inhibitor PP2 could totally block the recruitment and subsequent activation of Src by OPRM1 (supplemental figure) and the fact that chronic morphine could significantly up-regulate the Src activity in the phosphorylation of the substrate peptide and this activity was abolished by pretreatment of PP2 (Fig. 4) clearly indicate that chronic morphine treatment causes the recruitment and activation of Src kinase by OPRM1.
It is very likely that there are more signaling molecules involved in OPRM1-Src signaling complex. For example, β-arrestins have been shown to be critical for Src kinase activation (27). The initial role of β-arrestins is solely as a negative regulator to desensitize the activated GPCRs and mediate receptor internalization (1). However, recent studies have shown that β-arrestins might regulate GPCR signaling beyond their roles in desensitization and internalization by serving as platforms for Src kinase activation (46) and MAPK/ERK cascades (51). Thus, it is logical to assume that β-arrestins are responsible for Src activation during chronic opioid agonist treatment. However, our observations show that chronic morphine-induced Src activation is independent of β-arrestins. This was demonstrated by the ability of morphine to activate Src (Fig. 5A) and induce AC activation (Fig. 5C) in MEF cells that lack β-arrestin2 or both β-arrestin1 and -2. These results are not too striking because, with some GPCRs, Src may associate directly with the intracellular domains of the receptor (27). For example, the β3-adrenergic receptor does not bind β-arrestins and fails to endocytose upon agonist activation. In this case, the agonist induces the formation of a complex between Src and the receptor. This association seems to be mediated through Pro-rich domains in the third intracellular loop and carboxyl tail of the receptor, which serve as docking sites for the Src SH3 domain (41). However, such Pro-rich domains are not present in the intracellular loops and carboxyl tail sequence of OPRM1. Instead our studies suggest that the heterotrimeric G-proteins are involved in the recruitment and activation of Src kinase.
Our previous studies showed that the Gαi2 subunit is specifically required for opioid agonist-induced AC activation (31). Coincidentally our current studies showed that Src activation after chronic morphine treatment is also Gαi2-dependent (Fig. 5D). Such selectivity points to possible involvement of the Gα subunits in Src kinase activation, which has been reported previously (47). Although the exact role of Gα subunits on Src kinase activity remains to be determined, the Gα subunits could activate Src kinase by binding to the catalytic domain and changing the conformation of Src, leading to increased accessibility of the substrates to the active site (47). Alternatively the Gα subunits could serve as a scaffold that facilitates and stabilizes the signaling complex of the receptor, Src, and other signaling molecules involved. An excellent example is the interaction between the heterotrimeric G protein and RGS, thus regulating the GPCR signals. The ability of morphine administration to alter the RGS levels associated with the synaptic membrane has been reported (52). Opiate-induced changes in the RGS9 level contributing to the behavioral and neural plasticity associated with chronic opiate administration has been demonstrated with RGS9 knock-out mice (53). Because signaling proteins such as RGS could interact with other scaffolding molecules such as 14-3-3 and spinophilin (54), the observed AC activation could be the consequence of Gαi2 recruiting Src kinase and a specific RGS, for example RGS4 (55), to the vicinity of OPRM1, or the OPRM1 receptor and Gαi2 together decide the selectivity of a specific RGS (56). Such selectivity could determine the interaction with specific scaffolds and formation of a new signaling complex. Obviously more studies are needed to address the exact organization and new components in the OPRM1-Gαi2-Src signaling complex.
Protein targets that are phosphorylated by Src could contribute to AC activation and switching of opioid receptor signals. One possible target is the opioid receptor itself (Fig. 6). Tyr phosphorylation of the δ-opioid receptor has been reported previously (29). Our mutagenesis study suggests that Tyr336 within the NPXXY motif is the target of Src phosphorylation (Fig. 7, A–D). The mutation of this Tyr to Phe resulted in a significant decrease in AC activation (Fig. 8B). The importance of the NPXXY motif has been reported in many GPCRs. For example, mutation of Tyr in NPXXY leads to constitutive phosphorylation and internalization, but not signaling, of human B2 bradykinin receptor (57). Mutation of this site also causes the δ-opioid receptor to be unable to induce a robust activation of the MAPK pathway (58), suggesting that the tyrosine phosphorylation in NPXXY motif is important for this signaling pathway. It is also reasonable to suggest that a phosphorylated NPXXY motif serves as a new docking site to recruit SH2/SH3 domain-containing proteins, which together form a signal complex, for initiating a new set of signaling. Whether such SH2/SH3 domain-containing proteins are recruited by OPRM1 and participate in AC activation remains to be determined.
Another highly conserved motif among the Class A GPCRs of the rhodopsin subfamily is the DRY motif. It has been reported that this motif may be directly involved in regulating receptor conformation and G protein coupling/recognition, thus contributing to the constitutive activity of the receptor (59). Our mutagenesis study also found that the Y166F mutation in the DRY motif results in hyperphosphorylation of OPRM1 (Fig. 7C) and causes the significant blunting of AC activation after chronic morphine treatment (Fig. 8B). In the presence of 10 μm forskolin, cells expressing this receptor mutant showed a much higher intracellular cAMP level, suggesting that the Y166F mutant changed the receptor to a state resembling that after chronic morphine treatment, and the addition of the antagonist naloxone prior to chronic morphine treatment also resulted in much higher cAMP production. Similar results were also observed in OPRM1 when the aspartic acid residue (Asp164) was changed to glutamine (Gln) in the DRY motif (60). The increase in forskolin-stimulated AC activity in cells expressing the DRY mutant also supports the previous hypothesis of the involvement of “constitutive” receptor activity in the AC activation after chronic agonist treatment (61). Studies in human oxytocin receptor indicated that the D136N mutation in the DRY motif caused receptor switching from a Gαi-mediated pathway to a Gαq-mediated pathway (62), suggesting that the DRY motif is important not only in receptor activation but also in signal switching among different G protein couplings. It has been suggested that DRY and NPXXY motifs in rhodopsin may interact with each other in concert to provide the dual constraints of the receptor. Changes in either of the motifs will affect the other and will result in the conformational changes of the whole receptor (63). This is supported by our results that mutations in either the DRY or the NPXXY motif will significantly blunt the AC activation after chronic morphine treatment, and further the double mutations in these motifs totally abolished the chronic morphine-induced AC activation (Fig. 8B). Moreover the fact that all of the Tyr mutations, single or double, did not affect the acute morphine-induced AC inhibition (Fig. 8A) clearly indicates that Src activation and eventual phosphorylation of OPRM1 are unique signaling events required for the chronic agonist-induced AC activation (summarized in Fig. 9).
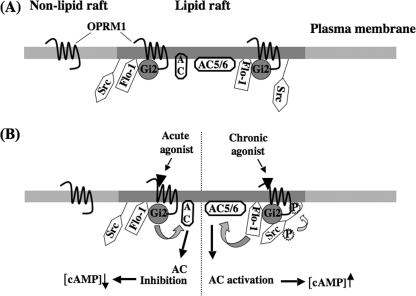
FIGURE 9.
Schematic representation of OPRM1-Gαi2-Src kinase signaling pathway in lipid rafts. A, lipid raft microdomains contain OPRM1, Gαi2, Src kinases, AC, and flotillin-1 (Flo-1), a lipid raft marker that is also known to anchor the signaling molecules, such as Src kinases (69) and G-proteins (L. Zhang and P.-Y. Law, unpublished observation) in lipid raft. There is another pool of OPRM1 located outside the lipid raft. B, under the acute agonist treatment, OPRM1 interacts with Gαi2 and causes the inhibition of adenylyl cyclase activity and the decrease of intracellular cAMP level (31). After chronic agonist treatment, Src kinase is recruited by OPRM1-Gαi2 signal complex, and it is activated by autophosphorylation at Tyr416. The activated Src will phosphorylate the OPRM1 at Tyr336. Although the exact mechanism has yet to be elucidated, the phosphorylated and activated OPRM1-Gαi2-Src signaling complex, either directly or indirectly by activating other protein kinases, phosphorylates AC isoforms, such as AC 5/6 (14, 15), and other signaling molecules (16), eventually leads to the observed AC activation.
It is also possible that the activated Src will phosphorylate other signaling molecules in addition to OPRM1 and mediate the switching of the opioid receptor signals. For example, activation of angiotensin II type 1 receptor and epidermal growth factor receptor leads to the activation of Src, which will phosphorylate phospholipase Cγ and the GPCR-kinase-interacting protein-1. GPCR-kinase-interacting protein-1 has been shown to be important for GPCR internalization and acts as an integrator of Src-dependent signal transduction activated by GPCRs and receptor tyrosine kinases (64). In the case of β2-adrenergic receptor, Src kinase is responsible for an agonist-induced rapid and transient tyrosine phosphorylation of G protein-coupled receptor kinase 2 (65). Further studies suggest that the tyrosine residue (Tyr350) on the carboxyl-terminal tail of β2-receptor is phosphorylated by some tyrosine kinase, which performs as a docking site to recruit and activate Src. Activated Src, in turn, phosphorylates and activates G protein-coupled receptor kinase 2. By this means, β2-receptor signaling is switched from the adenylyl cyclase pathway to the MAP kinase pathway (66). In our examination of the OPRM1 complex, additional Tyr-phosphorylated proteins were observed after chronic morphine treatment (Fig. 6A). The identities and the roles of these proteins in opioid receptor signal switching remain to be determined.
In summary, our current studies suggest that after the first wave of signals from agonist activation of the opioid receptor is blunted; an alternative second wave of signals could be initiated by activation of Src kinase, which was not observed in the initial wave of signals. The activated Src is recruited by the receptor and results in the Tyr phosphorylation of the receptor in a G protein-dependent and β-arrestin-independent manner. The OPRM1-Gαi2-Src signaling complex could recruit and/or phosphorylate other signaling molecules to accomplish specific signal transduction. In this respect, the opioid receptor transduces its initial signals as a classical GPCR. With prolonged agonist treatment, the recruitment of Src converts the opioid receptor into a receptor tyrosine kinase-like signaling complex. Within this complex containing activated Src kinase, the autophosphorylation of OPRM1 and the requirement of such phosphorylation in AC activation parallel the activation of receptor tyrosine kinases. Furthermore the Src activation and the subsequent Tyr phosphorylation of OPRM1 are more pronounced upon the addition of antagonist like naloxone after chronic morphine treatment; this might be the molecular basis for the hyperactivities observed during agonist withdrawal after chronic opiate administration in animal models. The eventual identification of all the Src kinase targets and the elucidation of the mechanism by which opioid receptors could activate Src would greatly facilitate the eventual understanding of chronic opioid drug effects.
Supplementary Material
Acknowledgments
We thank Dr. R. J. Lefkowitz (Duke University) for the MEF-WT1, MEF-βArr2KO, and MEF-βArr1&2KO cell lines.
This work was supported, in whole or in part, by National Institutes of Health Grants DA007339, DA016674, DA000564, DA011806, K05-DA70544 (to H. H. L.), and K05-DA00513 (to P.-Y. L.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains a supplemental figure.
Footnotes
The abbreviations used are: GPCR, G protein-coupled receptor; AC, adenylyl cyclase; HEK293, human embryonic kidney 293; OPRM1, μ-opioid receptor; HEKMT cells, HEK293 cells stably expressing hemagglutinin-tagged OPRM1; MEF, mouse embryonic fibroblast; pSrc, Tyr416-phosphorylated Src; PP2, 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine; PP3, 4-amino-7-phenylpyrazolo[3,4-d]pyrimidine; PTX, pertussis toxin; MAP, mitogen-activated protein; HA, hemagglutinin; WT, wild type; SYF cells, MEF cells with Src/Fyn/Yes knock-out; MES, 4-morpholineethanesulfonic acid; βArr, β-arrestin; KO, knock-out; ERK, extracellular signal-regulated kinase; RGS, regulator of G protein signaling; MAPK, mitogen-activated protein kinase; SH, Src homology.
References
- 1.Pierce, K. L., and Lefkowitz, R. J. (2001) Nat. Rev. Neurosci. 2 727–733 [DOI] [PubMed] [Google Scholar]
- 2.Nguyen, Q. D., Faivre, S., Bruyneel, E., Rivat, C., Seto, M., Endo, T., Mareel, M., Emami, S., and Gespach, C. (2002) FASEB J. 16 565–576 [DOI] [PubMed] [Google Scholar]
- 3.Lefkowitz, R. J., Pierce, K. L., and Luttrell, L. M. (2002) Mol. Pharmacol. 62 971–974 [DOI] [PubMed] [Google Scholar]
- 4.Smith, N. J., and Luttrell, L. M. (2006) Hypertension 48 173–179 [DOI] [PubMed] [Google Scholar]
- 5.Hall, R. A., Premont, R. T., and Lefkowitz, R. J. (1999) J. Cell Biol. 145 927–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lefkowitz, R. J., and Shenoy, S. K. (2005) Science 308 512–517 [DOI] [PubMed] [Google Scholar]
- 7.Fischer, J. A., Muff, R., and Born, W. (2002) Biochem. Soc. Trans. 30 455–460 [DOI] [PubMed] [Google Scholar]
- 8.Brady, A. E., and Limbird, L. E. (2002) Cell. Signal. 14 297–309 [DOI] [PubMed] [Google Scholar]
- 9.Law, P. Y., Wong, Y. H., and Loh, H. H. (2000) Annu. Rev. Pharmacol. Toxicol. 40 389–430 [DOI] [PubMed] [Google Scholar]
- 10.Law, P. Y., Hom, D. S., and Loh, H. H. (1982) Mol. Pharmacol. 22 1–4 [PubMed] [Google Scholar]
- 11.Sharma, K. S., Klee, W. A., and Nirenberg, M. (1977) Proc. Natl. Acad. Sci. U. S. A. 74 3365–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pineyro, G., and Archer-Lahlou, E. (2007) Cell. Signal. 19 8–19 [DOI] [PubMed] [Google Scholar]
- 13.Koob, G. F., and Bloom, F. E. (1988) Science 242 715–723 [DOI] [PubMed] [Google Scholar]
- 14.Avidor-Reiss, T., Nevo, I., Levy, R., Pfeuffer, T., and Vogel, Z. (1996) J. Biol. Chem. 271 21309–21315 [DOI] [PubMed] [Google Scholar]
- 15.Avidor-Reiss, T., Nevo, I., Saya, D., Bayewitch, M., and Vogel, Z. (1997) J. Biol. Chem. 272 5040–5047 [DOI] [PubMed] [Google Scholar]
- 16.Chakrabarti, S., Oppermann, M., and Gintzler, A. R. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 4209–4214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varga, E. V., Rubenzik, M., Grife, V., Sugiyama, M., Stropova, D., Roeske, W. R., and Yamamura, H. I. (2002) Eur. J. Pharmacol. 451 101–102 [DOI] [PubMed] [Google Scholar]
- 18.Schallmach, E., Steiner, D., and Vogel, Z. (2006) J. Mol. Neurosci. 29 115–122 [DOI] [PubMed] [Google Scholar]
- 19.Li, L. Y., and Chang, K. J. (1996) Mol. Pharmacol. 50 599–602 [PubMed] [Google Scholar]
- 20.Walwyn, W., Evans, C. J., and Hales, T. G. (2007) J. Neurosci. 27 5092–5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szucs, M., Boda, K., and Gintzler, A. R. (2004) J. Pharmacol. Exp. Ther. 310 256–262 [DOI] [PubMed] [Google Scholar]
- 22.Sun, Y., Huang, J., Xiang, Y., Bastepe, M., Juppner, H., Kobilka, B. K., Zhang, J. J., and Huang, X. Y. (2007) EMBO J. 26 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao, W., Luttrell, L. M., Medvedev, A. V., Pierce, K. L., Daniel, K. W., Dixon, T. M., Lefkowitz, R. J., and Collins, S. (2000) J. Biol. Chem. 275 38131–38134 [DOI] [PubMed] [Google Scholar]
- 24.Singer, C. A., Vang, S., and Gerthoffer, W. T. (2002) Am. J. Physiol. 282 G61–G68 [DOI] [PubMed] [Google Scholar]
- 25.Linseman, D. A., Heidenreich, K. A., and Fisher, S. K. (2001) J. Biol. Chem. 276 5622–5628 [DOI] [PubMed] [Google Scholar]
- 26.Schindelholz, B., and Reber, B. F. (1997) J. Neurosci. 17 8391–8401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luttrell, D. K., and Luttrell, L. M. (2004) Oncogene 23 7969–7978 [DOI] [PubMed] [Google Scholar]
- 28.Kam, A. Y., Chan, A. S., and Wong, Y. H. (2004) J. Pharmacol. Exp. Ther. 310 301–310 [DOI] [PubMed] [Google Scholar]
- 29.Kramer, H. K., Andria, M. L., Esposito, D. H., and Simon, E. J. (2000) Biochem. Pharmacol. 60 781–792 [DOI] [PubMed] [Google Scholar]
- 30.Narita, M., Kato, H., Kasukawa, A., Narita, M., Suzuki, M., Takeuchi, T., and Suzuki, T. (2006) Neuroreport 17 115–119 [DOI] [PubMed] [Google Scholar]
- 31.Zhang, L., Tetrault, J., Wang, W., Loh, H. H., and Law, P. Y. (2006) Mol. Pharmacol. 69 1810–1819 [DOI] [PubMed] [Google Scholar]
- 32.Zhao, H., Loh, H. H., and Law, P. Y. (2006) Mol. Pharmacol. 69 1421–1432 [DOI] [PubMed] [Google Scholar]
- 33.Allen, J. A., Halverson-Tamboli, R. A., and Rasenick, M. M. (2007) Nat. Rev. Neurosci. 8 128–140 [DOI] [PubMed] [Google Scholar]
- 34.Klinghoffer, R. A., Sachsenmaier, C., Cooper, J. A., and Soriano, P. (1999) EMBO J. 18 2459–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li, S., Okamoto, T., Chun, M., Sargiacomo, M., Casanova, J. E., Hansen, S. H., Nishimoto, I., and Lisanti, M. P. (1995) J. Biol. Chem. 270 15693–15701 [DOI] [PubMed] [Google Scholar]
- 36.Law, P. Y., Wong, Y. H., and Loh, H. H. (1999) Biopolymers 51 440–455 [DOI] [PubMed] [Google Scholar]
- 37.Audet, N., Paquin-Gobeil, M., Landry-Paquet, O., Schiller, P. W., and Pineyro, G. (2005) J. Biol. Chem. 280 7808–7816 [DOI] [PubMed] [Google Scholar]
- 38.Finn, A. K., and Whistler, J. L. (2001) Neuron 32 829–839 [DOI] [PubMed] [Google Scholar]
- 39.Qiu, Y., Law, P. Y., and Loh, H. H. (2003) J. Biol. Chem. 278 36733–36739 [DOI] [PubMed] [Google Scholar]
- 40.Kam, A. Y., Chan, A. S., and Wong, Y. H. (2003) J. Neurochem. 84 503–513 [DOI] [PubMed] [Google Scholar]
- 41.Roskoski, R., Jr. (2005) Biochem. Biophys. Res. Commun. 331 1–14 [DOI] [PubMed] [Google Scholar]
- 42.Sharma, K. S., Klee, W. A., and Nirenberg, M. (1975) Proc. Natl. Acad. Sci. U. S. A. 72 3092–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng, H. C., Nishio, H., Hatase, O., Ralph, S., and Wang, J. H. (1992) J. Biol. Chem. 267 9248–9256 [PubMed] [Google Scholar]
- 44.Shenoy, S. K., and Lefkowitz, R. J. (2003) Biochem. J. 375 503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lefkowitz, R. J., and Whalen, E. J. (2004) Curr. Opin. Cell Biol. 16 162–168 [DOI] [PubMed] [Google Scholar]
- 46.Luttrell, L. M., Ferguson, S. S., Daaka, Y., Miller, W. E., Maudsley, S., Della Rocca, G. J., Lin, F., Kawakatsu, H., Owada, K., Luttrell, D. K., Caron, M. G., and Lefkowitz, R. J. (1999) Science 283 655–661 [DOI] [PubMed] [Google Scholar]
- 47.Ma, Y. C., Huang, J., Ali, S., Lowry, W., and Huang, X. Y. (2000) Cell 102 635–646 [DOI] [PubMed] [Google Scholar]
- 48.Graham, F. L., Smiley, J., Russell, W. C., and Nairn, R. (1977) J. Gen. Virol. 36 59–74 [DOI] [PubMed] [Google Scholar]
- 49.Chakrabarti, S., Wang, L., Tang, W. J., and Gintzler, A. R. (1998) Mol. Pharmacol. 54 949–953 [DOI] [PubMed] [Google Scholar]
- 50.Violin, J. D., and Lefkowitz, R. J. (2007) Trends Pharmacol. Sci. 28 416–422 [DOI] [PubMed] [Google Scholar]
- 51.McDonald, P. H., Chow, C. W., Miller, W. E., Laporte, S. A., Field, M. E., Lin, F. T., Davis, R. J., and Lefkowitz, R. J. (2000) Science 290 1574–1577 [DOI] [PubMed] [Google Scholar]
- 52.Garzon, J., Rodriguez-Munoz, M., and Sanchez-Blazquez, P. (2005) Neuropharmacology 48 853–868 [DOI] [PubMed] [Google Scholar]
- 53.Zachariou, V., Georgescu, D., Sanchez, N., Rahman, Z., DiLeone, R., Berton, O., Neve, R. L., Sim-Selley, L. J., Selley, D. E., Gold, S. J., and Nestler, E. J. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 13656–13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abramow-Newerly, M., Roy, A. A., Nunn, C., and Chidiac, P. (2006) Cell. Signal. 18 579–591 [DOI] [PubMed] [Google Scholar]
- 55.Cavalli, A., Druey, K. M., and Milligan, G. (2000) J. Biol. Chem. 275 23693–23699 [DOI] [PubMed] [Google Scholar]
- 56.Roy, A. A., Lemberg, K. E., and Chidiac, P. (2003) Mol. Pharmacol. 64 587–593 [DOI] [PubMed] [Google Scholar]
- 57.Kalatskaya, I., Schussler, S., Blaukat, A., Muller-Esterl, W., Jochum, M., Proud, D., and Faussner, A. (2004) J. Biol. Chem. 279 31268–31276 [DOI] [PubMed] [Google Scholar]
- 58.Kramer, H. K., Andria, M. L., Kushner, S. A., Esposito, D. H., Hiller, J. M., and Simon, E. J. (2000) Brain Res. Mol. Brain Res. 79 55–66 [DOI] [PubMed] [Google Scholar]
- 59.Rovati, G. E., Capra, V., and Neubig, R. R. (2007) Mol. Pharmacol. 71 959–964 [DOI] [PubMed] [Google Scholar]
- 60.Li, J., Chen, C., Huang, P., and Liu-Chen, L. Y. (2001) Mol. Pharmacol. 60 1064–1075 [DOI] [PubMed] [Google Scholar]
- 61.Wong, Y. H., Conklin, B. R., and Bourne, H. R. (1992) Science 255 339–342 [DOI] [PubMed] [Google Scholar]
- 62.Favre, N., Fanelli, F., Missotten, M., Nichols, A., Wilson, J., di Tiani, M., Rommel, C., and Scheer, A. (2005) Biochemistry 44 9990–10008 [DOI] [PubMed] [Google Scholar]
- 63.Fritze, O., Filipek, S., Kuksa, V., Palczewski, K., Hofmann, K. P., and Ernst, O. P. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 2290–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haendeler, J., Yin, G., Hojo, Y., Saito, Y., Melaragno, M., Yan, C., Sharma, V. K., Heller, M., Aebersold, R., and Berk, B. C. (2003) J. Biol. Chem. 278 49936–49944 [DOI] [PubMed] [Google Scholar]
- 65.Sarnago, S., Elorza, A., and Mayor, F., Jr. (1999) J. Biol. Chem. 274 34411–34416 [DOI] [PubMed] [Google Scholar]
- 66.Fan, G., Shumay, E., Malbon, C. C., and Wang, H. (2001) J. Biol. Chem. 276 13240–13247 [DOI] [PubMed] [Google Scholar]
- 67.Law, P. Y., Hom, D. S., and Loh, H. H. (1983) Mol. Pharmacol. 24 413–424 [PubMed] [Google Scholar]
- 68.Bahia, D. S., Wise, A., Fanelli, F., Lee, M., Rees, S., and Milligan, G. (1998) Biochemistry 37 11555–11562 [DOI] [PubMed] [Google Scholar]
- 69.Stuermer, C. A., Lang, D. M., Kirsch, F., Wiechers, M., Deininger, S. O., and Plattner, H. (2001) Mol. Biol. Cell 12 3031–3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.