Abstract
GSH concentration is considerably lower in the nucleus than in the cytoplasm; however, it is significantly elevated during active cell proliferation. The main purpose of this study was to understand the mechanism underlying these variations in nuclear/cytoplasmic distribution of GSH. The rate-limiting step in the de novo GSH biosynthesis pathway is catalyzed by glutamate cysteine ligase (GCL), a heterodimer, composed of a catalytic subunit (GCLc) and a modulatory subunit (GCLm). In Drosophila, GCLc, but not GCLm, contains a nuclear localization signal (NLS). Drosophila S2 cells, constitutively expressing regular GCLc protein or expressing GCLc protein with a mutated NLS motif, were generated by transfection. In quiescent S2 cells, GCLc is aggregated in the perinuclear cytosol and the nucleus, whereas GLCm resides solely in the cytosol. In actively proliferating S2 cells, expressing the normal NLS motif, GCLc migrates from the perinuclear cytoplasm into the nucleus, and the nuclear GSH level becomes elevated; in contrast, in proliferating cells, expressing the mutated NLS motif, neither does the GCLc migrate into the nucleus nor does the nuclear GSH amount rise. In S2 cells expressing wild type GCLc, perturbation of cellular redox state by exposure to cadmium resulted in the migration of GCLc into the nucleus but not in cells expressing GCLc with the mutated NLS motif. Overall, results indicated that GSH biosynthesis in the nucleus is associated with migration of only the GCLc subunit from the cytoplasm into the nucleus, and this migration requires the presence of an intact NLS.
The tripeptide, γ-glutamylcysteinylglycine or GSH, is the most abundant intracellular nonprotein thiol. It serves multiple physiological functions, including maintenance of redox homeostasis, providing reducing equivalents for the elimination of reactive oxygen species, protection against electrophilic xenobiotics, maintenance of protein structure, and storage/transport of l-cysteine. GSH is synthesized de novo by two ATP-dependent consecutive reactions: ligation of l-glutamate to l-cysteine by the activity of glutamate-cysteine ligase (GCL2; EC 6.3.2.2), a rate-limiting step in the pathway, followed by the coupling of glycine to γ-glutamylcysteine by glutathione synthase (EC 6.3.2.3). The GCL holoenzyme is heterodimeric, consisting of a catalytic (GCLc) and a modifier (GCLm) subunit, each encoded by a unique gene. The intracellular ratios of GLCc and GCLm are, however, not necessarily equimolar and may be altered under conditions of oxidative stress. Although GCLc by itself is fully competent to catalyze the biosynthesis of GSH (1), dimerization with GCLm lowers the Km for glutamate and also decreases sensitivity to feedback inhibition by GSH (2).
Overexpression of GCL subunits in cultured cells has been reported to increase GSH production and confer enhanced protection against oxidative stress (3), apoptosis (4), and oxidant-induced DNA lesions (5). We have shown that overexpressions of GCLc or GCLm boost GSH biosynthesis and extend life span in Drosophila melanogaster (6). In contrast, inhibition of GCL activity results in decreased GSH levels, enhanced susceptibility to oxidative or nitrosative stress, increased DNA damage, and cell cycle arrest (7, 8). The homozygous knock-out for the catalytic GCLc subunit is embryonic lethal (9).
GSH is predominantly synthesized in the cytoplasm, but its levels greatly vary among the different intracellular compartments, such as the nucleus, mitochondria, endoplasmic reticulum, and cytosol (10–12). Currently available evidence suggests the existence of two alternate mechanisms for the differential distribution of GSH in subcellular compartments: (i) GSH may first be synthesized in the cytoplasm and then transported into the organelles either actively or via passive diffusion; (ii) GSH may be synthesized in those organelles that display activities of GCL and glutathione synthase (13, 14).
It has been demonstrated that GSH levels in the nucleus of proliferating cells are much higher than those in the confluent cells (15); however, the mechanism underlying this variation is presently unclear. In the present study, we provide evidence that GSH is synthesized in the nucleus and that this synthesis is dependent upon the shuttling of the GCLc, but not the GCLm, subunit from the cytoplasm to the nucleus. The ability of GCLc to migrate into the nucleus is due to the presence of a nuclear localization signal (NLS).
EXPERIMENTAL PROCEDURES
Generation of Drosophila S2 Cells Overexpressing GCLc Proteins—A coding region of the GCLc gene was cloned into the pIB/V5-His vector (Invitrogen) in frame with the V5 epitope and His6 (Fig. 1A), and the construct was transfected into S2 cells as recommended (Invitrogen), followed by the selection of stable transfectants with blasticidin. To generate a construct with altered nuclear localization signal (NLS) sequence, a fragment of the pGCLc/V5-His construct was substituted with a mutated fragment as indicated in Fig. 1. The mutated fragment was generated by splicing by overlapping extension PCR, using primers containing mismatches (Fig. 1B). The first set of two different PCR fragments was generated using mutated primers and outer primers upstream of the BamHI site and downstream of the XbaI site. The resulting overlapping PCR fragments were annealed and extended during a second round of PCR. The PCR product was digested with BamHI and XbaI endonucleases and ligated with the pGCLc/V5-His construct that had been digested with the corresponding enzymes, thus introducing the desired mutation. The generated construct was subsequently transfected into S2 cells, as indicated above. A control cell line was generated by transfecting S2 cells with the empty pIB/V5-His vector. All cell cultures were maintained by transferring to fresh medium every 3–5 days at 1:3–1:5 dilution, retaining one-third of the conditioned medium.
FIGURE 1.
Generation and structure of pGCLc/V5-His and pGCLcNLS/V5-His constructs. A, structural representation of the GCLc overexpressor constructs. The thin lines represent vector sequence, the open bars represent V5 epitope and His6 domains, and the gray-filled bars represent the GCLc coding regions. The mutated NLS region is dark gray. The arrow indicates polarity of transcription. B, mutagenesis scheme. At the top is a part of the GCLc gene sequence with substituted nucleotides indicated above; the arrows indicate primers that were used for PCR-mutagenesis. After the first PCR introducing the desired base changes, the resulting fragments were annealed, and the product was further amplified. The substituted nucleotides are highlighted, and substituted amino acids are in boldface type.
Experimentally Induced Stress and Cell Viability—Cell viability was determined by staining with trypan blue (Invitrogen) and assessed as the percentage of live cells relative to the total number of cells. Overnight cell cultures that reached 1 × 106 cells/ml density were exposed to 20 mm hydrogen peroxide, 10 mm paraquat, 2 mm CuSO4, or 0.5 mm CdCl2 and incubated at room temperature for different durations. Chemical reagents were purchased from Sigma.
Subcellular Fractionation—For immunoblot analysis, proteins were isolated from the nuclei and cytoplasm using the NE-PER® nuclear and cytoplasmic extraction kit (Pierce). Subcellular fractionation was also performed using a sucrose gradient, as previously reported (16). For HPLC analysis, nuclei were separated from cytoplasm following the method of Hasbold et al. (17). Briefly, nuclei were prepared by lysing whole cells with Hanks' balanced salt buffer supplemented with bovine serum albumin and nonionic detergent Nonidet P-40, followed by centrifugation and washing in the same buffer. The integrity of the collected nuclear fraction and the presence of unbroken cells was monitored microscopically with DAPI staining (Molecular Probes). The purity of subcellular fractions was evaluated by immunoblot analysis, as described below.
Immunoblot Analysis—Protein concentrations were determined by Bio-Rad protein assay reagent, and 5–10 μg of the protein extracts were then resolved by 10% SDS-PAGE, followed by transfer to polyvinylidene difluoride membrane (Millipore). Immunoblots were probed with anti-V5-HRP antibodies (Invitrogen) or with antibodies raised against recombinant GCLc and GCLm proteins, as described (6), and visualized using the ECL®+ Western blotting detection system (Amersham Biosciences) according to the manufacturer's instructions. To control for loading and for purity of fractions, anti-actin (MP Biomedicals), anti-HDAC3 (BD Biosciences), or anti-histone 3 (Upstate) antibodies were used. We also used anti-Mn-superoxide dismutase (mitochondrial) and anti-CuZn-superoxide dismutase (cytosolic) antibodies raised against recombinant proteins. The intensity of signals was analyzed by densitometric scanning, using the digital imaging analysis system with AlphaEase Stand Alone Software (Alpha Innotech Corp., San Leandro, CA).
Measurement of GSH, GSSG, γ-Glutamylcysteine (γ-GC), and Cysteinylglycine (CG) by HPLC—Amounts of aminothiols (GSH, GSSG, γ-GC, and CG) were measured in cytoplasmic and nuclear fractions, prepared from ∼106 to 107 S2 cells. Pellets containing nuclei were resuspended in 50 μl of 5% (w/v) freshly prepared ice-cold meta-phosphoric acid (MPA). The cytoplasmic fractions were adjusted to a volume of 150 μl by adding 50 μl of 20% (w/v) MPA. Samples were incubated for 30 min on ice and centrifuged at 18,000 g for 20 min at 4 °C. Supernatants were filtered using 0.45 μm PTFE Acrodisc® CR 4-mm syringe filters, obtained from Gelman Laboratory (Ann Arbor, MI), and filtrates were transferred to sampling vials and either analyzed immediately or stored at -80 °C for up to 1 month.
The procedure for the detection and quantification of aminothiols (GSH, GSSG, γ-GC, and CG) and the precautionary measures taken to minimize spontaneous GSH oxidation were similar to those described previously (18, 19). Briefly, aminothiols were separated by HPLC, fitted with a Shimadzu Class VP solvent delivery system and a reverse phase C18 Luna (II) column (3μ; 4.6 × 150 mm), obtained from Phenomenex (Torrance, CA). The mobile phase for isocratic elution consisted of 25 mm monobasic sodium phosphate, a 0.120 mm concentration of the ion-pairing agent 1-octane sulfonic acid, 1% (v/v) acetonitrile, pH 2.75, adjusted with 85% phosphoric acid. The flow rate was 0.7 ml/min. Under these conditions, the separation was completed in 30 min; GSSG was the last eluting peak, with a retention time of ∼22 min. Calibration standards were prepared in 5% (w/v) MPA. Aminothiols were detected with a model 5600 CoulArray® electrochemical detector (ESA Inc., Chelmsford, MA), equipped with an eight-channel analytical cell, using potentials from +500 mV in 100-mV increments. GSH, γ-GC, and CG were monitored at +800 mV and GSSG at +900 mV. Each sample was injected twice, and the average of the peak areas was used for quantification.
Enzyme Activity—GCL enzyme activity was determined by measuring γ-GC concentrations by HPLC as described above or by the method of Seelig, as adapted by Fraser et al. (20).
Flow Cytometry—For flow cytometric analysis, 1 × 106 cells were plated and allowed to grow overnight, followed by exposure to different stressors for specific lengths of time. Cells were pelleted, washed twice with phosphate-buffered saline, and stained with fluorescent dyes. Cell cycle distribution was determined by Vybrant® DyeCycle™ Orange stain (Molecular Probes). Flow cytometric analysis was performed on the FACSCalibur apparatus (BD Biosciences), using FlowJo software. Samples were acquired for 10,000 individual cells.
Immunostaining and Fluorescence Microscopy—Prior to staining, cells were washed with phosphate-buffered saline and fixed with a mixture of 2% formaldehyde and 1% glutaraldehyde, followed by blocking with 10% rabbit nonspecific serum in phosphate-buffered saline. Immunostaining was performed with affinity-purified anti-GCLc antibodies and secondary anti-rabbit antibodies labeled with Cy3 (Molecular Probes). For visualization of intracellular glutathione, cells were stained with monochlorobimane, as described by Bellomo et al. (10). After washing, cells were counter-stained with DAPI (Molecular Probes) for visualization of nuclei. Images were acquired by fluorescence microscopy (Nikon), using different filters and MetaMorph software.
Statistical Analysis—All experiments were performed at least three times unless otherwise indicated. Results, presented as means ± S.D., were analyzed by GraphPad Prism 4.0a or Microsoft Excel software.
RESULTS
In Silico Analysis of Drosophila GCLc Protein—To identify sequence motifs indicative of intracellular localization and function, computer analysis of the deduced amino acid sequences of GCLc and GCLm was carried out, employing the PSORT and InterPro Scan programs (Fig. 2). The catalytic subunit of Drosophila GCL (GCLc) had a 74% probability of nuclear localization, whereas the modulatory subunit sequence exhibited no evidence for nuclear localization, showing a 52% probability of cytosolic localization. The amino acid sequence of the GCLc gene revealed a stretch of KRDACRKEKFWFRKSSK between residues 469 and 485, comprising two basic amino acids (KR), 10 spacer residues, and another basic region consisting of 3 basic residues of 5 (RKSSK), which is a characteristic of the “bipartite” NLS motif (21–24). Another type of putative NLS, composed of 4 basic amino acids (Lys or Arg) and called the “pat4” motif (25), was located at positions 196–199. The N-terminal part of the GCLc protein contained a nucleic acid-binding OB-fold domain (23), which is encountered in proteins involved in the regulation of gene expression, synthesis of proteins and RNA, and telomere binding. The GCLc amino acid sequence also contained alanine/glutamine dipeptide repeats, a region rich with Asn/Gly repeats and four Asn-Gly-Ser triplets, often present in various regulatory domains associated with nuclear proteins, such as transcription factors, DNA- and RNA-binding proteins, and DNA damage checkpoint kinases (26). In short, this analysis indicated that the GCLc subunit included motifs associated with nuclear localization and nuclear function.
FIGURE 2.
Deduced amino acid sequence analysis of GCLc. The OB-fold domain is underlined. The putative NLS sequences are highlighted, and their essential amino acids are indicated in boldface type. A potential transmembrane region (TMR) is indicated with a dark background and includes part of a possible cleavage site (CS; boxed). The poly(alanine-glutamine) motif, Asn/Gly repeats, and Asn-Gly-Ser triplets are underlined. The GCLc motif signature is underlined by solid bars. Amino acid residues that were identified as substrate binding determinants in the Trypanosoma brucei GCLc homologue (36) are in boldface type and enlarged. The numbers represent amino acid positions, where 1 represents the methionine specified by the putative start codon.
Intracellular Localization of GCLc, GCLm, and Glutathione—Subcellular localization of GCLc and GCLm subunits was determined by immunostaining in a 24-h-old culture of Schneider S2 cells, using antibodies against recombinant GCLc and GCLm proteins. Microscopy of immunostained cells indicated that the catalytic subunit was preferentially localized in the perinuclear area (Fig. 3A), whereas the modulatory subunit was present throughout the cytoplasm, with no marked perinuclear distribution (Fig. 3B). The subcellular localization of GCL subunits was further investigated by immunoblot analysis of the subcellular fractions isolated from whole flies (10-day-old adult males). Consistent with the cell culture studies, GCLc protein was detected in both the 100 S cytosolic and nuclear fractions, whereas GCLm protein was largely restricted to the cytosolic fraction (Fig. 4). No significant amounts of GCLc or GCLm proteins were found in other organelle fractions. Hence, GCLc was inferred to reside in both nuclear and cytosolic fractions, whereas GCLm remained within the cytosol.
FIGURE 3.
Intracellular localization of GCLc (A) and GCLm (B) by fluorescence microscopy analysis. Shown in the left panels of A and B is immunostaining of S2 cells, performed with anti-GCLc and anti-GCLm antibodies. Staining with DAPI is shown in the middle, and overlays are shown on the right.
FIGURE 4.
Immunoblot analysis of subcellular fractions isolated from whole flies. After homogenization and lysis in a 0.25 m sucrose solution, organelles were separated by differential centrifugation. Nuclei (Nucl) were pelleted at ∼1000 × g, followed by precipitation of mitochondria (Mit) at ∼3000 × g. The cytosolic (Cyt) fraction was cleared from lysosomes, peroxisomes, and other organelles and membranes (Org + Memb) at 100,000 × g. The cytosol and protein lysates obtained from organelle fractions were adjusted to the same protein concentrations and loaded on a gel in ∼5-μg aliquots. Signals for GCLc and GCLm were obtained with antibodies developed to recombinant proteins. To control for purity of subcellular fractions, antibodies that recognize nuclear (histone 3; H3), mitochondrial (MnSOD), or cytosolic (CuZnSOD) proteins were used.
To investigate the distribution of intracellular GSH, cells were fixed and incubated with 80 μm monochlorobimane and counterstained with DAPI, as described under “Experimental Procedures.” GSH distribution in the monochlorobimanestained S2 cells was found to be uneven; cytoplasm stained more brightly than the nucleus (Fig. 6C), and this fluorescence was particularly strong in the perinuclear cytoplasm. Thus, the spatial pattern of GCLc localization was similar to that of GSH, a product of its activity.
FIGURE 6.
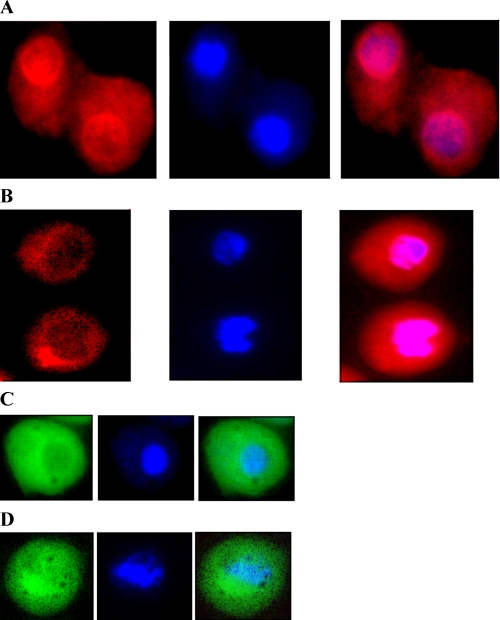
Intracellular localization of GCLc/V5-His (A), mutated GCLcNLS/ V5-His (B) proteins and glutathione (C and D) after exposure to cadmium determined by fluorescence microscopy. Cells were cultured for 5 h in the presence of 0.5 mm CdCI2 (A, B, and D) or without CdCl2 (C). In the left-hand panels is shown immunostaining of S2 cells, performed with anti-GCLc antibodies (A and B) or monochlorobimane (C and D). Staining with DAPI is shown in the middle, and overlays are on the right.
Generation of S2 Cells Overexpressing GCLc/V5-His and GCLcNLS/V5-His Proteins—The presence of GCLc in both the nucleus and the cytoplasm, together with the existence of an NLS motif in its coding domain, suggested that GCLc might be a nucleocytoplasmic shuttling protein. To investigate whether GCLc translocates from cytoplasm to the nucleus and whether the predicted NLS bipartite sequence is responsible for such migration, we generated transfectant cell lines, expressing either normal GCLc protein or a GCLc protein in which the NLS motif was mutated (as described under “Experimental Procedures”). As depicted in Fig. 1B, the NLS sequence was modified by the replacement of arginine with asparagine (Arg → Asn) and lysine with glutamine (Lys → Gln). The basic residues were replaced with hydrophilic and neutral residues in order to ensure that the mutated region had a hydropathy profile similar to that of the wild-type GCLc protein. Computer analysis, using the Kyte and Doolittle algorithm, confirmed that this had been achieved.
Both of the recombinant constructs, designated as GCLc/V5-His (wild type) and GCLcNLS/V5-His (mutant), contained V5-His tags for distinguishing their products from the endogenous ones, either on the basis of molecular size (Fig. 5A) or by the use of anti-tag (V5) antibodies (Fig. 5, B–D). In both cell lines, levels of recombinant proteins were found to be ∼4–10-fold higher than those of the endogenous GCLc (Fig. 5A), and the enzyme activity increased significantly (∼20%; Fig. 7, G and H). Notably, there were no differences in GCL enzyme activity in cells producing normal GCLc/V5-His and mutated GCLcNLS/V5-His protein, suggesting that disruption of the NLS signal has no effect on GCLc catalytic activity.
FIGURE 5.
Immunoblot analysis of GCLc transport to nucleus in response to cadmium and proliferation. A, analysis of protein preparations made from S2 cells transfected with pIB/V5-His vector only (V) and S2 cells expressing either GCLc/V5-His (GCLc) or mutated GCLcNLS/V5-His (NLS) recombinant proteins. Antibodies developed to Drosophila GCLc recognize both the endogenous (GCLc) and the recombinant proteins (GCLc/V5-His). Anti-actin antibodies were used to control for loading. B, immunoblot analysis of cytoplasmic (Cyt) and nuclear (Nucl) fractions obtained from S2 cells expressing recombinant GCLc/V5-His protein. Cells were untreated or treated for 8 h with 0.5 mm CdCl2, 6 h with 20 mm H2O2, or 24 h with 10 mm CuSO4. After harvesting, cells were fractionated, and 5-μg aliquots of cytoplasmic and nuclear protein extracts were loaded on a gel and developed with anti-V5 and anti-GCLm antibodies. The purity of the nuclear fractions was monitored by DAPI staining. C and D, analysis of nuclear and cytoplasmic fractions isolated from S2 cell transfectants expressing GCLc/V5-His (C) and mutated GCLcNLS/V5-His (D) proteins. Cells were cultured under normal conditions after a 1:10 dilution with fresh medium (right) or in the presence of 0.5 mm cadmium (left). Samples were taken at the indicated time intervals, and nuclei were isolated. 5 μg of the protein lysates were loaded, and immunoblot analysis with anti-V5 antibodies was conducted. Anti-GCLm (cytosolic) and anti-histone 3 (nuclear) antibodies were used to control for loading and purity.
FIGURE 7.
HPLC analysis of GSH, GSSG, and γ-GC in S2 cells overexpressing GCLc/V5-His (GCLc) and mutated GCLcNLS/V5-His (NLS) proteins. V, vector-only-transfected cells. 24 h fresh cell cultures with cell densities of ∼106 cells/ml were cultured in the absence or presence of 0.5 mm CdCl2 for 5 h, followed by separation of nuclei from cytoplasm. HPLC analysis was then used to determine GSH levels (A and B), GSSG levels (C and D), GSH/GSSG ratios (E and F), and γ-GC levels (G and H). The results are mean ± S.D. from three independent experiments. The statistically significant differences (p < 0.05) with respect to control vector-only-transfected cells are indicated by asterisks.
Nucleocytoplasmic Shuttling of GCLc—To understand the mechanisms underlying entry of GCLc into the nucleus, we investigated the effect of various treatments that could potentially perturb the cellular redox state on the subcellular localization of GCLc. Cells producing GCLc/V5-His were cultured in the presence of oxidants, such as hydrogen peroxide and paraquat, as well as the heavy metals, copper and cadmium, which can also increase oxidative stress, albeit by different mechanisms. 24-h-old cell cultures were exposed to these stressors for durations sufficient to cause 50% cell death, after which the cells were collected. Immunoblot analysis of cytoplasmic and nuclear fractions showed that in untreated cells and in cells exposed to copper, H2O2 (Fig. 5B), or paraquat (data not shown), GCLc was predominantly located in the cytoplasm. In contrast, in cadmium-treated cells, significant amounts of GCLc were detected in the nucleus (Figs. 5 (B and C) and 6A). It is worth pointing out that cadmium treatment is known to cause GSH depletion in the cells (27, 28). In cells expressing the recombinant GCLcNLS/V5-His protein, which lacks the nuclear localization signal, the amount of GCLc in the nucleus in response to cadmium treatment is considerably diminished (Fig. 5D, left), and the mutated GCLc remained largely in the cytoplasmic fraction. Fluorescence microscopy also indicated that compared with the wild type, the mutated GCLc aggregated predominantly in the perinuclear region, with a substantially lesser amount in the nucleus (Fig. 6B).
GSH concentration in the nucleus has been reported to be considerably higher in proliferating cells compared with those in quiescence (15). To examine proliferation-associated GCLc migration from the cytoplasm into the nucleus, cell cultures containing GCLc/V5-His or GCLcNLS/V5-His were grown to stationary (∼107 cells/ml), diluted 1:10 with fresh culture medium, and sampled at various time intervals. The levels of GCLc per cell remained relatively constant during the growth phase. In cells expressing the wild type GCLc/V5-His, the nuclear GCLc content increased with time, peaking at 5 h post-dilution, and dropped off thereafter (Fig. 5C, right). In contrast, in cells containing the mutated form, there was no accumulation of mutated GCLc in the nucleus (Fig. 5D, right). Overall, the results of these experiments showed that GCLc shuttles into the nucleus during cell proliferation or in response to cadmium treatment and that the bipartite NLS motif is essential for the movement of GCLc from the cytoplasm into the nucleus.
Effects of GCLc and GCLcNLS Expression on GSH Content and Redox State—The purpose of this experiment was to determine the effects of GCLc translocation to the nucleus on glutathione metabolism. A comparison of the amounts of γ-GC, a product of GCLc activity, GSH, GSSG, and CG, a breakdown product of GSH, was made between cell lines expressing mutated and wild type GCLc recombinant proteins and a vector-only-transfected cell line. Stationary cells were cultured for 4–5 h with or without cadmium, harvested, and separated into nuclear and cytoplasmic fractions. It should be noted that accumulation of wild type GCLc in the nucleus is maximal in response to cadmium treatment at 4–5 h (Fig. 5C). In each cell line, the amounts of GSH were 80–100-fold greater in the cytoplasmic than the nuclear fraction. GSH and GSSG levels were higher in the wild type GCLc-transfected cell line than in the mutated GCLc-transfected or vector-only cell lines. The GSH/GSSG ratio was also significantly higher in the nucleus of GCLc expressor versus the mutant and the vector only control, whereas the GSH/GSSG ratio increased in the cytoplasm of the mutant cell line.
Compared with the vector-only lines, expression of both mutated and wild type GCLc resulted in a small (10–20%) marginally significant increases in the level of γ-GC in the cytoplasm (Fig. 7G). Cadmium treatment caused a decrease in GSH/GSSG ratio in the cytoplasm of cells expressing the mutated GCLc, whereas in the nucleus, the largest decrease was in the GCLc/V5-His-expressing cells, which also had relatively higher GSH content. No differences in concentrations of CG, a breakdown product of GSH, were observed between the different cell lines (data not shown). Thus, it seems that GCLc transport into the nucleus results in an increase in GSH level as well as a proreducing shift in the glutathione redox state.
Exposure to cadmium resulted, as anticipated, in the depletion of GSH in the nucleus as well as cytoplasm (Fig. 7, A and B), but the levels of γ-GC in all cell lines were enhanced (Fig. 7, G and H), presumably through an up-regulation of GCL activity. Nuclear GSH content was not affected in cells expressing mutated GCLcNLS, and γ-GC levels were elevated (Fig. 7, F and H), suggesting that although GCLcNLS is not capable of being transported into the nucleus, its perinuclear location may facilitate the shuttling of γ-GC into the nucleus to help maintain GSH levels. On the other hand, the results also support the notion that GCLc transport to the nucleus could be driven by GSH deficiency and/or changes in the redox state of the nucleus. A notable effect of cadmium was that the amount of γ-GC significantly increased in both the cytoplasm and the nucleus in all three cell lines, suggesting that GCL activity is stimulated by cadmium. The increase in γ-GC concentration in the nucleus after cadmium treatment accords with the observed migration of GCLc into the nucleus, further strengthening the view that GCLc migration enhances the increase in nuclear GSH levels in response to cadmium exposure.
GCLc Localization and Cell Cycle—The objective of this experiment was to establish the relationship between intracellular GCLc distribution and cell proliferation. Cells were grown to stationary phase and diluted with fresh culture medium with or without stressors, such as cadmium, paraquat, and H2O2 (29), for 8 or 24 h or until 50% cell death had occurred in the control cultures. Subsequently, cells were subjected to fluorescence-activated cell sorting (flow cytometry) analysis. Following cadmium exposure, a relatively greater proportion of GCLcNLS-expressing cells were found to be in the S phase, compared with their GCLc-expressing counterparts. Exposure to H2O2 resulted in relatively greater arrest of GCLcNLS-expressing cells at the G2/M phase. Under normal conditions, GCLc overexpression had no significant impact on cell cycle progression, whereas GCLcNLS-expressing cells were characterized by a modest but reproducible increase in the G2/M population in the 8 h culture (Fig. 8A). Upon exposure to cadmium, cells expressing wild type GCLc had a relatively smaller proportion of cells in S phase, whereas cells overexpressing the GCLcNLS had a greater fraction in the S phase (Fig. 8B). Upon exposure to H2O2, cells expressing the mutated GCLcNLS had a tendency to become arrested at the G2/M phase (Fig. 8C).
FIGURE 8.
Cell cycle analysis of S2 cell cultures expressing GCLc/V5-His and mutated GCLcNLS/V5-His proteins. Cell cultures in a stationary stage were diluted with a fresh medium to a density of ∼0.5 × 106 cells/ml and allowed to grow for 24 h. Cells were then cultured in the presence (B, C, and E) or absence (A and D) of chemicals, and cell cycle distribution was determined by flow cytometry. Cells were exposed to 0.5 mm CdCI2 (B) or 20 mm H2O2 (C) for 8 h or 10 mm paraquat (E) for 24 h. Cell cycle analysis was performed with DyeCycle Orange (Invitrogen). Shown are representative histogram profiles for the gated populations of live cells. V, vector-only-transfected cell line; GCLc, cell line expressing normal recombinant GCLc/V5; NLS, cell line expressing GCLcNLS/V5 with a modified NLS motif. The proportion of cells in G1, S, and G2/M phases are shown in the tables beside each histogram. Values are average ± S.D. from three independent experiments.
In 24 h cultures, all cell lines had relatively larger proportions of cells in the S phase, compared with 8 h cultures (Fig. 8D). Paraquat exposure caused an increase in the proportion of cells arrested at G2/M (Fig. 8E). This effect was partially alleviated by the expression of normal GCLc but further exacerbated in cells expressing mutated GCLcNLS (Fig. 8E). These results clearly indicate that exposure to oxidant stressors results in increased cell cycle arrest in G2/M phase; GCLc and its ability to translocate to the nucleus can partially rescue this effect.
DISCUSSION
Results of this study demonstrate for the first time that the catalytic subunit of glutamate cysteine ligase migrates into the nucleus in proliferating cells as well as in response to stressors, specifically cadmium. An in silico analysis of the Drosophila GCLc protein indicated several attributes of nucleus-localized proteins: two NLS motifs, “bipartite” and “pat4,” an OB-fold domain, Asn/Gly dipeptide repeats, and Asn/Gly/Ser triplets (26). The classic SV40 type “bipartite” sequence (22) is associated with nucleocytoplasmic shuttling proteins, with a molecular mass exceeding the size exclusion limit of the nuclear pore (50 kDa). Unlike smaller proteins (<50 kDa), that can diffuse passively into the nucleus, larger proteins are transported by carrier proteins bound to a series of basic residues, the NLS sequence (23). Apparently, such a mechanism would be required for GCLc, with a molecular mass of 73 kDa, to be moved into the nucleus. This predicted nuclear localization of GCLc protein is not unique for Drosophila. Indeed, in silico nuclear localization signatures have been obtained for Saccharomyces and Neurospora GCLc subunits, which have a classical “bipartite” nuclear localization signal. A similar motif is present in mammalian orthologs, although the absence of a conserved basic amino acid would argue against its function as a possible nuclear localization signal (Fig. 9).
FIGURE 9.
A partial alignment of the amino acid sequences of GCLc proteins. Sequences that are homologous to the Drosophila nuclear localization sequence region are boxed. Basic essential amino acids are indicated in bold-face type.
Marcovic et al. (15) have shown that nuclear GSH levels are elevated during active cell division, ostensibly to provide the reducing environment necessary to prevent DNA damage and the consequent cell cycle arrest, thereby promoting normal proliferation. The present study provides a mechanistic explanation for the increase in the GSH level during cell proliferation, by demonstrating that the shuttling of GCLc into the nucleus is linked to the elevation of GSH level. In the present study, GCLc was detectable in both the nuclear and the cytoplasmic fractions (Fig. 4); however, when cultures were diluted into fresh medium, which provides stimulation for active cell proliferation, there was a significant influx of GCLc into the nuclear compartment and an increase in GSH levels (Figs. 5C, 6 (A and D), and 7B). Importantly, in mutant cells, there was no increase in nuclear GCLc or GSH levels compared with the vector-only cells, thereby suggesting that GCLc shuttling may be responsible for enhanced GSH levels in the nucleus of proliferating cells. An important, relevant question is why GCLc but not GCLm migrates into the nucleus. GCLc by itself possesses catalytic activity, although dimerization with GCLm lowers the Km for its substrates. It is plausible that the shuttling of GCLc into the nucleus provides the means to increase the level of GSH in the nucleus but to maintain it at a level that is much lower than in the cytoplasm. Nevertheless, the possibility that the increase in the intranuclear glutathione content could also be due, at least partially, to a passive diffusion from the cytoplasm cannot be ruled out on the basis of the present results.
The presence of GCLc in other cellular compartments cannot yet be ruled out, although in co-localization studies using mitotracker, no evidence for the presence of GCLc in mitochondria was observed (data not shown). In adult flies, GCLc was readily detectable in both the nuclear and cytoplasmic fractions derived from whole body (Fig. 4). We also studied the compartmentalization of GCLc protein in the head and found that GCLc was largely associated with the cytoplasmic fraction; in contrast, in the abdomen, a greater proportion of GCLc was associated with the nuclear fraction (data not shown). The head of a fly consists of mostly postmitotic cells, whereas the abdomen contains a significant proportion of replicating cells associated with the gut and the reproductive system. It is thus plausible that the relatively abundant amount of GCLc in the nuclear fraction in the abdomen is due to the higher proliferative activity associated with these tissues, a contention supported by the present results on proliferating S2 cells.
Treatment of S2 cells with cadmium, but not copper, H2O2, or paraquat, was observed to induce the entry of GCLc into the nucleus (Fig. 5, B and C). The mechanism of cadmium toxicity is presently not completely known. Reported effects of cadmium include inhibition of cellular respiration and oxidative phosphorylation, presumably through binding to mitochondrial proteins; increased oxidative stress due to inhibition of the activity of antioxidant enzymes, such as catalase and superoxide dismutases, enhancement of lipid peroxide production, and depletion of GSH by binding to sulfhydryl groups (27, 28); modulation of thiol redox balance in cells via alterations in the expression of enzymes of the thioredoxin and glutathione systems (30); and inhibition of cell cycle progression through its ability to repress cell cycle regulating proteins (31, 32). It is unclear which of these cadmium effects alters GCLc subcellular distribution. One possibility is that GCLc is recruited to the nucleus in response to reductions in GSH levels or GSH/GSSG ratios, caused by the chelation of GSH to cadmium (Fig. 7, B and F). In the transfected GCLc-expressing cells exposed to cadmium, depletion of GSH was found to be associated with increased levels of nuclear GCLc and γ-GC content, an intermediate in the GSH biosynthesis pathway. This set of events suggests that the entry of GCLc into the nucleus serves the purpose of enhanced GSH synthesis. One caveat of these HPLC studies is the relative purity of the fractions used, in particular due to the possible leakage of small molecular weight compounds from the nuclei. Nevertheless, equivalent results were obtained using fluorescence microscopy analysis of cadmium-treated cells (Fig. 6, C and D), strongly supporting the inferences drawn from our HPLC work.
Bidirectional trafficking of macromolecules between the nucleus and cytoplasm through the nuclear pore complex lies at the core of many fundamental cellular processes, such as signaling, response to stress, differentiation, and proliferation (21, 33–36). To understand the functional impact of GCLc shuttling, our strategy was to manipulate nuclear entry, initially inferred on the basis of in silico analysis. The essential basic residues in the bipartite nuclear localization signal were replaced, resulting in a (GCLcNLS) that could no longer gain access into the nuclear compartment, although it still showed the perinuclear localization, characteristic of the wild type form. This approach allowed us to confirm the significance of the NLS sequence in facilitating nuclear entry. However, gaining insights into the functional significance of nuclear shuttling was complicated by the fact that the S2 cells contain an endogenous gene encoding normal GCLc. Nevertheless, the inability of the transfected recombinant GCLcNLS to enter the nucleus resulted in a decrease in its capacity to mitigate cell cycle disruption, compared with the recombinant wild type. To avoid the complicating effects of the endogenous GCLc, we are currently generating P element transgenics containing wild type and mutant GCLc, which will be tested in a null background.
Acknowledgments
We are grateful to Drs. Federico Pallardó and Jose Vin a (University of Valencia, Spain) for a critical reading of the manuscript and to Sharon Waldrop (University of Texas Southwestern Medical Center) for technical assistance with flow cytometry.
This work was supported, in whole or in part, by National Institutes of Health, Grant RO1-AG15122 from NIA. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: GCL, glutamate-cysteine ligase; GCLc, glutamate-cysteine ligase catalytic subunit; GCLm, glutamate-cysteine ligase modulatory subunit; NLS, nuclear localization signal; HPLC, high performance liquid chromatography; γ-GC, γ-glutamylcysteine; CG, cysteinylglycine; DAPI, 4′,6-diamidino-2-phenylindole; MPA, meta-phosphoric acid.
References
- 1.Fraser, J. A., Kansagra, P., Kotecki, C., Saunders, R. D., and McLellan, L. I. (2003) J. Biol. Chem. 278 46369-46377 [DOI] [PubMed] [Google Scholar]
- 2.Huang, C. S., Anderson, M. E., and Meister, A. (1993) J. Biol. Chem. 268 20578-20583 [PubMed] [Google Scholar]
- 3.Das, G. C., Bacsi, A., Shrivastav, M., Hazra, T. K., and Boldogh, I. (2006) Mol. Carcinog. 45 635-647 [DOI] [PubMed] [Google Scholar]
- 4.Manna, S. K., Kuo, M. T., and Aggarwal, B. B. (1999) Oncogene 18 4371-4382 [DOI] [PubMed] [Google Scholar]
- 5.Shi, S., Hudson, F. N., Botta, D., McGrath, M. B., White, C. C., Neff-LaFord, H. D., Dabrowski, M. J., Singh, N. P., and Kavanagh, T. J. (2007) Cytometry A 71 686-692 [DOI] [PubMed] [Google Scholar]
- 6.Orr, W. C., Radyuk, S. N., Prabhudesai, L., Toroser, D., Benes, J. J., Luchak, J. M., Mockett, R. J., Rebrin, I., Hubbard, J. G., and Sohal, R. S. (2005) J. Biol. Chem. 280 37331-37338 [DOI] [PubMed] [Google Scholar]
- 7.Lu, Q., Jourd'Heuil, F. L., and Jourd'Heuil, D. (2007) J. Cell. Physiol. 212 827-839 [DOI] [PubMed] [Google Scholar]
- 8.Zegura, B., Lah, T. T., and Filipic, M. (2006) Mutat. Res. 611 25-33 [DOI] [PubMed] [Google Scholar]
- 9.Dalton, T. P., Dieter, M. Z., Yang, Y., Shertzer, H. G., and Nebert, D. W. (2000) Biochem. Biophys. Res. Commun. 279 324-329 [DOI] [PubMed] [Google Scholar]
- 10.Bellomo, G., Vairetti, M., Stivala, L., Mirabelli, F., Richelmi, P., and Orrenius, S. (1992) Proc. Natl. Acad. Sci. U. S. A 89 4412-4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, Z., and Lash, L. H. (1998) J. Pharmacol. Exp. Ther. 285 608-618 [PubMed] [Google Scholar]
- 12.Dixon, B. M., Heath, S. H., Kim, R., Suh, J. H., and Hagen, T. M. (2008) Antioxid. Redox Signal. 10 963-972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho, Y. F., and Guenthner, T. M. (1994) Toxicologist 14 178 [Google Scholar]
- 14.Lash, L. H. (1993) Pharmacologist 35 148 [Google Scholar]
- 15.Markovic, J., Borras, C., Ortega, A., Sastre, J., Vina, J., and Pallardo, F. V. (2007) J. Biol. Chem. 282 20416-20424 [DOI] [PubMed] [Google Scholar]
- 16.Radyuk, S. N., Klichko, V. I., Spinola, B., Sohal, R. S., and Orr, W. C. (2001) Free Radical Biol. Med. 31 1090-1100 [DOI] [PubMed] [Google Scholar]
- 17.Hasbold, J., and Hodgkin, P. D. (2000) Cytometry 40 230-237 [DOI] [PubMed] [Google Scholar]
- 18.Rebrin, I., Bayne, A. C., Mockett, R. J., Orr, W. C., and Sohal, R. S. (2004) Biochem. J. 382 131-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rebrin, I., Kamzalov, S., and Sohal, R. S. (2003) Free Radical Biol. Med. 35 626-635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraser, J. A., Saunders, R. D., and McLellan, L. I. (2002) J. Biol. Chem. 277 1158-1165 [DOI] [PubMed] [Google Scholar]
- 21.Cyert, M. S. (2001) J. Biol. Chem. 276 20805-20808 [DOI] [PubMed] [Google Scholar]
- 22.Gorlich, D., and Mattaj, I. W. (1996) Science 271 1513-1518 [DOI] [PubMed] [Google Scholar]
- 23.Nigg, E. A. (1997) Nature 386 779-787 [DOI] [PubMed] [Google Scholar]
- 24.Robbins, J., Dilworth, S. M., Laskey, R. A., and Dingwall, C. (1991) Cell 64 615-623 [DOI] [PubMed] [Google Scholar]
- 25.Theobald, D. L., Mitton-Fry, R. M., and Wuttke, D. S. (2003) Annu. Rev. Biophys. Biomol. Struct. 32 115-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katti, M. V., Sami-Subbu, R., Ranjekar, P. K., and Gupta, V. S. (2000) Protein Sci. 9 1203-1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stohs, S. J., and Bagchi, D. (1995) Free Radical Biol. Med. 18 321-336 [DOI] [PubMed] [Google Scholar]
- 28.Patrick, L. (2003) Altern. Med. Rev. 8 106-128 [PubMed] [Google Scholar]
- 29.Rizzino, A., and Blumenthal, A. B. (1978) In Vitro 14 437-442 [DOI] [PubMed] [Google Scholar]
- 30.Vido, K., Spector, D., Lagniel, G., Lopez, S., Toledano, M. B., and Labarre, J. (2001) J. Biol. Chem. 276 8469-8474 [DOI] [PubMed] [Google Scholar]
- 31.Yang, P. M., Chiu, S. J., Lin, K. A., and Lin, L. Y. (2004) Chem. Biol. Interact. 149 125-136 [DOI] [PubMed] [Google Scholar]
- 32.Zhou, T., Jia, X., Chapin, R. E., Maronpot, R. R., Harris, M. W., Liu, J., Waalkes, M. P., and Eddy, E. M. (2004) Toxicol. Lett. 154 191-200 [DOI] [PubMed] [Google Scholar]
- 33.Daniel, S., Bradley, G., Longshaw, V. M., Soti, C., Csermely, P., and Blatch, G. L. (2008) Biochim. Biophys. Acta 1783 1003-1014 [DOI] [PubMed] [Google Scholar]
- 34.Engel, R., Valkov, N. I., Gump, J. L., Hazlehurst, L., Dalton, W. S., and Sullivan, D. M. (2004) Exp. Cell Res. 295 421-431 [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez, J. A., Schuchner, S., Au, W. W., Fabbro, M., and Henderson, B. R. (2004) Oncogene 23 1809-1820 [DOI] [PubMed] [Google Scholar]
- 36.Saydam, N., Georgiev, O., Nakano, M. Y., Greber, U. F., and Schaffner, W. (2001) J. Biol. Chem. 276 25487-25495 [DOI] [PubMed] [Google Scholar]