Abstract
Although autophagy maintains normal neural function by degrading misfolded proteins, little is known about how neurons activate this integral response. Furthermore, classical methods of autophagy induction used with nonneural cells, such as starvation, simply result in neuron death. To study neuronal autophagy, we cultured primary cortical neurons from transgenic mice that ubiquitously express green fluorescent protein-tagged LC3 and monitored LC3-I to LC3-II conversion by immunohistochemistry and immunoblotting. Evaluation of different culture media led us to discover that culturing primary neurons in Dulbecco's modified Eagle's medium without B27 supplementation robustly activates autophagy. We validated this nutrient-limited media approach for inducing autophagy by showing that 3-methyl-adenine treatment and Atg5 RNA interference knockdown each inhibits LC3-I to LC3-II conversion. Evaluation of B27 supplement components yielded insulin as the factor whose absence induced autophagy in primary neurons, and this activation was mammalian target of rapamycin-dependent. When we tested if nutrient-limited media could protect neurons expressing polyglutamine-expanded proteins against cell death, we observed a strong protective effect, probably due to autophagy activation. Our results indicate that nutrient deprivation can be used to understand the regulatory basis of neuronal autophagy and implicate diminished insulin signaling in the activation of neuronal autophagy.
Most neurodegenerative disorders are characterized by the accumulation of misfolded proteins that coalesce into “inclusions” and become visible at the light microscope level in the brains and spinal cords of affected patients (1, 2). These inclusions manifest themselves pathologically in Alzheimer disease as extracellular plaques and neurofibrillary tangles, in Parkinson disease as Lewy bodies, and in poly(Q) repeat diseases as cytosolic and nuclear aggregates. A fundamental advance in our understanding of neurodegeneration has been the realization that protein misfolding is a common theme in many important neurological disorders, including Alzheimer disease, Parkinson disease, amyotrophic lateral sclerosis, prion diseases, and poly(Q) diseases. The mechanistic underpinning of this “proteinopathy” hypothesis stems from the exquisite susceptibility of postmitotic cells in the central nervous system to misfolded protein stress, since neural cells are not continually replenished by cell division, unlike most of their nonneural counterparts.
The ubiquitin-proteasome system is the main intracellular degradation pathway to remove short lived proteins and to eliminate peptides that exit from the protein-folding machinery of the endoplasmic reticulum with an aberrant conformation. However, many aggregate-prone proteins, such as poly(Q) proteins, are inefficiently degraded by the proteasome (3–5). Failure of adequate degradation of aggregate-prone proteins activates alternative protein turnover pathways in the cell, including macroautophagy (hereafter referred to as autophagy). Autophagy is a degradative process that begins with engulfment of cytosolic materials and/or organelles and progresses through a series of steps involving production of a double membrane bound structure, culminating in the delivery of the engulfed material to lysosomes (6). In the central nervous system, basal levels of autophagy are required for the continued health and normal function of neurons, since conditional inactivation of the autophagy pathway in neural cells in mice yields neuronal dysfunction and neurodegeneration characterized by the accumulation of proteinaceous material (7, 8). Furthermore, the presence of aggregate-prone proteins, not degraded by the proteasome, induces autophagy above basal levels, and activation of autophagy appears capable of clearing misfolded proteins, decreasing cytotoxicity, and preventing neurodegeneration in Drosophila and mouse models of misfolded protein stress (9–11).
Although numerous reports have documented the protective effects of inducing autophagy in different areas of the diseased brain in model organisms (reviewed in Ref. 12), little is known about how neurons activate this integral response. Indeed, classical methods of autophagy induction used with cultured nonneural cells, such as starvation, simply result in the death of cultured primary neurons. Furthermore, starvation elicits quite different effects in neurons and nonneural cells, both in vitro and in vivo (13, 14). To directly study neuronal autophagy, we devised a primary neuron culture system where we can induce autophagy activation by withdrawal of a key supplement from the culture media. After independently validating the activation of autophagy in our system through pharmacological and genetic inhibition, we identified insulin as the factor responsible for autophagy induction in primary cortical neurons grown in nutrient-limited media. Further characterization of autophagy induction in primary neurons subjected to nutrient deprivation indicated that such autophagy activation is mammalian target of rapamycin (mTOR)2-dependent. We then tested if the autophagy response induced by nutrient deprivation could counter misfolded protein stress by expressing a poly(Q)-expanded protein in primary neurons and found that nutrient limitation prevented neuron cell death caused by misfolded protein stress.
EXPERIMENTAL PROCEDURES
Expression and RNA Interference Constructs—The AR-QN expression constructs (where N represents the number of CAG repeats) have been described (15). The lentiviral vector, pRRL-sin-cppt-pgk-wpre-GFP (Add-gene plasmid number 12252) was modified to express a short hairpin sequence targeting positions 1022–1041 or 1340–1359 in the murine Atg5 mRNA. Briefly, the WPRE promoter and green fluorescent protein (GFP) sequence were replaced with a cytomegalovirus promoter and enhanced cyan fluorescent protein sequence from the plasmid pECFP-C1 (Clontech) by cloning into unique XcmI and SalI sites. The mouse U6 promoter and short hairpin RNA (shRNA) sequence were then PCR-subcloned just 5′ to the cytomegalovirus/enhanced cyan fluorescent protein sequence at the XhoI and StuI/EcoRV sites. The resulting vector was renamed pRRLsin-Atg5-shRNA1(or 2)-CFP. An identical strategy was taken to derive the pRRLsin-beclin-1-shRNA-CFP vector, except that the short hairpin sequence targeted positions 606–624 in the murine beclin-1 mRNA.
Cell Culture, Primary Neuron Culture, and Transfection—Primary cortical neurons were cultured from postnatal day zero C57Bl/6J pups or GFP-LC3 transgenic pups. Briefly, pups were decapitated into Hanks' medium without Ca2+ and Mg2+, and cortices were dissected in Neurobasal-A™ medium supplemented with 10 mm HEPES. After dissection, cortices were trypsinized for 25 min at 37 °C and dissociated. Neurons were plated in Neurobasal-A™ with B27 supplement at a density of 2.5 × 105 cells/well in 4-well chamber CC2 slides (Nalge-Nunc). After 3 days, neurons were cultured in complete media (CM) (Neurobasal-A™ with B27 supplement), or nutrient-limited media (NLM) (Dulbecco's modified Eagle's medium (DMEM) alone). For autophagy induction, neurons were cultured for 4 h. For autophagy inhibition studies, 3-methyladenine was added to a final concentration of 10 mm to each medium condition. For transfection experiments, neurons were cultured in CM for 3 days. On day 4, we transfected primary cortical neurons with 1 μg of plasmid DNA using Lipofectamine 2000 for 24 h following the manufacturer's protocol (Invitrogen). The next day, transfected neurons were switched to CM, NLM, a reformulated solution that we designated as “MIX,” or NLM-supplemented media for 4 h or, depending upon the experiment, in CM or NLM for 24 h. For insulin supplementation, insulin (Sigma) was reconstituted in dilute acetic acid (0.01 n, pH 2.0) and added to CM at a final concentration of 0.6 μm. Experiments involving animals were approved by and performed in accordance with University of Washington Institutional Animal Care and Use Committee guidelines.
Western Blot Analysis—Primary cortical neurons were harvested in radioimmune precipitation buffer lysis buffer (10 mm Tris, 0.1% SDS, 1% sodium deoxycholate, 0.01% Triton X-100, 150 mm NaCl) and homogenized by passing five times through a 26.5-gauge syringe. Fifty μg of protein lysates were run on a 12% BisTris gel (Invitrogen) and transferred to polyvinylidene difluoride membranes (Millipore) using a semidry transfer system (Invitrogen). The membranes were blocked with 0.01% Tween 20, phosphate-buffered saline (PBS-T) containing 5% nonfat dried milk at 4 °C overnight and then probed with a primary antibody overnight. After washing, membranes were incubated with horseradish peroxidase-conjugated anti-rabbit IgG (1:10,000; Vector) in 5% milk, PBS-T for 1 h at room temperature. After treatment with enhanced chemiluminescence (Upstate), the membranes were visualized by autoradiography.
Primary Antibodies—The primary antibodies used in this study were as follows: rabbit anti-LC3 (1:1500; Novus Biologicals), rabbit anti-phospho-AKT-Ser473, rabbit anti-AKT, rabbit anti-phospho-mTOR-Ser2448, rabbit anti-mTOR, rabbit anti-phospho-S6K-Ser421/424, rabbit anti-S6K, rabbit anti-cleaved caspase-3-Asp175 (1:1000; Cell Signaling Technologies), mouse anti-actin (1:5000; Sigma), and mouse anti-HA-594 (Invitrogen).
Immunocytochemistry, Autophagy Assays, Primary Neuron Toxicity Assays, and Imaging—Neurons were fixed with 4% paraformaldehyde for 10 min at room temperature, washed with phosphate-buffered saline, counterstained with DAPI, and coverslipped. For autophagy experiments, GFP puncta were counted in at least five fields for at least three sets of slides from three separate cultures, with fields showing >5 GFP puncta scored as positive for autophagy activation. For the toxicity studies, cells were fixed in 4% paraformaldehyde, permeabilized with 0.25% Triton X-100, blocked with Pro-Block, and then incubated overnight in rabbit anti-active caspase-3 and mouse-anti-MAP2. Cells were then incubated in anti-rabbit 594 (1:1000; Molecular Probes) and anti-mouse 647 (1:1000; Molecular Probes) and counterstained with DAPI, and active caspase-3 was measured after medium replacement. Image analysis was performed with a Zeiss Axiovert 200M microscope, using an air objective at ×40 magnification (numerical aperture, 0.75) at room temperature. Our imaging medium was Fluoro-gel mounting medium from Electron Microscopy Services, and we used the following fluorochromes: GFP (fluorescein isothiocyanate), AlexaFluor 488, AlexaFluor 594, AlexaFluor 647, and DAPI. For our camera, we used the Photometrics CoolSnap HQ. Images were acquired with SLIDEBOOK digital microscopy software (version 4), and subsequently imported into Adobe Photoshop. We processed imported images with the brightness/contrast adjustments and the high pass filter at 32.0 pixels. All experiments were done in triplicate or quadruplicate.
Statistical Analysis—All data were prepared for analysis with standard spreadsheet software (Microsoft Excel). All error bars shown in the figures are S.E. Statistical analysis was done using Microsoft Excel or the VassarStats site on the World Wide Web. For ANOVA analysis involving multiple sample comparisons, we performed post hoc testing to discriminate significance relationships.
RESULTS
Development of Specialized Media for Autophagy Activation in Cultured Primary Neurons—Microtubule-associated LC3 (light chain 3), a mammalian homologue of yeast Atg8, is conjugated to phosphatidylethanolamine prior to its localization to the isolation membrane of precursor structures that become autophagosomes, an essential step in the autophagy pathway (13). The conversion of unconjugated LC3 (or LC3-I) to phosphatidylethanolamine-conjugated LC3 (or LC3-II) is a very reliable method for assaying autophagy activation. The LC3-I to LC3-II conversion can be monitored by a change in subcellular distribution, going from a diffuse to punctate appearance when associated with autophagic vesicles or by observing a molecular weight shift upon Western blot analysis (16). To study autophagy in neurons, we cultured primary cortical neurons from transgenic mice that ubiquitously express GFP-tagged LC3 (14). The utility of the GFP-LC3 transgenic mice for tracking autophagy activation in a wide variety of tissues has been shown, and immunoelectron microscopy has demonstrated that GFP-LC3 localizes only to the membranes of autophagosomes (16).
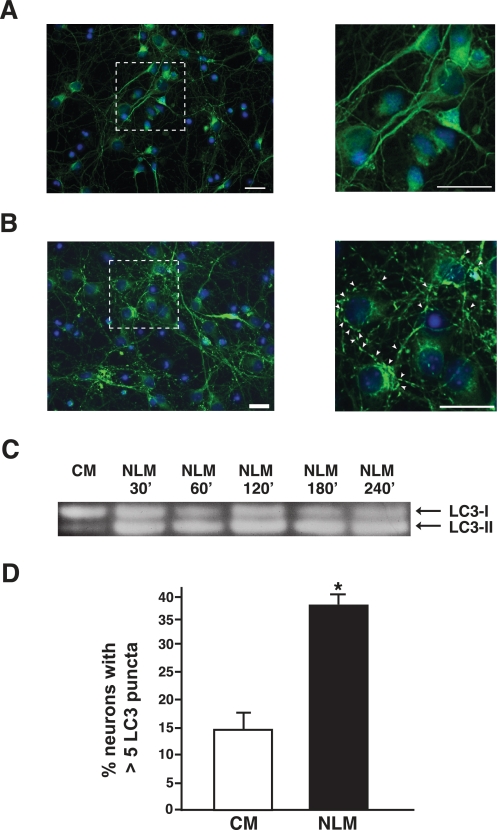
To establish primary cortical neuron cultures from postnatal day 0 GFP-LC3 transgenic pups, we followed a standard protocol and placed neurons in a CM of Neurobasal-A™ with B27 medium supplement and l-glutamine. Unlike other cell types, neurons do not tolerate most culture medium alterations, usually dying soon after the manipulation; hence, induction of autophagy in primary neurons using a standard starvation paradigm, as is typically done for nonneural cells in culture, is not feasible. Instead, we developed an NLM consisting of DMEM without B27 supplementation. The composition of DMEM is comparable with that of Neurobasal media, except that DMEM has a higher osmolarity, increased cysteine concentration, and increased glutamine concentration (17). B27 supplement provides a variety of lipid-rich compounds, proteins, and growth factors and was formulated to enhance primary neuron growth while inhibiting the growth of glia (17). Primary cortical neurons cultured in NLM for 4 h displayed a striking punctate LC3 distribution, consistent with prominent autophagosome formation, unlike primary neurons in CM, whose LC3 cytosolic distribution remained diffuse (Fig. 1, A and B). Induction of autophagy in NLM-cultured primary neurons was corroborated by Western blot detection of an endogenous LC3-I to LC3-II shift (Fig. 1C). Analysis of multiple independent primary neuron cultures by quantification of punctate structures confirmed the dramatic ability of the NLM culture conditions to induce autophagy (Fig. 1D).
FIGURE 1.
Autophagy is induced by nutrient deprivation in primary cortical neurons. A and B, analysis of GFP-LC3 distribution in primary cortical neurons from GFP-LC3 transgenic mice grown in different culture medium conditions. Primary neurons cultured in CM consisting of Neurobasal-A™ with B27 supplement display diffuse GFP-LC3 staining (A), but primary neurons cultured in NLM with just DMEM show prominent GFP-LC3 puncta formation (B), clearly discernible at higher magnification (arrowheads; see inset). Green, GFP-LC3; blue, DAPI. Scale bar, 20 μm. C, LC3 Western blot analysis of primary cortical neurons grown in CM and NLM. A shift in the LC3 isoform type is progressively observed in primary neurons cultured in NLM. D, quantification of GFP-LC3 puncta formation in primary neurons grown in different culture medium conditions. NLM significantly induces autophagosome formation in comparison with CM (p < 0.05, one-way ANOVA; error bars, S.E.).
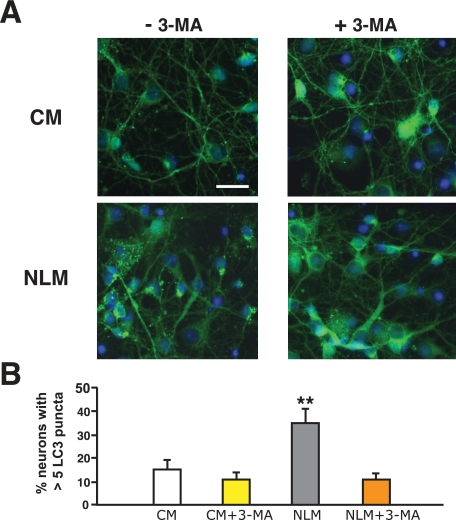
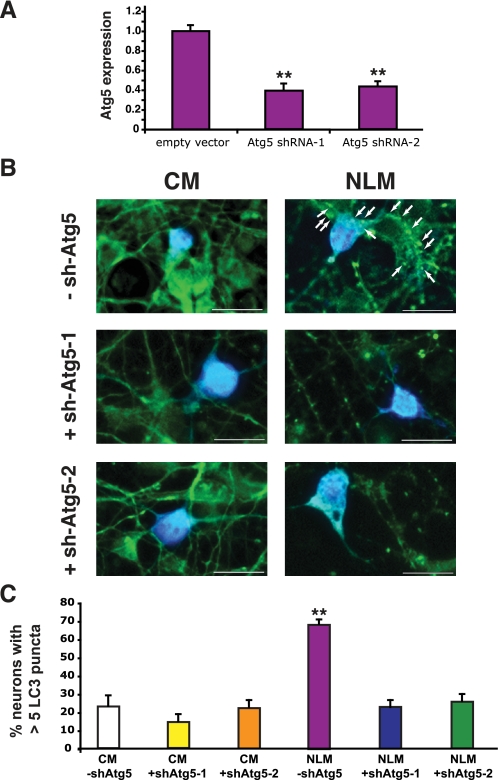
Validation of Neuronal Autophagy Induction by NLM—To confirm that these punctate structures were indicative of autophagy activation, we cultured primary cortical neurons from GFP-LC3 transgenic mice in NLM or in CM. These experiments were performed in the presence or absence of the class III PI3-kinase inhibitor 3-methyladenine (3-MA), since 3-MA inhibits the lipid kinase complex required for initial formation of the autophagosome isolation membrane and is thus an accepted pharmacological inhibitor of autophagy activation (18, 19). Neurons cultured in NLM displayed increased numbers of punctate GFP-LC3 structures, and as predicted, the punctate structures induced by NLM culture conditions no longer occurred upon the addition of 3-MA (Fig. 2). Inhibition of autophagy induction by 3-MA treatment was corroborated by Western blot analysis (supplemental Fig. 1). Although these data strongly suggested that our NLM method of autophagy induction in primary cortical neurons is valid, concerns about nonspecific effects of 3-MA pharmacological inhibition led us to perform an independent validation of our autophagy activation method. We therefore devised a RNA interference strategy for autophagy inhibition by generating shRNAs directed against the murine Atg5 gene (20) and validated them in Neuro2a cells (Fig. 3A). To permit tracking of Atg5 shRNA-expressing primary neurons, we inserted a CFP tag 3′ to the Atg5 shRNA in the knockdown constructs. Comparison of empty CFP vector-expressing primary neurons and Atg5 shRNA-CFP-positive primary neurons revealed decreased puncta in Atg5 shRNA-CFP-expressing neurons cultured in NLM (Fig. 3B). Quantification of puncta formation under these conditions confirmed that Atg5 shRNA knockdown prevented the appearance of GFP-LC3 puncta in NLM-cultured primary cortical neurons (Fig. 3C). To further validate the NLM method for autophagy induction, we also derived a beclin-1/Atg6 shRNA vector and noted reduced GFP-LC3 puncta in beclin-1 shRNA-CFP-expressing neurons cultured in NLM (supplemental Fig. 2).
FIGURE 2.
GFP-LC3 puncta formation induced by nutrient deprivation is inhibited by 3-methyladenine. A, primary cortical neurons from GFP-LC3 transgenic mice were cultured in CM or NLM, either in the absence of 3-methyladenine (-3-MA) or the presence of 3-methyladenine (+3-MA). GFP-LC3 puncta are infrequent in CM-cultured primary cortical neurons without 3-MA (upper left) but are common in NLM-cultured primary cortical neurons in the absence of 3-MA (bottom left). Inclusion of 3-MA in the culture media does not affect GFP-LC3 puncta frequency in CM cultured primary neurons (upper right) but markedly reduces GFP-LC3 puncta formation in NLM-cultured primary cortical neurons (bottom right). Green, GFP-LC3; blue, DAPI. Scale bar, 20 μm. B, quantification of GFP-LC3 puncta formation in primary neurons grown in different culture medium conditions with or without 3-MA. Although 3-MA has no effect upon GFP-LC3 puncta formation in primary neurons grown in CM (p = 0.47, one-way ANOVA), 3-MA treatment significantly reduces GFP-LC3 puncta formation in primary neurons grown in NLM (p < 0.01, one-way ANOVA; error bars, S.E.).
FIGURE 3.
GFP-LC3 puncta formation induced by nutrient deprivation is inhibited by Atg5 shRNA knockdown. A, validation of Atg5 shRNA knockdown constructs. The levels of Atg5 expression in Neuro2a cells transfected with two different Atg5 shRNA knockdown constructs are reduced to ∼37–42% of the Atg5 expression levels detected in Neuro2a cells transfected with the corresponding empty vector (p < 0.01 by one-way ANOVA). Data are based upon real time RT-PCR analysis of three independent sets of Neuro2a transfections per experiment. Error bars, S.E. B, primary cortical neurons from GFP-LC3 transgenic mice were cultured in CM or NLM and were transfected with either empty CFP vector (-sh-Atg5) or a Atg5-shRNA-CFP-tagged expression construct (+shAtg5-1 or +shAtg5-2). GFP-LC3 puncta are infrequent in CM cultured primary cortical neurons expressing CFP alone (upper left) but are common in NLM-cultured primary cortical neurons expressing CFP alone (upper right; GFP-LC3 puncta are indicated by arrows). GFP-LC3 puncta frequency in CM cultured primary neurons is unaffected by co-expression of a CFP-tagged Atg5 shRNA (middle left/lower left), but GFP-LC3 puncta formation is eliminated in NLM cultured primary cortical neurons expressing Atg5 shRNA-CFP (middle right/lower right). Green, GFP-LC3; light blue, CFP. Scale bar, 20 μm. C, quantification of GFP-LC3 puncta formation in primary neurons grown in different culture medium conditions with or without Atg5 shRNA knockdown. Although Atg5 knockdown has no significant effect upon GFP-LC3 puncta formation in primary neurons grown in CM (p > 0.05 by one-way ANOVA), Atg5 shRNA knockdown significantly reduces GFP-LC3 puncta formation in primary neurons grown in NLM (p < 0.01 for each Atg5 shRNA, one-way ANOVA; error bars, S.E.). Two different Atg5 shRNA constructs were used to rule out “off-target” effects.
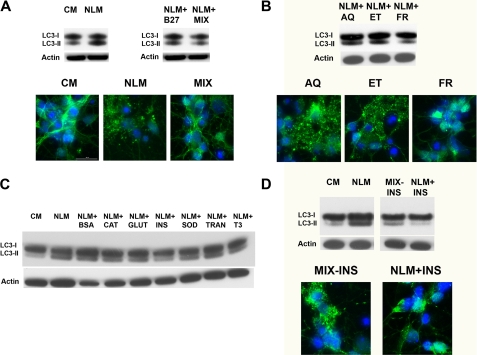
Evaluation of B27 Supplement Links Insulin Absence to Neuronal Autophagy Induction—Based upon these results, we reasoned that autophagy induction in primary cortical neurons was probably due to absence of the B27 supplement from the neuron growth media. To test this hypothesis, we added B27 supplement back to our NLM culture media and assessed primary cortical neurons grown in NLM + B27 supplement for autophagy activation. LC3 Western blot analysis revealed a decrease in the amount of lipidated LC3-II upon B27 supplementation (Fig. 4A). To investigate further which component(s) of the B27 supplement was most responsible for autophagy induction when withdrawn, we reconstituted our own version of B27 supplement by individually preparing each of the components (Table 1) and combining them, at the same concentrations as commercial B27, into a solution that we designated as MIX. Primary neurons cultured in NLM supplemented with this MIX displayed reduced LC3-II levels that were similar to LC3-II levels in primary neurons cultured in CM conditions, based upon Western blot analysis and fluorescence confocal microscopy (Fig. 4A). The MIX components could be further broken down into three main stock solutions: (i) an aqueous stock (AQ) containing vitamins, sugars, amino acids and minerals; (ii) an ethanolic stock (ET) containing fatty acids, hormones, and vitamin E; and (iii) a fresh stock (FR) containing antioxidants and hormones (Table 1). When we added the AQ, ET, or FR stocks to NLM for culturing of primary neurons, we found that supplementation of NLM with the fresh components prevented autophagy activation (Fig. 4B), suggesting that the component(s) most responsible for autophagy induction, when withdrawn, was contained in the fresh stock solution. We thus prepared each individual component of the fresh stock solution and supplemented the NLM with each one individually, prior to culturing primary neurons. Western blot analysis of neurons cultured under these different conditions indicated that the addition of insulin to NLM was most effective at reversing the LC3-II increase (Fig. 4C). To confirm the importance of insulin for preventing autophagy induced by NLM culture conditions, we prepared the MIX with all of its components except insulin and compared primary neurons cultured in CM, NLM, the MIX lacking insulin, or NLM supplemented with insulin. Both Western blot analysis and confocal microscopy revealed obvious evidence for LC3-II conjugation in primary neurons cultured in the MIX lacking insulin but minimal LC3-II conjugation in primary neurons cultured in NLM supplemented with insulin (Fig. 4D). This set of experiments demonstrated that insulin is the most important component of NLM for autophagy induction in cultured primary neurons.
FIGURE 4.
Analysis of B27 supplement components indicates that insulin is responsible for nutrient deprivation induction of autophagy in primary neurons. A, the addition of CM components back to NLM represses autophagy activation. Western blot analysis of primary cortical neurons grown in CM or NLM reveals a shift from LC3-I to LC3-II under NLM conditions (upper left). We added B27 supplement to NLM or reformulated a “MIX” consisting of the aqueous, ethanolic, and fresh components of B27 supplement (Table 1). Primary neurons cultured in NLM + B27 or NLM + MIX did not display induction of autophagy, based upon LC3 Western blot analysis (upper right) and confocal imaging analysis (bottom). B, “fresh” components of B27 repress autophagy induction. Primary cortical neurons were cultured in NLM combined with either B27 MIX AQ or ET or FR. LC3 Western blot analysis (top) and confocal imaging (bottom) both reveal induction of autophagy upon the addition of aqueous or ethanolic components and repression of autophagy activation upon the addition of the fresh components. C, evaluation of individual compounds comprising the fresh components of B27. Primary cortical neurons were cultured in NLM combined individually with each fresh component: bovine serum albumin (BSA), catalase (CAT), glutathione (GLUT), insulin (INS), superoxide dismutase (SOD), transferrin (TRAN), or tri-iodo-l-thyronine (T3). Comparison of LC3 Western blot analysis of primary neurons cultured with these different medium combinations revealed that the addition of insulin to NLM diminished LC3-I to LC3-II conversion to the greatest extent. D, insulin is the principal factor in B27 responsible for neuronal autophagy induction. When we compared primary neurons cultured in CM or NLM with primary neurons cultured in NLM lacking just insulin (MIX - INS) or in NLM supplemented with just insulin, LC3 Western blot analysis (top) and confocal imaging (bottom) revealed that the absence of insulin from NLM induced autophagy, whereas the addition of insulin to NLM repressed autophagy. All immunoblots were reprobed for β-actin to ensure equivalent loading.
TABLE 1.
Division of individual B27 supplement components into three stocks
| AQ | ET | FR |
|---|---|---|
| Biotin | Corticosterone | Bovine serum albumin |
| Carnitine | Linoleic acid | Catalase |
| Galactose | Linolenic acid | Glutathione |
| Putrescine | Progesterone | Insulin |
| Selenium | Retinyl acetate | Superoxide dismutase |
| d-l-α-Tocopherol | Transferrin | |
| Triodo-l-thyronine |
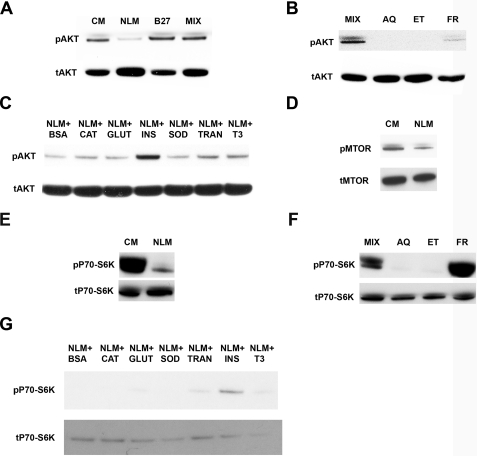
Induction of Neuronal Autophagy by NLM Is mTOR-dependent—The canonical insulin/insulin-like growth factor signaling pathway begins with the activation of a class I phosphatidylinositol 3-kinase that generates the second messenger molecule, phosphatidylinositol trisphosphate, which in turn activates Akt by positioning it for phosphorylation by PDK1 and PDK2 (21). Upon activation, Akt phosphorylates various substrates to promote cellular survival and growth. One key substrate for Akt phosphorylation is mTOR, whose role as a master regulator of autophagy and of protein translation is well established (22). To determine if NLM-induced neuronal autophagy involved the Akt signaling pathway, we cultured primary neurons under different medium conditions and performed Western blot analysis of Akt with an antibody directed against Akt phosphorylated at serine 473, since post-translation modification of Akt at this residue stabilizes the conformation of the enzyme (21). We also performed Western blot analysis with an antibody directed against another Akt epitope to detect all forms of Akt. This Western blot analysis revealed a decrease in active Akt in primary neurons cultured under NLM conditions and showed a recovery of Akt activation when B27 supplement or the MIX is added back to NLM (Fig. 5A). Consistent with our observations of autophagy induction, we found that Akt activation, as measured by phosphorylation, was present in primary neurons cultured in NLM supplemented with the fresh components (Fig. 5B) or NLM supplemented with just insulin (Fig. 5C). As mTOR activation is maintained as a downstream effect of Akt phosphorylation (21, 22), we analyzed mTOR phosphorylation status in primary neurons by Western blot analysis and documented decreased levels of phosphorylated mTOR in neurons cultured in NLM in comparison with neurons cultured in CM (Fig. 5D). To further confirm the activation state of mTOR, we evaluated the phosphorylation state of S6 kinase (p70-S6K), a direct substrate of mTOR. We found that levels of phosphorylated p70-S6K were markedly decreased in primary neurons cultured in NLM conditions (Fig. 5E). Western blot analysis of p70-S6K in primary neurons grown in the MIX or its components yielded strong activation of p70-S6K for neurons cultured in the MIX or in NLM supplemented with the fresh components, but minimal amounts of phosphorylated p70-S6K in neurons cultured in NLM supplemented with either aqueous or ethanolic components of the MIX (Fig. 5F). Finally, p70-S6K Western blot analysis of primary neurons cultured in NLM combined with individual components of the fresh stock confirmed that the absence of insulin is principally responsible for autophagy induction by NLM, since levels of phosphorylated p70-S6K were the highest, by far, in neurons cultured in NLM supplemented with insulin (Fig. 5G). Since these results suggested that autophagy induction in primary neurons involves Akt signaling and mTOR inhibition, we reasoned that direct inhibition of AKT signaling should also induce autophagy in primary neurons. Previous studies have shown that inhibition of the upstream class I phosphatidylinositol 3-kinase with pharmacologic inhibitors does not induce autophagy in HT-29 cells (18), and we confirmed this result in neurons (data not shown). We then decided to inhibit Akt activity itself, using the potent inhibitor X (Akt-X) (23). When we analyzed Akt-X-treated neurons for LC3-I to LC3-II conversion, we noted robust autophagy induction; however, Akt-X-treated neurons displayed high levels of phosphorylated p70-S6K (supplemental Fig. 3), suggesting that neuronal autophagy induction by direct Akt inhibition is mTOR-independent.
FIGURE 5.
Nutrient deprivation of primary neurons is accompanied by decreased activation of the Akt-mTOR signaling pathway. A, loss of Akt activation in neurons cultured in NLM. Western blot analysis of phosphorylated Akt (pAkt) and total Akt (tAkt) indicates that Akt activation is markedly decreased in primary cortical neurons cultured in NLM compared with CM. The addition of B27 supplement (B27) or the MIX to NLM yields a level of Akt activation that is comparable with CM. B, fresh components of B27 supplement yield Akt activation. When primary cortical neurons were cultured in NLM combined with either B27 MIX AQ or ET FR, the addition of the fresh components resulted in detectable Akt activation. C, evaluation of individual compounds comprising the fresh components of B27 for Akt activation. Primary cortical neurons were cultured in NLM combined individually with each fresh component: bovine serum albumin (BSA), catalase (CAT), glutathione (GLUT), insulin (INS), superoxide dismutase (SOD), transferrin (TRAN), or tri-iodo-l-thyronine (T3). Western blot analysis of phosphorylated Akt and total Akt indicates that Akt is most strongly activated in neurons cultured in NLM combined with insulin (NLM + INS). D, decreased mTOR activation in neurons cultured in NLM. Western blot analysis of phosphorylated mTOR (pMTOR) and total mTOR (tMTOR) shows that mTOR activation is decreased in primary cortical neurons cultured in NLM compared with CM. E, decreased S6 kinase activation in neurons cultured in NLM. Western blot analysis of phosphorylated S6 kinase (pP70-S6K) and total S6 kinase (pP70-S6K) reveals reduced S6 kinase activation in primary cortical neurons cultured in NLM compared with CM. F, fresh components of B27 supplement yield S6 kinase activation. When primary cortical neurons were cultured in NLM combined with either B27 MIX AQ, ET, or FR, the addition of the fresh components resulted in marked S6 kinase activation. G, evaluation of individual compounds comprising the fresh components of B27 for S6 kinase activation. Primary cortical neurons were cultured in NLM combined individually with different fresh components, as in C. Western blot analysis of phosphorylated S6 kinase (pP70-S6K) and total S6 kinase (pP70-S6K) demonstrates that S6 kinase is most strongly activated in neurons cultured in NLM combined with insulin (NLM + INS).
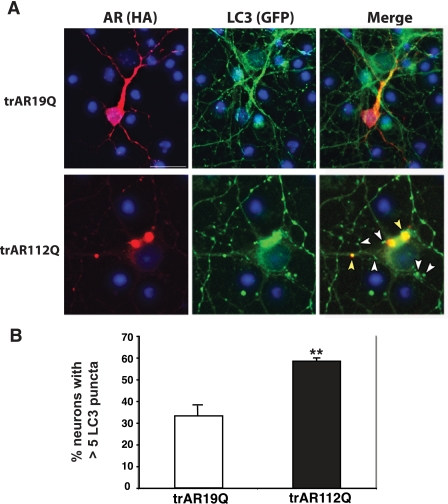
Induction of Autophagy by Nutrient Deprivation Can Prevent Polyglutamine Protein Stress in Primary Neurons—After validation of the NLM method to induce autophagy in primary neurons, we next asked whether misfolded proteins can induce autophagy. Several studies have shown that autophagy can mediate clearance of aggregateprone proteins involved in neurodegenerative disease, and autophagy activation in neurodegeneration has been demonstrated in several model systems (reviewed in Ref. 24). Spinal and bulbar muscular atrophy (Kennedy disease) is an inherited neurodegenerative disease caused by a poly(Q) repeat expansion in the androgen receptor (AR) gene (25). Importantly, poly(Q)-expanded AR has been shown to induce neurotoxicity in cell culture and in model organisms, including Drosophila and mice (26–30). Since amino-terminal truncation of poly(Q)-containing proteins enhances their toxicity (31–33), we expressed amino-terminally truncated poly(Q)-AR protein (trAR19Q or trAR112Q) in primary cortical neurons and noted that poly(Q)-AR yielded caspase-3 activation and nuclear condensation in a poly(Q) length-dependent manner, consistent with apoptotic activation and type I programmed cell death (supplemental Fig. 4). Cell death induced in primary cortical neurons by poly(Q)-AR is fully dependent upon Bax,3 further confirming that this neuron cell death process utilizes the apoptotic pathway. Since induction of autophagy may be a protective response against misfolded protein stress in primary neurons, we transfected GFP-LC3 cortical neurons with trAR19Q or with trAR112Q and measured autophagy induction. Recently, one report indicated that LC3 nonspecifically associates with aggregate-prone proteins, such as poly(Q)-expanded proteins (34), and indeed, an increase in coalesced LC3 in trAR112Q aggregates was noted in neurons expressing trAR112Q (Fig. 6). However, in primary neurons not yet undergoing apoptosis (caspase-3-negative), we observed a pronounced increase in punctate LC3 structures that did not co-localize with poly(Q)-containing aggregates (Fig. 6). This suggested that poly(Q)-expanded AR can induce autophagy prior to apoptotic cell death in primary neurons. To also ensure that neurite degeneration did not account for this GFP-LC3 puncta formation, we immunostained trAR112Q-expressing GFP-LC3 neurons for MAP2, and we observed prominent GFP-LC3 puncta in healthy neurites from trAR112Q-expressing neurons (supplemental Fig. 5).
FIGURE 6.
Polyglutamine-expanded AR induces autophagy in primary neurons. A, primary cortical neurons from GFP-LC3 transgenic mice were transfected with amino-terminally truncated AR with either 19 glutamines (trAR19Q) or 112 glutamines (trAR112Q), fused to a carboxyl-terminal HA tag. Anti-HA staining reveals expression of AR in perinuclear cytosol and neurite processes (left panels; red, HA; blue, DAPI). GFP-LC3 distribution remained diffuse in trAR16-expressing neurons, but in trAR112Q-expressing neurons, GFP-LC3 localized into discrete puncta. Merging of these images indicated that some GFP-LC3 puncta co-localized with trAR112Q aggregates (yellow arrowheads); however, most GFP-LC3 puncta were distinct from areas of AR112Q accumulation (white arrowheads). Scale bar, 20 μm. B, quantification of GFP-LC3 puncta formation in primary neurons expressing either amino-terminal truncated AR with either 19 glutamines (trAR19Q) or 112 glutamines (trAR112Q). Polyglutamine-expanded AR expression markedly increases the frequency of GFP-LC3 puncta (p < 0.01, one-way ANOVA; error bars, S.E.), indicating polyglutamine-length dependent autophagy activation.
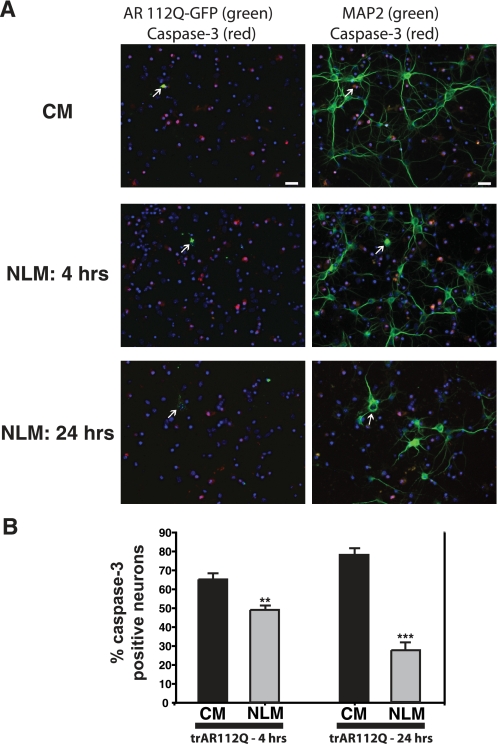
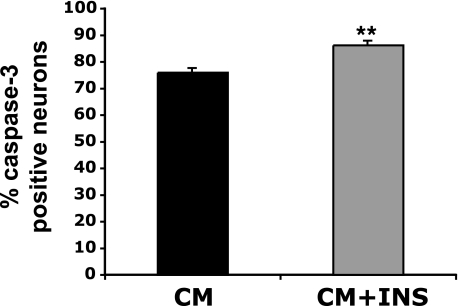
To determine if NLM culture conditions could yield enhanced activation of autophagy and thereby counter misfolded protein stress in primary neurons, we studied trAR112Q neurotoxicity in primary neurons cultured under different medium conditions. In this experiment, primary neurons expressing GFP-tagged poly(Q)-expanded AR protein were analyzed by immunostaining with an antibody against active caspase-3 and counting the number of poly(Q)-AR expressing neurons exhibiting caspase-3 activation. When we quantified caspase-3 activation in poly(Q)-AR-expressing neurons, we found that NLM culture conditions significantly reduced poly(Q)-AR neurotoxicity after 4 h (Fig. 7). The protective effect of NLM against poly(Q)-AR neurotoxicity was even more apparent at 24 h (Fig. 7). Since our results indicate that NLM induction of autophagy is neuroprotective principally due to insulin withdrawal, we decided to test if supplementation of CM with insulin would increase poly(Q) neurotoxicity. When we compared the levels of caspase-3 activation in trAR112Q-expressing primary neurons cultured in CM with or without insulin supplementation, we noted a significant increase in poly(Q) neurotoxicity upon insulin addition (Fig. 8). Our results thus suggest that insulin excess is correspondingly deleterious for neurons faced with poly(Q) proteotoxic stress.
FIGURE 7.
Nutrient deprivation prevents neuron cell death produced by expression of polyglutamine-expanded AR. A, primary neurons expressing GFP-tagged trAR112Q were cultured for 4 or 24 h in either CM or NLM and then assayed for apoptotic activation and neurotoxicity by immunostaining for caspase-3 activation and MAP2. Here we see representative low magnification images of these experiments. CM-cultured primary neuron expressing AR112Q-GFP (arrow) is positive for active caspase-3 and lacks MAP2-positive neurites. For neurons cultured in NLM for 4 h, AR112Q-GFP expression does not result in caspase-3 activation or loss of MAP2 reactivity (above left of neuron marked by an arrow). Culturing in NLM for 24 h yields even more robust protection for AR112Q-GFP-expressing neuron (arrow), since this neuron is negative for active caspase-3 but retains prominent MAP2 immunostaining. Left panels, AR112Q-GFP (fluorescein isothiocyanate; green), active caspase-3 (red), and DAPI (blue). Right panels, MAP2 (Cy5; green), active caspase-3 (red), and DAPI (blue). Scale bar, 20 μm. B, when we quantified caspase-3 activation in neurons expressing trAR112Q at 4 h post-transfection, we found that trAR112Q-expressing neurons cultured in NLM were significantly protected against apoptotic activation and neuron cell death (p < 0.01, one-way ANOVA). At 24 h post-transfection, the protective effect of culturing trAR112Q-expressing neurons in NLM was even more pronounced (p < 0.005, one-way ANOVA). Error bars, S.E.
FIGURE 8.
Insulin supplementation promotes trAR112Q neurotoxicity. Primary neurons expressing GFP-tagged trAR112Q were cultured for 24 h in either CM or CM supplemented with insulin (CM + INS). When we quantified caspase-3 activation, we noted a significant increase in trAR112Q-expressing neurons cultured in CM plus insulin. Increased AR112Q neurotoxicity in primary cortical neurons cultured in CM plus insulin was accompanied by decreased MAP2 staining and increased nuclear condensation (not shown).
DISCUSSION
Autophagy has emerged as a pathway of extreme importance in neurodegenerative disease and has a role in maintaining normal neural function by degrading aggregate-prone proteins, even when neurons are not exposed to mutant misfolded peptides or abnormally increased levels of altered conformers (7, 8). Despite its clearly demonstrated protective actions in normal neurons and neurological proteinopathies, whether neuronal autophagy might be deleterious in certain settings and how neuronal autophagy is regulated are crucial questions. To address these questions, a robust system for studying autophagy in primary neurons is needed. Neurons, unlike most other cell types, seldom experience nutrient stress, since the maintenance of normal central nervous system function is of paramount importance to an organism, promoting its ability to survive in a changing environment. Furthermore, since neurons have high bioenergetics demands and rely upon oxidative metabolism for energy production, starvation is not well tolerated. Hence, for cultured primary neurons, withdrawal of serum or nutrients rapidly produces cell death and cannot be employed to induce autophagy. To study the regulation of autophagy in primary neurons, we hypothesized that modification of standard neuron culture medium conditions might yield a reliable method for reproducible activation of the autophagy pathway. Evaluation of different culture medium combinations led us to discover that culturing primary cortical neurons in DMEM without B27 supplementation is a robust method for autophagy activation. We independently validated this so-called NLM culture approach for inducing autophagy by showing that 3-MA treatment, Atg5 RNA interference knockdown, and beclin-1/Atg6 RNA interference knockdown each inhibits the shift of GFP-tagged LC3 from a diffuse distribution to a punctate distribution in primary cortical neurons from GFP-LC3 transgenic mice. The GFP-LC3 shift from a diffuse to punctate distribution, which we visualized by confocal fluorescence microscopy, was accompanied by processing of endogenous LC3-I to its autophagosome-associated LC3-II isoform, based upon Western blot analysis. The facts that these two independent techniques corroborate one another and that previously published ultrastructural analysis of tissue from GFP-LC3 transgenic mice has localized GFP to autophagosomes (14, 16) indicate that this is a valid method for monitoring autophagy activation. Given the growing importance of autophagy for normal neuron function and neurodegenerative disease, we expect that selective nutrient deprivation for inducing neuronal autophagy will be a powerful approach for advancing this field. Toward that end, another group has recently shown that induction of autophagy in primary neurons can also be achieved with culture media lacking growth factors (35).
To better understand the basis of neuronal autophagy induction by NLM, we sought to identify the specific component(s) whose absence or alteration accounts for autophagy induction. Comparison of CM, containing Neurobasal™ with B27 supplementation, with NLM revealed a number of key differences: (i) NLM-DMEM has a higher osmolality, higher cysteine, and higher glutamine concentration than Neurobasal™-B27; (ii) B27 supplementation provides CM-cultured neurons with 20 unique components not present in NLM-DMEM; and (iii) NLM-DMEM contains ferrous salts that were removed from Neurobasal™ media at the time of its formulation (17). We reasoned that one or more of the 20 unique components of the B27 supplement was probably responsible for the autophagy induction. An algorithmic evaluation of the B27 supplement components led us to identify insulin as the key factor whose absence induced autophagy activation in cultured primary neurons. Further analysis of NLM-cultured neurons demonstrated that the absence of insulin activates autophagy in an mTOR-dependent manner, since NLM results in reduced phosphorylation of mTOR and its downstream substrates.
Although withdrawal of insulin from neuron culture media would appear to be a logical candidate for mediating autophagy induction, the impact of insulin absence for neurons is likely to be complex, since the insulin signaling pathway has so many different effects. It is well known that insulin is an important prosurvival signaling molecule in neurons (36) and that insulin can protect neurons from apoptotic cell death precipitated by serum withdrawal (37, 38). A number of studies have shown that insulin-like growth factor-1 can counter poly(Q) neurotoxicity (39, 40); however, the downstream effects of insulin and insulin-like growth factor-1 in neurons may differ, based upon differences in the nature or level of elicited receptor activation. Indeed, prolonged activation of Akt, the main downstream effector in the insulin signaling pathway, can also be detrimental, since prolonged Akt activation produces excessive ubiquitin-proteasome system action and insulin resistance in the central nervous system (36). Since life span extension in yeast, worms, flies, and mice has been linked to a decrease in insulin signaling (41), and interventions known to extend life span, such as dietary restriction, mitigate misfolded protein neurotoxicity (42–45), diminished insulin signaling can be neuroprotective. When we tested NLM for its ability to protect primary neurons expressing poly(Q)-expanded AR protein against apoptotic cell death, we observed a strong protective effect, probably due to NLM-induced autophagy activation. Autophagy induction may thus be required for neuroprotection imparted by reduced insulin signaling, just as autophagy activation is required for life span extension in worms carrying the daf-2 mutation that reduces insulin signaling (46).
Another important issue in neuronal autophagy induction is its regulatory basis. The NLM system, which we have developed for autophagy induction, is mTOR-dependent, and we found that NLM induction of such mTOR-dependent autophagy could protect primary cortical neurons from misfolded poly(Q) stress. Although rapamycin has been shown to activate mTOR-dependent autophagy that can be neuroprotective in HD Drosophila and mouse models (11), recent studies indicate that both mTOR-dependent and mTOR-independent activation of autophagy can clear misfolded poly(Q) protein aggregates (47, 48). Furthermore, one study in Neuro2a cells has even implicated the insulin signaling pathway, via IRS-2 (insulin receptor substrate 2), in mediating mTOR-independent autophagy activation in response to the expression of poly(Q)-expanded huntingtin protein (49). Although IRS-2-dependent clearance of poly(Q)-expanded huntingtin protein was documented in this study, the role of IRS-2 in promoting cellular survival was not assessed. Furthermore, regulation of autophagy activation at the level of the insulin receptor is bound to be complex, with the decision to activate or inactivate autophagy probably depending upon the nature and duration of the stimulus as well as the cellular milieu. The relative contributions of IRS-1 and IRS-2 to the ultimate downstream signal in neurons are unknown, and the role of p70 S6K feedback inhibition in modulating the IRS-1 output remains to be studied in neurons (50). The contribution of the insulin signaling pathway to neuronal autophagy activation is further complicated by the observation of mTOR-independent autophagy induction upon Akt inhibition. Taken together, our results and the work of others suggest that neurons are capable of activating autophagy via both mTOR-dependent and mTOR-independent pathways and that insulin signaling regulates this process. A key question for future studies will be to determine if mTOR-dependent and mTOR-independent pathways of autophagy activation are both neuroprotective or if the beneficial effects of one pathway predominate over the other pathway in neurons. Such information will better guide strategies for therapy development for the treatment of neurodegenerative proteinopathies.
Our work, for the first time, demonstrates that autophagy activation in primary neurons can be accomplished by subjecting cultured neurons to a specific type of nutrient stress. Unlike starvation protocols that deprive nonneural cells of glucose and/or amino acids to induce autophagy, our NLM strategy provides neurons with glucose and amino acids but withholds certain growth factors and hormones, most especially insulin. Although autophagy is a catabolic process initiated to yield biosynthetic building blocks and to provide the capacity to produce energy, our results underscore that autophagy is a cellular process that requires energy to occur. Hence, for neurons with limited capacities to produce their own energy, a selective starvation strategy is necessary for achieving autophagy induction. Since NLM induction of autophagy was shown to protect primary neurons from misfolded protein stress, our results indicate that the NLM protocol can be used to understand the regulatory basis of physiologically relevant neuronal autophagy. Furthermore, our findings demonstrate that reduced insulin signaling can be neuroprotective, especially when neurons are faced with proteotoxic stress.
Acknowledgments
We thank N. Mizushima and Z. Yue for providing GFP-LC3 transgenic mice and B. L. Sopher, A. C. Smith, and H. Burke for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants NS41648 (to A. R. L.), EY14997 (to A. R. L.), and T32-AG00057 (to J. E. Y.). This work was also supported by a grant from the Muscular Dystrophy Association. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
Footnotes
The abbreviations used are: mTOR, mammalian target of rapamycin; 3-MA, 3-methyladenine; CM, complete media; NLM, nutrient-limited media; GFP, green fluorescent protein; shRNA, short hairpin RNA; BisTris, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol; DAPI, 4′,6-diamidino-2-phenylindole; ANOVA, analysis of variance; CFP, cyan fluorescent protein; AQ, aqueous stock; ET, ethanolic stock; AR, androgen receptor; HA, hemagglutinin.
A. R. La Spada, unpublished results.
References
- 1.Alves-Rodrigues, A., Gregori, L., and Figueiredo-Pereira, M. E. (1998) Trends Neurosci. 21 516-520 [DOI] [PubMed] [Google Scholar]
- 2.Taylor, J. P., Hardy, J., and Fischbeck, K. H. (2002) Science 296 1991-1995 [DOI] [PubMed] [Google Scholar]
- 3.Bence, N. F., Sampat, R. M., and Kopito, R. R. (2001) Science 292 1552-1555 [DOI] [PubMed] [Google Scholar]
- 4.Holmberg, C. I., Staniszewski, K. E., Mensah, K. N., Matouschek, A., and Morimoto, R. I. (2004) EMBO J. 23 4307-4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatraman, P., Wetzel, R., Tanaka, M., Nukina, N., and Goldberg, A. L. (2004) Mol. Cell 14 95-104 [DOI] [PubMed] [Google Scholar]
- 6.Shintani, T., and Klionsky, D. J. (2004) Science 306 990-995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hara, T., Nakamura, K., Matsui, M., Yamamoto, A., Nakahara, Y., Suzuki-Migishima, R., Yokoyama, M., Mishima, K., Saito, I., Okano, H., and Mizushima, N. (2006) Nature 441 885-889 [DOI] [PubMed] [Google Scholar]
- 8.Komatsu, M., Waguri, S., Chiba, T., Murata, S., Iwata, J., Tanida, I., Ueno, T., Koike, M., Uchiyama, Y., Kominami, E., and Tanaka, K. (2006) Nature 441 880-884 [DOI] [PubMed] [Google Scholar]
- 9.Pandey, U. B., Nie, Z., Batlevi, Y., McCray, B. A., Ritson, G. P., Nedelsky, N. B., Schwartz, S. L., DiProspero, N. A., Knight, M. A., Schuldiner, O., Padmanabhan, R., Hild, M., Berry, D. L., Garza, D., Hubbert, C. C., Yao, T. P., Baehrecke, E. H., and Taylor, J. P. (2007) Nature 447 859-863 [DOI] [PubMed] [Google Scholar]
- 10.Ravikumar, B., Acevedo-Arozena, A., Imarisio, S., Berger, Z., Vacher, C., O'Kane, C. J., Brown, S. D., and Rubinsztein, D. C. (2005) Nat. Genet. 37 771-776 [DOI] [PubMed] [Google Scholar]
- 11.Ravikumar, B., Vacher, C., Berger, Z., Davies, J. E., Luo, S., Oroz, L. G., Scaravilli, F., Easton, D. F., Duden, R., O'Kane, C. J., and Rubinsztein, D. C. (2004) Nat. Genet. 36 585-595 [DOI] [PubMed] [Google Scholar]
- 12.Levine, B., and Kroemer, G. (2008) Cell 132 27-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klionsky, D. J., Cuervo, A. M., and Seglen, P. O. (2007) Autophagy 3 181-206 [DOI] [PubMed] [Google Scholar]
- 14.Mizushima, N., Yamamoto, A., Matsui, M., Yoshimori, T., and Ohsumi, Y. (2004) Mol. Biol. Cell 15 1101-1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor, J. P., Tanaka, F., Robitschek, J., Sandoval, C. M., Taye, A., Markovic-Plese, S., and Fischbeck, K. H. (2003) Hum. Mol. Genet. 12 749-757 [DOI] [PubMed] [Google Scholar]
- 16.Kabeya, Y., Mizushima, N., Ueno, T., Yamamoto, A., Kirisako, T., Noda, T., Kominami, E., Ohsumi, Y., and Yoshimori, T. (2000) EMBO J. 19 5720-5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brewer, G. J., Torricelli, J. R., Evege, E. K., and Price, P. J. (1993) J. Neurosci. Res. 35 567-576 [DOI] [PubMed] [Google Scholar]
- 18.Petiot, A., Ogier-Denis, E., Blommaart, E. F., Meijer, A. J., and Codogno, P. (2000) J. Biol. Chem. 275 992-998 [DOI] [PubMed] [Google Scholar]
- 19.Seglen, P. O., and Gordon, P. B. (1982) Proc. Natl. Acad. Sci. U. S. A. 79 1889-1892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qu, X., Yu, J., Bhagat, G., Furuya, N., Hibshoosh, H., Troxel, A., Rosen, J., Eskelinen, E. L., Mizushima, N., Ohsumi, Y., Cattoretti, G., and Levine, B. (2003) J. Clin. Invest. 112 1809-1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sale, E. M., and Sale, G. J. (2008) Cell Mol. Life Sci. 65 113-127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhaskar, P. T., and Hay, N. (2007) Dev. Cell 12 487-502 [DOI] [PubMed] [Google Scholar]
- 23.Thimmaiah, K. N., Easton, J. B., Germain, G. S., Morton, C. L., Kamath, S., Buolamwini, J. K., and Houghton, P. J. (2005) J. Biol. Chem. 280 31924-31935 [DOI] [PubMed] [Google Scholar]
- 24.Rubinsztein, D. C. (2006) Nature 443 780-786 [DOI] [PubMed] [Google Scholar]
- 25.La Spada, A. R., Wilson, E. M., Lubahn, D. B., Harding, A. E., and Fischbeck, K. H. (1991) Nature 352 77-79 [DOI] [PubMed] [Google Scholar]
- 26.Chevalier-Larsen, E. S., O'Brien, C. J., Wang, H., Jenkins, S. C., Holder, L., Lieberman, A. P., and Merry, D. E. (2004) J. Neurosci. 24 4778-4786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darrington, R. S., Butler, R., Leigh, P. N., McPhaul, M. J., and Gallo, J. M. (2002) Neuroreport 13 2117-2120 [DOI] [PubMed] [Google Scholar]
- 28.Katsuno, M., Adachi, H., Kume, A., Li, M., Nakagomi, Y., Niwa, H., Sang, C., Kobayashi, Y., Doyu, M., and Sobue, G. (2002) Neuron 35 843-854 [DOI] [PubMed] [Google Scholar]
- 29.Sopher, B. L., Thomas, P. S., Jr., LaFevre-Bernt, M. A., Holm, I. E., Wilke, S. A., Ware, C. B., Jin, L. W., Libby, R. T., Ellerby, L. M., and La Spada, A. R. (2004) Neuron 41 687-699 [DOI] [PubMed] [Google Scholar]
- 30.Takeyama, K., Ito, S., Yamamoto, A., Tanimoto, H., Furutani, T., Kanuka, H., Miura, M., Tabata, T., and Kato, S. (2002) Neuron 35 855-864 [DOI] [PubMed] [Google Scholar]
- 31.Ellerby, L. M., Hackam, A. S., Propp, S. S., Ellerby, H. M., Rabizadeh, S., Cashman, N. R., Trifiro, M. A., Pinsky, L., Wellington, C. L., Salvesen, G. S., Hayden, M. R., and Bredesen, D. E. (1999) J. Neurochem. 72 185-195 [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi, Y., Miwa, S., Merry, D. E., Kume, A., Mei, L., Doyu, M., and Sobue, G. (1998) Biochem. Biophys. Res. Commun. 252 145-150 [DOI] [PubMed] [Google Scholar]
- 33.Merry, D. E., Kobayashi, Y., Bailey, C. K., Taye, A. A., and Fischbeck, K. H. (1998) Hum. Mol. Genet. 7 693-701 [DOI] [PubMed] [Google Scholar]
- 34.Kuma, A., Matsui, M., and Mizushima, N. (2007) Autophagy 3 323-328 [DOI] [PubMed] [Google Scholar]
- 35.Boland, B., Kumar, A., Lee, S., Platt, F. M., Wegiel, J., Yu, W. H., and Nixon, R. A. (2008) J. Neurosci. 28 6926-6937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Heide, L. P., Ramakers, G. M., and Smidt, M. P. (2006) Prog. Neurobiol. 79 205-221 [DOI] [PubMed] [Google Scholar]
- 37.Ryu, B. R., Ko, H. W., Jou, I., Noh, J. S., and Gwag, B. J. (1999) J. Neurobiol. 39 536-546 [PubMed] [Google Scholar]
- 38.van der Heide, L. P., Hoekman, M. F., Biessels, G. J., and Gispen, W. H. (2003) J. Neurochem. 86 86-91 [DOI] [PubMed] [Google Scholar]
- 39.Humbert, S., Bryson, E. A., Cordelieres, F. P., Connors, N. C., Datta, S. R., Finkbeiner, S., Greenberg, M. E., and Saudou, F. (2002) Dev. Cell 2 831-837 [DOI] [PubMed] [Google Scholar]
- 40.Palazzolo, I., Burnett, B. G., Young, J. E., Brenne, P. L., La Spada, A. R., Fischbeck, K. H., Howell, B. W., and Pennuto, M. (2007) Hum. Mol. Genet. 16 1593-1603 [DOI] [PubMed] [Google Scholar]
- 41.Barbieri, M., Bonafe, M., Franceschi, C., and Paolisso, G. (2003) Am. J. Physiol. Endocrinol. Metab. 285 E1064-E1071 [DOI] [PubMed] [Google Scholar]
- 42.Duan, W., Guo, Z., Jiang, H., Ware, M., Li, X. J., and Mattson, M. P. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 2911-2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halagappa, V. K., Guo, Z., Pearson, M., Matsuoka, Y., Cutler, R. G., Laferla, F. M., and Mattson, M. P. (2007) Neurobiol. Dis. 26 212-220 [DOI] [PubMed] [Google Scholar]
- 44.Patel, N. V., Gordon, M. N., Connor, K. E., Good, R. A., Engelman, R. W., Mason, J., Morgan, D. G., Morgan, T. E., and Finch, C. E. (2005) Neurobiol. Aging 26 995-1000 [DOI] [PubMed] [Google Scholar]
- 45.Wang, J., Ho, L., Qin, W., Rocher, A. B., Seror, I., Humala, N., Maniar, K., Dolios, G., Wang, R., Hof, P. R., and Pasinetti, G. M. (2005) FASEB J. 19 659-661 [DOI] [PubMed] [Google Scholar]
- 46.Melendez, A., Talloczy, Z., Seaman, M., Eskelinen, E. L., Hall, D. H., and Levine, B. (2003) Science 301 1387-1391 [DOI] [PubMed] [Google Scholar]
- 47.Sarkar, S., Davies, J. E., Huang, Z., Tunnacliffe, A., and Rubinsztein, D. C. (2007) J. Biol. Chem. 282 5641-5652 [DOI] [PubMed] [Google Scholar]
- 48.Sarkar, S., Krishna, G., Imarisio, S., Saiki, S., O'Kane, C. J., and Rubinsztein, D. C. (2008) Hum. Mol. Genet. 17 170-178 [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto, A., Cremona, M. L., and Rothman, J. E. (2006) J. Cell Biol. 172 719-731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Um, S. H., Frigerio, F., Watanabe, M., Picard, F., Joaquin, M., Sticker, M., Fumagalli, S., Allegrini, P. R., Kozma, S. C., Auwerx, J., and Thomas, G. (2004) Nature 431 200-205 [DOI] [PubMed] [Google Scholar]